Abstract
Context
Pregnancy-associated plasma protein A2 (PAPPA2) is a protease that cleaves IGF-binding protein (IGFBP)-3 and IGFBP-5, liberating free IGF-I. Five patients from two families with genetic mutations in PAPPA2 presented with growth retardation, elevated total IGF-I, and IGFBP-3 but decreased free IGF-I.
Objective
To determine whether plasma transfusion or recombinant human (rh)PAPPA2 could increase free IGF-I in patients with PAPPA2 deficiency or idiopathic short stature (ISS).
Design
Single patient interventional study combined with in vitro experimentation.
Setting
Academic medical center.
Patients
Three siblings with PAPPA2 deficiency and four patients with ISS.
Interventions
An adult female with PAPPA2 deficiency received a 20 mL/kg plasma transfusion. PAPPA2, intact IGFBP-3, and free and total IGF-I levels were monitored during 2 weeks. rhPAPPA2 was added to serum from patients with PAPPA2 deficiency and ISS in vitro for 4 hours. Intact IGFBP-3 and free IGF-I levels were assayed via ELISA.
Main Outcome Measures
Free IGF-I concentrations.
Results
Plasma transfusion resulted in a 2.5-fold increase of free IGF-I levels on day 1 posttransfusion with a return to baseline during a 2-week period. In vitro studies demonstrated a dose-dependent increase in free IGF-I and decrease in intact IGFBP-3 after the addition of rhPAPPA2. The increase in free IGF-I was more pronounced in patients with PAPPA2 deficiency compared with those with ISS.
Conclusions
PAPPA2 plays a key role in regulation of IGF-I bioavailability. rhPAPPA2 is a promising therapy to increase free IGF-I levels both in patients with PAPPA2 deficiency as well as in patients with ISS.
Keywords: IGF-I, PAPPA2, short stature
PAPPA2 was given to a female with PAPPA2 deficiency via plasma transfusion, and recombinant PAPPA2 was added to serum from patients with PAPPA2 deficiency or ISS, resulting in increased free IGF-I.
The GH/IGF-I axis plays an essential role in human growth. Multiple genetic defects have been found throughout this axis that lead to significantly impaired growth, associated with IGF-I deficiency or IGF-I resistance [1]. IGF-I is the major mediator of GH action and is produced by the liver as well as locally in the growth plate. IGF-I circulates in ternary complex with IGF-binding proteins (IGFBPs) and the 85-kDa acid labile subunit (ALS). The binding of IGF-I to IGFBPs is requisite for association with ALS, as neither IGF-I nor IGFBP can independently associate with ALS [2, 3]. Of the six IGFBPs [4], IGFBP-3 is the main binding protein found in the ternary complexes, although IGFBP-5 also readily associates with both IGF-I and ALS [5]. Although the primary purpose of the ternary complex has been posited to increase the half-life of IGF-I in circulation, these binding proteins may also play an important role in the regulation of IGF-I bioavailability [6].
Levels of unbound, free IGF-I available for biological actions have been investigated in a number of populations, including children [7–10]. It is estimated that free IGF-I represents between 0.4% and 2% of total IGF-I in the serum [7, 8, 10]. Free IGF-I levels increase throughout childhood and peak in puberty, after which the levels fall [8–10]. Diagnostically, free IGF-I levels appear to be a sensitive indicator of GH deficiency but do not provide much additional information in the diagnostic workup beyond that of total IGF-I levels [8–10]. In one study of 16 prepubertal children treated with GH for GH deficiency, free IGF-I levels rapidly increased after the initiation of therapy and to a greater extent than did either total IGF-I or IGFBP-3 levels [10]. Interestingly, the percentage increase in free IGF-I levels after 1 month of treatment was a significant predictor of the increase in height velocity, whereas the increase in either total IGF-I or IGFBP-3 levels had no significant correlation. Taken together with their finding that peak free IGF-I levels correspond to the age of peak height velocity during puberty, the authors suggested that free IGF-I is a key biological mediator of GH action [10]. In another study involving 63 children treated with GH, the molar ratio of IGF-I to IGFBP-3, a proxy for free IGF-I levels, was significantly increased in children with an adequate response to GH therapy compared with nonresponders. These studies, albeit limited, support the hypothesis that increasing free IGF-I levels should lead to increased growth [11].
Pregnancy-associated plasma protein A2 (PAPPA2) is a metalloproteinase that specifically cleaves IGFBP-3 and IGFBP-5, thereby liberating IGF-I from its ternary complex, making it available for binding to cell surface IGF-I receptors and initiating growth-promoting activities [12]. Pappa2 knockout mice have postnatal growth retardation with elevated levels of total IGF-I and low levels of free IGF-I [13, 14]. Recently, the first human patients with genetic defects in PAPPA2 were reported [14]. Similar to the mouse model, the five affected children from two unrelated families presented with postnatal growth retardation and elevated levels of total IGF-I and IGFBPs, but low levels of free IGF-I and serum IGF bioactivity. The two identified homozygous mutations were shown to abrogate PAPPA2 protease function, resulting in a significant decrease in IGF-I bioavailability despite markedly increased total IGF-I levels [14]. These patients provide convincing human genetic evidence that PAPPA2 plays an important role in regulating IGF-I bioavailability and that defects in its function can affect human growth. Further human genetic evidence comes from large genome-wide association studies in which both PAPPA2 and its related gene, PAPPA, were identified to have nearby common genetic variants associated with adult stature in the general population [15]. Additionally, a more recent genome-wide association study found that rare damaging missense variants in stanniocalcin 2, an inhibitor of PAPPA and PAPPA2 [16], are associated with increased adult height [17]. It is thought that a lack of stanniocalcin 2 activity should lead to increased PAPPA and PAPPA2 activity, resulting in increased IGF-I bioavailability and thus increased growth.
Based on these insights, we sought to provide proof of principle evidence that PAPPA2 could serve as a novel growth-promoting therapeutic agent both in patients with PAPPA2 deficiency as well as in patients with idiopathic short stature (ISS). To achieve this goal, we took two complementary approaches. First, we performed a plasma transfusion treatment in a single adult patient with PAPPA2 deficiency to assess the biochemical response of PAPPA2 replacement therapy in vivo. Second, we performed in vitro studies in which we combined recombinant human (rh)PAPPA2 with serum from three patients with PAPPA2 deficiency as well as four patients with ISS and evaluated impact on integrity of IGFBPs and free IGF-I levels.
1. Materials and Methods
A. Subjects
Three patients (P1, female; P2 and P3, males) with a homozygous p.Ala1033Val missense mutation in PAPPA2 were included in this study. Details of their clinical presentation have been previously reported [14, 18]. We have previously demonstrated that this mutation leads to a PAPPA2 protein that is expressed at lower levels than wild-type and lacks any proteolytic activity [14]. Written informed consent was obtained from P1 and from the parents of P2 and P3, and written assent was obtained from P2 and P3. All procedures were performed as part of a research protocol approved by the Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board. P1, P2, and P3 had blood drawn for serum isolation and subsequent in vitro rhPAPPA2 enzyme activity experiments. The serum samples used for this study were obtained at ages 19 years, 14 years, and 10 years, respectively. Serum was isolated and stored at −80°C.
Patients diagnosed with ISS were recruited as part of a previously reported study examining the genetic basis of ISS [19]. Sera, isolated from blood samples, were stored in aliquots at −80°C. The four male ISS subjects were of comparable ages to subjects P2 and P3.
B. Plasma Transfusion Protocol
The plasma transfusion protocol was registered at ClinicalTrials.gov (no. NCT02412943). Fifteen samples of fresh-frozen plasma (FFP) were obtained from the local blood bank. PAPPA2 was measured by ELISA (Ansh Laboratories, Houston, TX). PAPPA2 was detectable in all the FFP samples with a mean value of 0.28 ± 0.06 ng/mL. Based on these data, it was estimated that a 20 mL/kg FFP transfusion would lead to an increase in serum PAPPA2 levels of ~0.11 ng/mL, assuming a plasma volume of 50 mL/kg and retention of all of the PAPPA2 in the plasma space. The analytical sensitivity of the PAPPA2 ELISA is 0.07 ng/mL.
P1 was given a total of 772 mL of FFP (19.3 mL/kg) from four separate units of FFP. PAPPA2 concentrations were measured from aliquots of each unit of FFP and ranged from 0.141 to 0.309 ng/mL. In total, the patient received ~170 ng of PAPPA2. At the time of the transfusion, P1’s hematocrit was 39%. Using an estimated total blood volume of 65 mL/kg for an adult female, her plasma volume was estimated to be 1586 mL [65 mL/kg × 40 kg × (1 − 0.39)]. Assuming that all of the transfused PAPPA2 remained within the plasma space, we calculated that her plasma PAPPA2 concentration should have increased by 0.11 ng/mL. Using a similar calculation, the expected increases in total and free IGF-I concentrations due to the transfusion are 119 ng/mL and 3.5 ng/mL, respectively.
At the time of the transfusion protocol, P1 was 18 years old. All study visits were performed in the outpatient clinical research center at CCHMC. On day −1, a baseline sample was obtained for a complete blood count, blood type and cross, total IGF-I, total IGFBP-3, PAPPA2, and free IGF-I. On day 0, repeat baseline measures were obtained and then the subject received a 20 mL/kg transfusion of FFP. She was observed in the clinic for 2 hours posttransfusion with no adverse events noted. She then returned to the outpatient clinic on days 1, 3, 7, 11, and 14 for repeat measurements of IGF-I, IGFBP-3, PAPPA2, and free IGF-I. The total IGF-I and IGFBP-3 levels for the transfusion protocol were performed in the CCHMC clinical laboratories using IDS-iSYS chemiluminescent immunoassays (Immunodiagnostic Systems, Tyne & Wear, United Kingdom).
C. rhPAPPA2 Expression and Purification
Wild-type PAPPA2 (Myc-DDK tagged)–human pappalysin 2 (PAPPA2), transcript variant 1 (NM_020318) in pCMV6 (Origene, Rockville, MD) was maintained in DH5-αEscherichia coli (Invitrogen, Carlsbad, CA). Plasmids were isolated with a plasmid maxi kit (Qiagen, Valencia, CA), and PAPPA2 Sanger sequencing was performed to confirm the presence of the transcript. HEK293T cells were cultured in 10% fetal bovine serum/DMEM at 37°C in a humidified incubator with 5% CO2. At 60% to 70% confluence, HEK293 cells were transfected with pCMV6-PAPPA2 plasmid (35 μg per p150) using PolyJet transfection reagent (SignaGen, Rockville, MD), following the manufacturer’s protocol. After 24 hours, transfected cells were placed in serum-free media (0.1% BSA/DMEM) for 48 to 72 hours. Conditioned media were collected and combined with equal parts 1× PBS and passed over a 100-kDa cutoff Amicon Ultra-15 centrifugal filter (Millipore, Darmstadt, Germany) twice to purify PAPPA2. The purity of rhPAPPA2 was analyzed by immunoblots. Proteins in conditioned media were separated on 4% to 20% SDS gradient gels (Bio-Rad Laboratories, Hercules, CA), transferred to nitrocellulose membranes (GE Healthcare/Amersham Biosciences, Germany), and the membrane was first stained with a MemCode reversible protein stain kit (Pierce, Rockford, IL) to check general protein profile prior to immunoblotting with anti-PAPPA2 mouse monoclonal antibody (Abcam, Cambridge, MA) [20]. A human PAPPA2 ELISA was employed to measure the concentration of rhPAPPA2. Isolated rhPAPPA2 protein preparations were stored at −20°C.
D. In Vitro rhPAPPA2 Enzyme Activities
The ability of the purified rhPAPPA2 to proteolyze IGFBP-3 and IGFBP-5 was evaluated. The source of the IGFBP-3 was media conditioned by human dermal fibroblasts, which we previously demonstrated secreted abundant IGFBP-3 [21]. rhIGFBP-5 was from GroPep (GroPep Bioreagents, Thebarton, Australia). rhPAPPA2 enzymatic activities were determined as follows: equal parts of fibroblast-conditioned media were incubated with nonconcentrated rhPAPPA2 or vector control–conditioned media for 24 to 48 hours at 37°C. Two hundred nanograms of rhIGFBP5 was similarly processed. Cleavage of IGFBP-3 and rhIGFBP-5 was visualized by immunoblotting with anti–IGFBP-3 or anti–IGFBP-5 mouse monoclonal antibodies (Santa Cruz Biotechnology, Dallas, Texas, and GroPep, respectively) [22, 23]. rhPAPPA2 protease activity on endogenous IGFBP-3 in human serum was assessed by incubating 300 μL of patient or control serum with 0 ng, 5 ng, or 50 ng of rhPAPPA2 at 37°C for 0, 30, 60, and 240 minutes. The 0-ng group received an equal volume of diluent. The mixtures were snap frozen and stored at −20°C for ELISA analysis. Commercially available PAPPA2, free IGF-1, and intact IGFBP-3 ELISA kits (Ansh Laboratories) were employed to measure protein in the serum and rhPAPPA2 mixtures according to the manufacturer’s protocols. Per the manufacturer, within-run and between-run percentage CV for PAPPA2 had a value of 4.25% and 1.94% for the low-serum control group (1.029 ng/mL) and 3.68% and 2.42% for the high-serum group (3.128 ng/mL). Intact IGFBP-3 had percentage CV for within run and between run for low control of 4.93% and 3.4% (22.069 ng/mL) and 2.88% and 3.05% for the high control (54.468 ng/mL). Additionally, free IGF-1 had a 4.14% within run and 4.81% between CV for low control (2.087 ng/mL) and 4.27% and 4.17% for high (8.191 ng/mL).
E. Statistical Analysis
Data were examined for distributional properties and detection of any outliers. Skewness was observed for both free IGF-I and intact IGFBP-3. Natural log and square root transformations were applied, respectively. No outlying values were detected and the 0 values were confirmed. Analysis was done on both raw and transformed values. Raw values are shown in all figures. A general linear mixed model was used to account for the repeated measures and a single missing data point. The initial approach was to examine a model for both of the outcome variables (bioavailable IGF-1 and intact IGFBP-3) and include time (0, 30, 60, and 240 minutes), dose (0 ng, 5 ng, and 50 ng) and group (ISS and PAPPA2 deficiency) in the model and examine the interaction. Time was considered as continuous in the model. In the event of significant interactions between time, dose and group, or time and dose we planned to consider separate models by dose. Analysis was done using SAS®, version 9.4 (SAS Institute, Cary, NC). A P value of <0.05 was considered statistically significant. Due to the nature of the study being to provide proof-of-principle evidence, no adjustment was made for multiple comparisons. Of note, the same results were obtained using both raw and transformed variables, so results from the analysis of the raw data are presented.
2. Results
A. Plasma Transfusion for in Vivo PAPPA2 Replacement
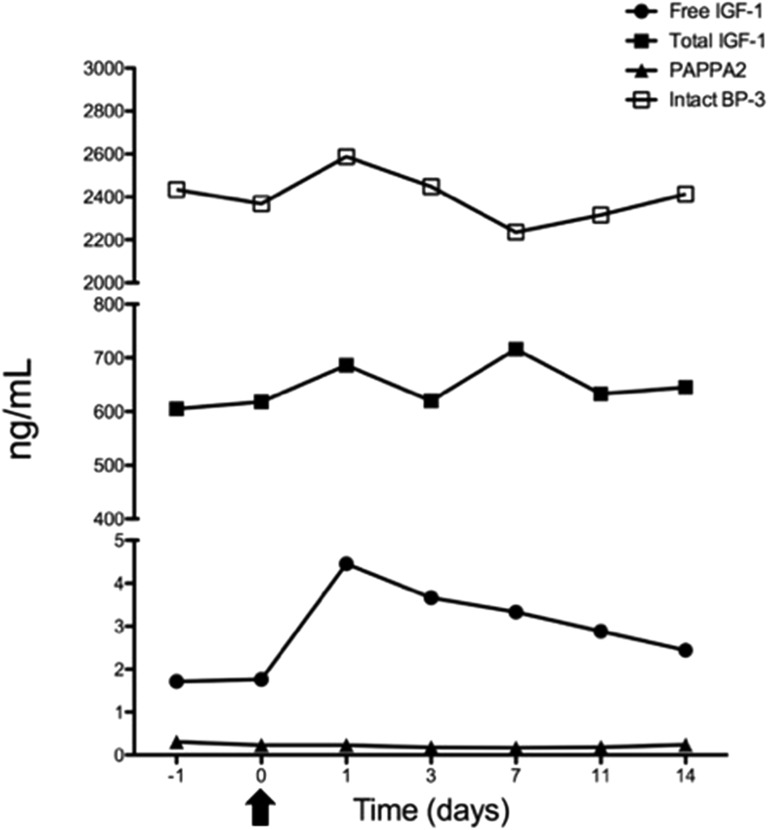
Prior to transfusion, P1 had a mean PAPPA2 concentration of 0.275 ng/mL. We have previously shown that this subject’s mutated PAPPA2 is not proteolytically active [14]. At day 1 posttransfusion, there was no significant measurable increase in PAPPA2 concentration (Fig. 1). However, despite the lack of measurable increase in PAPPA2, free IGF-I levels increased 2.5-fold, with a gradual return to baseline during the 14-day period (Fig. 1). A t test showed a statistically significant mean change between the baseline values (days −1 and 0) and postbaseline values (days 1 to 14) (P = 0.04). Examining the 95% CI for the postbaseline values showed that the baseline values fall outside the CI (mean, 3.35; 95% CI, 2.40, 4.30). There was no significant change in total IGF-I, total IGFBP-3, or intact IGFBP-3 throughout the study period (Fig. 1; Supplemental Table 1).
Figure 1.
Biochemical measurements during plasma transfusion experiment. Serum was collected 1 day prior to infusion (−1), at baseline on the day of transfusion (0), and on days 1, 3, 7, 11, and 14 after transfusion. Arrow indicates the transfusion on day 0.
B. In Vitro rhPAPPA2 Functional Validation
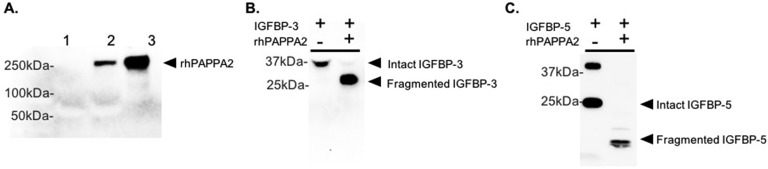
To further explore the effects of PAPPA2 on free IGF-I levels in vitro, we generated rhPAPPA2 peptides by overexpressing pCMV6-PAPPA2 in HEK293 cells. Secreted rhPAPPA2 was readily immunodetected in the conditioned media (Fig. 2A, lane 2) and was more prominent after concentration (Fig. 2A, lane 3). Retention of enzyme activity was confirmed by loss of detectable intact IGFBP-3 and rhIGFBP-5 when incubated with rhPAPPA2 (Fig. 2B and 2C), consistent with our previous report [14].
Figure 2.
A. Immunoblot analyses of rhPAPPA2 expression and proteolytic activities. (A) Conditioned media (CM) from vector-transfected (lane 1) or pCMV6-PAPPA2–transfected (lane 2) HEK293 cells. Lane 3, rhPAPPA2 after concentration. (B) Secreted intact IGFBP-3 in media conditioned by human dermal fibroblasts was incubated with or without rhPAPPA2 (~20 ng) for 24 h at 37°C. Addition of rhPAPPA2 resulted in cleavage of IGFBP-3. (C) rhPAPPA2 was incubated for 48 h at 37°C with rhIGFBP5. Immunoblot showing cleavage of IGFBP-5 in the presence of rhPAPPA2.
C. rhPAPPA2 Proteolyzes IGFBPs in Serum Samples
To validate our hypothesis that rhPAPPA2 will increase free IGF-I levels in patients with PAPPA2 deficiency who have low free IGF-I and high levels of total IGF-I, we combined purified rhPAPPA2 with serum samples obtained from the three affected siblings (P1, P2, and P3). We also explored whether rhPAPPA2 could have similar effects in serum samples from patients with ISS who are not PAPPA2 deficient.
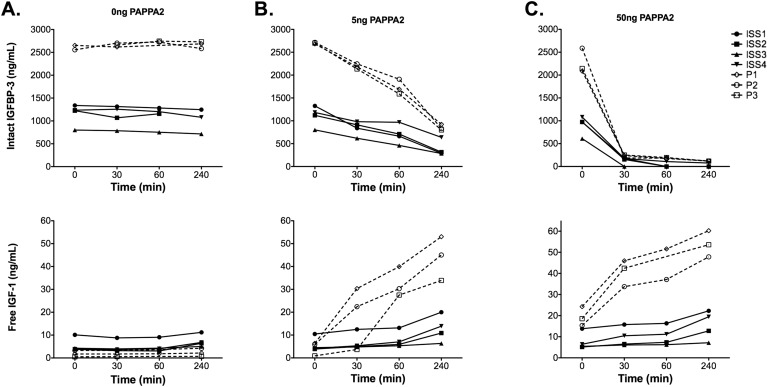
In serum samples from our PAPPA2-deficient patients, intact IGFBP-3 (2604 ± 69 ng/mL to 2666 ± 76 ng/mL; P = 0.42) and free IGF-I (1.85 ± 1.37 ng/mL to 2.36 ± 1.77 ng/mL; P = 0.57) levels remained stable during the course of 4 hours without the addition of rhPAPPA2 (Fig. 3). Addition of 5 or 50 ng of rhPAPPA2 to patient serum samples markedly and dose-dependently decreased intact IGFBP-3 levels compared with the baseline time point (Fig. 3). Concurrently, increased free IGF-I concentrations between 15- and 100-fold were detected (P = 0.001 for 5 ng and P = 0.008 for 50 ng) (Fig. 3; Table 1).
Figure 3.
Intact IGFBP-3 and free IGF-1 values in serum from PAPPA2-deficient patients (P1, P2, and P3) and ISS patients (ISS1, ISS2, ISS3, and ISS4) after 0, 30, 60, and 240 min of incubation at 37°C with rhPAPPA2. The 0 min time point occurred after incubation periods for each assay, thus resulting in different baseline free IGF-I and intact IGFBP-3 values for the same samples at the different doses of rhPAPPA2 (i.e., there was some enzyme action during the incubation). (A) Addition of 0 ng of rhPAPPA2. (B) Addition of 5 ng of rhPAPPA2. (C) Addition of 50 ng of rhPAPPA2.
Table 1.
Demographic and Baseline Hormonal Parameters for Patients Carrying the PAPPA2 Mutation and Patients Diagnosed with ISS
| P1 | P2 | P3 | ISS1 | ISS2 | ISS3 | ISS4 | |
|---|---|---|---|---|---|---|---|
| Sex | F | M | M | M | M | M | M |
| Age, y | 19.3 | 14.5 | 10.5 | 14 | 10.5 | 13.5 | 8.5 |
| Height, SD | −3.8 | −2.9 | −2.9 | −2.5 | −2.1 | −2.4 | −2.5 |
| Tanner stage | 5 | 2 | 1 | 1 | 1 | 2 | 1 |
| Total IGF-I, ng/mL | 566 | 573 | 547 | 299 | 169 | 102 | 137 |
| Total IGFBP-3, ng/mL | 7924 | 8997 | >10,000 | 4111 | 4319 | 3099 | 3658 |
| Free IGF-I, ng/mL | 1.75 | 3.27 | 0.53 | 10.11 | 3.78 | 4.28 | 3.85 |
| Intact IGFBP-3, ng/mL | 2653 | 2556 | 2493 | 1338 | 1224 | 801 | 1235 |
| Free IGF-I/IGF-I ratio, % | 0.3 | 0.6 | 0.1 | 3.3 | 2.2 | 4.2 | 2.8 |
| PAPPA2, ng/mL | 0.09 | 0.06 | 0.11 | 0.51 | 0.19 | 0.16 | 0.29 |
Tanner Stage was based on testicular volume for males and breast for females.
Similar trends were seen for patients with ISS, although the degree of increase in free IGF-I was more modest (1.7- to 5-fold) (Fig. 3). Note that the patients with ISS had lower starting total IGF-I, total IGFBP-3 and intact IGFBP-3, but higher free IGF-I levels, when compared with PAPPA2-deficient patients (Table 1). Hence, the baseline free-to-total IGF-I ratios were markedly higher in the ISS patients. Statistical analysis supported the significantly greater increase in free IGF-I for the PAPPA2 group compared with the ISS group (P = 0.0005 for 5 ng and P = 0.01 for 50 ng).
3. Discussion
To our knowledge, this is the first study to explore the potential use of PAPPA2 as a modulator of IGF-I bioavailability in patient samples. PAPPA2 has been shown to be a key regulator of IGF-I bioavailability, and the recently described patients with loss-of-function PAPPA2 mutations demonstrated postnatal growth retardation with low free IGF-I levels [14]. We hypothesized that administration of exogenous PAPPA2 would proteolyze IGFBP-3 molecules, thereby freeing up endogenous IGF-I from its ternary complex. Theoretically, higher levels of free IGF-I could lead to increased growth over time.
We used two complementary approaches to investigate the effect of augmenting PAPPA2 activity in patients with PAPPA2 deficiency, extending our study to include samples from patients with ISS. In the first approach, we delivered a relatively small dose of PAPPA2 via plasma transfusion to a single patient with PAPPA2 deficiency. Unfortunately, there was no detectable increase in the serum PAPPA2 concentration posttransfusion. There are a number of possible explanations for this lack of increase. It is possible that there was a small increase in PAPPA2 levels that fell below the discriminatory threshold of the assay. Our maximal expected increase was 0.11 ng/mL and the analytical sensitivity of the assay is only 0.07 ng/mL. It is also possible that the PAPPA2 was metabolized before the day 1 measurement or was distributed into other tissues and thus the expected increase in plasma levels was not observed. Despite this lack of measurable increase in PAPPA2, the free IGF-I levels did increase 2.5-fold, with a slow return to baseline. This suggests that even a small transient increase in PAPPA2 levels may be sufficient for a prolonged effect on free IGF-I levels. The transfusion did also include some free IGF-I, but we think the persistent increase in free IGF-I is unlikely to be due to transfused free IGF-I, as the half-life of free IGF-I in vivo is only a few minutes. Interestingly, intact IGFBP-3 levels did not fall during the course of the experiment, which questions whether the increase in free IGF-I levels was a consequence of PAPPA2 actions on IGFBP3. PAPPA2 is known to efficiently proteolyze IGFBP-5, which was not measured during this trial. Additional in vivo studies are needed to determine whether PAPPA2 replacement could have a sustained effect on increasing free IGF-I levels.
Our second approach involved in vitro studies, exploring the effect of rhPAPPA2 on intact IGFBP-3 and free IGF-I levels in serum from patients with either PAPPA2 deficiency or ISS. These results showed that PAPPA2 administration leads to rapid cleavage of IGFBP-3 and significant rises in free IGF-I concentrations, supporting our initial hypothesis. The increase in free IGF-I was significantly more prominent in patients with PAPPA2 deficiency as expected, as these patients have greater stores of bound IGF-I poised to be liberated from their ternary complexes. The ISS patients also demonstrated significant increases in their free IGF-I levels, albeit more modest, ranging from 1.7- to 5-fold. Interestingly, the serum sample from the one ISS patient with the highest free IGF-I to total IGF-I ratio at baseline has the smallest increase in free IGF-I upon the addition of PAPPA2. Presumably, this patient had a smaller fraction of bound IGF-I available for rhPAPPA2 actions. Although our analyses are all in vitro and involved a limited number of patient serum samples, the results do raise the intriguing possibility that PAPPA2 therapy could lead to a meaningful increase in free IGF-I levels in patients with short stature. Currently, there is a lack of information about the pharmacokinetics or pharmacodynamics of PAPPA2, and thus much additional work is necessary before PAPPA2 can be considered as a viable therapeutic option for short stature. Although the normal ranges for PAPPA2 or free IGF-I in childhood need to be established to fully interpret the clinical impact of our results, our data support the potential of the development of PAPPA2 as a novel growth-promoting therapy.
Supplementary Material
Acknowledgments
We thank the patients and their family members for participation in this study.
Financial Support: This work was supported by the 2015 Alexion Rare Disease Innovation Award at Cincinnati Children’s Hospital Medical Center. Alexion played no role in the design, conduct, and data analysis or manuscript preparation for this study. The study was also supported by National Institutes of Health Grant 1UL1 TR001425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical Trial Information: ClinicalTrials.gov no. NCT02412943 (registered 9 April 2015).
Disclosure Summary: A.D. and V.H. hold a patent on the use of PAPPA2 as a growth-promoting agent. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- ALS
acid labile subunit
- CCHMC
Cincinnati Children’s Hospital Medical Center
- FFP
fresh-frozen plasma
- IGFBP
IGF-binding protein
- ISS
idiopathic short stature
- P
patient
- PAPPA2
pregnancy-associated plasma protein A2
- rh
recombinant human
References and Notes
- 1. David A, Hwa V, Metherell LA, Netchine I, Camacho-Hübner C, Clark AJ, Rosenfeld RG, Savage MO. Evidence for a continuum of genetic, phenotypic, and biochemical abnormalities in children with growth hormone insensitivity. Endocr Rev. 2011;32(4):472–497. [DOI] [PubMed] [Google Scholar]
- 2. Janosi JBM, Ramsland PA, Mott MR, Firth SM, Baxter RC, Delhanty PJD. The acid-labile subunit of the serum insulin-like growth factor-binding protein complexes. Structural determination by molecular modeling and electron microscopy. J Biol Chem. 1999;274(33):23328–23332. [DOI] [PubMed] [Google Scholar]
- 3. Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol. 2001;170(1):63–70. [DOI] [PubMed] [Google Scholar]
- 4. Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20(6):761–787. [DOI] [PubMed] [Google Scholar]
- 5. Twigg SM, Baxter RC. Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit. J Biol Chem. 1998;273(11):6074–6079. [DOI] [PubMed] [Google Scholar]
- 6. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. [DOI] [PubMed] [Google Scholar]
- 7. Frystyk J, Skjaerbaek C, Dinesen B, Orskov H. Free insulin-like growth factors (IGF-I and IGF-II) in human serum. FEBS Lett. 1994;348(2):185–191. [DOI] [PubMed] [Google Scholar]
- 8. Hasegawa Y, Hasegawa T, Takada M, Tsuchiya Y. Plasma free insulin-like growth factor I concentrations in growth hormone deficiency in children and adolescents. Eur J Endocrinol. 1996;134(2):184–189. [DOI] [PubMed] [Google Scholar]
- 9. Juul A, Holm K, Kastrup KW, Pedersen SA, Michaelsen KF, Scheike T, Rasmussen S, Müller J, Skakkebaek NE. Free insulin-like growth factor I serum levels in 1430 healthy children and adults, and its diagnostic value in patients suspected of growth hormone deficiency. J Clin Endocrinol Metab. 1997;82(8):2497–2502. [DOI] [PubMed] [Google Scholar]
- 10. Kawai N, Kanzaki S, Takano-Watou S, Tada C, Yamanaka Y, Miyata T, Oka M, Seino Y. Serum free insulin-like growth factor I (IGF-I), total IGF-I, and IGF-binding protein-3 concentrations in normal children and children with growth hormone deficiency. J Clin Endocrinol Metab. 1999;84(1):82–89. [DOI] [PubMed] [Google Scholar]
- 11. Ballerini MG, Braslavsky D, Scaglia PA, Keselman A, Rodríguez ME, Martínez A, Freire AV, Domené HM, Jasper HG, Bergadá I, Ropelato MG. Circulating IGF-I, IGFBP-3 and the IGF-I/IGFBP-3 molar ratio concentration and height outcome in prepubertal short children on rhGH treatment over two years of therapy. Horm Res Paediatr. 2017;88(5):354–363. [DOI] [PubMed] [Google Scholar]
- 12. Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J Biol Chem. 2001;276(24):21849–21853. [DOI] [PubMed] [Google Scholar]
- 13. Conover CA, Boldt HB, Bale LK, Clifton KB, Grell JA, Mader JR, Mason EJ, Powell DR. Pregnancy-associated plasma protein-A2 (PAPP-A2): tissue expression and biological consequences of gene knockout in mice. Endocrinology. 2011;152(7):2837–2844. [DOI] [PubMed] [Google Scholar]
- 14. Dauber A, Muñoz-Calvo MT, Barrios V, Domené HM, Kloverpris S, Serra-Juhé C, Desikan V, Pozo J, Muzumdar R, Martos-Moreno GA, Hawkins F, Jasper HG, Conover CA, Frystyk J, Yakar S, Hwa V, Chowen JA, Oxvig C, Rosenfeld RG, Pérez-Jurado LA, Argente J. Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability. EMBO Mol Med. 2016;8(4):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, Willer CJ, Jackson AU, Vedantam S, Raychaudhuri S, Ferreira T, Wood AR, Weyant RJ, Segrè AV, Speliotes EK, Wheeler E, Soranzo N, Park JH, Yang J, Gudbjartsson D, Heard-Costa NL, Randall JC, Qi L, Vernon Smith A, Mägi R, Pastinen T, Liang L, Heid IM, Luan J, Thorleifsson G, Winkler TW, Goddard ME, Sin Lo K, Palmer C, Workalemahu T, Aulchenko YS, Johansson A, Zillikens MC, Feitosa MF, Esko T, Johnson T, Ketkar S, Kraft P, Mangino M, Prokopenko I, Absher D, Albrecht E, Ernst F, Glazer NL, Hayward C, Hottenga JJ, Jacobs KB, Knowles JW, Kutalik Z, Monda KL, Polasek O, Preuss M, Rayner NW, Robertson NR, Steinthorsdottir V, Tyrer JP, Voight BF, Wiklund F, Xu J, Zhao JH, Nyholt DR, Pellikka N, Perola M, Perry JR, Surakka I, Tammesoo ML, Altmaier EL, Amin N, Aspelund T, Bhangale T, Boucher G, Chasman DI, Chen C, Coin L, Cooper MN, Dixon AL, Gibson Q, Grundberg E, Hao K, Juhani Junttila M, Kaplan LM, Kettunen J, König IR, Kwan T, Lawrence RW, Levinson DF, Lorentzon M, McKnight B, Morris AP, Müller M, Suh Ngwa J, Purcell S, Rafelt S, Salem RM, Salvi E, Sanna S, Shi J, Sovio U, Thompson JR, Turchin MC, Vandenput L, Verlaan DJ, Vitart V, White CC, Ziegler A, Almgren P, Balmforth AJ, Campbell H, Citterio L, De Grandi A, Dominiczak A, Duan J, Elliott P, Elosua R, Eriksson JG, Freimer NB, Geus EJ, Glorioso N, Haiqing S, Hartikainen AL, Havulinna AS, Hicks AA, Hui J, Igl W, Illig T, Jula A, Kajantie E, Kilpeläinen TO, Koiranen M, Kolcic I, Koskinen S, Kovacs P, Laitinen J, Liu J, Lokki ML, Marusic A, Maschio A, Meitinger T, Mulas A, Paré G, Parker AN, Peden JF, Petersmann A, Pichler I, Pietiläinen KH, Pouta A, Ridderstråle M, Rotter JI, Sambrook JG, Sanders AR, Schmidt CO, Sinisalo J, Smit JH, Stringham HM, Bragi Walters G, Widen E, Wild SH, Willemsen G, Zagato L, Zgaga L, Zitting P, Alavere H, Farrall M, McArdle WL, Nelis M, Peters MJ, Ripatti S, van Meurs JB, Aben KK, Ardlie KG, Beckmann JS, Beilby JP, Bergman RN, Bergmann S, Collins FS, Cusi D, den Heijer M, Eiriksdottir G, Gejman PV, Hall AS, Hamsten A, Huikuri HV, Iribarren C, Kähönen M, Kaprio J, Kathiresan S, Kiemeney L, Kocher T, Launer LJ, Lehtimäki T, Melander O, Mosley TH Jr, Musk AW, Nieminen MS, O'Donnell CJ, Ohlsson C, Oostra B, Palmer LJ, Raitakari O, Ridker PM, Rioux JD, Rissanen A, Rivolta C, Schunkert H, Shuldiner AR, Siscovick DS, Stumvoll M, Tönjes A, Tuomilehto J, van Ommen GJ, Viikari J, Heath AC, Martin NG, Montgomery GW, Province MA, Kayser M, Arnold AM, Atwood LD, Boerwinkle E, Chanock SJ, Deloukas P, Gieger C, Grönberg H, Hall P, Hattersley AT, Hengstenberg C, Hoffman W, Lathrop GM, Salomaa V, Schreiber S, Uda M, Waterworth D, Wright AF, Assimes TL, Barroso I, Hofman A, Mohlke KL, Boomsma DI, Caulfield MJ, Cupples LA, Erdmann J, Fox CS, Gudnason V, Gyllensten U, Harris TB, Hayes RB, Jarvelin MR, Mooser V, Munroe PB, Ouwehand WH, Penninx BW, Pramstaller PP, Quertermous T, Rudan I, Samani NJ, Spector TD, Völzke H, Watkins H, Wilson JF, Groop LC, Haritunians T, Hu FB, Kaplan RC, Metspalu A, North KE, Schlessinger D, Wareham NJ, Hunter DJ, O'Connell JR, Strachan DP, Wichmann HE, Borecki IB, van Duijn CM, Schadt EE, Thorsteinsdottir U, Peltonen L, Uitterlinden AG, Visscher PM, Chatterjee N, Loos RJ, Boehnke M, McCarthy MI, Ingelsson E, Lindgren CM, Abecasis GR, Stefansson K, Frayling TM, Hirschhorn JN. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467(7317):832–838. [DOI] [PMC free article] [PubMed]
- 16. Jepsen MR, Kløverpris S, Mikkelsen JH, Pedersen JH, Füchtbauer EM, Laursen LS, Oxvig C. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J Biol Chem. 2015;290(6):3430–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marouli E, Graff M, Medina-Gomez C, Lo KS, Wood AR, Kjaer TR, Fine RS, Lu Y, Schurmann C, Highland HM, Rüeger S, Thorleifsson G, Justice AE, Lamparter D, Stirrups KE, Turcot V, Young KL, Winkler TW, Esko T, Karaderi T, Locke AE, Masca NG, Ng MC, Mudgal P, Rivas MA, Vedantam S, Mahajan A, Guo X, Abecasis G, Aben KK, Adair LS, Alam DS, Albrecht E, Allin KH, Allison M, Amouyel P, Appel EV, Arveiler D, Asselbergs FW, Auer PL, Balkau B, Banas B, Bang LE, Benn M, Bergmann S, Bielak LF, Blüher M, Boeing H, Boerwinkle E, Böger CA, Bonnycastle LL, Bork-Jensen J, Bots ML, Bottinger EP, Bowden DW, Brandslund I, Breen G, Brilliant MH, Broer L, Burt AA, Butterworth AS, Carey DJ, Caulfield MJ, Chambers JC, Chasman DI, Chen YI, Chowdhury R, Christensen C, Chu AY, Cocca M, Collins FS, Cook JP, Corley J, Galbany JC, Cox AJ, Cuellar-Partida G, Danesh J, Davies G, de Bakker PI, de Borst GJ, de Denus S, de Groot MC, de Mutsert R, Deary IJ, Dedoussis G, Demerath EW, den Hollander AI, Dennis JG, Di Angelantonio E, Drenos F, Du M, Dunning AM, Easton DF, Ebeling T, Edwards TL, Ellinor PT, Elliott P, Evangelou E, Farmaki AE, Faul JD, Feitosa MF, Feng S, Ferrannini E, Ferrario MM, Ferrieres J, Florez JC, Ford I, Fornage M, Franks PW, Frikke-Schmidt R, Galesloot TE, Gan W, Gandin I, Gasparini P, Giedraitis V, Giri A, Girotto G, Gordon SD, Gordon-Larsen P, Gorski M, Grarup N, Grove ML, Gudnason V, Gustafsson S, Hansen T, Harris KM, Harris TB, Hattersley AT, Hayward C, He L, Heid IM, Heikkilä K, Helgeland Ø, Hernesniemi J, Hewitt AW, Hocking LJ, Hollensted M, Holmen OL, Hovingh GK, Howson JM, Hoyng CB, Huang PL, Hveem K, Ikram MA, Ingelsson E, Jackson AU, Jansson JH, Jarvik GP, Jensen GB, Jhun MA, Jia Y, Jiang X, Johansson S, Jørgensen ME, Jørgensen T, Jousilahti P, Jukema JW, Kahali B, Kahn RS, Kähönen M, Kamstrup PR, Kanoni S, Kaprio J, Karaleftheri M, Kardia SL, Karpe F, Kee F, Keeman R, Kiemeney LA, Kitajima H, Kluivers KB, Kocher T, Komulainen P, Kontto J, Kooner JS, Kooperberg C, Kovacs P, Kriebel J, Kuivaniemi H, Küry S, Kuusisto J, La Bianca M, Laakso M, Lakka TA, Lange EM, Lange LA, Langefeld CD, Langenberg C, Larson EB, Lee IT, Lehtimäki T, Lewis CE, Li H, Li J, Li-Gao R, Lin H, Lin LA, Lin X, Lind L, Lindström J, Linneberg A, Liu Y, Liu Y, Lophatananon A, Luan J, Lubitz SA, Lyytikäinen LP, Mackey DA, Madden PA, Manning AK, Männistö S, Marenne G, Marten J, Martin NG, Mazul AL, Meidtner K, Metspalu A, Mitchell P, Mohlke KL, Mook-Kanamori DO, Morgan A, Morris AD, Morris AP, Müller-Nurasyid M, Munroe PB, Nalls MA, Nauck M, Nelson CP, Neville M, Nielsen SF, Nikus K, Njølstad PR, Nordestgaard BG, Ntalla I, O’Connel JR, Oksa H, Loohuis LM, Ophoff RA, Owen KR, Packard CJ, Padmanabhan S, Palmer CN, Pasterkamp G, Patel AP, Pattie A, Pedersen O, Peissig PL, Peloso GM, Pennell CE, Perola M, Perry JA, Perry JR, Person TN, Pirie A, Polasek O, Posthuma D, Raitakari OT, Rasheed A, Rauramaa R, Reilly DF, Reiner AP, Renström F, Ridker PM, Rioux JD, Robertson N, Robino A, Rolandsson O, Rudan I, Ruth KS, Saleheen D, Salomaa V, Samani NJ, Sandow K, Sapkota Y, Sattar N, Schmidt MK, Schreiner PJ, Schulze MB, Scott RA, Segura-Lepe MP, Shah S, Sim X, Sivapalaratnam S, Small KS, Smith AV, Smith JA, Southam L, Spector TD, Speliotes EK, Starr JM, Steinthorsdottir V, Stringham HM, Stumvoll M, Surendran P, ’t Hart LM, Tansey KE, Tardif JC, Taylor KD, Teumer A, Thompson DJ, Thorsteinsdottir U, Thuesen BH, Tönjes A, Tromp G, Trompet S, Tsafantakis E, Tuomilehto J, Tybjaerg-Hansen A, Tyrer JP, Uher R, Uitterlinden AG, Ulivi S, van der Laan SW, Van Der Leij AR, van Duijn CM, van Schoor NM, van Setten J, Varbo A, Varga TV, Varma R, Edwards DR, Vermeulen SH, Vestergaard H, Vitart V, Vogt TF, Vozzi D, Walker M, Wang F, Wang CA, Wang S, Wang Y, Wareham NJ, Warren HR, Wessel J, Willems SM, Wilson JG, Witte DR, Woods MO, Wu Y, Yaghootkar H, Yao J, Yao P, Yerges-Armstrong LM, Young R, Zeggini E, Zhan X, Zhang W, Zhao JH, Zhao W, Zhao W, Zheng H, Zhou W, Rotter JI, Boehnke M, Kathiresan S, McCarthy MI, Willer CJ, Stefansson K, Borecki IB, Liu DJ, North KE, Heard-Costa NL, Pers TH, Lindgren CM, Oxvig C, Kutalik Z, Rivadeneira F, Loos RJ, Frayling TM, Hirschhorn JN, Deloukas P, Lettre G; EPIC-InterAct Consortium; CHD Exome+ Consortium; ExomeBP Consortium; T2D-Genes Consortium; GoT2D Genes Consortium; Global Lipids Genetics Consortium; ReproGen Consortium; MAGIC Investigators . Rare and low-frequency coding variants alter human adult height. Nature. 2017;542(7640):186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cabrera-Salcedo C, Mizuno T, Tyzinski L, Andrew M, Vinks AA, Frystyk J, Wasserman H, Gordon CM, Hwa V, Backeljauw P, Dauber A. Pharmacokinetics of IGF-1 in PAPP-A2-deficient patients, growth response, and effects on glucose and bone density. J Clin Endocrinol Metab. 2017;102(12):4568–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang SR, Jacobsen CM, Carmichael H, Edmund AB, Robinson JW, Olney RC, Miller TC, Moon JE, Mericq V, Potter LR, Warman ML, Hirschhorn JN, Dauber A. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) gene as a cause of short stature. Hum Mutat. 2015;36(4):474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RRID. AB_2050158, http://antibodyregistry.org/search?q=MM0507-8M12.
- 21. Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med. 2003;349(12):1139–1147. [DOI] [PubMed] [Google Scholar]
- 22.RRID. AB_10988386, http://antibodyregistry.org/search.php?q=AB_10988386.
- 23.RRID. AB_2728746, http://antibodyregistry.org/search?q=RRID:AB_2728746.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.