Abstract
Introduction
Manual determination of insulin dosing largely fails to optimise glucose control in type 1 diabetes. Automated insulin delivery via closed-loop systems has improved glucose control in short-term studies. The objective of the present study is to determine the effectiveness of 6 months’ closed-loop compared with manually determined insulin dosing on time-in-target glucose range in adults with type 1 diabetes.
Methods and analysis
This open-label, seven-centre, randomised controlled parallel group clinical trial will compare home-based hybrid closed-loop versus standard diabetes therapy in Australia. Adults aged ≥25 years with type 1 diabetes using intensive insulin therapy (via multiple daily injections or insulin pump, total enrolment target n=120) will undertake a run-in period including diabetes and carbohydrate-counting education, clinical optimisation and baseline data collection. Participants will then be randomised 1:1 either to 26 weeks of MiniMed 670G hybrid closed-loop system therapy (Medtronic, Northridge, CA, USA) or continuation of their current diabetes therapy. The hybrid closed-loop system delivers insulin automatically to address basal requirements and correct to target glucose level, while bolus doses for meals require user initiation and carbohydrate estimation. Analysis will be intention to treat, with the primary outcome time in continuous glucose monitoring (CGM) target range (3.9–10.0 mmol/L) during the final 3 weeks of intervention. Secondary outcomes include: other CGM parameters, HbA1c, severe hypoglycaemia, psychosocial well-being, sleep, cognition, electrocardiography, costs, quality of life, biomarkers of vascular health and hybrid closed-loop system performance. Semistructured interviews will assess the expectations and experiences of a subgroup of hybrid closed-loop users.
Ethics and dissemination
The study has Human Research Ethics Committee approval. The study will be conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. Results will be disseminated at scientific conferences and via peer-reviewed publications.
Trial registration number
ACTRN12617000520336; Pre-results.
Keywords: type 1 diabetes, closed loop, adult
Strengths and limitations of this study.
Multicentre, randomised controlled parallel group trial of 26 weeks home-based hybrid closed-loop versus standard therapy.
Broad outcomes will be assessed in addition to glucose control: psychosocial, sleep, cognition, ECG, vascular health biomarkers and health economic measures.
The standard therapy comparator—multiple daily insulin injections or insulin pump therapy, without real-time continuous glucose monitoring—reflects current practice in Australia for most adults with type 1 diabetes, though this may not reflect standard care in other countries.
The study emphasises education and clinical optimisation for all participants prerandomisation, and the visit schedule is identical for both groups (by design, continuous glucose monitoring information is only available to the closed-loop group).
This study of adults aged ≥25 years has glucose end-points aligned with a concurrent study examining hybrid closed loop for young people aged 12 to <25 years, thereby facilitating comparison of metabolic outcomes between the two populations.
Introduction
Advances in type 1 diabetes insulin regimens and glucose monitoring have occurred over recent decades, facilitating improved glucose control and resulting in better health and quality of life.1–4 The long-term vascular complications of type 1 diabetes are reduced by intensive insulin therapy compared with less intensive therapy.1 2 Consequently, intensive insulin therapy—with subcutaneous administration via either multiple daily injections (MDI) or insulin pump therapy (IPT)—is a core strategy in current type 1 diabetes management.5 Nevertheless, even with modern therapies, only 20%–30% of adults with type 1 diabetes achieve HbA1c targets,6 7 and long-term vascular complications and reduced life expectancy continue to be a reality for people with type 1 diabetes.8 9
Insulin requirements can vary unpredictably. They are impacted by time of day, meals, exercise, illness and antecedent hypoglycaemia. Manual determination of insulin dosing by people with type 1 diabetes requires continuous vigilance to maintain glucose levels within a healthy range. Insulin dosing decisions carry cognitive and emotional burden, and may be inconsistent due to fatigue, distress, fluctuating glucose levels or coexistent fear of hypoglycaemia. Hence, manual determination of insulin dosing represents an imperfect strategy to optimise glucose control. Further advances in technology are required to improve the match of insulin delivered to individuals’ varying insulin requirements, and to minimise the burden of type 1 diabetes.
Closed-loop systems are designed to maintain glucose levels at a predetermined target by linking continuous glucose monitoring (CGM) information with an insulin dosing algorithm for automated subcutaneous insulin delivery by a pump.10 These systems are being developed to address the need for improving glucose control while reducing the burden associated with treatment regimens. There is increasing scientific literature of randomised controlled studies reporting improved glucose control with short-term use of closed-loop systems (up to 3 months) compared with conventional insulin pumps.11–15 A recent meta-analysis of outpatient randomised controlled trials with intervention periods ranging from 4 days to 12 weeks reported that single-hormone (insulin alone) closed-loop systems improve time-in-target glucose range and reduce time spent in hypoglycaemia compared with conventional IPT (with/without CGM).16 Overall, time-in-target glucose range had a mean (95% CI) absolute increase of 11.1% (6.9, 15.2), and the time spent in hypoglycaemia had an absolute reduction of 1.9% (0.4, 3.4). Studies in this meta-analysis used ‘hybrid closed-loop’ systems with automated insulin delivery to address basal requirements and correct to target glucose, and user-initiated bolus insulin to address carbohydrate consumption. Results from a short-term randomised crossover study challenging a closed-loop system with both moderate-intensity and high-intensity exercise indicated that closed-loop glucose control was safe; only a single episode of mild hypoglycaemia occurred and marked hyperglycaemic excursions were limited.17 In an uncontrolled study, there were no safety concerns when 14 participants used free-living closed-loop 24/7 for 6 months.18
For individuals with type 1 diabetes, both hypoglycaemia and hyperglycaemia can affect physical and emotional well-being, quality of life and activities of daily living such as driving.4 19–21 Moreover, type 1 diabetes places significant burden on caregivers, families, workplaces and health services.22–24 Closed-loop technology has shown promise to address the limitations of current therapy in relation to these burdens.25 Qualitative and small-scale quantitative substudies in closed-loop trials have shown user acceptability and treatment satisfaction are high with closed-loop systems in home settings, particularly for overnight use when there is minimal manual interaction for meals and activity.26–28 Although intrusive device alerts, device size and technical difficulties can negatively affect the overall experience, users typically report benefits outweighing annoyances, which they anticipate will be overcome with future iterations of the technology.27–29 However, the only published randomised closed-loop trial involving adults to have included established, validated psychological measures reported no between-group differences in treatment satisfaction or fear of hypoglycaemia.30
HbA1c, a measurement of average glycaemia during the preceding 10–12 weeks, predicts the risk of developing long-term complications and is valuable for assessing glycaemic trends in populations over time.1 2 31 However, HbA1c cannot provide information about glucose variability or time-in-target glucose range, and is even considered an unreliable indicator of an individual’s mean glucose.32 A recent large longitudinal registry study reported lower cardiovascular and all-cause mortality in individuals using IPT compared with MDI, even without between-group differences in HbA1c.33 The mortality difference observed may have been attributable to factors such as time-in-target glucose range or glucose variability (not reflected in HbA1c). Consequently, HbA1c may be of limited value in comparison with CGM when assessing an individual’s glucose levels in response to automated closed-loop insulin delivery.
With short-term randomised controlled studies of closed-loop systems (conducted in camp/hotel and home settings) demonstrating improvements in glucose control,16 it remains to be determined whether these findings are sustained in the longer term in the home setting and whether diabetes-related vascular complications may be influenced. Longer term randomised controlled home-based studies—with closed loop implemented day and night—are required. In addition, the impact of closed-loop insulin delivery on patient-reported outcomes such as fear of hypoglycaemia, treatment satisfaction, sleep quality and cognition remains a significant gap in the evidence base.34 Finally, the benefits associated with this new technology need to be balanced against its cost.
In Australia, the government presently subsidises the purchase of insulin, injection needles, blood glucose monitoring strips and insulin pump delivery consumables for people with type 1 diabetes.35 Insulin pumps are not government subsidised, but are available via either direct purchase or in conjunction with a private health insurance fund. CGM is government subsidised only for eligible individuals under 21 years of age.36 As a result, only a small fraction of adults with type 1 diabetes use CGM on a regular basis. Hence, standard diabetes therapy for adults in Australia currently involves subcutaneous intensive insulin therapy delivered via either MDI or pump, together with finger-prick blood glucose monitoring.
We hypothesise that hybrid closed-loop insulin delivery compared with manually determined insulin dosing (without CGM) will improve time-in-target glucose range for adults with type 1 diabetes. The overall aim of the study is to evaluate the effect of 6 months of hybrid closed-loop insulin delivery on glucose control, psychosocial well-being, sleep quality, cognition and markers of vascular disease risk compared with standard diabetes therapy for adults with type 1 diabetes.
Methods and analysis
Overview
This open-label, randomised controlled parallel group clinical trial will compare 26 weeks of hybrid closed-loop therapy versus ‘standard therapy’ for 120 adults (aged ≥25 years) with type 1 diabetes (protocol version 2.0, dated 29 March 2017). The standard therapy comparator consists of insulin delivered via either MDI or IPT, without real-time continuous glucose monitoring (RT-CGM), and was chosen to reflect current self-management of type 1 diabetes among adults in Australia.
The study is being conducted at seven university hospitals across Australia. The University of Melbourne is the coordinating academic institution, with St Vincent’s Hospital Melbourne (Melbourne) the study sponsor and lead clinical site. Other clinical sites are: Flinders Medical Centre (Adelaide), Royal Hobart Hospital (Hobart), Royal Melbourne Hospital (Melbourne), Sir Charles Gairdner Hospital (Perth), The Alfred and Baker Heart and Diabetes Institute (Melbourne) and Westmead Hospital (Sydney). Other academic institutions involved are Sydney University and Deakin University. In parallel, a similar study of younger people (aged 12 to <25 years) with type 1 diabetes is being undertaken in Australia; the hybrid closed-loop system and primary outcome are aligned for the two studies.
Study outcomes
The study outcomes are listed in box 1.
Box 1. Study outcomes.
Primary outcome
The proportion of time sensor glucose is in target range (3.9–10.0 mmol/L) with hybrid closed-loop versus standard therapy (multiple daily injections (MDI) or insulin pump therapy (IPT) without real-time continuous glucose monitoring (RT-CGM)), measured by masked CGM at 23–26 weeks postrandomisation.
Secondary outcomes
Hybrid closed-loop therapy versus standard therapy (overall and for each of baseline MDI and IPT separately) for the measures listed below.
-
Glucose control:
-
Masked CGM metrics for 24 hours/day, day (06:00–00:00) and night (00:00–06:00) (measured at mid-study, end of study and mid-study plus end of study combined):
Proportion of time spent 3.9–10.0 mmol/L (excluding the primary outcome).
Proportion of time spent <2.8 mmol/L.
Proportion of time spent <3.3 mmol/L.
Proportion of time spent <3.9 mmol/L.
Proportion of time spent 3.9–7.8 mmol/L.
Proportion of time spent >10.0 mmol/L.
Proportion of time spent >13.9 mmol/L.
Proportion of time spent >16.7 mmol/L.
SD and coefficient of variation.
Mean glucose.
Fasting capillary blood glucose.
HbA1c.
1,5-anhydroglucitol.
Symptomatic hypoglycaemia (with blood glucose <3.5 mmol/L) requiring carbohydrate rescue (n).
-
-
Clinical:
Change in total daily dose of insulin, and basal/bolus proportions.
Change in insulin-to-carbohydrate ratio.
Change in body weight.
-
Psychosocial, sleep and cognitive functioning:
Treatment satisfaction: the Diabetes Treatment Satisfaction Questionnaire (DTSQ) status and change versions.
Satisfaction with technology: Diabetes Management Experiences Questionnaire (DME-Q).
Fear of hypoglycaemia: Hypoglycaemia Fear Survey short form (HFS-SF).
Fear of hyperglycaemia: Hyperglycaemia Avoidance Scale (HAS).
Hypoglycaemia Awareness: Gold Score.
Diabetes distress: Problem Areas in Diabetes (PAID).
Diabetes-specific quality of life: DAWN Impact of Diabetes profile (DIDP).
Diabetes-specific positive well-being: Well-being Questionnaire (W-BQ28) Positive Diabetes Well-being Subscale.
Cognitive function: Prospective and Retrospective Memory Questionnaire (PRMQ) and Psychomotor Vigilance Task (PVT-192).
Driving: proportion of time-in-target glucose range while driving (Melbourne sites only).
Sleep quality: Actigraph data, Pittsburgh Sleep Quality Index, Karolinska Sleepiness Scale.
-
Electrocardiograph profile (via Holter monitor):
Corrected QT interval (QTc).
Heart rate.
Cardiac arrhythmias.
-
Human–technology interaction (participants using hybrid closed-loop system):
Participant perceptions of the hybrid closed-loop system assessed via short message service (SMS) data collection.
Participant expectations and experiences with the hybrid closed-loop system assessed via longitudinal semistructured interviews (Melbourne sites only).
-
Health economic:
Quality-adjusted life years calculated from the EQ-5D-5L.
Hypoglycaemic events and HbA1c.
Participant and family reporting on work interruption.
Reported time spent on training, education and support, by the type of health professional resource used.
Diabetes management consumables (glucose strips, ketone strips, batteries, sensors, site dressings, lancets, needles, insulin).
Resource utilisation tracked via linked administrative data from the Australian Medicare Benefits Schedule and Pharmaceutical Benefits Scheme.
-
Biochemical markers of vascular disease risk:
Cell adhesion molecules.
Oxidised low-density lipoprotein.
Myeloperoxidase.
MicroRNA signatures for arterial, renal and retinal complications.
Telomerase.
DNA methylation/acetylation.
Isoprostanes (blood and urine) and proteomics.
Clotting profile.
-
Hybrid closed-loop system performance parameters:
Proportion of time closed-loop active.
Unplanned exits from closed loop (n).
Sensor performance versus blood glucose metre as measured by mean absolute relative difference (MARD) and sensor failures (n).
Reported insulin delivery line failures (n).
Participant calls to the technical help line (n).
-
Safety:
Hospitalisations for diabetic ketoacidosis (n).
Severe hypoglycaemia, defined as hypoglycaemia requiring the assistance of another person to actively administer carbohydrate, glucagon, or take other corrective actions (n).
The primary study outcome is the proportion of sensor glucose time-in-target range (3.9–10.0 mmol/L) with hybrid closed-loop versus standard therapy, measured by masked CGM 23–26 weeks postrandomisation. This primary end-point was selected to provide the best indication of individual participants’ glucose control. The 3.9–10.0 mmol/L glucose range is aligned with outcome metrics proposed by the JDRF Artificial Pancreas Project Consortium, is consistent with available data relating glucose control and complication prevention, and represents a realistic glucose target.32 37 The secondary outcomes are listed in box 1, sections 1–9.
CGM study outcome data will be collected by identical methods for participants in both groups. Hence, participants assigned hybrid closed-loop therapy will wear two identical glucose sensors for 2 weeks mid-study and 3 weeks at end of study—one sensor providing RT-CGM information to the user and directly linking to the hybrid closed-loop system, and a second sensor collecting masked CGM study outcome data. The closed-loop system performance parameters chosen as study outcome measures are based on an international consensus report for outcome measures in closed-loop trials.37
For closed-loop technology to achieve long-term clinical benefits, then in addition to positively impacting biomedical outcomes, user acceptance, uptake and adaptations are required.28 38 Therefore, this study will assess aspects of psychosocial well-being via both subjective (questionnaires, interviews) and objective (actigraph, psychomotor task) methods. This holistic approach will progress understanding of the human factors involved, thereby enabling adaption of the technology in line with the person’s expectations and experiences.39 The study will also assess whether CGM has an impact on utilisation of health services and medications.
Eligibility
Inclusion and exclusion criteria for participation are listed in box 2.
Box 2. Eligibility.
Inclusion criteria
Type 1 diabetes (as defined by the American Diabetes Association)49 for at least 1 year.
-
Insulin regimen consisting of either:
Multiple daily injections (MDI) with ≥4 injections per day (including ≥3 rapid-acting insulin injections and ≥1 long-acting insulin injection).
Insulin pump therapy (IPT) established for ≥3 months.
Ages 25–70 years inclusive.
HbA1c ≤10.5% (≤91 mmol/mol).
Living in an area with internet and cellular phone coverage.
English speaking proficiency.
Exclusion criteria
Chronic kidney disease (estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m2).
Current use of real-time continuous glucose monitoring (RT-CGM) (defined as use >25% of the time during the past 3 months).
Use of any non-insulin glucose-lowering agent within the past 3 months.
Oral or injected steroid use within the past 3 months.
Pregnancy, or pregnancy planned within study period.
Untreated coeliac disease or other malabsorption.
Uncontrolled thyroid disease.
Clinically significant gastroparesis.
Uncontrolled hypertension (blood pressure: diastolic >100 or systolic >160 mm Hg).
History of myocardial infarction, severe uncontrolled heart failure, unstable angina, transient ischaemic attack, stroke, or thromboembolic disease in the past 3 months.
Poor visual acuity precluding use of the study technology.
Inability or unwillingness to meet protocol requirements.
Any severe or unstable medical or psychological condition which, in the opinion of the investigator, would compromise the ability to meet protocol requirements.
The minimum inclusion age of 25 years was chosen to reflect a general adult population with type 1 diabetes while avoiding potential confounders associated with adolescence and emerging adulthood. This decision was informed by results of previous type 1 diabetes CGM and closed-loop studies, where individuals aged <25 years differed from those aged ≥25 years.14 40
Use of RT-CGM >25% of the time precludes inclusion. This decision was informed by study findings that adults aged ≥25 years with type 1 diabetes using RT-CGM with warning alarms had improved glucose control without increase in biochemical hypoglycaemia only when RT-CGM was worn ≥5–6 days/week.40–42 When CGM is used less often, or without warning alarms, evidence suggests no glucose control benefit.
Study diabetes management devices
Hybrid closed-loop system
The study hybrid closed loop is the MiniMed 670G system, comprising a glucose sensor and transmitter coupled with an insulin pump containing a closed-loop algorithm (Medtronic, Northridge, CA, USA), and rapid-acting analogue insulin (either insulin aspart or insulin lispro) delivered subcutaneously. CGM data are transmitted to the pump every 5 min and the algorithm calculates the basal insulin dose (delivered at 5 min intervals) required to maintain the target glucose level. The algorithm uses a modified proportional integrative derivative model with insulin feedback based on an insulin delivery algorithm originally developed by Steil et al.43 The algorithm also incorporates a supervisory model predictive component aiming to avoid insulin overdelivery.44 For meals, the user estimates the amount of carbohydrate to be consumed (entering this into the pump) and checks their capillary blood glucose level. Using this information, an insulin bolus is calculated and delivered according to the individualised insulin-to-carbohydrate ratio and an insulin sensitivity factor determined by the algorithm (should a correction bolus be required).
The MiniMed 670G system has been deemed safe and effective for glucose control in a 3-month uncontrolled study45 46 and an exercise study.17 The system was approved for use by the US Food and Drug Administration in 2016.
Masked CGM
CGM data masked to both the participants and research team will be collected for study outcome measurements at three time points: baseline prerandomisation (3 weeks), mid-study (2 weeks) and end of study (3 weeks). For participants randomised to hybrid closed loop, this masked CGM data collection will be in addition to the system’s RT-CGM. The study uses Guardian Sensor 3 glucose sensors (Medtronic, Northridge, CA, USA). This sensor configuration has reported performance parameters of mean absolute relative difference±SD of 9.6%±9.0% and mean functional sensor life of 146±39 hours when used with a Medtronic MiniMed 640G insulin pump.47 By using a separate device to collect CGM study outcome data, the device under investigation is not also being used to evaluate its own performance.
For masked CGM data collection, the glucose sensor will be inserted and the sensor recorder will be connected by the study team. During masked CGM, participants will be required to test capillary blood glucose levels at least four times per day with a CONTOUR NEXT LINK metre (details below). Masked CGM data are collected retrospectively by uploading the recorder and the metre.
Blood glucose monitoring
All participants will be provided with a CONTOUR NEXT LINK 2.4 blood glucose metre (Ascensia, Parsippany, NJ, USA) which is able to transmit data directly to the MiniMed 670G insulin pump. Prerandomisation, and for participants randomised to standard therapy, the CONTOUR NEXT LINK 2.4 will be used in addition to their regular glucose metre during masked CGM. Use of the same glucose metre within the hybrid closed-loop system and for masked CGM calibration will standardise data collection.
Participants using MDI at enrolment will also be provided with an ACCU-CHEK Aviva Expert blood glucose metre (Roche Diagnostics, Mannheim, Germany), selected for its in-built ‘bolus calculator’. The bolus calculator uses the measured blood glucose level, calculated rapid-acting ‘insulin on board’, and the programmed insulin sensitivity factor and insulin-to-carbohydrate ratio to determine the recommended insulin bolus doses. The use of a metre with bolus calculator by those in the control group who continue with MDI will reflect the bolus calculators used by participants randomised to hybrid closed-loop therapy and by those using IPT randomised to standard diabetes therapy.
Diabetes management software
CareLink, an internet-based platform from Medtronic, will be used for uploading insulin pump, glucose sensor and glucose metre data. The hybrid closed-loop system data are uploaded to a computer via the system’s glucose metre USB connection; insulin pump, sensor and metre data are then accessible to study investigators.
Study design
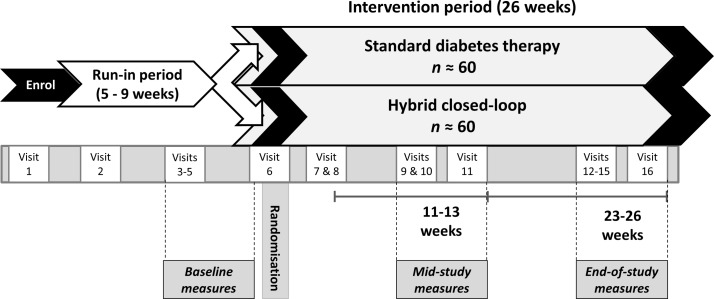
This is a prospective, open-label, parallel design randomised controlled study involving adults with type 1 diabetes (overall target n=120, with ≥40% using MDI and ≥40% using IPT). Study procedures will be undertaken by medical doctors with subspecialty training in endocrinology, diabetes nurse educators, dietitians and research nurses. Throughout the study, the time taken for participant education, training, clinical care and technical support will be recorded; the health professional time will be used in health economic analyses to determine implications for closed loop becoming a mainstream therapy. Adherence to study protocols will be assessed at each study visit; verbal and written reminders of study instructions will be provided to improve protocol adherence. Participants will continue their usual diabetes clinical care with their treating clinicians during study participation. Participants will be randomised 1:1 either to hybrid closed-loop therapy or to continue using their current standard diabetes therapy (either MDI or IPT) for 26 weeks (figure 1). Use of RT-CGM will not be permitted during run-in or by participants randomised to standard diabetes therapy (though CGM without live alerts, eg, Abbott FreeStyle Libre, is permissible).
Figure 1.
Study protocol overview.
Patient involvement
Investigator discussions with patients throughout provision of clinical care and during previous research studies were taken into consideration when designing this study protocol. The burden of the study intervention will be assessed via short message service data collection and during semistructured interviews (see box 1, sections 5a and 5b).
Sample size
The power calculation is for a parallel study design with two groups of equal size. It is based on SDs of the percentage time-in-target glucose range at 6 months (adjusted for baseline) observed for the subset of participants in two randomised clinical trials from the JDRF Study Group who had similar characteristics to participants being recruited here (Professor Roy Beck, personal communication). The SD (95% CI) for pump users was 9% (8%, 12%) and for MDI users was 10% (7%, 19%).
From an initial overall sample size of n=120, with a dropout rate of 10%, a common SD of 9% and a type I error rate of 5%, the power to detect a minimum absolute difference of 5% time-in-target glucose range would be 80%. A more conservative scenario with a dropout rate of 20%, and unequal SDs of 12% and 19% for pump and MDI users, respectively, increases the minimum detectable absolute difference to 9% with power of 80%.
Study schedule
The study will consist of 16 visits including the run-in and intervention periods. Key activities undertaken during each visit are shown in table 1. Participants will be provided with 24 hours’ telephone contacts for support if required. Health professionals will log all time taken training and communicating with the study participants.
Table 1.
Study visits
| Study visit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Weeks from randomisation | −3 | −2 | −1 | 0 | 1 | ~7 | 11 | 12 | 13 | 23 | 24 | 25 | 26 | 26 | 39 | ||
| Clinical assessment | X | X | X | X | |||||||||||||
| Time with health professional | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| HbA1c | X | X | X | X | |||||||||||||
| β-hCG, C-peptide | X | ||||||||||||||||
| CHO counting education | X | X | |||||||||||||||
| Insulin pump training | X | ||||||||||||||||
| Insulin dose review | X | X | X | X | |||||||||||||
| Logbook provision | X | ||||||||||||||||
| Logbook data collection | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Masked CGM insertion | X | X | X | X | X | X | X | X | |||||||||
| Glucose metre upload | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Psychosocial, sleep, cognitive functioning surveys | X | X | X | ||||||||||||||
| Cognitive performance device provision | X | X | X | ||||||||||||||
| Actigraphy and sleep diary provision | X | X | X | X | X | X | X | X | |||||||||
| Semistructured interviews | X | X | X | X | |||||||||||||
| Driving device and diary provision | X | X | X | X | X | X | X | X | |||||||||
| Holter monitor provision | X | X | X | ||||||||||||||
| Vascular disease risk markers | X | X |
CGM, continuous glucose monitoring; CHO, carbohydrate.
Run-in period
After enrolment, there will be a run-in period lasting at least 5 weeks. Participants will undergo initial medical, psychosocial and cognitive assessments. Their diabetes-related knowledge and carbohydrate-counting proficiency will be assessed and their insulin dosing will be optimised. Participants will be provided with detailed training and support to use the study glucose metres and masked CGM devices. Education will be provided by diabetes nurse educators and dietitians to optimise participants’ diabetes self-management including carbohydrate counting. The optimisation of carbohydrate counting is central to baseline optimisation for all participants in the study—this aims to achieve the best possible match of bolus insulin doses to the individuals’ requirements for the carbohydrate consumed for both groups, thereby testing the closed-loop aspect of the hybrid closed-loop system’s insulin delivery in comparison with standard therapy.
After provision of education, data will be collected for 3 weeks of baseline masked CGM, actigraphy (sleep data) and from the self-reported diabetes logbook. Driving log data (to associate with the CGM data) will also be collected during these 3 weeks for participants at the three clinical sites in Melbourne. At the end of the run-in period, the CGM data will be uploaded and checked to ensure data are available for at least 70% of the time.40 If the minimum required CGM data are not available, an additional week of CGM will be undertaken to fulfil the protocol requirements. At the end of the run-in, baseline blood and urine samples will be collected for measurement of HbA1c and biochemical markers of vascular disease risk.
Randomisation
Eligible participants will be randomised after completing the run-in. Group allocation will be a 1:1 ratio using minimisation with three variables, all of which are expected to be highly prognostic of the primary outcome. These minimisation variables are: (1) the proportion of time-in-target glucose range at baseline (dichotomised to ≤50% and >50%); (2) study centre (seven clinical sites); and (3) insulin delivery modality (MDI or IPT). Randomisation will be performed by an independent group of statisticians using central randomisation software, and will be implemented into an electronic participant record system.
The nature of the study groups does not allow blinding of participants or investigators.
Intervention period
After randomisation, there will be a 26-week intervention period.
Participants randomised to standard therapy will continue using their current insulin delivery modality (MDI or IPT, with bolus calculator in the glucose metre or pump, respectively) and will be instructed to refrain from using RT-CGM during the study.
Participants randomised to hybrid closed-loop therapy will receive general insulin pump and CGM education and training, plus instruction regarding usage of the study hybrid closed-loop system. This education and training period may take up to 4 weeks (likely longer for those using MDI than IPT at baseline). The hybrid closed-loop system will be programmed with participants’ usual insulin-to-carbohydrate ratios and insulin sensitivity factors, as well as their usual basal rates (or the basal rates determined by their clinicians for those participants transitioning from MDI). Participants will be provided with a 24 hours’ technical help telephone contact for the hybrid closed-loop system.
Participants at the three clinical sites in Melbourne who are randomised to hybrid closed-loop therapy will undergo four semistructured interviews to assess their expectations of, and experiences with, the technology. These interviews will be conducted at randomisation, then at 11, 26 and 39 weeks postrandomisation.
Participants will have mid-study data collected between 11 and 13 weeks postrandomisation. Two weeks of masked CGM data, cognitive assessments and actigraphy will be collected, plus driving data for participants at the Melbourne sites. Clinical review with assessment of diabetes management and carbohydrate counting, and adjustment of therapy and further education as required, will be undertaken 13 weeks postrandomisation. At this visit, psychosocial questionnaires will be completed and venous samples for HbA1c will be collected.
Participants will have end-of-study data collected between 23 and 26 weeks postrandomisation. Three weeks of masked CGM data, cognitive assessments and actigraphy will be collected, plus driving data for participants at the Melbourne sites. At the end of the 3-week period, the CGM data will be uploaded and checked for available data at least 70% of the time. If 70% of CGM data are not available, an additional week of CGM data will be collected. At the end-of-study visit (26 weeks postrandomisation), psychosocial questionnaires will be completed, and venous and urine samples will be collected for HbA1c and biochemical markers of vascular disease risk. Participants in the hybrid closed-loop group will change back to using their usual insulin delivery modality (MDI or IPT). Doctor visit data from the Medicare Benefits Schedule and insulin prescription data from the Pharmaceutical Benefits Scheme will be accessed for study participants.
Statistical methods
The primary analysis will assess differences in the proportion of time-in-target glucose sensor range (3.9–10.0 mmol/L) with hybrid closed-loop versus standard therapy, measured by masked CGM at 23–26 weeks postrandomisation on an intention-to-treat basis using analysis of covariance (ANCOVA) with adjustment for baseline time-in-target range. A p value threshold of <0.05 will be used to determine statistical significance.
Model fit will be evaluated by exploration of residuals. If the model is of poor fit, the outcome variable will be transformed and the model refitted and evaluated. If unsuccessful, non-parametric analysis will be performed.
Analysis of continuous secondary outcomes will also use ANCOVA with adjustment for baseline time-in-target range, whereas Poisson or negative binomial regression will be used for count outcomes and logistic regression will be used for binary outcomes. Subgroup analysis by baseline insulin delivery modality will be performed by inclusion of an interaction term in the regression modelling or by a stratified analysis when non-parametric methods are used.
No adjustment for multiplicity is planned. All results for primary and secondary outcomes will be reported.48 No interim analysis is planned.
Health economic evaluation
An economic evaluation will determine the incremental cost of home-based hybrid closed-loop versus standard diabetes therapy in Australia. This analysis will quantify costs directly associated with hybrid closed-loop and standard diabetes therapy plus other impacts on the health system (box 1). Outcomes will be assessed in quality-adjusted life years for changes in health-related quality of life, and for the likely long-term impact of changes in glucose control on long-term outcomes using a type 1 diabetes simulation model.
Safety assessments
Safety parameters to be assessed include severe hypoglycaemia, ketoacidosis and unplanned hospitalisations directly related to the study (box 1).
Effectiveness assessments
Effectiveness parameters to be assessed include glucose control, clinical measures, psychosocial and cognitive functioning, human–technology interaction, health economic measures and biochemical markers of vascular disease risk (box 1).
Closed-loop system performance parameters
Closed-loop system performance parameters to be assessed relate to the system overall, to individual system components and to system usability (box 1).
Trial oversight
The study will be conducted in accordance with the principles of the Declaration of Helsinki and guidelines for Good Clinical Practice (GCP).
The day-to-day study management will be the responsibility of the investigators at each clinical site. The principal investigator and study project manager will maintain regular correspondence with all investigators and study coordinators. The principal investigator, with the sites’ lead investigators, will assume responsibility for the progress of the study in accordance with agreed timelines and milestones with the study funders. A combined data safety and monitoring board (DSMB) will be established for this study and the aligned study, independent from the study investigators, comprising adult and paediatric physicians experienced in statistics and clinical trials. The study project manager will liaise with the study teams in all centres to establish procedures and ensure that the study is carried out according to the protocol and to standards of GCP, with robust systems for reporting adverse events. The study project manager will be responsible for the central preparations of data for presentation to the DSMB.
Ethics and dissemination
The study has received ethics approval from the lead site Human Research Ethics Committee. Other clinical sites provide oversight through local governance committees. Any substantial amendments to the study protocol will be reported to the lead site ethics committee for approval prior to implementation, and updated on the trial registry, with the study investigators being advised in writing.
All potential participants will be provided with written and verbal information regarding the study, the procedures involved and all potential risks related to participating. A study investigator will obtain written informed consent from each participant prior to commencing study procedures. All personal information about potential and enrolled participants will be deidentified to protect confidentiality before, during and after the trial. Standard operating procedures for reporting all adverse events, device-related adverse events and severe adverse events will be in place. The Human Research Ethics Committees and the Therapeutic Goods Administration of Australia will be informed of any serious adverse events and any unexpected device-related adverse events.
Screening and recruitment commenced in May 2017. It is anticipated that the study visits will be completed by May 2019. The results of the study will be disseminated at national and international conferences and by peer-reviewed publications. Participants will be provided with a summary of the study results by their site’s lead investigator.
Supplementary Material
Acknowledgments
We thank Professor Roman Hovorka and Professor Roy Beck for their expert advice regarding study design.
Footnotes
Contributors: SAM, MIdB, PGC, AJJ, ACK, JS, GMW, TWJ and DNO designed the study. SAM and DNO drafted the manuscript. SAM, MIdB, VS, MHL, BP, GRA, LAB, MGB, FJC, PMC, NDC, PGC, EAD, JMF, CH, DJHW, JCH, AJJ, JK, ACK, BRK, KK, RJM, RWM, JAN, CS, JS, SNS, ST, GMW, SV, TWJ and DNO contributed to the writing and/or critical review of the study protocol and reviewed the manuscript for intellectual content. DNO is the principal investigator and guarantor.
Funding: This study is funded by the Australian Research Council (with the funding administered by JDRF Australia), and by the National Health and Medical Research Council of Australia. Material support is being provided via a grant from the Medtronic External Research Program. SAM is supported by a JDRF Early-Career Patient-Orientated Diabetes Research Award. MIdB was supported by a Raine Clinical Research Fellowship. AJJ is supported by an NHMRC Practitioner Fellowship. JS is supported by core funding to the Australian Centre for Behavioural Research in Diabetes provided by Diabetes Victoria and Deakin University.
Disclaimer: The study funders and sponsor did not have any role in study design or contribution to the manuscript, and they will not be involved in collection, management, analysis or interpretation of the data. The study funders will not have any role in writing the study report or the decision to submit the report for publication.
Competing interests: MIdB and NDC report receiving speaker honoraria from Medtronic. DJHW reports receiving speaker and advisory board honoraria from Medtronic. RWM reports receiving conference travel and accommodation support from Medtronic. JS reports that the ACBRD has received honoraria from Medtronic in relation to her speaking engagements and role in advisory boards. DNON reports receiving speaker honoraria and research grants from Medtronic.
Patient consent: Not required.
Ethics approval: St Vincent’s Hospital Melbourne Human Research Ethics Committee (lead site, approval number HREC-D 088/16).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. 10.1056/NEJMoa052187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nathan DM. DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9–16. 10.2337/dc13-2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev 1999;15:205–18. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018 . Diabetes Care 2018;41(Suppl 1):S64–74. 10.2337/dc18-S008 [DOI] [PubMed] [Google Scholar]
- 6. Beck RW, Tamborlane WV, Bergenstal RM, et al. The T1D Exchange clinic registry. J Clin Endocrinol Metab 2012;97:4383–9. 10.1210/jc.2012-1561 [DOI] [PubMed] [Google Scholar]
- 7. McKnight JA, Wild SH, Lamb MJ, et al. Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med 2015;32:1036–50. 10.1111/dme.12676 [DOI] [PubMed] [Google Scholar]
- 8. Huo L, Harding JL, Peeters A, et al. Life expectancy of type 1 diabetic patients during 1997-2010: a national Australian registry-based cohort study. Diabetologia 2016;59:1177–85. 10.1007/s00125-015-3857-4 [DOI] [PubMed] [Google Scholar]
- 9. Huo L, Shaw JE, Wong E, et al. Burden of diabetes in Australia: life expectancy and disability-free life expectancy in adults with diabetes. Diabetologia 2016;59:1437–45. 10.1007/s00125-016-3948-x [DOI] [PubMed] [Google Scholar]
- 10. Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia 2016;59:1795–805. 10.1007/s00125-016-4022-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care 2014;37:1931–7. 10.2337/dc13-2911 [DOI] [PubMed] [Google Scholar]
- 12. Nimri R, Muller I, Atlas E, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care 2014;37:3025–32. 10.2337/dc14-0835 [DOI] [PubMed] [Google Scholar]
- 13. Thabit H, Tauschmann M, Allen JM, et al. Home Use of an Artificial Beta Cell in Type 1 Diabetes. N Engl J Med 2015;373:2129–40. 10.1056/NEJMoa1509351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharifi A, De Bock MI, Jayawardene D, et al. Glycemia, Treatment Satisfaction, Cognition, and Sleep Quality in Adults and Adolescents with Type 1 Diabetes When Using a Closed-Loop System Overnight Versus Sensor-Augmented Pump with Low-Glucose Suspend Function: A Randomized Crossover Study. Diabetes Technol Ther 2016;18:772–83. 10.1089/dia.2016.0288 [DOI] [PubMed] [Google Scholar]
- 15. Bally L, Thabit H, Kojzar H, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol 2017;5:261–70. 10.1016/S2213-8587(17)30001-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weisman A, Bai JW, Cardinez M, et al. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–12. 10.1016/S2213-8587(17)30167-5 [DOI] [PubMed] [Google Scholar]
- 17. Jayawardene DC, McAuley SA, Horsburgh JC, et al. Closed-Loop Insulin Delivery for Adults with Type 1 Diabetes Undertaking High-Intensity Interval Exercise Versus Moderate-Intensity Exercise: A Randomized, Crossover Study. Diabetes Technol Ther 2017;19:340–8. 10.1089/dia.2016.0461 [DOI] [PubMed] [Google Scholar]
- 18. Kovatchev B, Cheng P, Anderson SM, et al. Feasibility of Long-Term Closed-Loop Control: A Multicenter 6-Month Trial of 24/7 Automated Insulin Delivery. Diabetes Technol Ther 2017;19:18–24. 10.1089/dia.2016.0333 [DOI] [PubMed] [Google Scholar]
- 19. Cox DJ, Kovatchev BP, Anderson SM, et al. Type 1 diabetic drivers with and without a history of recurrent hypoglycemia-related driving mishaps: physiological and performance differences during euglycemia and the induction of hypoglycemia. Diabetes Care 2010;33:2430–5. 10.2337/dc09-2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiang JL, Kirkman MS, Laffel LM, et al. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37:2034–54. 10.2337/dc14-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hendrieckx C, Halliday JA, Bowden JP, et al. Severe hypoglycaemia and its association with psychological well-being in Australian adults with type 1 diabetes attending specialist tertiary clinics. Diabetes Res Clin Pract 2014;103:430–6. 10.1016/j.diabres.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 22. Goss J. Projection of Australian health care expenditure by disease, 2003 to 2033. Cat. no. HWE 43. Canberra: AIHW, 2008. [Google Scholar]
- 23. Colagiuri S, Brnabic A, Gomez M, et al. DiabCo$t Australia Type 1: Assessing the burden of Type 1 Diabetes in Australia. Canberra: Diabetes Australia, 2009. [Google Scholar]
- 24. Tao B, Pietropaolo M, Atkinson M, et al. Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLoS One 2010;5:e11501 10.1371/journal.pone.0011501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barnard KD, Hood KK, Weissberg-Benchell J, et al. Psychosocial assessment of artificial pancreas (AP): commentary and review of existing measures and their applicability in AP research. Diabetes Technol Ther 2015;17:295–300. 10.1089/dia.2014.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barnard KD, Wysocki T, Allen JM, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Res Care 2014;2:e000025 10.1136/bmjdrc-2014-000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barnard KD, Wysocki T, Thabit H, et al. Psychosocial aspects of closed- and open-loop insulin delivery: closing the loop in adults with Type 1 diabetes in the home setting. Diabet Med 2015;32:601–8. 10.1111/dme.12706 [DOI] [PubMed] [Google Scholar]
- 28. Hendrieckx C, Poole LA, Sharifi A, et al. "It Is Definitely a Game Changer": A Qualitative Study of Experiences with In-home Overnight Closed-Loop Technology Among Adults with Type 1 Diabetes. Diabetes Technol Ther 2017;19:410–6. 10.1089/dia.2017.0007 [DOI] [PubMed] [Google Scholar]
- 29. Barnard KD, Wysocki T, Ully V, et al. Closing the Loop in Adults, Children and Adolescents With Suboptimally Controlled Type 1 Diabetes Under Free Living Conditions: A Psychosocial Substudy. J Diabetes Sci Technol 2017;11:1080–8. 10.1177/1932296817702656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kropff J, DeJong J, Del Favero S, et al. Psychological outcomes of evening and night closed-loop insulin delivery under free living conditions in people with Type 1 diabetes: a 2-month randomized crossover trial. Diabet Med 2017;34:262–71. 10.1111/dme.13268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DCCT Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995;44:968–83. [PubMed] [Google Scholar]
- 32. Beck RW, Connor CG, Mullen DM, et al. The Fallacy of Average: How Using HbA1c Alone to Assess Glycemic Control Can Be Misleading. Diabetes Care 2017;40:994–9. 10.2337/dc17-0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steineck I, Cederholm J, Eliasson B, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ 2015;350:h3234 10.1136/bmj.h3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barnard KD, Venkat MV, Close K, et al. PsychDT Working Group: Report Psychosocial Aspects of Artificial Pancreas Systems. J Diabetes Sci Technol 2015;9:925–8. 10.1177/1932296815588332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Diabetes Services Scheme. Product and supply 2017. https://www.ndss.com.au/product-and-supply (accessed 22 June 2017).
- 36. National Diabetes Services Scheme. Continuous Glucose Monitoring 2017. https://www.ndss.com.au/cgm (accessed 22 June 2017).
- 37. Maahs DM, Buckingham BA, Castle JR, et al. Outcome Measures for Artificial Pancreas Clinical Trials: A Consensus Report. Diabetes Care 2016;39:1175–9. 10.2337/dc15-2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonder-Frederick LA, Shepard JA, Grabman JH, et al. Psychology, technology, and diabetes management. Am Psychol 2016;71:577–89. 10.1037/a0040383 [DOI] [PubMed] [Google Scholar]
- 39. Gonder-Frederick LA, Grabman JH, Shepard JA. Human Factor Considerations for Artificial Pancreas Research. Diabetes Technol Ther 2016;18:762–4. 10.1089/dia.2016.0403 [DOI] [PubMed] [Google Scholar]
- 40. Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–76. 10.1056/NEJMoa0805017 [DOI] [PubMed] [Google Scholar]
- 41. Beck RW, Hirsch IB, Laffel L, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–83. 10.2337/dc09-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 2009;52:1250–7. 10.1007/s00125-009-1365-0 [DOI] [PubMed] [Google Scholar]
- 43. Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab 2011;96:1402–8. 10.1210/jc.2010-2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grosman B, Ilany J, Roy A, et al. Hybrid Closed-Loop Insulin Delivery in Type 1 Diabetes During Supervised Outpatient Conditions. J Diabetes Sci Technol 2016;10:708–13. 10.1177/1932296816631568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes. JAMA 2016;316:1407–8. 10.1001/jama.2016.11708 [DOI] [PubMed] [Google Scholar]
- 46. Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther 2017;19:155–63. 10.1089/dia.2016.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Christiansen MP, Garg SK, Brazg R, et al. Accuracy of a Fourth-Generation Subcutaneous Continuous Glucose Sensor. Diabetes Technol Ther 2017;19:446–56. 10.1089/dia.2017.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet 2005;365:1591–5. 10.1016/S0140-6736(05)66461-6 [DOI] [PubMed] [Google Scholar]
- 49. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018 . Diabetes Care 2018;41(Suppl 1):S11–S24. 10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.