Serum respiratory syncytial virus (RSV)–specific antibody levels and viral load were correlated with disease severity in 131 infants with primary RSV infection. Higher levels of immunoglobulin G to fusion protein were associated with lower severity of illness. Viral load was not associated with severity.
Keywords: Maternal antibody, RSV infection, infants, virus load, disease severity
Abstract
Background
Maternally derived serum antibody and viral load are thought to influence disease severity in primary respiratory syncytial virus (RSV) infection. As part of the AsPIRES study of RSV pathogenesis, we correlated various serum antibody concentrations and viral load with disease severity.
Methods
Serum neutralizing antibody titers and levels of immunoglobulin G (IgG) to RSV fusion protein (F), attachment proteins of RSV group A and B, the CX3C region of G, and nasal viral load were measured in 139 full-term previously healthy infants with primary RSV infection and correlated with illness severity.
Results
Univariate analysis showed no relationship between measures of serum antibody and severity. However, a multivariate model adjusting for age at the time of infection found a significant 0.56 decrease in severity score for each 2-fold increase in antibody concentration to RSV F. The benefit of antibody was greatest in infants ≤ 2 months of age. Additionally, estimated antibody titer at birth was correlated with age at infection, suggesting that higher antibody titers delay infection. Viral load did not differ by illness severity.
Conclusion
Our data support the concept of maternal immunization with an RSV vaccine during pregnancy as a strategy for reducing the burden of RSV infection in full-term healthy infants exposed to RSV during their first winter.
Respiratory syncytial virus (RSV) is the most common cause of serious lower respiratory tract infection in the first year of life, resulting in 52000–82000 bronchiolitis hospitalizations and 22000–44000 pneumonia hospitalizations annually in the United States [1–3]. More than half of newborns are infected during their first winter, and although 1%–2% require hospitalization and approximately 15% are seen in emergency departments or physician offices, the majority have relatively mild disease [1]. Risk factors for more-severe illness include congenital heart disease, pulmonary or neurologic conditions, immunosuppression, prematurity, and young age at time of infection [3]. Less consistent risk factors include lack of breast feeding and exposure to tobacco smoke.
In addition, serum RSV-specific antibody and viral load are thought to influence clinical manifestations of RSV infection. Several studies but not all have noted that higher levels of cord blood RSV-specific antibody are associated with diminished risk of infection or delayed hospitalization [4–13]. However, only 1 study has directly shown that illness severity is diminished in infants with higher levels of serum antibody when measured at time of infection [13]. In addition, studies of the relationship between illness severity and viral load have yielded conflicting results [14–23].
The AsPIRES study of RSV disease pathogenesis was designed to identify factors associated with severe illness in healthy full-term infants during primary infection, including RSV-specific serum antibodies and nasal viral load during infection. We report results in 139 infants manifesting very mild to severe RSV disease.
METHODS
Study Subjects
RSV-infected infants were enrolled from 3 cohorts. A birth cohort was enrolled at University of Rochester Medical Center’s (URMC’s) Strong Memorial Hospital and Highland Hospital and at Rochester General Hospital (RGH) between 15 August and 1 February 2012–2015 and followed by passive and active surveillance for RSV infection during the winter (ie, from 1 November to 1 April). A supplemental cohort was enrolled in pediatric offices or emergency departments at the URMC’s Golisano Children’s Hospital or RGH when new respiratory symptoms were present at routine or illness visits. The third cohort was enrolled on admission to the hospital with documented RSV infection. Eligible infants were full-term (ie, delivered after gestation week 36), healthy infants born after 1 May and <10 months of age at infection. The institutional review boards of the University of Rochester and RGH approved the study. A parent provided written informed consent.
RSV-infected infants underwent evaluation by 2 members of the study team (a physician and a nurse). Demographic data, illness symptoms, physical findings, and results of laboratory and radiograph testing were recorded. A summary breastfeeding score was assigned, with 0 points defined as never breastfed; 1, as breastfed on <50% of occasions; 2, as breastfed on ≥50% of occasions; and 3, as exclusively breastfed. Midturbinate nasal swab specimens were obtained using a medium-sized flocked swab (catalog number 501CS01; Copan Diagnostics, Murrieta, CA) and placed in 2 mL of sterile UV-inactivated water for quantitative RSV reverse transcription polymerase chain reaction (RT-PCR) analysis. A total of 2–3 mL of heparinized blood was collected for antibody measurements. Illness severity was assigned using a 10-point continuous global respiratory severity score (GRSS), as previously described [24].
RT-PCR
The initial diagnostic RT-PCR and a quantitative RT-PCR specific for group A and B RSV were performed as previously described [25]. Viral titers are reported as plaque-forming units per milliliter equivalents.
Antibody Titers
Heparinized blood was immediately separated, and plasma was stored at −80°C. The level of IgG to RSV fusion protein (F) and attachment proteins of RSV group A (Ga) and B (Gb) was determined by enzyme immunoassay (EIA) as described elsewhere [26]. We also assessed IgG specificity to the central conserved region of G proteins, using a monoclonal antibody (mAb) blocking assay. For this assay, the Fab fragment of L9, a mAb specific for this region, was prepared according to manufacturer’s instructions (Pierce Fab Preparation Kit, catalog number 44985; Thermo Fisher Scientific, Rockford, IL) [27–29]. The L9 Fab was incubated with dilutions of plasma in a competitive EIA, using EIA plates coated with purified Ga or Gb. L9 Fab binding was detected with alkaline phosphatase–conjugated rabbit antimouse antibody, and the titer was recorded as the log2 dilution yielding 50% inhibition, denoted as the GaL9 competing antibody (Gal9CA) and the GbL9 competing antibody (GbL9CA).
Neutralization Assay
Titers were determined for the infecting RSV group, using a modification of previously described microneutralization assays (MNAs) specific to group A viruses (MNA_A) or to group B viruses (MNA_B) [26]. Because heparin directly neutralizes RSV by blocking virus attachment to glycosaminoglycans on HEp-2 cells, plasma was pretreated with heparanase (Dade Hepzyme; Siemans Healthcare Diagnostics, Marburg, Germany) for 30 minutes at 37°C, followed by heat inactivation of heparanase for 1 hour at 56°C [27]. Two-fold pretreated serum dilutions, starting at a 1:25 dilution, were incubated for 30 minutes with group A or B virus at room temperature before adding HEp-2 cells. Preliminary experiments confirmed that conversion of plasma to serum by using this method eliminated the neutralizing effect of heparin. The titers of EIA and neutralizing antibodies are reported as the log2 dilution per milliliter of serum, after adjustment using a standard serum to control for interassay and intraassay variation.
Statistical Analysis
Group comparisons and marginal correlation analyses were used to interrogate associations between clinical and laboratory variables, viral load, and GRSS. For binary variables (ie, sex, delivery mode, infecting virus group, and tobacco smoke exposure), a 2-sample Welch t test was used to determine whether there was a significant mean difference in the GRSS between 2 patient groups defined by this variable. For continuous variables (ie, age at infection, birth weight, breastfeeding score, each antibody titer, and viral load), a Spearman correlation test was used to examine dependence of 2 continuous variables. A P value of <.05 was considered statistically significant.
We also conducted bivariate linear regression analyses to study linear associations between the GRSS (the response variable) and various antibody titers (covariates) while controlling for effects of age at infection. Multivariate linear regression analyses were performed to study the collective linear relationship between the GRSS (the response variable) and important clinical variables and antibody titers (covariates). For regression analyses, regression t tests were used to determine significant associations between covariates and response variables. Additionally, we estimated antibody titers at birth on the basis of the titer at infection and then investigated the association between these estimated antibody titers at birth and age at infection by use of the Spearman correlation test.
All analyses were performed with SAS (version 9.3; SAS Institute, Cary, NC) and R programming language (version 3.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Subjects
One hundred thirty-nine RSV-infected infants were enrolled, and of these 131 had blood specimens of sufficient volume to complete antibody assays (birth cohort, n = 19; supplemental cohort, n = 37; and hospital cohort, n = 75). Overall, 81 infants (62%) were hospitalized, including 4 from the birth cohort and 2 from the supplemental cohort. Demographic and clinical characteristics of these infants are shown in Table 1 according to enrollment strategy. For the entire group, mean and median ages at infection were 3.3 and 2.8 months, respectively, with a range of 0.5 to 9.4 months. The sex of just over half was female, 28% were delivered by cesarean section, 22% were never breastfed, and 34% were exclusively breastfed. Eight percent required intensive care, 5% required ventilator support, and none died. Group A viruses were more common than group B viruses. RSV-infected infants were enrolled a mean duration (±SD) of 3.8 ± 0.2 days after illness onset.
Table 1.
Demographic, Clinical, and Viral Characteristics of 131 Respiratory Syncytial Virus (RSV)–Infected Subjects
| Characteristics | Birth Cohort (n = 19) |
Supplemental Cohort (n = 37) |
Hospital Cohort (n = 75) |
Total (n = 131) |
|---|---|---|---|---|
| Age at infection, mo | ||||
| Mean ± SE | 2.3 ± 0.2 | 4.7 ± 0.3 | 2.9 ± 0.3 | 3.3 ± 0.2 |
| Median (range) | 2.5 (0.7–4.5) | 4.4 (1.1–8.9) | 2.0 (0.5–9.4) | 2.8 (0.5–9.4) |
| Gestational age, wk | ||||
| Mean ± SE | 38.8 ± 0.3 | 39.1 ± 0.2 | 38.8 ± 0.2 | 38.9 ± 0.1 |
| Range | 37–41 | 37–42 | 36–41 | 36–42 |
| Male sex, no. (%) | 9 (47) | 18 (49) | 36 (48) | 63 (48) |
| Cesarean section delivery, no. (%) | 9 (47) | 4 (11) | 24 (32) | 37 (28) |
| Birth weight, kg, mean ± SE | 3.3 ± 0.2 | 3.3 ± 0.1 | 3.4 ± 0.1 | 3.4 ± 0.1 |
| Maternal age, y, mean ± SE | 31. 3 ± 1.2 | 28.8 ± 0.7 | 28.1 ± 0.7 | 28.8 ± 0.5 |
| Hospitalized, no. (%) | 4 (21) | 2 (6) | 75 (100) | 81 (62) |
| ICU admission, no. (%) | 1 (5) | 1 (3) | 8 (11) | 10 (8) |
| Ventilator support | 1 (5) | 1 (3) | 4(5) | 6 (5) |
| Breastfeeding | ||||
| Score,a mean ± SE | 2.0 ± 0.3 | 1.6 ± 0.2 | 1.6 ± 0.1 | 1.63 ± 0.11 |
| None, no. | 3 | 8 | 8 | 29 |
| Some, no. | 6 | 17 | 17 | 58 |
| Exclusive, no. | 10 | 12 | 12 | 44 |
| Tobacco smoke exposure, no. (%) | 0 | 13 (35) | 32 (43) | 45 (34) |
| RSV group | ||||
| Infecting RSV group, no.b | ||||
| Group A | 14 | 20 | 43 | 77 |
| Group B | 5 | 17 | 29 | 51 |
| GRSS | 2.6 ± 0.5 | 2.1 ± 0.3 | 6.2 ± 0.2 | 4.5 ± 0.2 |
Abbreviations: GRSS, Global Respiratory Severity Score; ICU, intensive care unit; SE, standard error.
aSee Methods for scoring parameters.
bInfecting RSV group could not be determined for 3 subjects in the hospital cohort.
Demographic Data, Antibody Levels, and Illness Severity
We first performed univariate analysis to assess the relationship of demographic data and each RSV antibody measurement to disease severity (Table 2). There were no differences in GRSS according to virus group, sex, or birth weight. However, a higher GRSS was significantly correlated with younger age (r = −0.32; P < .001) or a history of exposure to tobacco smoke (mean GRSS [±SD], 5.22 ± 0.37 vs 4.21 ± 0.30; P = .04). There was a trend toward increased severity among infants delivered by cesarean section as compared to those delivered vaginally (mean GRSS [±SD], 5.21 ± 0.45 vs 4.3 ± 0.28; P = .09) and among infants with lower breastfeeding scores (r = −0.14; P = .1).
Table 2.
Associations Between the Global Respiratory Severity Score (GRSS) and Demographic Characteristics and Laboratory Variables
| Variable | Correlation Coefficient | P a |
|---|---|---|
| Age at infection | −0.32 | <.001 |
| Birth weight | 0.03 | .70 |
| Breastfeeding score | −0.14 | .10 |
| Anti–RSV F titer (for all RSV infections) | 0.11 | .19 |
| Anti–RSV Ga titer (for all RSV infections) | 0.09 | .29 |
| Anti–RSV Gb titer (for all RSV infections) | 0.15 | 0.08 |
| Neutralizing antibody titer for group A RSV infections | 0.11 | .35 |
| Neutralizing antibody titer for group B RSV infections | 0.01 | .95 |
| GRSS, Mean ± SE | ||
| Sex | .55 | |
| Male | 4.41 ± 0.34 | |
| Female | 4.70 ± 0.33 | |
| Tobacco smoke exposure | .04 | |
| Yes | 5.22 ± 0.37 | |
| No | 4.21 ± 0.30 | |
| Delivery mode | .09 | |
| Vaginal | 4.30 ± 0.28 | |
| Cesarean section | 5.21 ± 0.45 | |
| RSV group | .45 | |
| A | 4.69 ± 0.31 | |
| B | 4.32 ± 0.39 |
Abbreviation: F, fusion protein; Ga, attachment protein of group A RSV; Gb, attachment protein of group B RSV; RSV, respiratory syncytial virus; SE, standard error.
aThe Spearman correlation test was used for continuous covariates, and the 2-sample Welch t test was used for binary covariates.
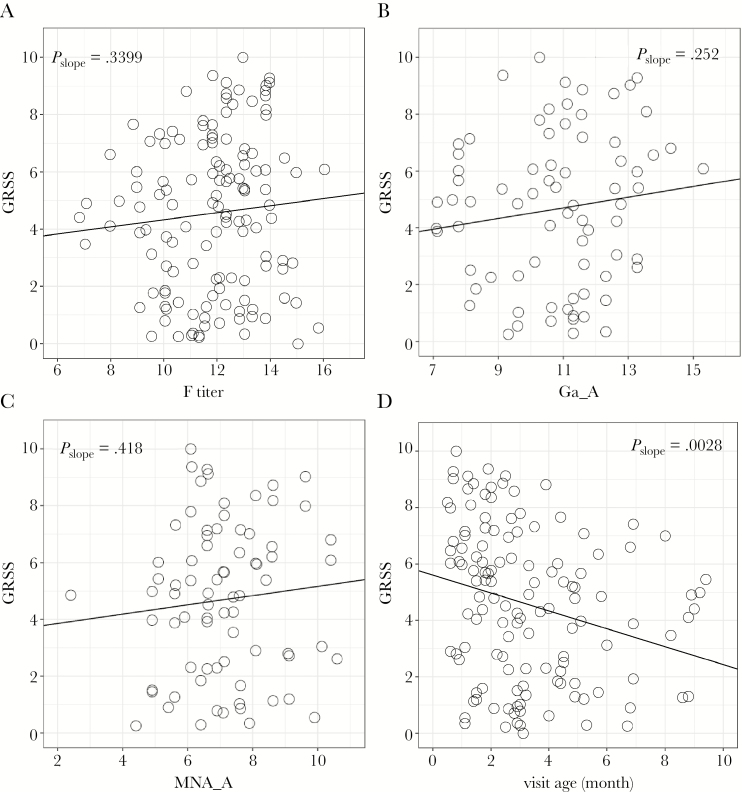
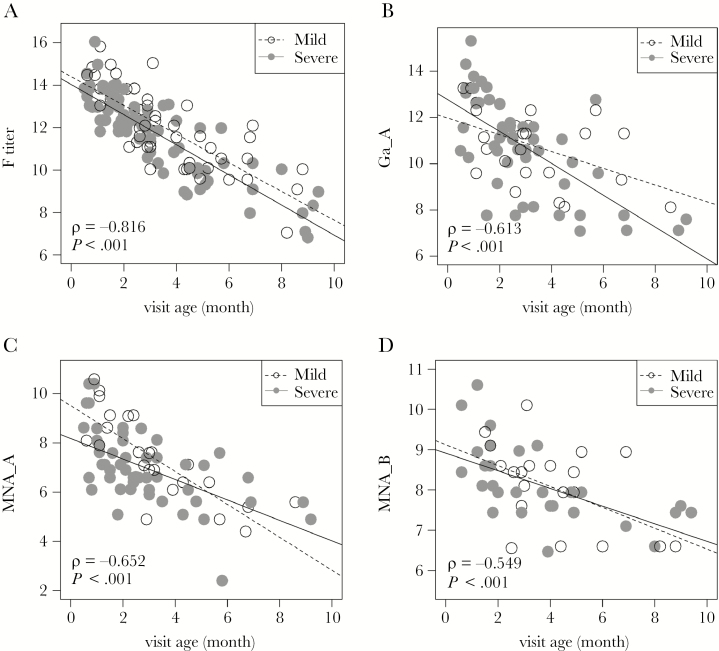
For all antibody measurements, titers increased slightly as disease severity increased, although none of the trends were significant (Figure 1, Table 2, and Supplementary Figure 1). Since RSV F is highly conserved among isolates of both major viral groups, titers of antibody to F were plotted against severity for group A and B RSV infections combined (Figure 1A). However, RSV G proteins are poorly conserved between strains, and thus antibody to G protein was plotted only for infection with the homologous virus group (Figure 1B and Supplementary Figure 1A). Similarly, neutralizing antibody titers to group A and B virus, when plotted for the homologous infecting virus group, were slightly higher as severity increased (Figure 1C and Supplementary Figure 1B). However, it should be noted that there was a significant decline in severity score with older age at the time of infection (r = −0.318; P < .003; Figure 1D) and a significant decline in antibody titers with older age at infection (P < .001 for each antigen and neutralization antibody titer; Figure 2 and Supplementary Figure 2).
Figure 1.
Relationship of the global respiratory severity score (GRSS) and anti–respiratory syncytial virus (RSV) antibody titers. Reported slope P values were calculated by a regression t test. A, Antibody to RSV fusion protein (F) for all infants. B, Antibody to attachment protein of group A RSV (Ga) for group A infections (Ga_A). C, Neutralizing antibody to group A RSV for group A infection (MNA_A). D, Relationship between GRSS and age at time of infection.
Figure 2.
Relationship of antibody titers and age at the time of infection. Disease severity was categorized as mild (global respiratory severity score [GRSS] ≤ 3.5; open circles) or severe (GRSS > 3.5; closed circles). Reported ρ and P values were calculated by the Spearman correlation test. A, Antibody to respiratory syncytial virus (RSV) fusion protein (F). B, Antibody to attachment protein of group A RSV (Ga) for group A virus infection (Ga_A). C, Neutralizing antibody to group A RSV for group A infection (MNA_A). D, Neutralizing antibody to group B RSV for group B infection (MNA_B).
Since older age was associated with both lower antibody titers and less severe illness, we performed bivariate analysis using linear regression with age at infection and each antibody titer separately as coindependent variables and GRSS as the dependent outcome variable (Table 3). In this analysis, there was a significant inverse relationship between antibody titer to RSV F and disease severity (correlation coefficient, −0.56; P = .009), with each 2-fold increase in antibody titer associated with a 0.56-unit decline in GRSS. None of the other antibody measures (Ga, Gb, GaL9CA, GbL9CA, MNA_A, and MNA_B) were significantly associated with severity in bivariate analysis (Supplementary Figure 3). When breastfeeding score and tobacco smoke exposure were included in an expanded multivariate analysis, higher antibody RSV F titers and older age at infection remained significantly and independently associated with decreased severity (P = .02 and P < .0006, respectively), while tobacco smoke exposure and breastfeeding were not significant.
Table 3.
Results of Bivariate and Multivariate Analysis of Association of Severity with Antibody Titers in Acute Illness Serum
| Analysis | Estimate | P |
|---|---|---|
| Bivariate | ||
| Analysis 1 | ||
| Age at infection | −0.70 | .0001 |
| Anti–RSV F titer | −0.56 | .009 |
| Analysis 2 | ||
| Age at infection | −0.35 | .007 |
| Anti–RSV Ga titer | −0.08 | .61 |
| Analysis 3 | ||
| Age at infection | −0.41 | .007 |
| Anti–RSV Gb titer | −0.15 | .40 |
| Analysis 4 | ||
| Age at infection | −0.44 | .019 |
| Anti–RSV Ga titer for group A infection | −0.11 | .59 |
| Analysis 5 | ||
| Age at infection | −0.21 | .35 |
| Anti–RSV Gb titer for group B infection | 0.05 | .87 |
| Analysis 6 | ||
| Age at infection | −0.55 | .005 |
| Neutralizing antibody titer for group A infection | −0.31 | .22 |
| Analysis 7 | ||
| Age at infection | −0.28 | .16 |
| Neutralizing antibody titer for group B infection | −0.23 | .63 |
| Multivariate | ||
| Age at infection | −0.64 | <.0001 |
| Antibody titer to RSV F | −0.50 | .02 |
| Breastfeeding score | −0.10 | .62 |
| History of tobacco smoke exposure | 0.56 | .29 |
Abbreviations: F, fusion protein; Ga, attachment protein of group A RSV; Gb, attachment protein of group B RSV; RSV, respiratory syncytial virus.
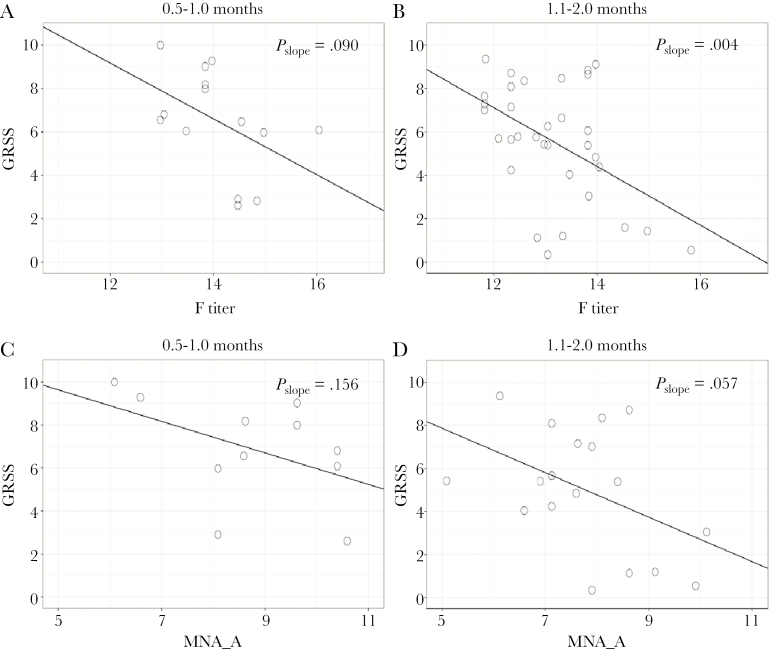
Since maternally transferred antibody titers fall continuously after birth, it seemed plausible that the beneficial effect on severity may be limited to the first few months of life, when titers are in a range typically found in adults. In univariate analysis limited to infants 0.5–2 months of age at the time of infection, there was a significant inverse relationship between severity and F-specific antibody titers (Figure 3A and 3B). For all infants ≤ 2 months of age, there was a 0.9-unit decrease in GRSS for each 2-fold increase in anti-F antibody (P = .013; Supplementary Figure 3A). There was a similar, nonsignificant trend of higher group A neutralization antibody titers in less severely ill infants between 0.5–1 month and 1–2 months (Figure 3C and 3D and Supplementary Figure 3B). We saw no such relationship with severity for Ga titers in the youngest infants, but there was a trend for higher L9FabCA titers in less ill infants ≤1 month of age (Supplementary Figure 3C and 3D). We did not find a similar relationship for neutralizing antibody titers in group B RSV infections.
Figure 3.
Relationship between antibody titer and severity (global respiratory severity score [GRSS]) for infants ≤ 2 months of age. GRSS is plotted against antibody to respiratory syncytial virus (RSV) fusion protein (F) for infants 0.5–1.0 months of age (A) and infants 1.1–2.0 months of age (B) and against neutralizing antibody for group A RSV infections (MNA_A) for the same age groups (C and D).
Estimated Antibody Titers at Birth and Time of RSV Infection
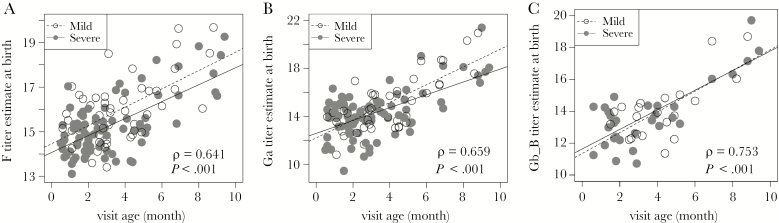
Glezen et al noted that time to hospitalization with RSV infection was directly correlated with cord blood RSV-neutralizing antibody titer [4]. Thus, we sought to determine whether our data were consistent with this relationship. Since cord blood was not available for most subjects, we estimated titers at birth by using titer at the time of infection and assuming an IgG half-life of 28 days. Using estimated titers, we found that, for each antibody titer, there was a significant direct correlation with age at infection for infants with mild disease (GRSS ≤ 3.5) and those with severe disease (GRSS > 3.5; Figure 4 and Supplementary Figure 4). It is notable that estimated neutralization antibody titers were most tightly correlated with age at infection (Supplementary Figure 4A and 4B).
Figure 4.
Relationship of estimated antibody titers at birth and time to respiratory syncytial virus (RSV) infection in mild (global respiratory severity score [GRSS] ≤ 3.5; open circles) or severe (GRSS > 3.5; closed circles) illness. The estimated antibody titer at birth is plotted against the age at which RSV infection was identified. Reported ρ and P values were calculated by the Spearman correlation test. A, Antibody to RSV fusion protein (F). B, Antibody to attachment protein of group A RSV (Ga) for group A virus infection (Ga_A). C, Antibody to attachment protein of group B RSV (Gb) for group A virus infection (Ga_B).
Viral Load and RSV Diseases Severity
Previous attempts to correlate viral load with RSV disease severity have yielded mixed results. Therefore, we analyzed the relationship between nasal viral load in the initial nasal swab samples and disease severity. Univariate analysis found no relationship between viral load and sex, RSV group, mode of delivery, tobacco smoke exposure, age at infection, or breastfeeding score (data not shown). However, viral load was significantly higher (P < .001) in samples collected earlier in the course of illness symptoms, regardless of illness severity (Supplementary Figure 1). Multivariate analysis using age at infection, tobacco smoke exposure, antibody to F, and viral load adjusted for time after illness onset showed that, while increased anti-F titer (; P = .04) and visit age (; P = .001) were significantly associated with a lower GRSS, higher viral load was not significant (P = .54).
DISCUSSION
The importance of maternally acquired antibody in primary RSV infection has been studied by a number of investigators, using a variety of antibody assays, with the goal of identifying correlates of immunity that might inform vaccine development and immunization strategies. The relationship between RSV-specific serum antibodies in young infants undergoing their first RSV infection is particularly germane to predicting success of maternal immunization during pregnancy. This strategy aims to provide protection early in life, either by modifying disease severity directly or by delaying infection until an older age when infection is more likely to be less severe. The majority of published studies used cord blood specimens or maternal samples as a proxy, rather than blood specimens collected at the time of infection, to address this question. A variety of illness outcomes were used, including incidence, time to infection, or RSV-associated hospitalization. Only a few used illness severity as the outcome. Most used a case-control study design and generally controlled for high-risk conditions expected to affect RSV infection rates or illness severity.
In a frequently cited report, Glezen et al noted that higher cord blood RSV neutralizing antibody was associated with reduced risk of hospitalization with RSV infection and was directly correlated with older age at hospitalization, suggesting that high levels of antibody at birth delayed the onset of severe illness [4]. In a more recent retrospective case-control study in >400 RSV-infected Danish infants, Stensballe et al noted an inverse correlation between the cord blood neutralizing antibody titer and the risk of RSV hospitalization and calculated a 26% reduction in hospitalization for every 2-fold increase in neutralizing antibody titer [11]. Similar results were reported in a small study of southwestern Native American infants but not in a study in native Alaskan infants [7, 10]. The serum antibody titer in specimens collected at the time of RSV infection was inversely correlated with the risk of RSV hospitalization in small case-control studies by Roca et al in Mozambique and by Piedra et al in Houston, Texas [8, 9]. However, the latter study included older children and adults experiencing secondary infections, and thus other components of adaptive immunity may have influenced the protection noted. In infants and older children with cardiopulmonary disease, Wang et al found an inverse correlation between preseason RSV antibody titers and the overall risk of RSV infection but not with hospitalization [6]. Holberg et al reported that a higher cord blood antibody titer diminished the risk of lower respiratory tract infection but only in infants from lower socioeconomic class with minimal breastfeeding [5]. In a recent case-control study from Kenya, the cord blood neutralizing antibody titer was not different between controls and those with severe RSV disease [12]. However, in a recent analysis of serum antibody titers in specimens collected at the time of RSV infection, Capella et al found that higher levels of antibody to prefusion F and G proteins but not to postfusion F were associated with lower disease severity among hospitalized infants [13]. Consistent with the literature, they noted that the majority of neutralizing antibodies in human sera are directed to epitopes on prefusion F. While most observational studies suggest beneficial effects of antibody, the most compelling evidence comes from clinical trials of hyperimmune RSV-neutralizing immunoglobulin and the neutralizing monoclonal antibody palivizumab. When administered prophylactically to high-risk infants, hospitalization was reduced by approximately half [30, 31]. Of note, palivizumab levels greater than the targeted 40 µg/mL were associated with decreased severity in high-risk infants hospitalized with RSV [32].
As noted, few studies considered illness severity as an outcome, and only 2 found a significant relationship between disease severity and antibody level [4, 13]. The primary objective of our study was to assess the relationship between levels of various RSV antibodies at the time of infection and disease severity, using the full spectrum of clinical illness, from very mild to most severe. Age at infection was the most significant factor influencing illness severity, and in marginal analysis RSV-specific antibodies were not associated with reduced severity. Because both antibody level at the time of infection and illness severity were inversely correlated with age at time of infection, we performed bivariate and multivariate linear regression modeling, using age at infection and antibody titer as variables and GRSS as the dependent variable. In this analysis, only antibody to RSV F was statistically inversely correlated with severity. When adjusted for age, each 2-fold increase in anti-F antibody level was associated with a 0.56-unit decrease in GRSS. However, when we limited the analysis to infants aged ≤2 months, the beneficial relationship between antibody and disease severity was evident for anti–F-specific antibody and neutralizing antibody in univariate analysis, with the effect greatest for anti-F antibody. This result is of interest since our EIA measures antibody binding to postfusion F that contains the neutralizing site recognized by palivizumab but does not display the more potent neutralizing sites found on prefusion F [33]. This may be relevant since a recent RSV F phase II vaccine study in women of childbearing age was shown to induce higher titers of palivizumab-like antibody, as well as neutralizing antibody [34]. This vaccine is currently in a clinical trial involving pregnant women [35].
We also sought to determine whether antibody to the central conserved region of the G proteins influenced disease severity. This region of G contains a CX3C chemokine motif that binds to the RSV receptor (CX3CR) on primary human bronchial epithelial cells and also affects migration of T cells and natural killer cells that express CX3CR [29, 36]. Administration of mAbs specific to this region of G is highly protective from virus challenge in a murine model of RSV infection, resulting in markedly reduced pulmonary inflammation [37, 38]. However, we did not find evidence that antibody binding to this region, as determined by a competitive blocking assay with a site-specific mAb, Fab, was associated with reduced severity.
Analogous to findings by Glezen et al [4], we found a direct correlation between higher estimated cord blood antibody titer and older age at the time of infection. In contrast to our severity-associated antibody analysis, this relationship was evident for each of the antibody measurements and seemed to be true for older infants also. Collectively, these data suggest that an important effect of antibody is to prevent or delay infection until an older age, when the infant is likely to be less ill. Once infection is established, the efficacy of antibody may be relatively limited, primarily to the first few months of life. However, this is important, as this is the age with the greatest risk of hospitalization. It also may be consistent with the lack of effectiveness of monoclonal antibody to treat RSV infection [39].
Several previous studies have noted a direct correlation between viral load and disease severity [13, 16, 19, 21, 40]. In earlier studies, DeVincenzo et al noted that intubated infants had a 1.15-log10 greater viral load than nonintubated hospitalized infants, and an increased nasal viral load was associated with a longer hospital stay [16, 40]. In a subsequent analysis by the same investigators, involving 219 RSV-infected infants in both inpatient and outpatient settings, viral load did not predict severity, although among those who were hospitalized, viral load on day 3 but not at enrollment was a predictor of respiratory failure [40]. However, similar to a number of other studies, our study did not reveal a correlation between higher viral load at enrollment and increased disease severity [15, 17, 18, 20, 22]. The inconsistency of results may be due to technical difficulties of precisely quantifying virus load at a mucosal surface, or perhaps optimal analysis may require sampling over several days.
This study has several limitations. The population was limited to full-term healthy infants, and thus we cannot assume that these results are predictive of similar results in premature infants or those with underlying medical conditions. Although there was no direct evidence, it is possible that a few older infants with moderately high antibody titers were having a second RSV infection. Notably, we did not measure antibody to prefusion F, which may have increased the strength of the association between antibody titer and disease severity. In addition, the use of a competitive binding assay to assess CX3C epitope antibody may not accurately reflect direct binding of serum antibodies to this epitope. Finally, our failure to associate viral load with severity may be due to not collecting multiple timed nasal specimens and to difficulty adjusting for dilutional factors in nasal swab samples.
In summary, our data suggest that RSV-specific maternally derived antibody has a modest protective effect on disease severity most evident in the first months of life but is also associated with later acquisition of infection. These results add to existing data supporting the concept of maternal immunization as a strategy for reducing the burden of RSV infections in full-term healthy infants during their first winter.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Amy Murphy, RN, Melissa Bowman, RN, Doreen Frances, RN, and Mary Criddle, RN, for enrolling and evaluating subjects; and Maryanne Formica, MT, for performing laboratory assays.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant HHSN272201200005C).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hall CB, Weinberg GA, Blumkin AK et al. . Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013; 132:e341–8. [DOI] [PubMed] [Google Scholar]
- 2. Zhou H, Thompson WW, Viboud CG et al. . Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 2012; 54:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meissner HC. Viral bronchiolitis in children. N Engl J Med 2016; 374:62–72. [DOI] [PubMed] [Google Scholar]
- 4. Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98:708–15. [DOI] [PubMed] [Google Scholar]
- 5. Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol 1991; 133:1135–51. [DOI] [PubMed] [Google Scholar]
- 6. Wang EE, Law BJ, Robinson JL et al. . PICNIC (Pediatric Investigators Collaborative Network on Infections in Canada) study of the role of age and respiratory syncytial virus neutralizing antibody on respiratory syncytial virus illness in patients with underlying heart or lung disease. Pediatrics 1997; 99:E9. [DOI] [PubMed] [Google Scholar]
- 7. Bulkow LR, Singleton RJ, Karron RA, Harrison LH; Alaska RSV Study Group Risk factors for severe respiratory syncytial virus infection among Alaska native children. Pediatrics 2002; 109:210–6. [DOI] [PubMed] [Google Scholar]
- 8. Roca A, Abacassamo F, Loscertales MP et al. . Prevalence of respiratory syncytial virus IgG antibodies in infants living in a rural area of Mozambique. J Med Virol 2002; 67:616–23. [DOI] [PubMed] [Google Scholar]
- 9. Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 2003; 21:3479–82. [DOI] [PubMed] [Google Scholar]
- 10. Eick A, Karron R, Shaw J et al. . The role of neutralizing antibodies in protection of American Indian infants against respiratory syncytial virus disease. Pediatr Infect Dis J 2008; 27:207–12. [DOI] [PubMed] [Google Scholar]
- 11. Stensballe LG, Ravn H, Kristensen K et al. . Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J Allergy Clin Immunol 2009; 123:398–403. [DOI] [PubMed] [Google Scholar]
- 12. Nyiro JU, Sande CJ, Mutunga M et al. . Absence of association between cord specific antibody levels and severe respiratory syncytial virus (RSV) disease in early infants: a case control study from coastal Kenya. PLoS One 2016; 11:e0166706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Capella C, Chaiwatpongsakorn S, Gorrell E et al. . Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis 2017; 216:1398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buckingham SC, Bush AJ, Devincenzo JP. Nasal quantity of respiratory syncytical virus correlates with disease severity in hospitalized infants. Pediatr Infect Dis J 2000; 19:113–7. [DOI] [PubMed] [Google Scholar]
- 15. Wright PF, Gruber WC, Peters M et al. . Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. J Infect Dis 2002; 185:1011–8. [DOI] [PubMed] [Google Scholar]
- 16. DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis 2005; 191:1861–8. [DOI] [PubMed] [Google Scholar]
- 17. Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis 2008; 62:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franz A, Adams O, Willems R et al. . Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol 2010; 48:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Houben ML, Coenjaerts FE, Rossen JW et al. . Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol 2010; 82:1266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luchsinger V, Ampuero S, Palomino MA et al. . Comparison of virological profiles of respiratory syncytial virus and rhinovirus in acute lower tract respiratory infections in very young Chilean infants, according to their clinical outcome. J Clin Virol 2014; 61:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hasegawa K, Jartti T, Mansbach JM et al. . Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis 2015; 211:1550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piedra FA, Mei M, Avadhanula V et al. . The interdependencies of viral load, the innate immune response, and clinical outcome in children presenting to the emergency department with respiratory syncytial virus-associated bronchiolitis. PLoS One 2017; 12:e0172953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan XL, Li YN, Tang YJ et al. . Clinical characteristics and viral load of respiratory syncytial virus and human metapneumovirus in children hospitaled for acute lower respiratory tract infection. J Med Virol 2017; 89:589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caserta MT, Qiu X, Tesini B et al. . Development of a global respiratory severity score for respiratory syncytial virus infection in infants. J Infect Dis 2017; 215:750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE, Falsey AR. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis 2013; 207:1424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falsey AR, Walsh EE. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis 1998; 177:463–6. [DOI] [PubMed] [Google Scholar]
- 27. Walsh EE, Schlesinger JJ, Brandriss MW. Purification and characterization of GP90, one of the envelope glycoproteins of respiratory syncytial virus. J Gen Virol 1984; 65 (Pt 4):761–7. [DOI] [PubMed] [Google Scholar]
- 28. Walsh EE, Falsey AR, Sullender WM. Monoclonal antibody neutralization escape mutants of respiratory syncytial virus with unique alterations in the attachment (G) protein. J Gen Virol 1998; 79 (Pt 3):479–87. [DOI] [PubMed] [Google Scholar]
- 29. Johnson SM, McNally BA, Ioannidis I et al. . Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog 2015; 11:e1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Groothuis JR, Simoes EA, Hemming VG. Respiratory syncytial virus (RSV) infection in preterm infants and the protective effects of RSV immune globulin (RSVIG). Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics 1995; 95:463–7. [PubMed] [Google Scholar]
- 31. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–7. [PubMed] [Google Scholar]
- 32. Forbes ML, Kumar VR, Yogev R, Wu X, Robbie GJ, Ambrose CS. Serum palivizumab level is associated with decreased severity of respiratory syncytial virus disease in high-risk infants. Hum Vaccin Immunother 2014; 10:2789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Graham BS, Modjarrad K, McLellan JS. Novel antigens for RSV vaccines. Curr Opin Immunol 2015; 35:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. August A, Glenn GM, Kpamegan E et al. . A Phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine 2017; 35:3749–59. [DOI] [PubMed] [Google Scholar]
- 35. RSV F vaccine maternal immunization study in healthy third-trimester pregnant women. Clinicaltrialsgov 2017; NCT02247726. [Google Scholar]
- 36. Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol 2006; 176:1600–8. [DOI] [PubMed] [Google Scholar]
- 37. Collarini EJ, Lee FE, Foord O et al. . Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol 2009; 183:6338–45. [DOI] [PubMed] [Google Scholar]
- 38. Boyoglu-Barnum S, Todd SO, Meng J et al. . Mutating the CX3C motif in the G protein should make a live respiratory syncytial virus vaccine safer and more effective. J Virol 2017; 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramilo O, Lagos R, Sáez-Llorens X et al. ; Motavizumab Study Group Motavizumab treatment of infants hospitalized with respiratory syncytial virus infection does not decrease viral load or severity of illness. Pediatr Infect Dis J 2014; 33:703–9. [DOI] [PubMed] [Google Scholar]
- 40. El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis 2011; 204:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.