Abstract
Background
Analysis of the International Association for the Study of Lung Cancer (IASLC) Malignant Pleural Mesothelioma (MPM) database revealed that clinical (cTNM) staging minimally stratified survival and was discrepant with pathological (pTNM) staging. To improve prognostic classification of MPM, alternative staging models based on quantitative parameters were explored.
Methods
An institutional review board–approved MPM registry was queried to identify patients with available pathological and preoperative imaging data. Qualifying patients were randomly assigned to training and test sets in a 1:2 ratio. Computed cTNM and pTNM staging (AJCC Cancer Staging Manual, 7th ed.) were compared. Quantitative image analysis included tumor volume assessed from three-dimensional reconstruction of computed tomography scans (VolCT) and maximal fissural thickness (Fmax). Survival was estimated using the Kaplan-Meier method, and the relationship with VolCT was examined by Cox regression analysis to identify optimized cut-points. Performance of cTNM and quantitative models derived was compared in the test set using Harrell’s C index.
Results
A total of 472 patients met inclusion criteria. TNM staging was concordant with pathological TNM staging in 171 of 472 (36.2%), understaged in 209 (44.2%), and overstaged in 92 (19.4%) patients. The most concordant feature was involvement of interlobar fissures. A quantitative clinical staging model comprising VolCT and Fmax (c-index = 0.638, 95% confidence interval [CI] = 0.603 to 0.673) performed statistically significantly better as a prognostic classifier when compared in the test set with cTNM (c-index = 0.562, 95% CI = 0.525 to 0.599, P = .001).
Conclusions
Improved prognostic performance may be achievable by quantitative clinical staging combining VolCT and Fmax, providing a cost-effective and clinically relevant surrogate for clinical TNM stage.
Malignant pleural mesothelioma (MPM) is a highly aggressive malignancy of the pleura most frequently associated with asbestos exposure (1).The disease usually develops after a long latency following asbestos exposure (2). Treatment ranges from chemotherapy or supportive care for advanced disease to aggressive surgery-based multimodality regimens for fit patients with limited disease (3). Histological subtype, lymph node status, and pathologically determined TNM stage are prognostic of overall survival (OS) (4–6); however, these prognostic parameters are lacking for patients who either have not yet undergone, or are not candidates for, surgical resection.
In the case of most malignant tumors, clinical staging using noninvasive multimodality imaging is an important means of predicting pathological stage and prognosis. Clinical stage, therefore, plays a vital role in patient management and permits selection or stratification of patients for therapeutic clinical trials. However, clinical TNM staging of patients with MPM is not prognostic (7) and does not sufficiently stratify patients to determine homogeneous cohorts for enrollment to treatment protocols. For example, the two largest randomized trials that established combination platinum antifolate chemotherapy as standard of care did not include stage among inclusion criteria (8,9). Challenges to accurate clinical staging include the irregular and complex morphology and distribution of MPM within the affected thoracic cavity, which has made it impractical to include a T classification criterion based on the size of the tumor, as is the case for most solid malignancies. For example, T classification of lung cancer is primarily defined by quantitative determination of tumor diameter (10). By contrast, T classification of MPM is entirely qualitative, using multiple binary criteria based on identifying tumor invasion through specific tissue planes into adjacent structures. Clinical staging of MPM thus requires the radiologist’s subjective assessment of such invasion, leading to inter- and intra-observer variability and to inconsistent clinical staging that lacks prognostic accuracy (7,11,12).
The current study considers two potential quantitative parameters to augment clinical staging of MPM. Tumor volume assessed from three-dimensional reconstruction of computed tomography (CT) scans (VolCT) (12) may represent a practical measure of tumor size by accommodating its diffuse, irregular morphology. VolCT has been found to be prognostic of OS in patients with MPM who undergo surgery and to improve assessment of response to nonsurgical therapy (13–15). The feasibility of incorporating VolCT into clinical staging of MPM was recently demonstrated in a North American multi-institutional pilot study (12). A second potential quantitative parameter, also correlated with patient prognosis (16,17), is the maximal thickness of disease in the interlobar fissures, which can be accurately measured on CT due to high contrast with the adjacent lung. Other groups have similarly explored the utility of quantitative measures such as pleural thickness (18) and diaphragmatic tumor thickness (19) to augment T classification.
We compared the performance of clinical staging using current American Joint Committee on Cancer (AJCC) criteria (20) to that of alternative staging models based on quantitive clinical assessment, including VolCT and maximal fissural thickening, among patients who underwent surgery for MPM at a single center of expertise. Our objective was to identify a model that could statistically significantly improve the pretreatment prognostic classification of MPM, and thereby provide a surrogate clinical stage to enhance therapeutic decision-making and surgical planning, predict prognosis of nonsurgical patients, and support meaningful patient selection for or stratification within clinical trials.
Methods
Subjects
With approval from the Institutional Review Board, we retrospectively reviewed data from the institutional mesothelioma Patient Data Registry (protocol number 2005p 001520; informed consent was waived for the registry protocol; however, most of the patients were consented to a comprehensive specimen collection and data protocol). We audited the records of patients with MPM who underwent surgery at Brigham and Women’s Hospital, Boston, Massachusetts, between 2001 and 2014. We identified patients who had macroscopic complete resection and an available preoperative (maximum 30 days prior to surgery) computed tomography scan. DICOM format CT imaging data were obtained along with demographics, laboratory findings, pertinent history, treatment details, pathological findings, and vital status. The study was compliant with provisions of the Health Insurance Portability and Accountability Act.
Radiological Assessment
Four hundred seventy-two patients who underwent macroscopically complete surgical resection (EPP, P/D, and eP/D) (21) and had diagnostic quality preoperative imaging scans within 30 days prior to surgery were included in the study. For patients who had completed neoadjuvant chemotherapy, the scan following the last cycle was used. Of these, 303 patients (64.1%) underwent CT scans on the Sensation 64-MDCT scanner (Siemens Medical Solutions, Erlangen, Germany) with 120 kVp, 0.6 mm collimation, high-speed mode, a pitch equivalent of a 1.5-slice interval of 5 mm, and reconstructed in a slice thickness of 5 mm. Intravenous contrast consisting of 55 mL of contrast material (Ultravist 370 [iopromide], Bayer HealthCare, Berlin, Germany [formerly Schering]) was administered intravenously to 197 (41.7%) patients at 4 mL/s using an automated power injector.
For the remaining 169 (35.8%) patients, diagnostic quality CT scans were obtained for anatomic correction as part of an integrated PET-CT study (Discovery ST system, GE Healthcare, Chicago, USA) using 140 kVp and 75 mA, and reconstructed with 3.75 mm slice thickness at 3.25 mm intervals. No intravenous contrast was administered. The metabolic information from PET-CT studies was not considered in the current analysis.
Analysis of CT images was accomplished by an experienced thoracic radiologist (RRG). Qualitative and quantitative assessment of each scan was performed using barcoded data collection forms and following a standardized protocol. The CT images were analyzed qualitatively using the AJCC Cancer Staging Manual (7th ed.) (20). Each individual AJCC classification criterion defining T2–T4, N1–N3, and M1 was assessed for each patient, scored on a case report form as either involved or not involved by tumor, and compared with corresponding pathological assessment. Clinical stage was determined based on published stage groupings (20) and compared with pathological stage.
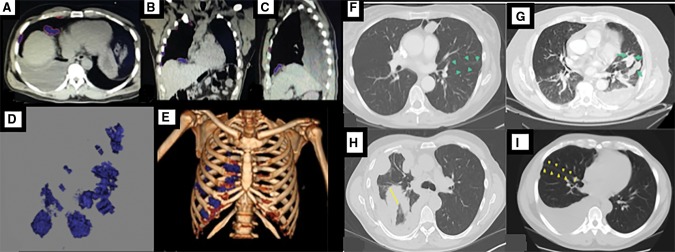
Quantitative analysis was performed on a PACS workstation. Volumetric assessment of the tumor was performed using Vitrea Enterprise suite 6.0 (Vital, MN) by semiautomatic segmentation of tumor area on serial axial images using Hounsfield thresholding (default 20–80) with manual correction to exclude pleural fluid and normal adjacent tissue, and integration across images (Figure 1, A–E) (12,14). The integrated measurement caliper was used to quantify interlobular fissural thickening at its maximum thickness evident on axial CT images (Fmax) (Figure 1, F–I).
Figure 1.
Volumetric assessment in a patient with right-sided mesothelioma. A–C) Segmentation of the tumor in axial, coronal, and sagittal planes is shown. D–E) Tumor volume and relative distribution in the thorax are shown. Axial computed tomography images of patients with malignant pleural mesothelioma showing (F) no fissural involvement, (G) fissural involvement, (H) maximal fissural thickness (Fmax) of 56 mm, and (I) Fmax of 5 mm.
Pathological Review
Detailed pathological staging information was obtained from the electronic medical record. Similar to the clinical staging review, each individual classification criterion defining T2–T4, N1–N3, and M1 was scored on a case report form as involved or not involved by tumor, and pathological stage was determined from these data. Criteria involving anatomical sites not mentioned in the pathology report as either involved or uninvolved were scored as uninvolved for staging purposes.
Statistical Analysis
The primary objective of this study was to determine if quantitative assessment of CT data could provide improved prognostic classification of patients with MPM undergoing surgery-based multimodality therapy, relative to clinical staging using the current AJCC system. Patients in the study cohort were randomly assigned to a training set and a test set in a 1:2 ratio. The training set was used to generate survival models incorporating the quantitative parameters (Fmax and/or VolCT). The performance of candidate quantitative models was evaluated in the test set, in comparison with clinical AJCC staging.
OS was defined as the interval from the date of the surgery to the date of death or last follow-up. Patients who remained alive or were lost to follow-up were censored at the date of last contact. Kaplan-Meier analysis was used to estimate the survival functions for patient subgroups. Cox regression was used to estimate the hazard ratios associated with stage levels in each of the staging models. Dummy variables were created representing stages II, III, and IV, with stage I serving as reference. Predictive performance was measured with Harrell’s C index and compared among staging models using the somersd package in Stata (22). Stata version 13.1 (StataCorp LP, College Station, TX) was used for all statistical calculations. All P values were two-sided, and P values of less than .05 were considered statistically significant.
Results
Study Cohort
The study cohort included 472 patients for whom imaging scans were available within 30 days prior to macroscopic complete resection of their tumor. Baseline demographics, disease characteristics, and exposure history for the cohort are described in Table 1.
Table 1.
Patient demographics, imaging, treatment, and pathological characteristics of the study cohort*
| Demographics and characteristics | Total (n = 472) |
|---|---|
| Demographics | |
| Sex | |
| Male | 369 |
| Female | 103 |
| Side | |
| Left | 214 |
| Right | 258 |
| Median age at surgery (range), y | 64 (18–84) |
| Asbestos exposure (self report) | |
| Negative | 103 |
| Positive | 283 |
| Unknown | 86 |
| Preoperative imaging | |
| Median interval from scan to surgery (range), d | 6 (0–30) |
| Intravenous contrast | |
| No | 275 |
| Yes | 197 |
| Effusion size | |
| None | 165 |
| Small | 160 |
| Moderate | 119 |
| Large | 28 |
| Effusion loculated | |
| No | 112 |
| Yes | 195 |
| Prior talc sclerosis | |
| No | 355 |
| Yes | 117 |
| Median tumor volume, cm3 | 248 (0–5471) |
| Median fissure maximal thickness (range), mm | 7 (0–59) |
| Treatment | |
| Neoadjuvant chemotherapy | |
| No | 406 |
| Yes | 66 |
| Procedure | |
| Extrapleural pneumonectomy | 317 |
| Pleurectomy/decortication | 35 |
| Extended pleurectomy/decortication | 120 |
| Heated intra-operative chemotherapy | |
| No | 98 |
| Yes | 374 |
| Adjuvant chemotherapy | |
| No | 269 |
| Yes | 202 |
| nknown | 1 |
| Adjuvant radiotherapy | |
| No | 378 |
| Yes | 93 |
| Unknown | 1 |
| Pathology | |
| WHO subtype classification | |
| Epithelioid | 286 |
| Sarcomatoid | 23 |
| Biphasic | 159 |
| Desmoplastic | 3 |
| Papillary (well-differentiated) | 1 |
| Clinical stage (AJCC) | |
| Stage I | 72 |
| Stage II | 129 |
| Stage III | 186 |
| Stage IV | 85 |
| Pathological stage (AJCC) | |
| Stage I | 40 |
| Stage II | 68 |
| Stage III | 231 |
| Stage IV | 133 |
AJCC = American Joint Committee on Cancer; WHO = World Health Organization.
AJCC Staging
The majority of the patients were categorized as having advanced stage (III and IV) according to clinical and pathological AJCC criteria (Table 2). Clinical staging underestimated pathological stage for 209 (44.2%) and overestimated pathological stage for 92 (19.4%) patients. Concordant staging was observed for 171 of 472 (36.2%) patients. Correspondingly, concordance between pathological and clinical detection of tumor involvement at the level of individual classification criteria was low and variable (Supplementary Table 1, available online). One exception, detection of confluent visceral pleural tumor including fissures, demonstrated twice the concordance rate (253 of 385 patients detected, 65.7%) of the next most concordant criterion (metastasis in ipsilateral bronchopulmonary or hilar lymph nodes, 112/336, 33.3%).
Table 2.
Concordance between AJCC clinical stage and pathological stage (entire cohort)*
| Pathological AJCC stage | Clinical AJCC stage |
||||
|---|---|---|---|---|---|
| I | II | III | IV | Total | |
| I | 16 | 10 | 9 | 5 | 40 |
| II | 12 | 28 | 22 | 6 | 68 |
| III | 31 | 67 | 93 | 40 | 231 |
| IV | 13 | 24 | 62 | 34 | 133 |
| Total | 72 | 129 | 186 | 85 | 472 |
AJCC = American Joint Committee on Cancer.
Cohort patients randomly assigned to the training set (n = 159, 33.7%) and test set (n = 313, 66.3%) were found to be comparable in terms of demographic, exposure, prognostic factors, therapy applied, and imaging parameters that could potentially affect clinical assessment of the imaging data (presence of pleural effusion, use of intravenous contrast, and prior talc pleurodesis) (Supplementary Table 2, available online).
Quantitative Image Analysis
Median VolCT was 248 cc, with a range from 0 to 5471 cc. Because fissural involvement was the clinical AJCC parameter most concordant with pathological assessment and because fissural thickness was unambiguously quantifiable (median = 7 mm, range = 0–59 mm) on CT images because of high contrast with surrounding lung parenchyma, Fmax was also explored as a potential quantitative classification parameter.
Categorical transformations of VolCT and Fmax were derived for the purpose of generating practical quantitative models that could represent a surrogate clinical stage. Univariate hazard ratio associated with varying VolCT cut-points in the training set shows local maxima at 150, 500, and 1200 cc (Supplementary Figure 1, available online), defining a four-level VolCT model. Fmax dichotomized at 5 mm statistically significantly stratified OS in training set patients with low (≤500 cc) overall tumor volume (P = .01) (Supplementary Figure 2A, available online), but high (>500 cc) tumor volume was associated with thick fissures (>5 mm) in most patients (88.9%) (Supplementary Figure 2B, available online). Thus, a four-level bivariate model (quantitative clinical stage) was defined, with stage grouping based primarily on categorical VolCT, with upstaging by one level if tumor volume was 500 cc or less and Fmax was greater than 5 mm (Table 3).
Table 3.
Quantitative clinical stage groupings*
| Quantitative stage groupings | VolCT (cc) and Fmax (mm) |
|---|---|
| Stage I | VolCT ≤ 150 and Fmax ≤ 5 |
| Stage II | VolCT ≤ 150 and Fmax > 5 or VolCT > 150 and VoCT ≤ 500 and Fmax ≤ 5 |
| Stage III | VolCT > 150 and VoCT ≤ 500 and Fmax > 5 or VolCT > 500 and VoCT ≤ 1200 |
| Stage IV | VolCT > 1200 |
Fmax = maximal fissural thickness; VolCT = tumor volume assessed from three-dimensional reconstruction of computed tomography scans.
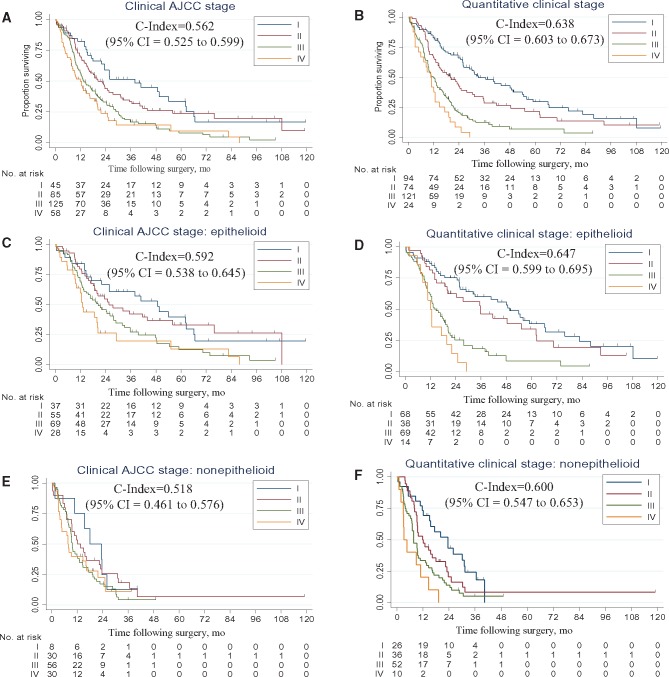
Model performance was evaluated in the 313 patients randomized to the test set. The baseline model (clinical AJCC stage) was associated with modest discriminative performance (c-index = 0.562, 95% confidence interval [CI] = 0.525 to 0.599) (Figure 2A). The univariate quantitative model based on VolCT alone showed statistically significantly better discrimination (c-index = 0.629, 95% CI = 0.593 to 0.665, P = .004) (Supplementary Figure 3, available online). The bivariate quantitative model (quantitative clinical stage) also demonstrated statistically significantly enhanced discriminative ability compared with the baseline model (c-index = 0.638, 95% CI = 0.603 to 0.673, P = .001) (Figure 2B). Model performance was similar when excluding the 42 patients in the test set who had received neoadjuvant chemotherapy (baseline c-index = 0.586, 95% CI = 0.546 to 0.627; univariate c-index: 0.636, 95% CI = 0.598 to 0.674, P = .03 vs baseline; bivariate c-index = 0.648, 95% CI = 0.612 to 0.685, P = .005, vs baseline). Statistically significant performance enhancement was also observed when comparing the bivariate model with the baseline in subsets of the test cohort comprising epithelioid (n = 189; baseline c-index = 0.592, 95% CI = 0.538 to 0.645; bivariate c-index = 0.647, 95% CI = 0.599 to 0.695, P = 0.03) (Figure 2, C and D) and nonepithelioid (n = 124; baseline c-index = 0.518, 95% CI = 0.461 to 0.576; bivariate c-index = 0.600, 95% CI = 0.547 to 0.653, P = .02) (Figure 2, E and F) tumor histology.
Figure 2.
Kaplan-Meier survival curves depicting estimated survival functions. Results are presented according to the (A)AJCC Cancer Staging Manual (7th ed.) clinical stage, (B) quantitative clinical stage, (C) AJCC Cancer Staging Manual (7th ed.) clinical stage: epitheloid subtype, (D) quantitative clinical stage: epithelpoid subtype, (E) AJCC Cancer Staging Manual (7th ed.) clinical stage: nonepitheloid subtype, and (F) quantitative clinical stage: nonepitheloid subtype. C-indexes are shown, with their corresponding 95% confidence intervals in parentheses. AJCC = American Joint Committee on Cancer; CI = confidence interval.
Quantitative clinical staging was concordant with AJCC pathological staging for 90 of 313 (28.7%), overestimated pathological stage for 42 (13.4%), and underestimated pathological stage for 181 (57.8%) patients in the test set (Table 4). Discriminative performance was not statistically different between quantitative clinical staging (c-index = 0.638, 95% CI = 0.603 to 0.673) and pathological AJCC staging (c-index = 0.607, 95% CI = 0.570 to 0.643, P = .22).
Table 4.
Counts of observed patients representing each possible pairwise combination of quantitative clinical stage and pathological AJCC stage (test set)
| Pathological AJCC stage | Quantitative clinical stage |
||||
|---|---|---|---|---|---|
| I | II | III | IV | Total | |
| I | 14 | 7 | 4 | 0 | 25 |
| II | 19 | 13 | 13 | 0 | 45 |
| III | 45 | 29 | 57 | 18 | 149 |
| IV | 16 | 25 | 47 | 6 | 94 |
| Total | 94 | 74 | 121 | 24 | 313 |
AJCC = American Joint Committee on Cancer.
Discussion
This study derives and validates a practical quantitative clinical staging strategy for MPM. Quantitative clinical staging performs statistically significantly better as a prognostic classifier when compared head to head in an independent data set with clinical staging by current AJCC criteria. It should also be noted that performance of the AJCC model in this study may have benefited from optimal circumstances (single experienced radiologist, standardized data form and collection protocol) and appears superior to other large published analyses (7,11,12). In notable contrast to clinical AJCC staging, quantitative clinical staging 1) identified twice as many stage I patients, 2) identified few stage IV patients who survived beyond two years, and 3) stratified survival among nonepithelioid patients.
For most solid tumors, tumor size can be accurately and reproducibly quantified by measuring diameters of spherical or ovoid lesions both upon gross dissection (for pathological stage) and on two-dimensional images at the resolution of available technology (for clinical stage). By contrast, the irregular and inconsistent morphology of MPM complicates quantification to a degree that has necessitated MPM-specific modifications of RECIST criteria (23) and has precluded tumor size consideration in TNM staging (20).
Instead, AJCC T classification of MPM is based on qualitative assessment of invasion of structures within the hemithorax by the tumor. Current TNM criteria were based on Rusch (1995) (24) and are theoretically consistent with microscopic examination of sampled margins of extrapleural pneumonectomy specimens. In practice, however, complete pathological staging of MPM is impractical because of the number of margins that must be assessed for invasion, the fact that many of them involve vital structures that must be retained by the patient, and the increasing proportion of fragmented and unoriented specimens generated by lung-sparing surgery. These limitations also challenge the notion that pathological staging represents a “gold standard” against which the accuracy of clinical staging may be assessed. On the other hand, modern imaging technology provides noninvasive access to all relevant margins, but at orders-of-magnitude lower resolution. These considerations may account for the low level of concordance between clinical and pathological staging observed in this and prior studies (25–27).
Some criteria, such as endothoracic fascial invasion and mediastinal fat invasion, may be best assessed intraoperatively, prompting the IASLC Staging Committee to consider a combined metric representing “best” stage (7), improving performance but adding an additional degree of subjectivity to MPM staging. A practical and objective approach is needed to evaluate MPM patients so that effective management strategy can be considered. Concordance between quantitative clinical stage and pathological AJCC stage was low, with 58% of patients understaged by the quantitative model, as reflected in larger numbers of patients being classified to stages I and II. The two systems demonstrated similar discriminative performance, but quantitative clinical stage does not require surgical resection and may therefore be applicable to a larger subset of patients with MPM.
VolCT was first described as prognostic for MPM in 1998 (13). At that time, the specialized equipment and time-consuming manual tumor segmentation that were required rendered VolCT impractical for clinical use. Rapid proliferation of digital radiology and image processing technologies has overcome these limitations, allowing for semi-automatic quantification of tumors on hybrid PACS workstations. This has made possible the use of VolCT in the context of standard radiology workflow for the purposes of response assessment and prognosis (14,15).
Measuring the degree of tumor presence in interlobar fissures is a convenient and unambiguous quantitative method due to the high degree of contrast between normal lung parenchyma and involved fissural pleura. In the current study, qualitative involvement of fissures demonstrated the highest rate of concordance among AJCC criteria between clinical and pathological assessment. Fmax provided additive prognostic information for patients with smaller-volume tumors, improving model performance relative to VolCT alone, as assessed in the test set.
If further validated in a multi-institutional and multi-observer setting, quantitative clinical staging would represent a cost-effective and ubiquitously available strategy for MPM staging because it is based entirely on information derived from CT scan, the primary imaging modality employed for evaluation of MPM worldwide. Where available, FDG-PET and MRI may further augment quantitative clinical staging with additional prognostic (metabolic, anatomical, and histological) information (28–31). The need for quantitative radiographic assessment as a prognostic marker is being actively explored (18,19). The IASLC/IMIG staging committee has recently published measurements of pleural thickness along three defined sites in the thorax on axial CT scan images, noting correlation with T classification (TNM, 7th ed.), nodal status, and survival (18). A recent paper by de Perrot and colleagues reported the prognostic significance of unidimensional measurement of diaphragmatic tumor thickness in patients treated with preoperative radiotherapy (32). If validated, these measures may also prove useful to augment clinical staging.
It will also be of interest to determine whether the proposed quantitative strategy may assist in assessing response to nonsurgical therapy. Monitoring treatment response in MPM is challenging due to the circumferential rind-like morphology and unique growth pattern. Two-dimensional quantification using unidimensional measurements of the long axis, including RECIST 1.0 (up to five lesions per organ), RECIST 1.1 (up to two lesions per organ), and even bidimensional measurements (WHO; up to five lesions) are poorly suited for response assessment in phase II/ III therapeutic trials and routine clinical practice (33).
These shortcomings have led to the development of mesothelioma-specific modified RECIST criteria (tumor thickness at two positions on three separate CT sections for up to six target lesions) (23). However, response assessment continues to be highly problematic, prompting exploration of 3D or VolCT assessment of MPM (33–37), and there remains an unmet need to develop quantifiable metrics for clinical trials. The quantitative approach combining VolCT and Fmax may allow for a more accurate comparison of outcomes in patients who undergo nonsurgical treatments.
This study has several limitations. First, it was performed using retrospectively collected data, and the treatment strategies applied were not assigned in a randomized fashion. Second, we limited our initial exploration to quantitative models based on imaging data derived from CT scans only. There was some variability in the slice thickness among available scans. The CT scans, which were acquired with PET scans, were acquired without breathing instructions. The degree to which these parameters affect the volumetric assessment has not been assessed. Future work will explore the additive power conferred by other imaging modalities such as MRI and FDG-PET. Third, choosing the cut-points for VolCT based on local maxima may not be as optimal statistically as a nomogram or multivariable prognostic index, without increasing the burden for clinicians, as ease of use is an essential hallmark of an ideal prognostic model (28). Fourth, because all images were reviewed by a single radiologist, inter-reader variability could not be addressed and could potentially affect universal translation. However, because the proposed algorithm bins tumor volume into four categories, moderate inter-reader variability would be expected to impact classification only for volumes in the vicinity of cut-points. Further validation studies involving multiple radiologists will also be required to determine whether the proposed surrogate staging strategy is sufficiently reproducible to be clinically relevant.
In conclusion, our results show that quantitative clinical staging based on VolCT and Fmax improves prognostic stratification among stages in comparison with AJCC clinical staging. The proposed system may represent a straightforward and objective approach to determining appropriate treatment strategy and to risk stratification of patients entering clinical trials.
Note
Presented at the 16th World Conference on Lung Cancer in 2015 (Denver, CO).
Supplementary Material
References
- 1. Skammeritz E, Omland LH, Johansen JP et al. , Asbestos exposure and survival in malignant mesothelioma: A description of 122 consecutive cases at an occupational clinic. Int J Occup Environ Med. 2011;24:224–236. [PubMed] [Google Scholar]
- 2. Bianchi C, Giarelli L, Grandi G et al. , Latency periods in asbestos-related mesothelioma of the pleura. Eur J Cancer Prev. 1997;62:162–166. [PubMed] [Google Scholar]
- 3. Wolf AS, Daniel J, Sugarbaker DJ.. Surgical techniques for multimodality treatment of malignant pleural mesothelioma: Extrapleural pneumonectomy and pleurectomy/decortication. Semin Thorac Cardiovasc Surg. 2009;212:132–148. [DOI] [PubMed] [Google Scholar]
- 4. Sugarbaker DJ, Flores RM, Jaklitsch MT et al. , Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: Results in 183 patients. J Thorac Cardiovasc Surg. 1999;1171:54–63, discussion 63–65. [DOI] [PubMed] [Google Scholar]
- 5. Sugarbaker DJ, Strauss GM, Lynch TJ et al. , Node status has prognostic significance in the multimodality therapy of diffuse, malignant mesothelioma. J Clin Oncol. 1993;116:1172–1178. [DOI] [PubMed] [Google Scholar]
- 6. Rusch VW, Venkatraman ES.. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg. 1999;685:1799–1804. [DOI] [PubMed] [Google Scholar]
- 7. Rusch VW, Giroux D, Kennedy C et al. , Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol. 2012;711:1631–1639. [DOI] [PubMed] [Google Scholar]
- 8. Vogelzang NJ, Rusthoven JJ, Symanowski J et al. , Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;2114:2636–2644. [DOI] [PubMed] [Google Scholar]
- 9. van Meerbeeck JP, Gaafar R, Manegold C et al. , Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: An intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;2328:6881–6889. [DOI] [PubMed] [Google Scholar]
- 10. Rami-Porta R, Bolejack V, Crowley J et al. , The IASLC Lung Cancer Staging Project: Proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;107:990–1003. [DOI] [PubMed] [Google Scholar]
- 11. Rusch VW, Gill R, Mitchell A et al. , A Multicenter study of volumetric computed tomography for staging malignant pleural mesothelioma. Ann Thorac Surg. 2016;1024:1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gill RR, Naidich DP, Mitchell A et al. , North American Multicenter Volumetric CT Study for Clinical Staging of Malignant Pleural Mesothelioma: Feasibility and logistics of setting up a quantitative imaging study. J Thorac Oncol. 2016;118:1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pass HI, Temeck BK, Kranda K et al. , Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1998;1152:310–317, discussion 317–318. [DOI] [PubMed] [Google Scholar]
- 14. Gill RR, Richards WG, Yeap BY et al. , Epithelial malignant pleural mesothelioma after extrapleural pneumonectomy: Stratification of survival with CT-derived tumor volume. Am J Roentgenol. 2012;1982:359–363. [DOI] [PubMed] [Google Scholar]
- 15. Liu F, Zhao B, Krug LM et al. , Assessment of therapy responses and prediction of survival in malignant pleural mesothelioma through computer-aided volumetric measurement on computed tomography scans. J Thorac Oncol. 2010;56:879–884. [DOI] [PubMed] [Google Scholar]
- 16. Gill RR, Richards WG, Yeap BY et al. , Quantitative clinical T classification criteria for malignant pleural mesothelioma. J Thorac Oncol. 2013;8(S2):S313. [Google Scholar]
- 17. Gill RR, Richards WG, Yeap BY et al. , Novel clinical assessment of malignant pleural mesothelioma. J Thorac Oncol. 2013;8(S2):S939. [Google Scholar]
- 18. Nowak AK, Chansky K, Rice DC et al. , The IASLC Mesothelioma Staging Project: Proposals for revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for pleural mesothelioma. J Thorac Oncol. 2017;1112:2089–2099. [DOI] [PubMed] [Google Scholar]
- 19. de Perrot M, Dong Z, Bradbury P et al. , Impact of tumour thickness on survival after radical radiation and surgery in malignant pleural mesothelioma. Eur Respir J. 2017;493. [DOI] [PubMed] [Google Scholar]
- 20. Edge SB, Byrd DR, Compton CC et al. , AJCC Cancer Staging Manual. 7th ed.New York: Springer; 2010. [Google Scholar]
- 21. Rice D, Rusch V, Pass H et al. , Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: A consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol. 2011;68:1304–1312. [DOI] [PubMed] [Google Scholar]
- 22. Newson RB. Comparing the predictive powers of survival models using Harrell's C or Somers' D. Stata J. 2010;103:339. [Google Scholar]
- 23. Byrne MJ, Nowak AK.. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;152:257–260. [DOI] [PubMed] [Google Scholar]
- 24. Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest. 1995;1084:1122–1128. [DOI] [PubMed] [Google Scholar]
- 25. Richards WG, Godleski JJ, Yeap BY et al. , Proposed adjustments to pathologic staging of epithelial malignant pleural mesothelioma based on analysis of 354 cases. Cancer. 2010;1166:1510–1517. [DOI] [PubMed] [Google Scholar]
- 26. Cao C, Krog Andvik SK, Yan TD et al. , Staging of patients after extrapleural pneumonectomy for malignant pleural mesothelioma—institutional review and current update. Interact Cardiovasc Thorac Surg. 2011;125:754–757. [DOI] [PubMed] [Google Scholar]
- 27. Nakas A, Black E, Entwisle J et al. , Surgical assessment of malignant pleural mesothelioma: Have we reached a critical stage? Eur J Cardiothorac Surg. 2010;376:1457–1463. [DOI] [PubMed] [Google Scholar]
- 28. Heng DY, Xie W, Regan MM et al. , External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013;142:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coolen J, De Keyzer F, Nafteux P et al. , Malignant pleural disease: Diagnosis by using diffusion-weighted and dynamic contrast-enhanced MR imaging—initial experience. Radiology. 2012;2633:884–892. [DOI] [PubMed] [Google Scholar]
- 30. Gill RR, Umeoka S, Mamata H et al. , Diffusion-weighted MRI of malignant pleural mesothelioma: Preliminary assessment of apparent diffusion coefficient in histologic subtypes. Am J Roentgenol. 2010;1952:W125–W130. [DOI] [PubMed] [Google Scholar]
- 31. Francis RJ, Byrne MJ, van der Schaaf AA et al. , Early prediction of response to chemotherapy and survival in malignant pleural mesothelioma using a novel semiautomated 3-dimensional volume-based analysis of serial 18F-FDG PET scans. J Nucl Med. 2007;489:1449–1458. [DOI] [PubMed] [Google Scholar]
- 32. de Perrot M, Dong Z, Bradbury P et al. , Impact of tumour thickness on survival after radical radiation and surgery in malignant pleural mesothelioma. Eur Respir J. 2017;493:1601428. [DOI] [PubMed] [Google Scholar]
- 33. van Klaveren RJ, Aerts JG, de Bruin H et al. , Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer. 2004;431:63–69. [DOI] [PubMed] [Google Scholar]
- 34. Labby ZE, Nowak AK, Dignam JJ et al. , Disease volumes as a marker for patient response in malignant pleural mesothelioma. Ann Oncol. 2013;244:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nowak AK, Francis RJ, Phillips MJ et al. , A novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin Cancer Res. 2010;168:2409–2417. [DOI] [PubMed] [Google Scholar]
- 36. Frauenfelder T, Tutic M, Weder W et al. , Volumetry: An alternative to assess therapy response for malignant pleural mesothelioma? Eur Respir J. 2011;381:162–168. [DOI] [PubMed] [Google Scholar]
- 37. Lee HY, Hyun SH, Lee KS et al. , Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: Prediction of therapeutic response and prognostic implications. Ann Surg Oncol. 2010;1710:2787–2794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.