We showed no significant correlation between antiretroviral hair concentrations and measures of HIV persistence or soluble markers of inflammation, suggesting that higher cumulative ARV exposure may not impact the HIV reservoir or inflammation. Further study in real-world populations are indicated.
Keywords: HIV, antiretroviral therapy, hair concentrations, measures of HIV persistence, HIV-related inflammation
Abstract
Data on the relationship of antiretroviral exposure to measures of human immunodeficiency virus (HIV) persistence are limited. To address this gap, multiple viral, immunologic, and pharmacologic measures were analyzed from individuals with sustained virologic suppression on therapy (median 7 years) in the AIDS Clinical Trials Group A5321 cohort. Among 110 participants on tenofovir-(TFV)-disoproxil-fumarate (TDF)/emtricitabine (FTC)-containing regimens, we found no significant correlation between hair concentrations of individual antiretrovirals (ARVs) in the regimen and measures of HIV persistence (plasma HIV-1 RNA by single copy assay, cell-associated-DNA, cell-associated RNA) or soluble markers of inflammation. These findings suggest that higher systemic ARV exposure may not impact HIV persistence or inflammation.
Among people living with human immunodeficiency virus (HIV) on treatment, strong relationships have been shown between systemic exposure to antiretroviral (ARV) therapy (ART) and virologic suppression in plasma (eg, HIV RNA < 40 copies/mL) [1, 2]. Lower levels of ARVs in sanctuary sites may be associated with persistent HIV-1 replication on ART [3], although maintenance of the reservoir may be due to proliferation of infected cells instead of ongoing replication [4]. Suboptimal ART adherence, even with suppression of viremia, has also been associated with higher levels of inflammation [5]. To date, however, studies have not examined the relationship between cumulative exposure to ARVs and measures of HIV-1 persistence or inflammation.
Plasma levels measure short-term exposure to ARVs and are susceptible to day-to-day variation [6]. Cumulative measures of ARV exposure (such as drug levels in peripheral blood mononuclear cells [PBMCs], dried blood spots, or hair) are increasingly used to quantify exposure, whether in the context of HIV treatment or pre-exposure prophylaxis. Drug concentrations in hair reflect uptake from the systemic circulation over weeks to months [7], and can be used to measure cumulative exposure to all ARVs, regardless of whether they are activated intracellularly (such as nucleoside/nucleotide reverse transcriptase inhibitors [NRTIs]) or not (eg, non-NRTIs [NNRTIs], protease inhibitors [PIs], and integrase strand transfer inhibitors [INSTIs]).
Assays have been developed and validated to measure hair concentrations for a number of ARVs. Multiple studies have shown strong relationships between various ARV levels in hair and suppression of plasma viremia [1, 8]. The AIDS Clinical Trials Group (ACTG) HIV Reservoirs Cohort Study (A5321) collected multiple samples for measures of HIV-1 persistence, inflammation, and ARV exposure among participants with sustained suppression of plasma viremia [9]. We present here the first analysis examining the relationship between long-term ARV exposure (using hair levels) and measurements of HIV-1 persistence and inflammation among individuals with long-standing virologic suppression.
METHODS
Study Population
ACTG A5321 is a longitudinal cohort that includes participants who started ART during chronic HIV infection in the context of ACTG clinical trials, achieved HIV RNA levels <50 copies/mL after 6 months, and had documented sustained virologic suppression with no documented breakthroughs [9]. Multiple viral, immunologic, and pharmacologic measures are collected prospectively. The current analysis examines participants who were on tenofovir (TFV) disoproxil fumarate (TDF)/emtricitabine (FTC)-based ART regimens at study entry. Participants provided written informed consent, with opt-in for hair collection, and ethics approval was obtained from all participating institutions.
Study Procedures
Hair Concentrations
Small samples of hair (approximately 50 strands) were cut from the scalp at the entry visit [1]. Hair concentrations of various ARVs were measured via validated liquid chromatography/tandem mass spectrometry-based methods in the University of California San Francisco Hair Analytical Laboratory. Our assays have been peer reviewed and approved by the Division of AIDS’ Clinical Pharmacology and Quality Assurance Program. Briefly, hair is cut into 1–2 mm length segments and 5 mg is weighed and processed. The ARV is extracted with an organic solvent, along with an internal standard, and then analyzed. For this analysis, ARV concentrations were measured in the 1.5 cm of hair closest to the participant’s scalp, representing approximately 6 weeks’ exposure.
Measures of HIV-1 Persistence
Residual low-level plasma viremia using a single copy assay (iSCA) was measured using real-time PCR with a probe for a highly conserved region of integrase in the HIV-1 pol gene [10]; the lower limit of quantification of this assay for a 4-mL plasma sample is 0.4 copies/milliliters (mL). Cell-associated RNA (CA-RNA) and cell-associated DNA (CA-DNA) were measured via published methods at the University of Pittsburgh ACTG Virology Specialty Laboratory [9].
Immunologic Measures
To evaluate levels of soluble biomarkers of inflammation and immune activation, frozen plasma samples from each participant were thawed and analyzed. Plasma concentrations of interleukin (IL)-6, high-sensitivity CRP (hsCRP), soluble CD14 (sCD14), soluble CD163 (sCD163), interferon gamma-induced protein (IP)-10, and tumor necrosis factor (TNF)-α were quantified using enzyme-linked immunosorbent assay (ELISA) kits (R&D, Minneapolis, MN) at an ACTG Immunology Specialty Laboratory [9].
Statistical Analysis
Rank-based (Spearman) correlation coefficients assessed relationships between hair levels of ARVs (the average rank of TFV/FTC levels and concentrations of each individual drug) at study entry with HIV-1 persistence measurements (iSCA, CA-RNA, and CA-DNA) and soluble inflammatory markers. Hair levels, reservoir measures, and soluble biomarkers were each assessed as continuous measures. For HIV-1 DNA values <3 and CA-RNA values <19 copies/106 CD4+ T cells (below assay limits based on the number of cells analyzed), and iSCA <0.4 copies/mL, results were imputed to a lower number and analyzed as the lowest rank. The relationship between exposure reservoir measures was examined separately by sex. Differences in TFV concentrations with an accompanying pharmacoenhancer, cobicistat or ritonavir, in the regimen (versus an unboosted anchor ARV) were assessed by the Wilcoxon rank sum test; the rank sum test also evaluated differences in drug concentrations by iSCA <0.4 versus ≥0.4 copies/mL. Data analyses were performed using SAS 9.4.
RESULTS
Demographics
Hair samples at A5321 entry were analyzed for 110 participants on TDF/FTC (Table 1); besides TDF/FTC, 49% were on an NNRTI, 25% on an INSTI, and 26% on a boosted PI. The median age of participants was 48 years (range 23–69) and 24% female. The distribution of self-reported race/ethnicity was 15% black, 59% white, 23% Hispanic, and 3% Asian. The median duration on ART prior to entry was 7 years (range 4–16). Although all participants had sustained virologic suppression via commercial HIV-1 RNA assays, 48% had residual low-level viremia (≥0.4 copies/mL) by iSCA. Median CA-DNA and CA-RNA levels were 48 (interquartile range [IQR], 14–142) and 564 (IQR, 229–1236) copies/106 CD4+ cells, respectively (Table 1).
Table 1.
Demographics of Participants in the A5321 Hair Study (n = 110)
| Participant Characteristic | N or Median (%, range or IQR) |
|---|---|
| Age at study entry (years) | 48 (range 23–69) |
| Sex | |
| Male | 84 (76%) |
| Female | 26 (24%) |
| Race/ethnicity | |
| White non-Hispanic | 65 (59%) |
| Black non-Hispanic | 17 (15%) |
| Hispanic | 25 (23%) |
| Asian/Pacific Islander | 3 (3%) |
| Years of ART at study entry | 7 (range 4–16) |
| CD4+ cell count (cells/mm3) | |
| Pre-ART | 250 (IQR, 101–359) |
| Study entry | 661 (IQR, 494–839) |
| Pre-ART plasma HIV-1 RNA (log10cps/mL) | 4.6 (IQR, 4.2–5.1) |
| ART regimen at A5321 entry (all TDF-FTC based) | 46 (42%) EFV; 7 (6%) RPV; 1 (1%) NVP; 11 (10%) ATV/r; 18 (16%) DRV/r; 5 (5%) EVG/cobi; 22 (20%) RAL |
| Measures of viral persistence | |
| Plasma HIV-1 RNA (iSCA, copies/mL) | 52% < 0.4; 48% ≥ 0.4 |
| Cell associated HIV-1 RNA (copies/106 CD4+ cells) | 48 (IQR, 14–142) |
| Cell associated HIV-1 DNA (copies/106 CD4+ cells) | 564 (IQR, 229–1236) |
Abbreviations: ART, antiretroviral therapy; ATV/r, atazanavir/ritonavir; DRV/r, darunavir/ritonavir; EFV, efavirenz; EVG/cobi, elvitegravir/cobicistat; IQR, interquartile range; iSCA, single copy assay; NVP, nevirapine; RPV, rilpivirine.
Hair ARV Concentrations
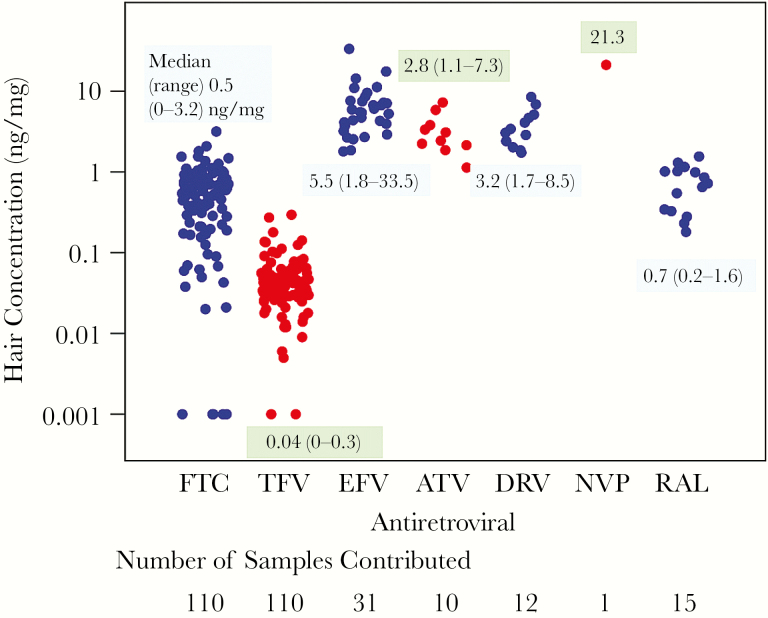
Figure 1 shows the distribution of TFV and FTC hair concentrations among the 110 participants and the distribution of each anchor drug in hair for the subset of participants on each ARV. In participants with measureable TFV and FTC, concentrations varied over 100-fold. Concentrations of third agents were detectable in all participants and varied over a narrower range. Median concentrations of TFV in hair were 25% higher in participants on cobicistat or ritonavir than in those on an unboosted anchor (P = .015).
Figure 1.
Interindividual variability in antiretroviral (ARV) concentrations seen even among virologically suppressed individuals. ARV concentrations in hair below the lower limit of quantification were set to 0.001. Abbreviations: ATV, atazanavir; DRV, darunavir; EFV, efavirenz; FTC, emtricitabine; NVP, nevirapine; RAL, raltegravir; TFV, tenofovir.
Correlation Between Hair Concentrations of ARVs and HIV-1 Persistence
A significant correlation was not detected between TFV and FTC concentrations in hair and low-level viremia measured by iSCA (r = 0.09 and r = −0.04, respectively); results were comparable when examining iSCA <0.4 versus ≥0.4 copies/mL (P = .18 and P = .88 for TFV and FTC, respectively). Similarly, a significant correlation was not detected between rank-based TFV concentrations in hair and either CA-RNA (r = −0.02) or CA-DNA (r = −0.1), or between FTC concentrations in hair and either CA-RNA (r = 0.01) or CA-DNA (r = −0.05). Finally, significant correlations were not found between hair concentrations of any of the NNRTI, PI, or INSTI (efavirenz, raltegravir, atazanavir, or darunavir) and the 3 measures of HIV-1 persistence (Supplementary Table 1). When stratifying analyses by sex, no significant correlations were observed between hair levels and HIV-1 persistence measures in either men or women.
Correlation Between Hair Concentrations of ARVs and Soluble Markers of Inflammation
Significant correlations were not detected between ranked TFV and FTC concentrations in hair and the 6 soluble biomarkers (Supplementary Table 2).
DISCUSSION
This is the first study to report the relationship between cumulative measures of ARVs and measures of HIV-1 persistence or inflammation. We found no significant correlation among ARV hair levels and 3 different measures of HIV-1 persistence and 6 soluble markers of inflammation in participants on suppressive ART. We observed marked interindividual variability in ARV exposure, which has been reported previously [11], but not among individuals with sustained virologic suppression. Finally, we confirm previous findings [12] that concomitant pharmacoenhancers result in higher TFV levels.
Previous studies have examined the impact of ART intensification (adding agents to currently suppressive ART) on persistent viremia and inflammation. In general, studies adding raltegravir and/or maraviroc to suppressive ART regimens early in HIV infection or in the context of chronic suppressive therapy [13, 14] have failed to show consistent or sustained impacts on markers of HIV-1 persistence. Similarly, in this cohort of individuals with sustained virologic suppression on ART at traditional doses, those exhibiting higher ARV concentrations in hair did not demonstrate lower measures of HIV persistence.
An ongoing debate has focused on whether low-level replication of HIV continues in tissues of individuals with suppression of viremia on ART. The fact that higher ARV concentrations in our study did not correlate with lower levels of viremia by iSCA (or lower levels of HIV transcription by CA-RNA) suggests that residual proviral transcripts and viremia are produced by stable reservoirs of infected cells that are not influenced by higher exposure to ARVs. However, it is not known whether higher hair ARV concentrations are associated with higher local levels of ARVs in sanctuary sites, such as the lymph node, where ongoing replication has been postulated to occur. Previous studies have demonstrated that ARV concentrations in sanctuary sites (lymphatic tissues [3], genital tissues, the central nervous system [CNS]) are lower (when normalized by weight of tissue or number of cells) than in the plasma. Ongoing work in A5321 will define the relationship between local concentrations of ARVs in various reservoir sites (CNS, genital tract, rectal tissue) and local reservoir measures (eg, cell-associated RNA in the CNS) to help resolve this debate.
Of note was the wide interindividual variability in ARV hair concentrations seen among participants with sustained virologic suppression (over 100-fold for TFV and FTC) (Figure 1), indicating that current ART regimens can be highly effective despite variable cumulative exposure. Such variability has been noted previously [11], but these studies were performed in cohorts with a range of virologic outcomes, whereas A5321 participants had sustained, well-documented virologic suppression. Prior studies have not observed variability in hair ARV levels by hair color or melaninization [14]. The variability in ARV levels seen among A5321 participants is likely due to interindividual variability in ARV pharmacokinetics, but also may reflect variability in adherence over the 6 weeks of exposure measured by hair levels. A prior study examined the association between self-reported suboptimal adherence and a variety of soluble biomarkers of inflammation and immune activation, finding an inverse relationship between adherence and some biomarkers [5]. Our analysis, which assessed actual drug exposure and not adherence, did not duplicate this finding in this highly selected population. Therefore, further study of the relationship between adherence, drug exposure, and inflammation is needed in a more routine clinic population, including examining correlates of poor adherence such as stress and substance use. Finally, as observed in previous analyses, cumulative TFV concentrations are higher when regimens are boosted with ritonavir or cobicistat than without pharmacoenhancement, a finding of increasing concern because higher ARV levels may lead to greater toxicity [12].
Our study has several limitations, including the small numbers of participants examined using individual ARVs other than FTC and TFV. In addition, at the time of the A5321 entry, the most commonly used INSTI was raltegravir, so data on either elvitegravir- or dolutegravir-based therapy is unavailable. In addition, the HIV persistence metrics were measured in peripheral samples (plasma and PBMCs) only. Because A5321 enrolled participants with sustained virologic suppression, we cannot assess whether drug levels are associated with HIV persistence in patients with more variable virologic outcomes. Finally, we did not collect data on self-reported adherence in A5321, which may have helped to elucidate some of the between-patient variability observed in hair concentrations.
In conclusion, we found no significant relationship between cumulative ARV exposure in chronically suppressed HIV-infected individuals on ART, as quantified by ARV concentrations in hair spanning a 100-fold range, and measures of either viral persistence or inflammation. This lack of correlation suggests that increasing exposure to currently available ARVs would not have a significant impact on HIV-1 persistence or inflammation. However, it is not known whether ARV concentrations in hair adequately reflect ARV concentrations in tissues. Future studies need to investigate this question and the impact of higher systemic exposure to currently available INSTIs and newer ARVs on HIV persistence and inflammation. Such data are required to support future cure strategies focused on discovering more potent ARV or ARV combinations, rather than approaches that reverse HIV-1 latency and promote the clearance of stable reservoirs of HIV-infected cells [15].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We would like to acknowledge the wonderful contributions Christopher Hensel, Laboratory Data Manager for A5321, made to the study and we mourn his untimely passing. We would also like to thank A5321 participants and their families. A full list of staff and the financial support at sites that enrolled participants is given in the Supplementary Notes.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (grant numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701). Hair assays for A5321 were supported by NIAID/NIH (grant number 2R01AI098472 to M. G.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 2017. Paper 2084.
References
- 1. Gandhi M, Ameli N, Bacchetti P et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis 2011; 52:1267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moholisa RR, Schomaker M, Kuhn L et al. Effect of lopinavir and nevirapine concentrations on viral outcomes in protease inhibitor-experienced HIV-infected children. Pediatr Infect Dis J 2016; 35:e378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fletcher CV, Staskus K, Wietgrefe SW et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A 2014; 111:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bui JK, Sobolewski MD, Keele BF et al. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog 2017; 13:e1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castillo-Mancilla JR, Brown TT, Erlandson KM et al. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 2016; 63:1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nettles RE, Kieffer TL, Parsons T et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis 2006; 42:1189–96. [DOI] [PubMed] [Google Scholar]
- 7. Beumer JH, Bosman IJ, Maes RA. Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Pract 2001; 55:353–7. [PubMed] [Google Scholar]
- 8. Baxi SM, Greenblatt RM, Bacchetti P et al. ; Women’s Interagency HIV Study (WIHS) Nevirapine concentration in hair samples is a strong predictor of virologic suppression in a prospective cohort of HIV-infected patients. PLoS One 2015; 10:e0129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gandhi RT, McMahon DK, Bosch RJ et al. ; ACTG A5321 Team Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cillo AR, Vagratian D, Bedison MA et al. Improved single-copy assays for quantification of persistent HIV-1 viremia in patients on suppressive antiretroviral therapy. J Clin Microbiol 2014; 52:3944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fabbiani M, Di Giambenedetto S, Bracciale L et al. Pharmacokinetic variability of antiretroviral drugs and correlation with virological outcome: 2 years of experience in routine clinical practice. J Antimicrob Chemother 2009; 64:109–17. [DOI] [PubMed] [Google Scholar]
- 12. Cattaneo D, Baldelli S, Minisci D et al. Effects of cobicistat on tenofovir durability: is it time to rethink TAF trials?18th Workshop on Clinical Pharmacology of Antiviral Therapy, June 14–17, 2017, Chicago. Abstract O_02. [Google Scholar]
- 13. McMahon D, Jones J, Wiegand A et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis 2010; 50:912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gandhi RT, Zheng L, Bosch RJ et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med 2010; 7:pii: e1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Margolis DM, Garcia JV, Hazuda DJ, Haynes BF. Latency reversal and viral clearance to cure HIV-1. Science 2016; 353:aaf6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.