Abstract
This article comments on:
Makavitskaya M, Svistunenko D, Navaselsky I, et al. 2018. Novel roles of ascorbate in plants: induction of cytosolic Ca2+ signals and efflux from cells via anion channels. Journal of Experimental Botany 69, 3477–3489.
Keywords: Anion channels, anion current, Arabidopsis, ascorbate, Ca2+ signaling, cytosolic Ca2+, hydroxyl radical, NADPH oxidase, ROS
Reactive oxygen species (ROS) and intracellular Ca2+ signaling interact with and amplify each other: the major ROS-producing plasma membrane enzyme NADPH-oxidase (NOX) is activated by cytosolic Ca2+, and in turn ROS activate Ca2+ influx across the plasma membrane. For the latter, NOX-produced superoxide anions need to be converted to hydroxyl radicals. Makavitskaya et al. (2018) have now demonstrated that salt stress promotes ascorbate efflux, which, via reduction of the apoplastic copper and iron ions, assists in the generation of hydroxyl radicals, thus inducing a rise in intracellular Ca2+ in the roots.
Aerobic metabolism inevitably generates ROS, which could be very destructive to biomolecules and structures. Consequently, the main components of the antioxidant system appeared at almost the same moment as the ROS-producing ones, approximately 3.8–3.6 billion years ago (Inupakutika et al., 2016). Stress-induced metabolic changes result in increased ROS production (oxidative stress component), which may be balanced or not by an increase in antioxidant activity, leading either to adaptation or death, respectively (Demidchik et al., 2010; Morales and Munné-Bosch, 2016; Choudhury et al., 2017). Compared to animals, plants generally show 10–1000 times higher resistance to ROS (as H2O2) (Haliwell and Gutteridge, 2015). At this point, ROS may be viewed as a burden, but the early evolutionary appearance of the NADPH-oxidases (NOX), a class of enzymes whose principal function is ‘deliberate’ ROS production, forces one to look on ROS generation from a different angle. NOX enzymes have been found in all multicellular eukaryotes, including plants, animals and fungi (Inupakutika et al., 2016). The function of plant homologs (RBOH: Respiratory Burst Oxidase Homologs) was originally attributed to pathogen defense in the hypersensitive response. However, later on, roles of RBOH enzymes in growth and morphogenesis were established and, finally, in ROS and Ca2+ signaling in response to different abiotic stresses (reviewed by Baxter et al., 2014; Kaur et al., 2014; Choudhury et al., 2017; see also Turkan, 2017).
Plant NOX function and regulation: the role of intracellular Ca2+
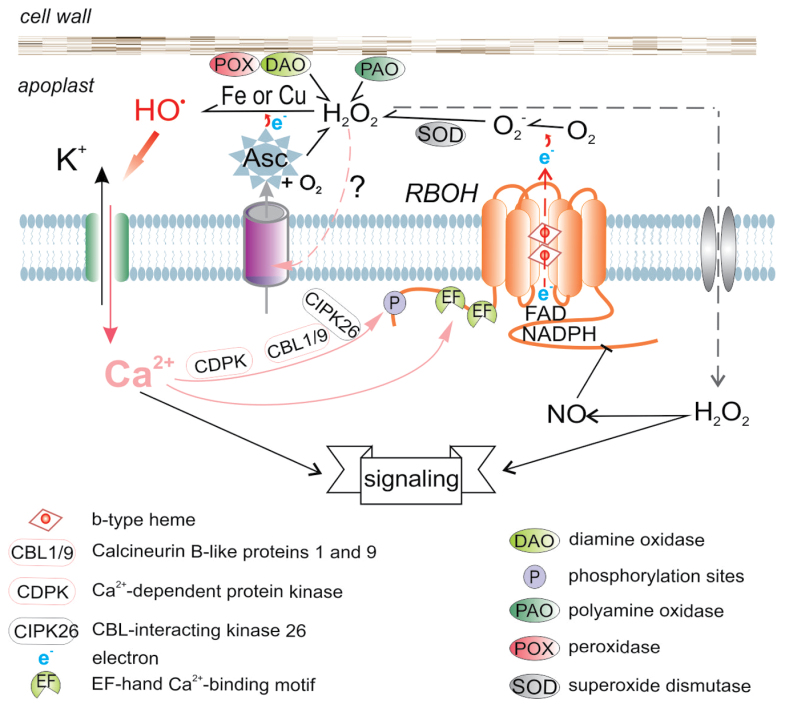
Plant NOX are situated in the plasma membrane and, similarly to animal homologs, are formed by six transmembrane domains. The catalytic part consists of NADPH and FAD-binding domains in the N-terminus and two b-type hemes located between transmembrane domains III and V (Box 1). Unlike most of their animal counterparts (except mammalian Nox5 and Duox), plant NOX possess two specific Ca2+-binding sites (EF-hand motifs) in their N-terminus (Suzuki et al., 2011). This underlies a specific type of plant RBOH regulation. Activation of plant RBOH critically depends on phosphorylation and cytosolic Ca2+ binding to the residues located in the N-terminus. Activation of Ca2+ influx conductance by HO• (less commonly by H2O2), as for the first time demonstrated by Foreman et al. (2003), and activation of RBOH by inflowing Ca2+ provides a feed-forward mechanism for ROS and Ca2+ crosstalk (Box 1) (for early experimental demonstrations see Takeda et al., 2008, Demidchik et al., 2009). Crosstalk between ROS and Ca2+ signaling as well as ROS activation of Ca2+-permeable channels has also been shown in animal cells (Zhang et al., 2016), but it seems to occur less frequently than in plants. In particular, a self-amplifying circuit of certain RBOH species combined with a Ca2+-permeable channel (of non-identified molecular nature or encoded by a member of the glutamate receptor or cyclic nucleotide-gated channel families) is involved in plant growth and development, phytohormone signaling, hypersensitive responses to pathogens and responses to abiotic stress (reviewed by Demidchik and Shabala, 2018).
Box 1. Regulation of NOX activity and operation of the ROS-Ca2+ self-amplifying loop in plants
Plant NADPH-oxidase (RBOH) is an integral plasma membrane protein which mediates a single-electron transmembrane transfer from NADPH to molecular oxygen, generating the superoxide-anion (O2–). The electron-transport chain from NADPH to O2 includes FAD and b-type hemes, located in the RBOH C-terminus and between transmembrane domains, respectively. The latter is converted to peroxide (H2O2) spontaneously or catalysed by superoxide dismutase (SOD). In the presence of reduced transient valency metals, copper (Cu+) or iron (Fe2+), H2O2 is reduced to the hydroxyl radical (HO•) by means of the Fenton reaction. Apoplastic Fe and Cu form part of low molecular weight complexes with organic acids or of metalloproteins, cell wall-bound peroxidases (POX, iron) and diamine oxidase (DAO, copper). Ascorbate in the apoplast is mainly exported from the cytosol.
Makavitskaya et al. (2018) recorded an anionic current mediating ascorbate efflux from roots for the first time. Ascorbate may directly interact with O2, generating H2O2, but it is far more essential for the generation of HO•, maintaining Fe, and especially Cu, in their reduced forms. In turn, HO• activates multiple conductances across the plasma membrane, which mediate K+ efflux and Ca2+ influx. Incoming Ca2+ activates the RBOH synergistically by direct interaction with Ca2+-binding sites (EF-hands) in the N-terminus or via Ca2+-dependent phosphorylation of different residues either by CDPK or the CBL-CIPK couple (Qu et al., 2017). This generates a positive feedback loop between generation of ROS and Ca2+ signals. Conversely, S-nitrosylation of the cysteine residue in the C-terminus was shown to inhibit AtRBOHD activity; as this residue is conserved in the other 18 AtRBOH members, such a regulatory mechanism may be hypothesized to be universal for the family (Kaur et al., 2018). In Arabidopsis, NO generation is rapidly stimulated by H2O2, so it is tempting to think that this mechanism may serve as a feedback loop, terminating the RBOH-generated ROS signal.
Different ROS species have different capabilities in the activation of Ca2+ conductance. For instance, extracellular H2O2 only induced activation of Ca2+-permeable channels in the root elongation zone, whereas HO• also induced multiple conductances in the mature root zone (Demidchik et al., 2007; Pottosin et al., 2014). In the apoplast HO• is generated through the Fenton reaction, via conversion of H2O2 by reduced transient valency metal ions, Fe2+ and Cu+. Copper is a much better catalyst of the Fenton reaction (reacting much more rapidly with H2O2) as compared to iron (Haliwell and Gutteridge, 2015). This is also confirmed by the data in the present study (Fig. 1B, Makavitskaya et al., 2018). It should also be noted that standard redox potentials for the pairs Cu2+/Cu+ and Fe3+/Fe2+ differ by 600 mV, i.e. under physiological redox conditions copper will be several orders of magnitude more oxidized compared to iron. This difference will be compensated by reducing conditions in the presence of high concentrations of ascorbate (Asc).
Export of reducing power: ascorbate efflux and signaling
Ascorbate is a very abundant cellular compound. About 90% of the available pool is cytosolic, yet it is exported to the apoplast across the plasma membrane and its concentration in this compartment may reach several millimolar (Akram et al., 2017). Its role as a powerful antioxidant (H2O2 scavenger) in the cytosol is well established, but this process employs a sophisticated four-enzyme system, which may be less abundant in the apoplast (Podgorska et al., 2017). As dicotyledonous plants can only transport iron across the plasma membrane in a reduced (Fe2+) form, export of ascorbate to the apoplast and its role in the reduction of Fe3+ to Fe2+ have been shown to be essential for iron uptake (Grillet et al., 2014). Yet, ferrous compounds potentially possess pro-oxidant activity, as Fenton reaction catalysts assisting the generation of HO•. Therefore, rather than acting as an anti-oxidant, apoplastic ascorbate may be involved in HO• production and the induction of HO•-sensitive Ca2+ conductance.
In line with this hypothesis, Makavitskaya et al. (2018) demonstrated that the addition of ascorbate to Arabidopsis roots induced cytosolic Ca2+ to increase in a dose-dependent manner. An ascorbate-induced increase in cytosolic Ca2+ was potentiated by externally applied Cu (as low as 0.001–0.01 mM) and Fe (1 mM) and suppressed by the HO• scavenger thiourea, specific Cu and Fe chelators and low external Ca2+. Collectively, these results imply that an ascorbate-induced increase in cytosolic Ca2+ is caused by Ca2+ influx via HO•-activated plasma membrane conductance, where HO• is generated by means of the Fenton reaction. Despite a higher affinity for copper as compared to iron, naturally occurring apoplastic Fe and Cu centers displayed comparable roles as Fenton catalysts in Arabidopsis roots. Their absence in cell wall-free preparations (root protoplasts) precluded a rise in ascorbate-induced cytosolic Ca2+. Up to this point, HO• generation in the discussed work was achieved at least partially by the artificial, external application of Fenton reagents. It was previously demonstrated that acute salt stress provokes very significant generation of HO• by Arabidopsis roots (Demidchik et al., 2010). Application of the electron spin resonance (ESR) technique by Makavitskaya et al. (2018) allowed the sensitive detection of ascorbate (as the ascorbyl anion radical, Asc•–), released by Arabidopsis NaCl-stressed roots. Another important finding of this study was a direct recording of ascorbate efflux by means of the patch-clamp technique. Whereas import of the oxidized form of ascorbate for its recycling in the cytosol is relatively well understood (Akram et al., 2017), to our knowledge this is the first time that the mechanism of ascorbate export to the apoplast has been addressed and successfully resolved. Ascorbate efflux measured as Asc•– formation and whole-cell current (patch-clamp measurements) were suppressed by A9C with the same potency (Makavitskaya et al., 2018), which suggests that the current is important for ascorbate efflux. The nature of this ionic current remains to be elucidated, though its rapid kinetics are reminiscent of the R-type anion current which plays an important role in stomatal closure. The major contributor to the R-type current in Arabidopsis guard cells is AtALMT12, which belongs to the ALMT (Aluminum-activated Malate Transporter) family. Another member of the ALMT family is predominantly expressed in Arabidopsis roots, with up-regulation by low external pH and, importantly, H2O2 (Sharma et al., 2016). Thus, future experiments with AtALMT1 knockout mutants should enable its role in salt-induced ascorbate efflux from roots to be elucidated.
Another open question is how ascorbate export is stimulated under stress (i.e. how the activity of transporters is potentiated). One interesting possibility may be feed-forward regulation by ROS (Box 1). The ESR method, which detects oxidized Asc•– as a measure of exported ascorbate, cannot be used for the evaluation of the ROS-induced ascorbate efflux because of the confounding effect of Asc•– formed from direct ascorbate oxidation by added ROS. An alternative technique, however, based on measurements of 14C-labelled ascorbate efflux, has been used to demonstrate rapid (peak at 2 min) and massive (up to 20% of cellular ascorbate) ascorbate release from plant cell cultures, induced by 1–10 mM H2O2 (Parsons and Fry, 2010). The exact mechanism by which ROS stimulates ascorbate efflux is unknown. It may be a direct activation of the anionic transporter (which could be directly addressed in a patch-clamp study) or mediated by some ROS-associated factors (e.g. intracellular Ca2+ signaling) and/or be a consequence of electrocoupling with the ROS-induced K+ efflux (anion, and ascorbate in particular, efflux will be driven by K+ efflux for electroneutrality).
Conclusion
The ROS-Ca2+ self-amplifying loop is gaining shape and has been shown to be involved in diverse plant responses. Ascorbate efflux could potentially intensify the operation of such a loop, assisting the formation of powerful hydroxyl radicals. However, whether such efflux is a common component of the self-amplifying mechanism and how it is co-ordinated in time and space with the operation of RBOH and/or ROS-activated plasma membrane channels will need to be addressed in future studies.
References
- Akram NA, Shafiq F, Ashraf M. 2017. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Frontiers in Plant Science 8, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A, Mittler R, Suzuki N. 2014. ROS as key players in plant stress signalling. Journal of Experimental Botany 65, 1229–1240. [DOI] [PubMed] [Google Scholar]
- Choudhury FK, Rivero RM, Blumwald E, Mittler R. 2017. Reactive oxygen species, abiotic stress and stress combination. The Plant Journal: for cell and molecular biology 90, 856–867. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V. 2010. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Science 123, 1468–1479. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala S. 2018. Mechanisms of cytosolic calcium elevation in plants: the role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS-Ca2+ Hub’. Functional Plant Biology 45, 9–27. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Davies JM. 2007. Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. The Plant Journal: for cell and molecular biology 49, 377–386. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shang Z, Shin R, et al. 2009. Plant extracellular ATP signaling by plasma membrane NADPH oxidase and Ca2+ channels. The Plant Journal 58, 903–913. [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, et al. 2013. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446. [DOI] [PubMed] [Google Scholar]
- Grillet L, Ouerdane L, Flis P, Hoang MT, Isaure MP, Lobinski R, Curie C, Mari S. 2014. Ascorbate efflux as a new strategy for iron reduction and transport in plants. The Journal of Biological Chemistry 289, 2515–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. 2015. Free radicals in biology and medicine. 5th edn Oxford University Press. [Google Scholar]
- Inupakutika MA, Sengupta S, Devireddy AR, Azad RK, Mittler R. 2016. The evolution of reactive oxygen species metabolism. Journal of Experimental Botany 67, 5933–5943. [DOI] [PubMed] [Google Scholar]
- Kaur G, Guruprasad K, Temple BRS, Shirvanyants DG, Dokholyan NV, Pati PK. 2018. Structural complexity and functional diversity of plant NADPH oxidases. Amino Acids 50, 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Sharma A, Guruprasad K, Pati PK. 2014. Versatile roles of plant NADPH oxidases and emerging concepts. Biotechnology Advances 32, 551–563. [DOI] [PubMed] [Google Scholar]
- Makavitskaya M, Svistunenko D, Navaselsky I, et al. 2018. Novel roles of ascorbate in plants: induction of cytosolic Ca2+ signals and efflux from cells via anion channels. Journal of Experimental Botany 69, 3477–3489. [DOI] [PubMed] [Google Scholar]
- Morales M, Munné-Bosch S. 2016. Oxidative stress: a master regulator of plant trade-offs?Trends in Plant Science 21, 996–999. [DOI] [PubMed] [Google Scholar]
- Parsons HT, Fry SC. 2010. Reactive oxygen species-induced release of intracellular ascorbate in plant cell-suspension cultures and evidence for pulsing of net release rate. The New phytologist 187, 332–342. [DOI] [PubMed] [Google Scholar]
- Podgórska A, Burian M, Szal B. 2017. Extra-cellular but extra-ordinarily important for cells: apoplastic reactive oxygen species metabolism. Frontiers in Plant Science 8, 1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin I, Velarde-Buendía AM, Bose J, Zepeda-Jazo I, Shabala S, Dobrovinskaya O. 2014. Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: implications for plant adaptive responses. Journal of Experimental Botany 65, 1271–1283. [DOI] [PubMed] [Google Scholar]
- Qu Y, Yan M, Zhang Q. 2017. Functional regulation of plant NADPH oxidase and its role in signaling. Plant Signaling & Behavior 12, e1356970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma T, Dreyer I, Kochian L, Piñeros MA. 2016. The ALMT family of organic acid transporters in plants and their involvement in detoxification and nutrient security. Frontiers in Plant Science 7, 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. 2011. Respiratory burst oxidases: the engines of ROS signaling. Current Opinion in Plant Biology 14, 691–699. [DOI] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. 2008. Local positive feedback regulation determines cell shape in root hair cells. Science 319, 1241–1244. [DOI] [PubMed] [Google Scholar]
- Turkan I. 2017. Emerging roles for ROS and RNS—versatile molecules in plants. Journal of Experimental Botany 68, 4413–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W. 2016. ROS and ROS-mediated cellular signaling. Oxidative Medicine and Cellular Longevity 2016, 4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]