Abstract
Plasmodium falciparum parasites causing placental malaria express the VAR2CSA type of the clonally variant antigen family erythrocyte membrane protein 1 (PfEMP1). This enables evasion of preexisting immunity and results in placental accumulation of infected erythrocytes. We present data on seasonal variation in levels of VAR2CSA-specific immunoglobulin G (IgG) and IgG specific for a placental malaria–unrelated PfEMP1 protein among Ghanaian women at their first antenatal visit. Our results indicate that placental malaria does not require recent exposure to infected mosquitoes, in contrast to malaria in general. This has implications for the impact of insecticide-treated bed nets on placental malaria incidence and for antenatal care in woman with preexisting immunity.
Keywords: IgG, PfEMP1, placental malaria, Plasmodium falciparum, VAR2CSA
People living in areas with stable transmission of Plasmodium falciparum acquire substantial immunity to malaria during childhood [1]. This protection is largely mediated by immunoglobulin G (IgG) antibodies targeting the asexual blood-stage parasites, which are responsible for all the clinical symptoms of malaria [2]. IgG targeting P. falciparum antigens on the infected erythrocyte surface, in particular members of the clonally variant antigen family erythrocyte membrane protein 1 (PfEMP1), appear to be of particular importance [3]. PfEMP1 mediates adhesion of mature infected erythrocytes to receptors in the vasculature of the infected individual. This tissue sequestration facilitates parasite evasion of phagocytic destruction of infected erythrocytes in the spleen. However, it can also lead to severe disease precipitated by inflammation, circulatory disturbances, and high parasite loads. PfEMP1-specific IgG is thought to be protective by interfering with infected erythrocyte sequestration and by opsonizing infected erythrocytes for improved phagocytosis.
Although P. falciparum parasites have evolved to subvert and delay acquisition of immunity by switching among the approximately 60 different PfEMP1 proteins encoded in the parasite genome [3], girls living in stable-transmission areas have acquired substantial protection from malaria (apart from placental infection) well before they reach childbearing age. Clinical disease episodes are rare by then, but carriage of low-density parasitemia, suppressed by the immune response to densities often too low to be detected by rapid diagnostic tests or standard examination of blood films, remains common. This notwithstanding, pregnancy is accompanied by a sudden reappearance of susceptibility to malaria, particularly among primigravidae. It is now well established that this is due to parasites expressing a particular type of PfEMP1. It is called VAR2CSA, and it enables sequestration of infected erythrocytes in the placenta by binding to an adhesion receptor (oncofetal chondroitin sulfate) available there but not elsewhere in the body. Expression of VAR2CSA is therefore incompatible with parasite survival in a nonpregnant person. Because VAR2CSA is not only functionally distinct but also antigenically distinct from all other PfEMP1 types, PfEMP1-specific protective immunity acquired prior to the first pregnancy does not protect against infected erythrocytes expressing VAR2CSA on their surface. However, once the immune system is exposed to VAR2CSA during the first placental infection, protective immunity to this antigen type develops. Placental malaria is therefore mainly a problem during the first few pregnancies. Once acquired, protection is maintained between pregnancies by specific memory B cells, rather than by VAR2CSA-specific IgG, which normally declines to relatively low levels shortly after delivery. This low durability of the specific IgG response once the antigen is gone is not restricted to VAR2CSA. It is rather the rule for most PfEMP1-specific IgG responses and, indeed, for IgG responses to many other malaria antigens, as well. With these facts in mind, we assessed the relative importance in the etiology of placental malaria of infections acquired from mosquitoes during pregnancy versus subclinical parasitemia established before conception.
METHODS
Study Site and Participants
The study was conducted in the town of Asutsuare, Ghana, located about 85 km northeast of Accra. P. falciparum transmission intensity in this area is seasonally variable, peaking during and immediately after the rainy season [4]. Pregnant women, who consented to participate in the study after receiving oral and written information about its aims at their first or second antenatal visit, were recruited consecutively from July 2006 to September 2008. Women with any form of pregnancy complication were excluded from participation. Data regarding age, gestational age, pregnancy history, number of remembered previous malaria episodes, clinical data (temperature, hemoglobin level, blood type, and parasitemia level), and a venous anticoagulated (via ethylenediaminetetraacetic acid) blood sample (4 mL) were collected from all participants. Some plasma samples were inadvertently lost during storage, before antibody analysis. Plasma samples from 30 healthy men and 30 nonpregnant women from the same community were included for comparison, and 10 Danish healthy adults without previous exposure to P. falciparum were included as negative controls.
Ethics Statement
This study was reviewed and approved by the ethics committee of the Noguchi Memorial Institute for Medical Research, University of Ghana (permission no. NMIMR-IRB CPN 014/05/-06).
P. falciparum–Specific Plasma Antibody Levels
The plasma levels of IgG antibodies to 2 recombinant PfEMP1 proteins were measured by enzyme-linked immunosorbent assay as described in detail previously [5]. One protein (FV2) represented the full-length ectodomain of the VAR2CSA-type antigen IT4VAR04. The other protein (FV6) represented the full-length ectodomain of another PfEMP1 protein (HB3VAR06), which is a commonly recognized PfEMP1 antigen that is not associated with placental malaria.
Statistical Analysis
Monthly variation (January–December) in rainfall was analyzed by 1-way analysis of variance (test statistic: F). The difference in antibody levels in women with and without parasitemia was analyzed by the Mann-Whitney test (T). Intergroup differences (men, pregnant women, and nonpregnant women; and women with fetuses of different gestational ages) were evaluated by Kruskal-Wallis 1-way analysis of variance on ranks (H), followed by the Dunn post hoc test, to isolate statistically significant pair-wise differences. The association between FV2-specific and FV6-specific IgG responses was evaluated by Spearman rank-order correlation analysis (rs). Associations between antibody levels and donor parity were evaluated by the Cuzik test for trend (z). P values <.05 were considered statistically significant.
RESULTS
Baseline Characteristics of the Study Participants and Study Site
A total of 807 pregnant women were recruited into the study. Recruitment was fairly even over the study period (Supplementary Figure 1). Samples from 644 women were available for antibody measurements. The characteristics of the latter women are summarized in Table 1. The temporal distribution (by calendar month) of recruitment was similar for women with and those without detectable peripheral parasitemia (P(T) > .15).
Table 1.
Characteristics of Study Participants
| Parameter | Value |
|---|---|
| Age, y | 25 (20–30) |
| Parity (n = 634) | 2 (1–3) |
| 0 | 175 |
| 1 | 129 |
| 2 | 119 |
| >2 | 211 |
| First antenatal visit | |
| Evaluated | 631 |
| Yes | 580 |
| No | 51 |
| Self-reported prophylaxis | |
| Evaluated | 627 |
| Yes | 245 |
| No | 382 |
| Gestational age, mo (n = 632)a | 5 (4–6) |
| Peripheral parasitemia detected | |
| Evaluated | 644 |
| Yes | 104b |
| No | 540 |
| Axillary temperature, °C (n = 622) | 36.9 (36.5–37.1) |
| Hemoglobin level, g/L (n = 632) | 11.0 (9.9–11.8) |
| Hemoglobin genotype AS | |
| Evaluated | 633 |
| Yes | 60 |
| No | 573 |
Data are median value (interquartile range) or no. of participants.
aBased on time since reported last menstruation.
bRange, 80–21000 asexual parasites/µL.
As proxy marker for seasonal variation in P. falciparum transmission intensity in our study area at the time the study was conducted, we collected and analyzed monthly rainfall data during 2006–2008 from the meteorological records of the nearby town of Akuse. The data supported our assumption of marked seasonal variation, as significant overall and annual seasonal variations in rainfall were evident during the study period (P(F) < .001), with peaks in April–May, during the long rainy season, and in September, during the short rainy season (Supplementary Figure 2).
Plasma Levels of PfEMP1-Specific IgG Among Study Participants
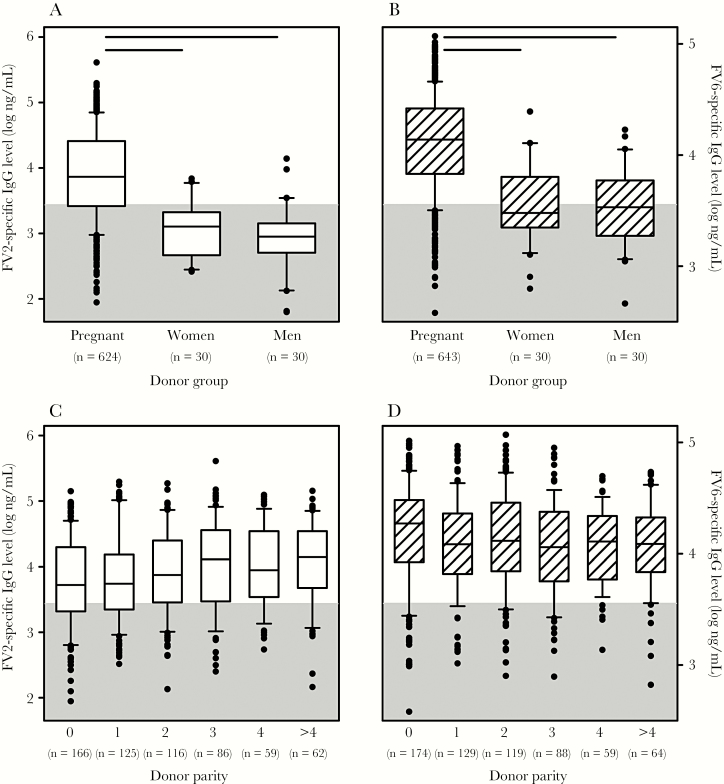
Plasma levels of FV2-specific (Figure 1A) and FV6-specific IgG (Figure 1B) differed significantly among the study groups (P(H) < .001). Post hoc analysis (by the Dunn test) showed that the pregnant women had significantly higher plasma levels of FV2- and FV6-specific IgG than the men and the nonpregnant women from the same area (P < .05). In contrast, the levels did not differ significantly between men and nonpregnant women for either antigen. Whereas about 40% of the men and nonpregnant women had FV6-specific IgG levels above the cutoff, only about 15% had levels of FV2-specific IgG above the negative cutoff. There was a moderate positive correlation between FV2-specific and FV6-specific IgG plasma levels (P < .0001 and rs = .60; n = 634).
Figure 1.
Plasma levels of Plasmodium falciparum family erythrocyte membrane protein 1 (PfEMP1)–specific immunoglobulin G (IgG). A, Levels of FV2-specific IgG in plasma from Ghanaian pregnant women, nonpregnant women, and men. Median values (center lines), interquartile ranges (boxes), interdecile ranges (bars), and outliers (points) are shown. The number of donors in each group is indicated along the bottom of the panel. Statistically significant differences (P < .05) are indicated by horizontal bars along the top of the panel. B, Levels of FV6-specific IgG in plasma from Ghanaian pregnant women, nonpregnant women, and men. Measures of central tendency and dispersion are as described in panel A. C, Relationship between plasma levels of FV2-specific IgG and parity among pregnant women. D, Relationship between plasma levels of FV6-specific IgG and parity among pregnant women. The cutoff for positivity (calculated as the mean level [plus 2 standard deviations] measured in 10 nonexposed Danish control donors) is indicated by gray shading in all panels.
Levels of FV2-specific IgG depended significantly and positively on parity (P(z) < .0001; Figure 1C) but did not depend significantly on either gestational age at recruitment (P(H) = .7) or calendar month of recruitment (P(H) = .1). In contrast, FV6-specific IgG levels tended to decrease with increasing parity (P(z) = .01; Figure 1D), did not vary with gestational age at recruitment (P(H) = .06), but showed marked variation with calendar month of recruitment (P(H) < .001). Essentially identical results were obtained when these analyses were restricted to primigravidae. Median levels of FV2-specific and FV6-specific IgG were lower in women with self-reported prophylaxis (3.78 and 4.04, respectively) than in women who reported no use of prophylaxis (3.91 and 4.22, respectively). The difference was highly significant for FV6-specific IgG (P(T) < .001) and borderline significant for FV2-specific IgG (P(T) = .05).
DISCUSSION
The incidence of clinical episodes of malaria varies markedly with seasonal changes in the intensity of parasite transmission, whereas the prevalence of infection (whether symptomatic or not) generally shows much less seasonal variation [6, 7]. These findings suggest that chronic parasitemia in semiimmune individuals is often suppressed by the immune response to low levels that do not cause symptoms, whereas clinical disease is associated with mosquito-mediated acquisition of parasites capable of expressing new antigenic types not controlled by preexisting immunity [8]. However, recent reports indicate that placental P. falciparum malaria may be an important (partial) exception to this rule [9, 10]. Their clinical, parasitological, and histological evidence suggests that placental malaria often follows when already infected women become pregnant, rather than when women who are already pregnant become infected owing to mosquito bites. This scenario is supported by occasional case reports [11].
The PfEMP1-based interpretation of the above findings is that placental malaria may be caused by asymptomatic infections present before conception (in addition to newly acquired infections), as the parasites seize the opportunity to escape preexisting immunity when the placenta develops, effectuated by switching to expression of VAR2CSA-type PfEMP1. To test this hypothesis directly, we selected a study area with marked seasonal variation in rainfall (and, hence, likely variation in transmission intensity; Supplementary Figure 2) and 2 PfEMP1 antigens, IT4VAR04 (FV2) and HB3VAR06 (FV6). The former antigen is a VAR2CSA-type PfEMP1, whereas the latter is a protein associated with rosetting and severe malaria in children but not with placental malaria.
VAR2CSA-specific IgG levels increase during pregnancy, but decline again within months of delivery, as maintenance of acquired protection against placental malaria appears to rely primarily on memory B cells [5]. Thus, only limited systematic variation with seasonal fluctuations in transmission intensity would be expected, if indeed placental infection often arises from preexisting, nonplacental parasitemia switching to expression of VAR2CSA-type PfEMP1 without the need for acquisition of new infection. Indeed, we did not detect much seasonal variation in plasma levels of VAR2CSA-specific IgG among the pregnant women we studied. In contrast, FV6-specific IgG levels would be expected to show some seasonal variation, as boosting should generally require new infection. Again, this is what we observed. Nevertheless, we also found that FV2- and FV6-specific IgG levels correlated to some extent. Although this limits the power of our investigative approach, it is not surprising. Thus, some of the parasites in a rapidly expanding population expressing VAR2CSA-type PfEMP1 during placental malaria are likely to switch to other PfEMP1 types, thereby boosting any preexisting immunological memory to those variants. This was indeed observed in a previous longitudinal study [5]. Furthermore, new infections acquired from mosquito bites undoubtedly also contribute to placental malaria [10].
Our study has several limitations. The complex rainfall pattern in the study area makes it difficult to analyze in detail the causal relationship between temporal variation in transmission patterns and antibody levels. This problem is complicated further by the many variables other than transmission intensity that potentially contribute to the observed variation in antibody levels. All this notwithstanding, our study supports the notion that placental malaria often arises from parasites already present at the time of conception, rather than from new infection in women already pregnant. This mode of developing placental malaria has not often been considered in the literature but should not be unexpected, as low-grade, asymptomatic parasitemia is common among African adults [12]. It might explain, at least partially, the negative impact of low parasitemia levels during early pregnancy on pregnancy outcome, even before a fully functional placenta has developed [13]. Perhaps even more importantly, this etiology might also contribute to the modest protective efficacy of insecticide-treated bed nets against placental malaria as compared to their efficacy against malaria in children [14, 15].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all participants, for donating samples and data for this study, and Sophia Ampah, for excellent technical assistance.
Financial support. This work was supported by the Consultative Committee for Development Research (grants 900-RH and 12-081RH), the Danish Council for Independent Research (grant DFF-4183-00539), and Copenhagen University Hospital (Rigshospitalet; grant E-22071-01).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Crompton PD, Moebius J, Portugal S, et al. . Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol 2014; 32:157–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature 1961; 192:733–7. [DOI] [PubMed] [Google Scholar]
- 3. Hviid L, Jensen AT. PfEMP1—a parasite protein family of key importance in Plasmodium falciparum malaria immunity and pathogenesis. Adv Parasitol 2015; 88:51–84. [DOI] [PubMed] [Google Scholar]
- 4. Ofori M, Ansah E, Agyepong I, Ofori-Adjei D, Hviid L, Akanmori B. Pregnancy-associated malaria in a rural community of ghana. Ghana Med J 2009; 43:13–8. [PMC free article] [PubMed] [Google Scholar]
- 5. Ampomah P, Stevenson L, Ofori MF, Barfod L, Hviid L. Kinetics of B cell responses to Plasmodium falciparum erythrocyte membrane protein 1 in Ghanaian women naturally exposed to malaria parasites. J Immunol 2014; 192:5236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenwood BM, Bradley AK, Greenwood AM, et al. . Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg 1987; 81:478–86. [DOI] [PubMed] [Google Scholar]
- 7. Dodoo D, Theander TG, Kurtzhals JA, et al. . Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect Immun 1999; 67:2131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lines J, Armstrong JR. For a few parasites more: inoculum size, vector control and strain-specific immunity to malaria. Parasitol Today 1992; 8:381–3. [DOI] [PubMed] [Google Scholar]
- 9. Walker PG, Griffin JT, Cairns M, et al. . A model of parity-dependent immunity to placental malaria. Nat Commun 2013; 4:1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berry I, Walker P, Tagbor H, et al. . Seasonal dynamics of malaria in pregnancy in West Africa: evidence for carriage of infections acquired before pregnancy until first contact with antenatal care. Am J Trop Med Hyg 2018; 98:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giobbia M, Tonon E, Zanatta A, Cesaris L, Vaglia A, Bisoffi Z. Late recrudescence of Plasmodium falciparum malaria in a pregnant woman: a case report. Int J Infect Dis 2005; 9:234–5. [DOI] [PubMed] [Google Scholar]
- 12. Dal-Bianco MP, Köster KB, Kombila UD, et al. . High prevalence of asymptomatic Plasmodium falciparum infection in Gabonese adults. Am J Trop Med Hyg 2007; 77:939–42. [PubMed] [Google Scholar]
- 13. Schmiegelow C, Matondo S, Minja DTR, et al. . Plasmodium falciparum infection early in pregnancy has profound consequences for fetal growth. J Infect Dis 2017; 216:1601–10. [DOI] [PubMed] [Google Scholar]
- 14. Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev 2004; CD000363. [DOI] [PubMed] [Google Scholar]
- 15. Eisele TP, Larsen DA, Anglewicz PA, et al. . Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect Dis 2012; 12:942–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.