Reduced influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) viruses observed in some birth cohorts of adults during the 2015–2016 influenza season may reflect long-term effects of early exposure to specific A(H1N1) viruses.
Keywords: Influenza, influenza vaccine, vaccine effectiveness
Abstract
Background
The effectiveness of influenza vaccine during 2015–2016 was reduced in some age groups as compared to that in previous 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09 virus)–predominant seasons. We hypothesized that the age at first exposure to specific influenza A(H1N1) viruses could influence vaccine effectiveness (VE).
Methods
We estimated the effectiveness of influenza vaccine against polymerase chain reaction–confirmed influenza A(H1N1)pdm09-associated medically attended illness from the 2010–2011 season through the 2015–2016 season, according to patient birth cohort using data from the Influenza Vaccine Effectiveness Network. Birth cohorts were defined a priori on the basis of likely immunologic priming with groups of influenza A(H1N1) viruses that circulated during 1918–2015. VE was calculated as 100 × [1 – adjusted odds ratio] from logistic regression models comparing the odds of vaccination among influenza virus–positive versus influenza test–negative patients.
Results
A total of 2115 A(H1N1)pdm09 virus–positive and 14 696 influenza virus–negative patients aged ≥6 months were included. VE was 61% (95% confidence interval [CI], 56%–66%) against A(H1N1)pdm09-associated illness during the 2010–2011 through 2013–2014 seasons, compared with 47% (95% CI, 36%–56%) during 2015–2016. During 2015–2016, A(H1N1)pdm09-specific VE was 22% (95% CI, −7%–43%) among adults born during 1958–1979 versus 61% (95% CI, 54%–66%) for all other birth cohorts combined.
Conclusion
Findings suggest an association between reduced VE against influenza A(H1N1)pdm09-related illness during 2015–2016 and early exposure to specific influenza A(H1N1) viruses.
(See the Editorial commentary by Cheng and Subbarao, on pages 176–8.)
In 2009, antigenically distinct influenza A(H1N1) viruses emerged to cause a pandemic. Since 2009, 2009 pandemic influenza A(H1N1) viruses (influenza A[H1N1]pdm09 viruses) have replaced A(H1N1) viruses that circulated prior to the 2009 pandemic as a cause of seasonal influenza. An early identified A(H1N1)pdm09 virus (A/California/07/09) was chosen as the reference strain for a monovalent pandemic vaccine and as the A(H1N1) component of seasonal influenza vaccines from 2010–2011 through 2016–2017. Beginning in 2009, vaccination against A(H1N1)pdm09 provided protection against A(H1N1)pdm09-associated illness across a wide age range [1–3]. A(H1N1)pdm09 viruses that continue to circulate have acquired mutations in the hemagglutinin gene that caused the emergence of new A(H1N1)pdm09 genetic groups, including 6B (predominant in 2013–2014) [4] and 6B.1 (predominant in 2015–2016) [5]. The vast majority of circulating A(H1N1)pdm09 viruses in 2013–2014 and 2015–2016 belonging to genetic groups 6B and 6B.1 were antigenically similar to the A/California/07/2009 reference virus, based on hemagglutination-inhibiting antibody (HIA) assays, using postinfection ferret antisera [4, 5]. However, during the 2015–2016 influenza season, reduced vaccine effectiveness (VE) against A(H1N1)pdm09 was observed among some age groups of patients enrolled in the Influenza Vaccine Effectiveness (Flu VE) Network study [6], and some A(H1N1)pdm09 viruses reacted poorly with human sera from vaccinated individuals [5]. To explore age-related differences in A(H1N1)pdm09-specific VE, we defined birth cohorts on the basis of exposure to different groups of A(H1N1) viruses and estimated VE according to birth cohort. We compared VE for each birth cohort during 5 influenza seasons after 2009.
METHODS
A(H1N1) Virus–Exposed Birth Cohorts
To explore the hypothesis that patients’ initial infections with specific A(H1N1) viruses might influence A(H1N1)pdm09 VE, we retrospectively constructed a series of patient birth cohorts based on likely immunologic priming with groups of A(H1N1) viruses that circulated from 1918 through 1957 and from 1977 through 2015, represented by 7 reference A(H1N1) viruses [7, 8] (Table 1 and Figure 1A). We assumed that a majority of individuals had been infected with viruses similar to the first reference A(H1N1) strain to which they were exposed. For most individuals, initial A(H1N1) virus infections likely occurred early in life; serologic data suggest that a majority of persons experience their first influenza virus infection by age 3 years [9]. Because A(H1N1) viruses did not circulate during 1957–1977, individuals born during 1958–1979 were presumed to have been exposed to A/USSR/90/1977-like viruses after reemergence of A(H1N1) viruses in 1977. For individuals born before 1955, cohorts were defined on the basis of likely exposure to A(H1N1) viruses similar to 3 epidemic strains. For individuals born from 1955 to 2003, serologic data were used to determine immunologic priming, based on elevated HIA titers against reference A(H1N1) viruses (Centers for Disease Control and Prevention [CDC], unpublished data). Serologic data indicated mixed patterns of immunologic priming during intervals between circulation of reference A(H1N1) strains. To minimize mixed patterns of immunologic priming and maintain reasonable sample sizes in each cohort, we excluded from each birth cohort individuals born during the 2 years before circulation of a new reference A(H1N1) strain. We conducted sensitivity analyses including intervals between reference A(H1N1) strains in birth cohorts.
Table 1.
Chronology of Reference Influenza A(H1N1) Viruses, Interruption of A(H1N1) Virus Circulation, and A(H1N1)-Exposed Birth Cohorts
| Year of Emergence | Reference A(H1N1) Virus | Birth Cohorta |
|---|---|---|
| 1918 | A/South Carolina/1/1918 | 1918–1931 |
| 1934 | A/Puerto Rico/8/1934 | 1934–1944 |
| 1947 | A/Fort Monmouth/1/1947 | 1947–1955 |
| 1957 | Noneb | … |
| 1958 | Nonec | … |
| 1968 | Noned | … |
| 1977 | A/USSR/90/1977 | 1958–1979 |
| 1986 | A/Taiwan/1/1986 | 1982–1991 |
| 1999 | A/New Caledonia/20/1999 | 1994–2006 |
| 2009 | A/California/7/2009(H1N1)pdm09 | 2009–2010 |
| 2013 | A/Bolivia/559/2013(H1N1)pdm09 group 6B | 2013–2014 |
| 2015 | A/Michigan/45/2015(H1N1)pdm09 group 6B.1 | 2015–2016 |
Abbreviation: pdm09, 2009 pandemic.
Birth cohorts of individuals for whom the specified reference virus was the probable influenza A(H1N1) virus to which the individuals were first exposed.
Interruption of A(H1N1) virus circulation occurred.
A(H2N2) virus circulation.
A(H3N2) virus circulation.
Figure 1.
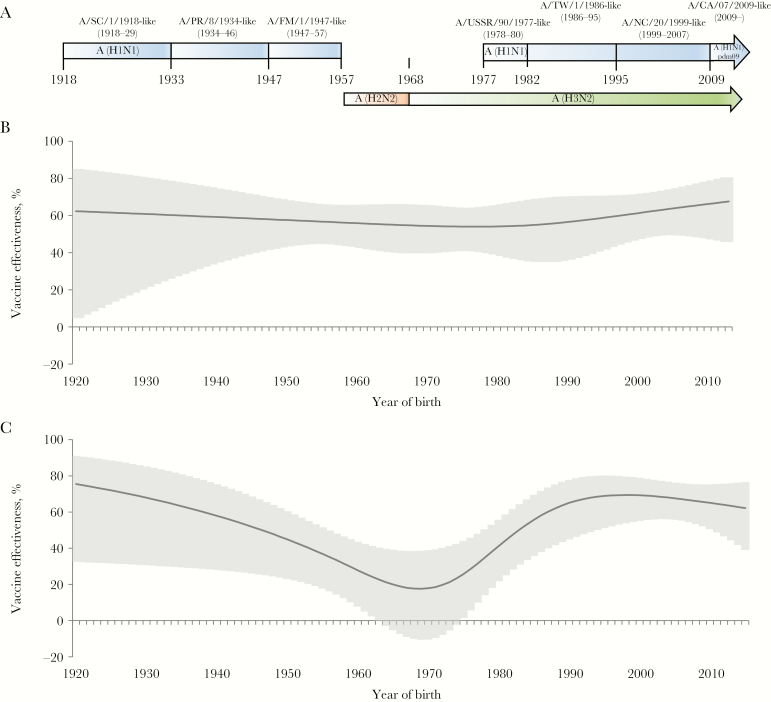
A, Approximate periods of exposure to influenza A(H1N1) viruses between 1918 and 2015, with reference viruses and periods of A(H2N2) and A(H3N2) virus circulation. B and C, Adjusted effectiveness of inactivated influenza vaccines against 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09 virus)–associated illness, by patient birth year, during the 2013–2014 season (B) and the 2015–2016 season (C). Vaccine effectiveness estimates and 95% confidence intervals were calculated with respect to patient birth year, using a cubic spline with 5 knots. CA, California; FM, Fort Monmouth; NC, New Caledonia; PR, Puerto Rico; SC, South Carolina; TW, Taiwan.
Calculation of VE
To evaluate the effectiveness of influenza vaccine with respect to A(H1N1) virus birth cohort, we analyzed data from patients enrolled in the Flu VE Network study from the 2010–2011 through the 2015–2016 seasons; the 2014–2015 season was excluded because of limited circulation of A(H1N1)pdm09 viruses. Methods for the Flu VE Network study have been described previously [3, 6]. Briefly, patients who presented to participating healthcare facilities within 7 days of illness onset and met symptom criteria (cough or fever [during 2010–2011 and 2011–2012], or at least cough [beginning in 2012–2013]) were enrolled. Demographic data were collected by study staff during an enrollment interview. Respiratory specimens (ie, nasal and throat swab specimens) were collected and tested for influenza virus at network laboratories with reverse transcription–polymerase chain reaction (RT-PCR), using a standardized protocol developed by the CDC [10]. Positive specimens were tested for influenza virus type and subtype. A(H1N1)pdm09 virus–positive patients and influenza virus–negative patients were included in this analysis. We excluded patients who tested positive for A(H3N2) or B virus infection, patients with an inconclusive RT-PCR result, and influenza virus–negative patients enrolled outside of periods of A(H1N1)pdm09 virus circulation at each site. Patients were considered vaccinated for the current season if medical or immunization records indicated receipt of ≥1 dose of inactivated influenza vaccine at least 14 days before symptom onset; patients vaccinated <14 days before illness onset and those who received live attenuated influenza vaccine were excluded. Patients aged ≥9 years were also considered vaccinated if they provided the month and location of receipt of inactivated influenza vaccine that was not documented in immunization records. The effectiveness of influenza vaccine against A(H1N1)pdm09-associated illness was calculated using a test-negative study design [11, 12], comparing odds of vaccination among cases of A(H1N1)pdm09 to the odds of vaccination among influenza virus–negative patients (controls). VE and 95% confidence intervals (CIs) were calculated as [1 − adjusted odds ratio] × 100, where adjusted odds ratios were calculated from multivariable logistic regression models including age, sex, race/ethnicity, interval from onset to enrollment, presence of any high-risk health condition, subjective general health status, study site, and calendar time (dichotomous variables representing 2-week intervals by season). We investigated trends in VE with respect to year of age, using a cubic spline function with 5 knots for age, as previously described [13]. VE against A(H1N1)pdm09-associated illness was estimated for each birth cohort born after 1933, in stratified multivariate regression models; interaction terms were included to test for statistically significant differences in birth cohort–specific VE by season. VE estimates were considered statistically significant if 95% CIs excluded 0 and interaction terms with P values of <.05 indicated statistically significant differences in VE with respect to birth cohort or season.
RESULTS
During 5 influenza seasons from 2010–2011 through 2015–2016 (excluding 2014–2015), the Flu VE Network enrolled >28000 patients with medically attended acute respiratory illness. A total of 2115 patients tested positive for A(H1N1)pdm09 virus and 14696 tested negative for influenza virus during weeks of the season with laboratory-confirmed A(H1N1)pdm09 cases. Numbers of A(H1N1)pdm09 virus–positive cases ranged from 49 in 2012–2013 to 964 in 2013–2014. Table 2 presents descriptive statistics for A(H1N1)pdm09 virus–positive cases and influenza virus–negative control patients.
Table 2.
Descriptive Characteristics of Study Participants With Illness Not Due to Influenza and Those With Illness Due to 2009 Pandemic Influenza A(H1N1) Virus (A[H1N1]pdm09 Virus), Overall and by Influenza Season(s)
| Characteristic | Influenza Virus Negative, No. (n = 14 696) | A(H1N1)pdm09 Virus Positive | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall, No. (n = 2115) | From 2010–2011 Through 2012–2013, No. (%) (n = 454) | During 2013–2014, No. (%) (n = 964) | During 2015–2016, No. (%) (n = 697) | |||||
| Site | ||||||||
| Michigan | 2597 | 423 | 143 | (31.5) | 118 | (12.2) | 162 | (23.2) |
| New York | 163 | 25 | 25 | (5.5) | 0 | 0 | ||
| Pennsylvania | 2720 | 494 | 13 | (2.9) | 261 | (27.1) | 220 | (31.6) |
| Tennessee | 374 | 67 | 67 | (14.8) | 0 | 0 | ||
| Texas | 2306 | 220 | 48 | (10.6) | 123 | (12.8) | 49 | (7.0) |
| Washington | 3212 | 329 | 68 | (15.0) | 176 | (18.3) | 85 | (12.2) |
| Wisconsin | 3324 | 557 | 90 | (19.8) | 286 | (29.7) | 181 | (26.0) |
| Age, y | ||||||||
| 0.5–8 | 3564 | 330 | 64 | (14.1) | 131 | (13.6) | 135 | (19.4) |
| 9–17 | 1919 | 165 | 51 | (11.2) | 71 | (7.4) | 43 | (6.2) |
| 18–35 | 3075 | 527 | 150 | (33.0) | 241 | (25.0) | 136 | (19.5) |
| 36–49 | 2325 | 468 | 91 | (20.0) | 223 | (23.1) | 154 | (22.1) |
| 50–64 | 2488 | 493 | 82 | (18.1) | 227 | (23.5) | 184 | (26.4) |
| ≥65 | 1325 | 132 | 16 | (3.5) | 71 | (7.4) | 45 | (6.5) |
| Male sex | 6167 | 940 | 195 | (43.0) | 426 | (44.2) | 319 | (45.8) |
| Race/ethnicitya | ||||||||
| White, non-Hispanic | 10880 | 1572 | 280 | (61.7) | 772 | (80.1) | 520 | (74.6) |
| Black, non-Hispanic | 1260 | 241 | 80 | (17.6) | 75 | (7.8) | 86 | (12.3) |
| Hispanic | 1290 | 135 | 39 | (8.6) | 61 | (6.3) | 35 | (5.0) |
| Other, non-Hispanic | 1198 | 139 | 29 | (6.4) | 56 | (5.8) | 54 | (7.7) |
| ≥1 high-risk condition | 9749 | 622 | 112 | (24.7) | 265 | (27.5) | 245 | (35.2) |
| Interval from illness onset to enrollment, d | ||||||||
| 0–2 | 4540 | 970 | 210 | (46.3) | 449 | (46.6) | 311 | (44.6) |
| 3–4 | 5782 | 765 | 172 | (37.9) | 325 | (33.7) | 268 | (38.5) |
| 5–7 | 4374 | 380 | 72 | (15.9) | 190 | (19.7) | 118 | (16.9) |
| Self-/ household exposure to smokeb | 3009 | 453 | 119 | (26.2) | 186 | (19.3) | 148 | (21.2) |
| Influenza season | ||||||||
| 2010–2011 | 2095 | 301 | 301 | (66.3) | 0 | 0 | ||
| 2011–2012 | 2171 | 104 | 104 | (22.9) | 0 | 0 | ||
| 2012–2013 | 2376 | 49 | 49 | (10.8) | 0 | 0 | ||
| 2013–2014 | 3595 | 964 | 0 | 964 | (100) | 0 | ||
| 2015–2016 | 4459 | 697 | 0 | 0 | 697 | (100) | ||
Data were missing for 96 patients.
Data were missing for 125 patients.
A total of 7002 influenza virus–negative patients (48%) and 597 A(H1N1)pdm09-positive patients (28%) had received ≥1 dose of seasonal inactivated influenza vaccine at least 14 days prior to illness onset. After adjustment for patient age, sex, race/ethnicity, and presence of high-risk conditions, VE against A(H1N1)pdm09 virus–associated illness was statistically significantly lower during the 2015–2016 season (47%; 95% CI, 36%–56%), compared with that during the 2010–2011 through 2013–2014 seasons (61%; 95% CI, 56%–66%; P < .01 for the difference in VE, by period; Table 3). VE estimates stratified by year of birth showed substantial variation during 2015–2016 (Figure 1C) but not during 2013–2014 (Figure 1B) or 2010–2013 (data not shown).
Table 3.
Adjusted Vaccine Effectiveness (VE) Estimates for Inactivated Influenza Vaccines Against 2009 Pandemic Influenza A(H1N1) Virus (A[H1N1]pdm09 Virus), by Influenza Season and Birth Cohort, 2010–2016
| Birth Cohort, Influenza Season | Age, y | A(H1N1)pdm09 Virus Positivea | A(H1N1)pdm09 Virus Negativea | Adjusted VE, % (95% CI)b | |||
|---|---|---|---|---|---|---|---|
| Overallc | |||||||
| 2010–2013 | 0.5–78 | 78/454 | (17.2) | 2979/6642 | (44.9) | 69 | (59–76) |
| 2013–2014 | 0.5–79 | 260/964 | (27) | 1765/3595 | (49.1) | 56 | (47 to 63) |
| 2015–2016 | 0.5–81 | 259/697 | (37.2) | 2258/4459 | (50.6) | 47 | (36 to 56) |
| 1934–1944 | |||||||
| 2010–2013 | 66–78 | 9/16 | (56.3) | 319/423 | (75.4) | 51 | (−82 to 87) |
| 2013–2014 | 67–79 | 34/52 | (65.4) | 228/277 | (82.3) | 51 | (−16 to 80) |
| 2015–2016 | 71–81 | 12/21 | (57.1) | 256/315 | (81.3) | 68 | (10 to 89) |
| 1947–1955 | |||||||
| 2010–2013 | 56–65 | 12/32 | (37.5) | 391/602 | (65) | 72 | (33 to 88) |
| 2013–2014 | 58–66 | 44/98 | (44.9) | 262/388 | (67.5) | 58 | (27 to 75) |
| 2015–2016 | 60–68 | 45/78 | (57.7) | 307/429 | (71.6) | 41 | (−4 to 67) |
| 1958–1979 | |||||||
| 2010–2013 | 31–54 | 31/144 | (21.5) | 739/1670 | (44.3) | 61 | (38 to 75) |
| 2013–2014 | 34–55 | 94/367 | (25.6) | 458/969 | (47.3) | 56 | (40 to 67) |
| 2015–2016 | 36–57 | 113/259 | (43.6) | 523/1063 | (49.2) | 22 | (−7 to 43) |
| 1982–1991 | |||||||
| 2010–2013 | 19–30 | 7/100 | (7) | 248/776 | (32) | 80 | (52 to 92) |
| 2013–2014 | 22–31 | 27/150 | (18) | 140/409 | (34.2) | 53 | (21 to 72) |
| 2015–2016 | 24–33 | 18/85 | (21.2) | 204/524 | (38.9) | 54 | (17 to 74) |
| 1994–2006 | |||||||
| 2010–2013 | 3–18 | 9/107 | (8.4) | 701/1993 | (35.2) | 82 | (63 to 91) |
| 2013–2014 | 7–19 | 18/145 | (12.4) | 245/739 | (33.2) | 64 | (38 to 79) |
| 2015–2016 | 9–21 | 16/87 | (18.4) | 331/895 | (37) | 63 | (32 to 79) |
| 2009– | |||||||
| 2010–2013 | 0.5–3 | 0/7 | 374/705 | (53.1) | … | ||
| 2013–2014 | 0.5–4 | 18/73 | (24.7) | 304/548 | (55.5) | 65 | (34 to 82) |
| 2015–2016 | 0.5–6 | 34/114 | (29.8) | 479/907 | (52.8) | 64 | (42 to 77) |
Data are no. of participants vaccinated against A(H1N1)pdm09 virus/no. evaluated (%).
Logistic regression models adjusted for site, age (natural cubic spline, using age in months at enrollment), high-risk status, interval from onset to enrollment, calendar time (biweekly intervals and season), sex, and race/Hispanic ethnicity.
Patients born before 1934 were excluded from the birth cohort analysis, owing to small numbers of A(H1N1)pdm09 virus–positive patients.
VE, by A(H1N1)-Exposed Birth Cohort
For analysis of the A(H1N1)pdm09 VE stratified by birth cohort, we combined data from the 2010–2011 season through the 2012–2013 season. We identified one cohort, born during 1958–1979 (patient age range during the 5 influenza seasons, 31–57 years), with a statistically significantly lower VE against A(H1N1)pdm09-associated illness during the 2015–2016 season (22%; 95% CI, −7%–43%) as compared to that from 2010–2011 through 2012–2013 (61%; 95% CI, 38%–75%; P < .01 for the difference in VE) and during 2013–2014 (56%; 95% CI, 40%–67%; P < .01 for the difference in VE; Table 3 and Figure 2). Exclusion the 1958–1979 birth cohort yielded an overall VE against A(H1N1)pdm09-associated illness of 61% (95% CI, 54%–66%) during 2015–2016.
The 1958–1979 birth cohort includes those whose initial exposure to influenza A(H1N1) viruses would not have occurred until 20 years of age. For subsets of this cohort, initial exposure to influenza A viruses would have been to A(H2N2) and A(H3N2) viruses (Table 1 and Figure 1A). A reduced VE against A(H1N1)pdm09 during 2015–2016 was observed in patients born during 1958–1966 (13%; 95% CI, −43%– 46%), who were initially exposed to A(H2N2) virus, and among those born during 1968–1975 (30%; 95% CI, −21%–60%), who were initially exposed to A(H3N2) virus prior to reemergence of A(H1N1) viruses. For patients born during 1977–1979, who were likely exposed to A(H1N1) during their initial infection, the estimated A(H1N1)pdm09-specific VE during 2015–2016 was 26% (95% CI, −116%–75%).
Reduced VE against A(H1N1)pdm09-associated illness during 2015–2016 was not observed in A(H1N1)-exposed birth cohorts born before 1955 or after 1981 (Table 3 and Figure 1C). During 2015–2016, the adjusted VE against A(H1N1)pdm09-associated illness was 68% (95% CI, 10%–89%) among patients born during 1934–1944 (age range, 71–81 years) and was not statistically significantly lower (41%; 95% CI, −4%–67%; P = .2 for the difference in VE stratified by birth cohort) among those born during 1947–1955 (age range, 60–68 years). Among younger cohorts, the VE against A(H1N1)pdm09 was 54% (95% CI, 17%–74%) among patients born during 1982–1991 and 63% (95% CI, 32%–79%) among those born during 1994–2006. In these birth cohorts, VE estimates against A(H1N1)pdm09 viruses were similar in 2013–2014, when 99% of A(H1N1)pdm09 viruses characterized by US virologic surveillance belonged to HA genetic group 6B, and in 2015–2016, when 93% of A(H1N1)pdm09 viruses belonged to genetic group 6B.1 and only 6% belonged to group 6B. Among children aged ≥6 months born in or after 2009, the adjusted VE against A(H1N1)pdm09-associated illness was 65% (95% CI, 34%–82%) during 2013–2014 and 64% (95% CI, 42%–77%) during 2015–2016.
DISCUSSION
In this analysis of 5 seasons of data from the Flu VE study, the VE against A(H1N1)pdm09-associated illness was significantly lower in 2015–2016 than during the 4 seasons following the 2009 pandemic. Decreased VE was consistent with observations that circulating A(H1N1)pdm09 viruses in 2015–2016 (belonging to genetic group 6B.1) were poorly inhibited by some groups of postvaccination adult human sera [5]. As a result, the A(H1N1) vaccine component was updated to an A/Michigan/45/2015 group 6B.1 virus for the 2017 southern hemisphere [14] and 2017–2018 northern hemisphere [15] vaccine formulations. The need to update the A(H1N1)pdm09 vaccine component was not indicated by traditional hemagglutination inhibition assays, using postinfection ferret antisera, which did not distinguish between the A/California/07/2009(H1N1)pdm09 reference strain and circulating A(H1N1)pdm09 group 6B and 6B.1 viruses [14]. Reduced VE associated with changes in circulating viruses may signal the need for updating vaccine components even when antigenic changes are not detected with ferret antisera. These results reinforce the need for human serologic data and VE studies, in addition to traditional ferret studies, to monitor changes in influenza viruses and inform vaccine strain selection.
Compared to the A(H1N1)pdm09-specific VE from 2010–2011 through 2013–2014, a significantly lower VE against A(H1N1)pdm09-associated illness during the 2015–2016 season appeared to be attributable to a reduced VE in the age cohort of adults born between 1958 and 1979. Adults aged 36–57 years accounted for nearly one third of confirmed A(H1N1)pdm09 cases among Flu VE Network enrollees during 2015–2016, and the decreased VE in this age cohort contributed to a lower overall VE against A(H1N1)pdm09-related illness compared to previous seasons. The decreased VE was specific to individuals in the affected cohort; excluding individuals born during 1958–1979 from the analysis, the VE against A(H1N1)pdm09 in 2015–2016 (approximately 60%) was similar to VE estimates in previous postpandemic seasons. Based on the hypothesis that initial infections with influenza A viruses may also provide heterosubtypic protection [16], we compared the VE among individuals in the 1958–1979 birth cohort, who were likely exposed to initial infection with A(H2N2) or A(H3N2) viruses prior to A(H1N1) viruses. However, we did not observe statistically significant differences in VE in these 2 groups and observed similar point estimates for VE (despite wide 95% CIs) for the subgroup born 1977–1979, which was likely exposed to A(H1N1) virus during their initial infection. The association with birth cohort was observed after the emergence of A(H1N1)pdm09 group 6B.1; the VE did not vary significantly by age cohort during the 2013–2014 season, when nearly all A(H1N1)pdm09 viruses were group 6B viruses.
An association between reduced VE and birth cohort was also observed during the 2015–2016 influenza season in Canada, where the overall VE against A(H1N1)pdm09 was 43% but the VE among adults born during 1957–1976 was 25% [17]. However, the association with birth cohort was not identified by other VE studies in 2015–2016, in which A(H1N1)pdm09 group 6B.1 viruses predominated, including among hospitalized adults in the United States (CDC, unpublished data). Interim 2015–2016 VE estimates from studies in the United Kingdom [19] and Europe [20] reported a VE against A(H1N1)pdm09 similar to that seen in previous seasons. A higher vaccination coverage among US adults in this age group and a larger sample size may have aided detection of this association between VE and birth cohort in the Flu VE Network study. Because birth cohort divisions are based on hypothesized time of initial infection but actual timing of infection varies among individual patients, larger numbers of patients may be needed to observe differences in VE stratified by birth cohort. On the other hand, the observed variability may have occurred by chance.
Limited serologic data suggest that some adults born during 1958–1979 (age range in 2015–2016, 36–57 years) have decreased antibody titers against A(H1N1)pdm09 group 6B and 6B.1 viruses [21, 22]. In this birth cohort, many individuals show immunologic priming with A/USSR/90/1977-like viruses, the first group of A(H1N1) viruses to which persons born during 1958–1979 were exposed. In some A/USSR/90/1977-primed individuals, vaccination with A(H1N1)pdm09 viruses may induce antibodies against shared epitopes present on early A(H1N1)pdm09 viruses [21]. Amino acid or glycosylation changes in these specific epitopes later A(H1N1)pdm09 viruses from genetic groups 6B and 6B.1 may result in decreased antibody titers in humans. In one analysis by Linderman et al [22], low reactivity to A(H1N1)pdm09 group 6B viruses (including those with an K163Q mutation) was observed in 42% of individuals born during 1965–1979 (and likely exposed to A/USSR/90/1977). In a prospective study, Petrie et al [23] found that decreased HIA titers to A(H1N1)pdm09 group 6B viruses predicted higher A(H1N1)pdm09 attack rates in 2013–2014, compared with HIA titers of ≥1:40 against A(H1N1)pdm09 group 6B viruses. However, the majority of individuals with HIA titers of ≥1:40 against A/California/07/2009 also had titers of ≥1:40 against group 6B viruses [23]. The lack of an observed association between birth cohort and A(H1N1)pdm09-specific VE during 2013–2014, when group 6B predominated, suggests that additional changes in 6B.1 viruses may have contributed to the age effect observed during 2015–2016. Specifically, the addition of a glycosylation site at HA position 162 in group 6B.1 viruses may further reduce or block antibody binding to the epitope containing Q163, resulting in reduced VE among groups of adults with dominant antibody responses targeting this epitope.
Initial exposures to specific influenza viruses may have long-lasting effects on immune responses [24, 25]. Postvaccination response is dominated by the expansion of preexisting memory B cells; a high serum titer before vaccination results in a more dominant boost effect of preexisting antibodies and the emergence of fewer vaccine antigen-elicited antibodies [26]. The A(H1N1)pdm09 pandemic was marked by elevated attack rates in children and young adults, who lacked cross-reactive antibodies to A(H1N1)pdm09 viruses. In contrast, a substantial percentage of adults aged >60 years had cross-reactive antibodies that provided protection against A(H1N1)pdm09-related illness [27, 28]. Cross-reactive antibodies in older adults were likely the result of infection early in life with A(H1N1) viruses with which A(H1N1)pdm09 viruses shared antigenic characteristics [29].
Although birth cohort effects on antibody responses were described by Francis in 1960 [30], there have been few reports of the association between reduced VE and birth cohort [17]. Age-specific differences in VE have been frequently observed, with the typical pattern being lower VE among adults aged ≥65 years [13]. However, the attribution of a lower VE to a birth cohort effect is subject to several limitations. First, like all observational studies of VE, differences between groups of vaccinated and unvaccinated individuals may influence results, and adjustments may not control all potential sources of confounding. Second, analyses of Flu VE Network data stratified by birth cohort resulted in small sample sizes that reduce the precision around VE estimates. This limits our ability to detect smaller age cohort effects, especially for the seasons prior to 2013–2014, when few confirmed patients with A(H1N1)pdm09-related illness were enrolled. In addition, patterns of immunologic priming with seasonal A(H1N1) viruses, especially before 1957, are based on limited serologic data, while relative proportions of individuals primed to reference strains during specific periods are unknown. More-recent human serologic data suggest overlapping patterns of priming to A(H1N1) viruses during antigenic transitions (CDC, unpublished data); for this reason, we excluded birth cohorts of individuals born ≤2 years before A(H1N1) virus transitions, but some misclassification of priming patterns is unavoidable. Inclusion of transition years in birth cohorts resulted in similar estimates (Supplementary Table 1). Previous studies have suggested that vaccination in years prior to the current year may influence VE [13, 31, 32]. However, we observed a reduced VE in the 1958–1979 birth cohort whether or not the individual had been vaccinated in the prior season (data not shown). Finally, Flu VE Network populations may differ from the US population in ways related to exposures to earlier A(H1N1) viruses, and we cannot exclude the possibility that the observed associations with birth cohort were due to factors other than early exposures to A(H1N1) viruses.
Replacement of the A/California/07/2009(H1N1)pdm09 vaccine reference strain with A/Michigan/45/2015 (group 6B.1) should lead to improved VE against circulating A(H1N1)pdm09 viruses. Ongoing monitoring of VE against A(H1N1)pdm09-related illness will evaluate whether the improved antibody response to circulating A(H1N1)pdm09 viruses is enough to overcome the effects of immunologic priming in the affected birth cohorts [30, 33].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention through cooperative agreements with the Kaiser Permanente–Washington Health Research Institute (U01 IP000466), the University of Michigan (U01 IP000474), the Marshfield Clinic Research Institute (U01 IP000471), Baylor Scott and White Health (U01 IP000473), the University of Pittsburgh (U01 IP000467), Vanderbilt University (U01 IP000184), and the University of Rochester (U01 IP000183); by the National Institutes of Health (grant UL1TR001857 to the University of Pittsburgh); and by the National Center for Advancing Translational Sciences (award UL1TR000445 to the Vanderbilt University Medical Center).
Potential conflicts of interest. M. L. J. has received research support from Sanofi Pasteur unrelated to the submitted work. A. S. M. has received grants from the Centers for Disease Control and Prevention (CDC) and Sanofi Pasteur and has served as a consultant for GSK, Sanofi Pasteur, Novavax, Novartis, and Protein Sciences. E. A. B. and H. Q. M. have received grants from the CDC and MedImmune. M. G. received grant funding from the CDC during the conduct of this study and grants from MedImmune/AstraZeneca outside the submitted work. R. K. Z. has received grants from Sanofi Pasteur, Pfizer, and Merck. M. P. N. received grants from Pfizer and Merck. H. K. T. has received research funding from the National Institutes of Health, the CDC, MedImmune, Sanofi, and Gilead and has served as an advisor for Vax Innate and Sequirus. All other authors report no potential conflicts.
Presented in part: 9th Meeting of Options for the Control of Influenza, Chicago, Illinois, 24–28 August 2016.
References
- 1. Gaglani M, Pruszynski J, Murthy K et al. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013–2014 in the United States. J Infect Dis 2016; 213:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Griffin MR, Monto AS, Belongia EA et al. ; U.S. Flu-VE Network Effectiveness of non-adjuvanted pandemic influenza A vaccines for preventing pandemic influenza acute respiratory illness visits in 4 U.S. communities. PLoS One 2011; 6:e23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Treanor JJ, Talbot HK, Ohmit SE et al. ; US Flu-VE Network Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012; 55:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Recommended composition of influenza virus vaccines for use in the 2015 southern hemisphere influenza season. Weekly Epidem Rec 2014; 89:441–52. [PubMed] [Google Scholar]
- 5. Recommended composition of influenza virus vaccines for use in the 2016–2017 northern hemisphere influenza season. Weekly Epidem Rec 2016; 91:121–32. [PubMed] [Google Scholar]
- 6. Jackson ML, Chung JR, Jackson LAet al. Influenza vaccine effectiveness in the United States--2015/16 season. N Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang SS, Lin Z, Banner D et al. Immunity toward H1N1 influenza hemagglutinin of historical and contemporary strains suggests protection and vaccine failure. Sci Rep 2013; 3:1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nelson MI, Viboud C, Simonsen L et al. Multiple reassortment events in the evolutionary history of H1N1 influenza A virus since 1918. PLoS Pathog 2008; 4:e1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bodewes R, de Mutsert G, van der Klis FR et al. Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands. Clin Vaccine Immunol 2011; 18:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spencer S, Chung J, Thompson M et al. Factors associated with real-time RT-PCR cycle threshold values among medically attended influenza episodes. J Med Virol 2016; 88:719–23. [DOI] [PubMed] [Google Scholar]
- 11. Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013; 31:3104–9. [DOI] [PubMed] [Google Scholar]
- 12. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 13. McLean HQ, Thompson MG, Sundaram ME et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis 2015; 211:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Recommended composition of influenza virus vaccines for use in the 2017 southern hemisphere influenza season. Weekly Epidem Rec 2016; 91:469–84. [PubMed] [Google Scholar]
- 15.Recommended composition of influenza virus vaccines for use in the 2017–2018 northern hemisphere influenza season. Weekly Epidem Rec 2017; 92:117–28. [PubMed] [Google Scholar]
- 16. Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 2016; 354:722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skowronski D, Chambers C, Sabaiduc S et al. Beyond antigenic match: possible agent-host and immuno-epidemiological influences on influenza vaccine effectiveness during the 2015–16 season in Canada. J Infect Dis 2017; 216:1487–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chambers C, Skowronski DM, Sabaiduc S et al. Interim estimates of 2015/16 vaccine effectiveness against influenza A(H1N1)pdm09, Canada, February 2016. Eurosurv 2016; 21:30168. [DOI] [PubMed] [Google Scholar]
- 19. Pebody R, Warburton F, Ellis J et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Eurosurv 2016; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kissling E, Valenciano M. Early influenza vaccine effectiveness results 2015–16: I-MOVE multicentre case-control study. Eurosurv 2016; 21. [DOI] [PubMed] [Google Scholar]
- 21. Huang KY, Rijal P, Schimanski L et al. Focused antibody response to influenza linked to antigenic drift. J Clin Invest 2015; 125:2631–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linderman SL, Chambers BS, Zost SJ et al. Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013-2014 influenza season. Proc Natl Acad Sci U S A 2014; 111:15798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petrie JG, Parkhouse K, Ohmit SE, Malosh RE, Monto AS, Hensley SE. Antibodies against the current influenza A(H1N1) vaccine strain do not protect some individuals from infection with contemporary circulating influenza A(H1N1) virus strains. J Infect Dis 2016; 214:1947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ross TM, Lin CJ, Nowalk MP et al. Influence of pre-existing hemagglutination inhibition titers against historical influenza strains on antibody response to inactivated trivalent influenza vaccine in adults 50-80 years of age. Hum Vaccin Immunother 2014; 10:1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Webby R. Understanding immune responses to the influenza vaccine. Nat Med 2016; 22:1387–8. [DOI] [PubMed] [Google Scholar]
- 26. Lee J, Boutz DR, Chromikova V et al. Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat Med 2016; 22:1456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hancock K, Veguilla V, Lu X et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945–52. [DOI] [PubMed] [Google Scholar]
- 28. Reed C, Katz JM, Hancock K, Balish A, Fry AM; H1N1 Serosurvey Working Group. Prevalence of seropositivity to pandemic influenza A/H1N1 virus in the United States following the 2009 pandemic. PloS One 2012; 7:e48187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu R, Ekiert DC, Krause JC, Hai R, Crowe JE Jr, Wilson IA. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 2010; 328:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Francis T. On the doctrine of original antigenic sin. Proc Amer Phil Soc 1960; 104:572–8. [Google Scholar]
- 31. Ohmit SE, Thompson MG, Petrie JG et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skowronski DM, Chambers C, Sabaiduc S et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monto AS, Malosh RE, Petrie JG, Martin ET. The doctrine of original antigenic sin: separating good from evil. J Infect Dis 2017; 215:1782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.