The magnitude of CD4 and CD8 T-cell responses to CMV antigens correlated strongly with markers of immune activation, and significantly predicted onset of frailty in nonfrail HIV-uninfected men. These responses may influence immune activation in people with chronic CMV infection.
Keywords: aging, chronic inflammation, immune activation, frailty, HIV infection
Abstract
Background
Both aging and treated human immunodeficiency virus (HIV)-infected populations exhibit low-level chronic immune activation of unknown etiology, which correlates with morbidity and mortality. Cytomegalovirus (CMV) infection is common in both populations, but its relation to immune activation is unknown.
Methods
T cells from men who have sex with men (22 virologically suppressed HIV+, 20 HIV−) were stimulated with peptides spanning 19 CMV open reading frames, and intracellular cytokine responses were assessed. Soluble and cellular inflammatory markers were assessed by multiplex electrochemiluminescence and flow cytometry, respectively. Frailty was assessed by the Fried criteria.
Results
All men had responses to CMV. Proportions of CMV-responsive T cells correlated strongly (r ≥ 0.6 or ≤ −0.6; P < .05) with immunologic markers, depending on donor HIV and frailty status. Markers significantly correlated in some groups after adjustment for multiple comparisons included interferon-γ, tumor necrosis factor-α, interleukin-6, and several chemokines in serum, and the proportion of activated T cells. The magnitude of the CD4 IL-2 response significantly predicted onset of frailty in HIV− nonfrail men, but not in HIV+ nonfrail men.
Conclusions
T-cell responses to CMV may strongly influence chronic immune activation in HIV-uninfected and virologically suppressed HIV-infected men, and may predict frailty in HIV-uninfected men.
Chronic inflammation and immune activation may play a significant role in the pathogenesis of age-related morbidity in both human immunodeficiency virus (HIV)-infected (HIV+) and HIV-uninfected (HIV−) populations. Circulating concentrations of inflammatory mediators, such as interleukin 6 (IL-6) and C-reactive protein (CRP), increase with age and independently predict the onset of important age-related conditions such as cardiovascular disease, osteoporosis, and frailty [1]. People with treated, virologically suppressed HIV infection demonstrate higher levels of inflammation and immune activation [2, 3], and have earlier occurrence of frailty and other common age-related outcomes, than similar HIV-uninfected individuals. These differences may be partly due to a higher prevalence of comorbidities and harmful behaviors [4–6], but the actual mechanisms are poorly understood.
Chronic cytomegalovirus (CMV) infection has long been suspected to contribute to age-related declines in immunity. CMV seroprevalence increases with age [7], and is accompanied by a skewing of the T-cell repertoire towards CMV antigens (often comprising 10% or more of the T-cell pool) [8–10]. This can result in a net shrinking of the T-cell pool and compromised immune function [11, 12]. CMV-seropositivity is also associated with a higher risk of age-related conditions such as frailty, and with mortality [13–18]. Like the older general adult population, people with or at risk for HIV infection have an extremely high CMV seroprevalence and strong T-cell responses to CMV antigens [19]. In addition, we have shown that HIV+ men have a higher prevalence of frailty at earlier ages than similar HIV− men [4]. Despite these data, it remains unclear how, or if, chronic CMV infection affects the aging process in populations with or without HIV infection. In particular, although the cellular immune response to CMV is known to be unusually large and broad, the relation of this response to chronic immune activation has not been well studied.
We hypothesized that increased CMV-specific T-cell responses could contribute to chronic inflammation and immune activation, and thus to frailty, in both HIV− and virologically suppressed HIV+ people. To explore this hypothesis, we studied the relationships among T-cell cytokine responses to a large panel of CMV antigens, serologic and cellular markers of systemic inflammation and immune activation, and frailty in HIV+ and HIV− men followed in the Multicenter AIDS Cohort Study (MACS).
METHODS
Study Population and Clinical Data
The MACS has followed HIV+ and HIV− men semiannually since 1984 [20, 21]. At each study visit, men fill out questionnaires covering medical history, undergo a brief physical examination, and provide whole blood for hematology and serology, and for storage of plasma, serum, and viable peripheral blood mononuclear cells (PBMCs). Plasma HIV RNA concentrations were measured by the Roche Taqman assay (sensitive to 20 copies/mL), and CD4 and CD8 T-cell subsets by standardized flow cytometry [22, 23]. Since 2007, the Fried Frailty Phenotype (FFP) [24] has been assessed at each study visit [4]. Briefly, this phenotype is present when 3 or more of 5 criteria are expressed: slow walking speed, low grip strength, exhaustion, low physical activity, and weight loss [24].
The present study included 42 men (20 HIV−, 22 HIV+) at the Baltimore-Washington, DC MACS site who had: (1) known frailty status and (2) stored serum and viable PBMCs available for study. To minimize misclassification, frailty was defined as expression of the FFP at 2 consecutive study visits, and nonfrailty as its nonexpression at 2 consecutive visits. To eliminate the effect of HIV viremia, only HIV+ men with undetectable viremia receiving combination antiretroviral therapy (cART) were included. To avoid depletion of the specimen repository, men with the greatest numbers of stored vials of serum and PBMC were selected for study. Of 23 HIV− men who met the definition of frailty, 22 had adequate PBMC for study and 10 of these were selected for study. For HIV+ men, the corresponding figures were 26, 19, and 11. Chronic or acute medical conditions were assessed based on questionnaire and medical records.
Assessment of T-Cell Characteristics and Responses to CMV
As described [25], cryopreserved PBMCs were thawed, washed, cultured overnight, and stimulated for 6 hours with 1 of 26 pools of overlapping 15-mer peptides covering 19 CMV open reading frames (ORFs): unique long (UL) 28, 32, 36, 48, 55, 82, 83, 86, 94, 99, 103, 122, 123, 151, and 153; and unique short (US) 3, 24, 29, and 32); all graciously provided by Dr. Louis Picker [8]. Cultures included costimulatory monoclonal anti-CD28 and anti-CD49d (BD Biosciences, San Jose, CA) and, for the final 5 hours of incubation, brefeldin A (Sigma Aldrich, St. Louis, MO). Staphylococcal enterotoxin B and no antigen were used as positive and negative controls, respectively. Cells were stored overnight at 4°C, washed with phosphate-buffered saline (PBS), and stained with LIVE/DEAD Fixable Aqua Dead Cell (Invitrogen, Eugene, OR), then fixed, permeabilized, and stained for 45 minutes with premixed monoclonal antibodies (anti-human interferon γ [IFN-γ]–fluorescein isothiocyanate [FITC], CD69-phycoerythrin [PE], CD8-peridinin chlorophyll-Cy5.5, and CD3-allophycocyanin), as well as antibodies to interleukin 2 (IL-2)–PE-Cy7, tumor necrosis factor-α (TNF-α)–Alexa Fluor 700, and CD4-V450 (all antibodies, including isotype controls, from BD Biosciences). Samples were analyzed on an LSRII flow cytometer (BD Biosciences), using FACSDiva software, V. 6.13 (BD Biosciences). CD3+/CD4+ and CD3+/CD8+ cells expressing CD69 and IFN-γ, TNF-α, and/or IL-2 were quantified using FlowJo software (Tree-Star, Ashland, OR). A response of stimulated cells that was ≥0.1% above staining with fluorescence-minus-one (FMO) controls was considered a positive response. Activated T cells were assessed by staining an aliquot of thawed PBMC with antibodies to CD38 and HLA-DR (BD Biosciences) and defined as cell expressing both markers.
Measurement of Serologic Inflammatory Markers
Serum levels of 15 inflammation-regulating cytokines and chemokines (IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, IFN-γ, TNF-α, GM-CSF, CCL2/MCP-1, CCL4/MIP-1β, CCL11/eotaxin, CCL13/MCP-4, CCL17/TARC, CXCL8/IL-8, CDL22 [MDC], CCL26 [eotaxin-3], CCL3 [MIP-1α], and CXCL10/IP-10) were measured by multiplex electrochemiluminescence (MSD, Gaithersburg, MD) on stored serum as described [26]. CRP was measured using high-sensitivity nephelometry (Quest Laboratories, Chantilly, VA).
Statistical Analysis
Summary statistics were described for the study population as a whole and for subgroups defined by HIV status (+/−), frailty status (+/−), or both HIV and frailty status (−/−, +/−, −/+, and +/+). Significance of differences between groups was assessed by Wilcoxon Rank-sum test or Fisher’s exact test, as appropriate. Associations between the 9 T-cell responses and the 14 inflammatory biomarkers detected in all donors were assessed by Spearman’s rank correlation coefficients for the 4 subgroups defined by HIV and frailty status. Correlations of 0.6 or greater in absolute magnitude were considered strong, and those with P ≤ .05 were considered significant. When at least 1 of the 2 frailty groups had a significant correlation, we applied Fisher’s z transformation to formally test whether correlations differed significantly by frailty status, after stratifying by HIV serostatus.
The Benjamini-Hochberg procedure [27] was used to account for multiple tests within each HIV/frailty stratum by controlling the false discovery rate. Because total T-cell responses are sums, and thus correlates, of CD4- and CD8-specific responses, the 3 total T-cell responses were adjusted separately from the 6 CD4- and CD8-specific responses. For Spearman correlation coefficients, we controlled the false discovery rate at 20% due to the exploratory nature of this analysis and correlations among the variables. For Fisher’s z transformation, we chose a lower false discovery rate, 5%, because we targeted only significant correlations, which reduced the total number of tests.
To explore the relationship between CMV T-cell response and future development of the frailty phenotype, we categorized the CD4-specific IL-2 response into tertiles; we then compared the top tertile to the 2 lower tertiles for time to first occurrence of the frailty phenotype, by Kaplan-Meier estimators and the nonparametric log-rank test, after stratifying by HIV serostatus. We also compared these 2 groups for the proportion of follow-up visits per individual that manifested the frailty phenotype, using the Wilcoxon rank-sum test.
RESULTS
Study Population
Demographic and clinical characteristics of the 42 men included in the study are summarized in Table 1. The median age of all study participants was 61 years; HIV− men were slightly older than the HIV+ men (P = .03) and had significantly higher CD4 and lower CD8 T-cell counts. By design, all HIV+ men had undetectable HIV viral loads. All men for whom serological data were available were CMV-seropositive. The HIV+ and HIV− men did not differ significantly in educational level, titer of anti-CMV IgG antibody, and prevalence of hepatitis C virus (HCV) infection, although the latter was more common in HIV+ men. Other clinical conditions, aside from history of AIDS, also did not differ by HIV status (data not shown).
Table 1.
Demographic Characteristics of the Men Studied (N = 42)
| Variable | Total | HIV Status | |||||
|---|---|---|---|---|---|---|---|
| (N = 42) | HIV+ (n = 22) | HIV− (n = 20) | P* | ||||
| Median | IQR | Median | IQR | Median | IQR | ||
| Age, y | 61 | 9 | 58 | 10 | 63 | 7 | .0310 |
| Education, y | 16 | 4 | 16 | 2 | 16 | 4 | .5082 |
| CD4 /μL | 1038 | 551 | 715 | 612 | 1139 | 301 | .0044 |
| CD8 /μL | 800 | 721 | 1167 | 648 | 604 | 217 | .0002 |
| HCV | 7 | 6 | 1 | .096 | |||
| Ethnicity | |||||||
| White | 33 | 16 | 17 | .46 | |||
| Black | 9 | 6 | 3 | ||||
| Smoking | |||||||
| Current | 9 | 7 | 2 | .25 | |||
| Former | 18 | 8 | 10 | ||||
| Never smoked | 15 | 7 | 8 | ||||
| Depressive symptoms | 14 | 7 | 7 | 1.0 | |||
| Hypertension | 28 | 13 | 15 | .34 | |||
| Diabetes | 9 | 3 | 6 | .44 | |||
| Dyslipidemia | 31 | 16 | 15 | 1.0 | |||
| Kidney disease | 3 | 3 | .23 | ||||
| Liver disease | 6 | 5 | 1 | .18 | |||
| Nadir CD4 /μL | 288 | 157 | |||||
| CD4/CD8 ratio | 0.82 | 0.47 | |||||
| Years of cART | 17 | 5 | |||||
| Years untreated | 12 | 7 | |||||
| HIV RNA (copies/mL) | 40 | n/a | |||||
| History of AIDS | 4 | 4 | |||||
Abbreviations: cART, combination antiretroviral therapy; IQR, interquartile range; HIV+, human immunodeficiency virus infected; HIV−, HIV-uninfected; n/a, not applicable.
a P value comparing HIV+ and HIV− subjects.
Measures of Systemic Inflammation
Most cytokines and all chemokines tested were detectable in the serum of all subjects studied. The data are summarized in Table 2. For the cytokines (Table 2), TNF-α concentrations were significantly higher in HIV+ than HIV− men, and IL-6 levels were significantly higher in frail men than in nonfrail men, both overall and within HIV− and HIV+ men. Other cytokines did not differ significantly by any stratification by HIV or frailty status. For chemokines (Table 2), no significant differences were present. CRP levels (Table 2) were significantly higher in frail men than in nonfrail men, both overall and within each HIV serostatus group.
Table 2.
Median Serum Concentrations of Cytokines and Chemokines in the Study Population, Stratified by HIV Status, Frailty Status, or Botha
| Variable | Total | HIV Status | Frailty Status | HIV and Frailty Status |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 42) | HIV− (n = 20) | HIV+ (n = 22) | P | Nonfrail (n = 21) | Frail (n = 21) | P | HIV− Nonfrail (n = 10) | HIV− Frail (n = 10) | HIV+ Nonfrail (n = 11) | HIV+ Frail (n = 11) | P | |
| Cytokines | ||||||||||||
| GM-CSF | 0.42a (0.39)b | 0.28 (0.50) | 0.47 (0.58) | .26 | 0.54 (0.79) | 0.32 (0.39) | .14 | 0.54 (0.64) | 0.17 (0.28) | 0.53 (0.77) | 0.42 (0.37) | .30 |
| IFN-γ | 4.95 (3.41) | 5.15 (3.64) | 4.81 (3.28) | .96 | 4.64 (2.92) | 5.14 (3.42) | .85 | 4.90 (3.54) | 5.19 (3.73) | 4.02 (2.92) | 4.83 (7.37) | .90 |
| IL-1 | 0.28 (0.31) | 0.28 (0.17) | 0.28 (0.34) | .67 | 0.23 (0.11) | 0.50 (0.76) | .05 | 0.25 (0.10) | 0.35 (0.16) | 0.23 (0.08) | 0.54 (0.48) | .16 |
| IL-10 | 0.25 (0.38) | 0.25 (0.36) | 0.25 (0.42) | .87 | 0.11 (0.32) | 0.30 (0.43) | .09 | 0.25 (0.34) | 0.22 (0.52) | 0.02 (0.31) | 0.36 (0.34) | .12 |
| IL-12p70 | 0.00 (0.02) | 0.00 (0.01) | 0.00 (0.03) | .76 | 0.00 (0.20) | 0.00 (0.00) | .43 | 0.00 (0.20) | 0.00 (0.00) | 0.00 (0.02) | 0.00 (0.04) | .70 |
| IL-2 | 0.34 (0.65) | 0.35 (0.51) | 0.34 (0.84) | .57 | 0.26 (0.22) | 0.68 (0.68) | .05 | 0.25 (0.12) | 0.68 (0.40) | 0.29 (0.22) | 0.75 (0.98) | .24 |
| IL-6 | 0.92 (1.04) | 1.01 (1.28) | 0.80 (1.10) | .47 | 0.63 (0.61) | 1.24 (1.48) | .01 | 0.70 (0.65) | 1.61 (2.01) | 0.61 (0.89) | 0.98 (0.94) | .04 |
| IL-8 | 12.62 (10.65) | 13.35 (9.21) | 11.86 (11.87) | 1.00 | 10.42 (7.65) | 15.79 (13.07) | .40 | 10.86 (6.56) | 15.54 (10.90) | 10.42 (10.29) | 16.16 (43.57) | .86 |
| TNF-α | 2.58 (1.33) | 2.27 (1.19) | 2.65 (1.55) | .04 | 2.40 (0.60) | 2.78 (1.92) | .22 | 2.19 (1.06) | 2.35 (1.58) | 2.63 (0.83) | 3.73 (1.54) | .09 |
| Chemokines, CRP, and Activated T Cells | ||||||||||||
| Eotaxin | 240.5 (163.3) | 258.8 (153.3) | 234.8 (170.5) | .84 | 292.6 (199.6) | 210.9 (107) | .024 | 306.7 (199.6) | 222.7 (133.1) | 292.6 (247.3) | 210.9 (153.2) | .15 |
| IP-10 | 274.8 (191) | 247 (93.6) | 364.4 (326.3) | .07 | 277.1 (147.8) | 272.5 (287.1) | .91 | 247 (119.3) | 248.3 (205.8) | 366.6 (333.4) | 298.9 (408.6) | .19 |
| MCP-1 | 274.4 (153.9) | 281.2 (123.4) | 236.6 (148.8) | .29 | 293.8 (113) | 228.6 (110.2) | .08 | 310.7 (93) | 258.2 (78.6) | 276.5 (144.3) | 201.9 (159.7) | .24 |
| MCP-4 | 167.3 (106.5) | 171 (82.1) | 161.6 (118.9) | .39 | 168.3 (77.7) | 147 (116.6) | .60 | 173.9 (47.4) | 160.3 (107.1) | 167.6 (118.2) | 132.1 (178.5) | .80 |
| MDC | 1406.1 (915.7) | 1543.9 (753) | 1268.2 (1001.1) | .13 | 1400.5 (789.6) | 1479.1 (1072.9) | .85 | 1426.7 (685.6) | 1777.4 (956.3) | 1289.8 (1001.3) | 1246.7 (1138.4) | .52 |
| MIP-1 | 129.8 (104.9) | 142 (88.8) | 128.1 (109) | .84 | 114.7 (73.7) | 167.3 (88.9) | .20 | 116.6 (78.5) | 167.7 (88.9) | 114.7 (109) | 147.5 (121.9) | .61 |
| TARC | 502.1 (471) | 504.6 (431.2) | 490.4 (474.3) | .24 | 511.5 (257.3) | 395.6 (694.9) | .83 | 504.6 (209) | 537 (784.8) | 511.5 (499.7) | 395.6 (458.9) | .70 |
| CRP c | 1.75 (8.50) | 1.80 (8.65) | 1.35 (8.20) | .54 | 0.90 (1.30) | 7.80 (11.00) | .01 | 1.75 (1.90) | 8.45 (12.40) | 0.50 (1.30) | 6.30 (11.40) | .03 |
| Activated CD4 | 4.3 (4.1) |
3.8 (3.9) |
4.3 (2.8) |
.88 | 4.3 (3.8) |
3.8 (5.5) |
.59 | 5.1 (3.6) |
3.6 (5.5) |
4.2 (4.7) |
4.6 (2.9) |
.92 |
| Activated CD8 | 17.1 (17.9) | 14.2 (27.3) | 19.2 (12.4) | .39 | 17.5 (11) | 15.4 (24.4) | .63 | 15.8 (15.7) | 7.2 (27.5) | 19.3 (6) |
18.2 (19.1) | .8 |
Abbreviations: CRP, C-reactive protein; IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumor necrosis factor-α; HIV+, human immunodeficiency virus infected; HIV− , HIV-uninfected.
aData
in pg/mL unless otherwise noted.
bIQR, interquartile range.
cData in mg/mL.
Bold = P ≤ .05.
T-Cell Responses to CMV
All men exhibited positive CD4 and CD8 T-cell responses to the panel of CMV peptide pools. Consistent with our previous report on a subgroup of these subjects [25], percentages of T cells producing IFN-γ and TNF-α were generally similar and were greater than percentages producing IL-2, and CD8 T-cell responses were greater than those of CD4 T cells, except for IL-2 responses (Supplementary Table 1); overall responses did not differ significantly by HIV status or frailty status (data not shown).
Correlations Between Systemic Inflammatory Markers and T-Cell Responses to CMV
Correlations between inflammatory markers and the sum of all T-cell responses to the 19 CMV ORF pools were analyzed either: (1) among the entire study population; or with the population stratified by (2) HIV status alone or frailty status alone or (3) both HIV status and frailty status. Age distributions were similar by HIV status within all groupings (data not shown). Analyses were performed for each cytokine response (IFN-γ, TNF-α, and IL-2) and for each T-cell subset (CD4 or CD8). Here, we present only correlations that were strong (r ≥ 0.6 or ≤ −0.6) and statistically significant (P < .05).
The overall study population exhibited no strong and significant correlations. After stratification by HIV status or by frailty status, the CD8 IL-2 response was strongly correlated with serum IL-8 concentration among HIV− men (r = 0.63, P = .0031), and the total IFN-γ response (CD4 + CD8) with the percentage of activated CD4 T cells among HIV+ men (r = 0.60, P = .0043). Nonfrail men showed strong correlations between the CD8 IFN-γ response and serum IL-8 levels (r = 0.64, P = .0017) and the CD8 TNF-α response and serum MIP-1β (r = 0.60, P = .0043). The last 3 of these correlations were significant after adjustment for multiple comparisons.
In contrast, after stratification by both HIV status and frailty status, 41 strong and significant correlations between CMV responses and measures of systemic inflammation were present, as shown in Table 3 (P values for correlations are in Supplementary Table 2). These differed for the different HIV-frailty groups, as follows:
Table 3.
Correlations Between Cytomegalovirus (CMV)-Induced T-Cell Responses and Markers of Systemic Inflammation and Immune Activation that were Large (≥0.6 or ≤ −0.6) and Significant (P < .05), Before and After Adjustment for Multiple Comparisons
| T-cell responses (to all 19 CMV ORFs) | Inflammatory markers | IFN-γ | IL-2 | TNF-α | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV/Frailty | HIV/Frailty | HIV/Frailty | |||||||||||||||||
| −/− | −/+ | P for diff b | +/− | +/+ | P for diff | −/− | −/+ | P for diff | +/− | +/+ | P for diff | −/− | −/+ | P for diff | +/− | +/+ | P for diff | ||
| r | r a | r | r | r | r | r | r | r | r | r | r | ||||||||
| CD4-specific | IFN-γ | 0.09 | 0.67 | .18 | 0.5 | 0 | 0.18 | 0.6 | 0.51 | 0.12 | 0.1 | 0.58 | 0.67 | 0.17 | .2 | ||||
| IL-6 | −0.14 | 0.16 | 0.45 | 0.16 | 0.22 | −0.07 | 0.75 | −0.01 | .049 | −0.14 | −0.03 | 0.58 | 0.3 | ||||||
| IP-10 | −0.26 | 0.22 | 0.52 | 0.15 | −0.15 | 0.26 | 0.15 | −0.01 | −0.36 | −0.02 | 0.71 | 0.4 | .35 | ||||||
| MCP-4 | −0.33 | −0.6 | 0.06 | 0.22 | −0.12 | −0.65 | .22 | 0.05 | −0.11 | −0.28 | −0.21 | 0.25 | 0.45 | ||||||
| MDC | 0.1 | −0.66 | .09 | 0.35 | 0.09 | −0.33 | −0.47 | 0.15 | −0.15 | −0.04 | −0.48 | 0.44 | 0.32 | ||||||
| TARC | −0.24 | −0.72 | .22 | 0.09 | −0.05 | −0.52 | −0.55 | 0.14 | 0.23 | −0.14 | −0.47 | 0.34 | 0 | ||||||
| Activated CD4 | 0.08 | 0.07 | 0.43 | 0.36 | 0.56 | 0.02 | 0.65 | 0.18 | .24 | 0.07 | 0.18 | 0.33 | 0.66 | .37 | |||||
| Activated CD8 | 0.02 | 0.07 | 0.46 | −0.32 | 0.47 | −0.2 | 0.66 | 0.02 | .12 | 0.02 | 0.13 | 0.59 | 0.15 | ||||||
| CD8-specific | TNF-α | 0.18 | −0.28 | −0.61 | 0.08 | .11 | −0.04 | −0.44 | −0.49 | 0.12 | 0.01 | −0.43 | −0.49 | 0.1 | |||||
| IL-8 | −0.49 | −0.28 | −0.73 | 0.33 | .011 | −0.75 | −0.37 | .27 | 0.32 | 0.03 | −0.64 | −0.52 | .73 | −0.56 | 0.2 | ||||
| Eotaxin | −0.02 | 0.08 | 0.32 | 0.09 | −0.25 | 0.73 | .027 | 0.25 | −0.18 | −0.04 | 0.24 | 0.28 | −0.01 | ||||||
| IP-10 | −0.45 | −0.37 | −0.47 | −0.35 | −0.61 | 0.25 | 0.12 | −0.15 | −0.65 | −0.01 | .15 | −0.26 | −0.25 | ||||||
| MCP-1 | −0.38 | 0.16 | 0.01 | 0.3 | −0.66 | 0.41 | .022 | 0.51 | 0.22 | −0.53 | 0.36 | 0.02 | 0.28 | ||||||
| MCP-4 | −0.31 | 0.39 | −0.7 | 0.43 | .008 | −0.33 | −0.08 | 0.35 | 0.36 | −0.41 | 0.14 | −0.57 | 0.38 | ||||||
| MDC | −0.19 | −0.18 | −0.67 | −0.17 | .20 | −0.25 | −0.39 | 0.29 | 0.06 | −0.26 | −0.65 | .34 | −0.44 | −0.14 | |||||
| MIP-1β | −0.7 | −0.41 | .42 | −0.35 | 0.29 | −0.43 | −0.33 | −0.4 | 0.3 | −0.72 | −0.66 | .83 | −0.45 | 0.31 | |||||
| TARC | 0.43 | 0.33 | −0.47 | 0.21 | 0.25 | 0.13 | 0.62 | 0.54 | .81 | 0.38 | −0.02 | −0.25 | 0.25 | ||||||
| CRP | −0.4 | 0.12 | 0.16 | 0.57 | −0.35 | −0.37 | −0.51 | 0.47 | −0.57 | −0.04 | −0.16 | 0.62 | .08 | ||||||
| Activated CD4 | −0.3 | 0.18 | 0.41 | 0.3 | 0.12 | −0.18 | 0.34 | 0.27 | −0.35 | 0.4 | 0.63 | 0.31 | .40 | ||||||
| Total (CD4+CD8) | IFN-γ | −0.01 | 0.76 | .06 | 0.31 | −0.32 | −0.02 | 0.7 | .1 | 0.07 | −0.21 | 0.05 | 0.77 | .07 | 0.26 | −0.15 | |||
| IL-6 | −0.44 | 0.19 | 0.14 | 0.35 | −0.12 | −0.27 | 0.67 | 0.43 | .48 | −0.43 | −0.36 | 0.29 | 0.4 | ||||||
| IL-8 | −0.52 | −0.78 | .38 | −0.53 | 0.31 | −0.41 | −0.47 | −0.06 | 0.13 | −0.48 | −0.38 | −0.42 | 0.12 | ||||||
| MCP-4 | −0.45 | −0.32 | −0.42 | 0.39 | −0.25 | −0.68 | .28 | 0.25 | 0.25 | −0.49 | 0.04 | −0.33 | 0.34 | ||||||
| MDC | −0.18 | −0.94 | .004 | −0.3 | −0.24 | −0.36 | −0.56 | 0.29 | −0.42 | −0.16 | −0.66 | .24 | −0.05 | 0.03 | |||||
| MIP-1β | −0.71 | −0.72 | 0.97 | −0.14 | 0.16 | −0.14 | −0.31 | −0.06 | 0.2 | −0.5 | −0.47 | −0.23 | 0.08 | ||||||
| Activated CD4 | −0.44 | 0.17 | 0.85 | 0.37 | .08 | 0.47 | 0.18 | 0.62 | 0.16 | .26 | −0.18 | 0.53 | 0.8 | 0.47 | .24 | ||||
| Activated CD8 | −0.26 | 0.13 | 0.66 | 0.02 | .12 | 0.38 | −0.08 | 0.42 | 0.45 | −0.07 | 0.35 | 0.69 | 0.16 | .17 | |||||
Abbreviations: CRP, C-reactive protein; IFN-γ, interferon-γ; IL, interleukin; ORF, open reading frame; TNF-α, tumor necrosis factor-α.
a Associations between T-cell responses (T-cell subset in left-most column, cytokine response in top row, of table) and inflammatory biomarkers (second column of table), as assessed by Spearman’s rank correlation coefficients. Biomarkers for which no correlations were significant are not shown.
b Comparison of correlation coefficients between nonfrail and frail men was done after Fisher r-to-z transformation and was performed only when there was a statistically significant correlation in at least 1 of the 2 groups.
Bold text indicates statistically significant (P < .05) correlations or P values. Black boxes indicate values that were statistically significant after adjustment for multiple comparisons.
Adjustments for multiple comparisons: For correlation coefficients, adjustments were performed within each HIV status-frailty status subgroup, by maintaining a false discovery rate of 0.2. Because total T-cell responses are sums, and thus correlates, of CD4- and CD8-specific responses, total T-cell responses were adjusted separately from CD4- and CD8-specific responses.
For P values, adjustments were performed within each HIV status group stratified by frailty, by maintaining a false discovery rate of .05; again, adjustment was done separately for CD4- and CD8-specific responses, and for total T-cell responses.
HIV−/nonfrail (n = 10 men): Seven correlations were strong and significant. All were with CD8 T-cell responses, and all were negative correlations with chemokines. The strongest was between the CD8 IL-2 response and IL-8 (r = −0.75). None was significant after adjustment for multiple comparisons.
HIV+/nonfrail (n = 11): Seventeen correlations were strong and significant; again 3 were between CD8 responses and chemokines and were negative. However, 7 were between both CD4 and CD8 T-cell responses and activated T cells (both CD4 and CD8) and were positive; of these, correlations between the total T-cell response (IFN-γ and TNF-α) and activated CD4 T cells remained significant after adjustment for multiple comparisons. Positive correlations were also present with TNF-α, IL-6, and IFN-γ cytokines associated with the presence or onset of frailty.
HIV−/frail (n = 10): Fifteen strong and significant correlations were present. Again, most were negative correlations with serum chemokine levels, but positive correlations with serum IFN-γ were present and remained significant for the total IFN-γ and TNF-α responses, after adjustment for multiple comparisons.
HIV+/frail (n = 11): this group had only 2 strong and significant correlations: between the CD4 TNF-α response and activated CD4 T cells (r = 0.66), and between the CD8 TNF-α response and serum CRP (r = 0.62).
Differences in Correlations by Frailty Status
For T-cell responses that exhibited a strong correlation within an HIV-frailty category, we tested whether the correlations differed by frailty status. As shown in Table 3, of 20 such comparisons, 6 were statistically significant (P < .05), and 1 remained significant after adjustment for multiple comparisons (namely, the differences in correlation between the total [CD4+CD8] T-cell IFN-γ response and MDC concentration in HIV− frail men).
Exploratory Analysis of the Relationship Between T-Cell Responses to CMV and Development of Frailty
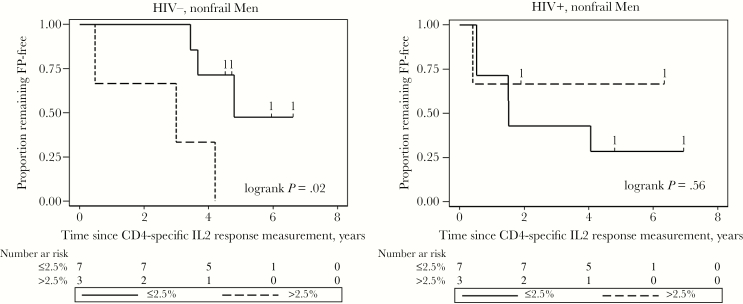
Given the strong correlation observed between the CD4 IL-2 response and serum IL-6 in the HIV+/nonfrail subgroup, and the fact that IL-6 is a predictor of frailty [1], we asked whether the CD4 IL-2 response could predict the onset of frailty in nonfrail men. Table 4 shows the CD4 IL-2 responses and follow-up of the nonfrail men. The overall median follow-up was 12 study visits. As shown in Figure 1, the highest tertile of the CD4 IL2 response had significantly faster onset of frailty than the lower 2 tertiles for HIV− men, but not for HIV+ men. Proportions of follow-up visits exhibiting the frailty phenotype showed the same pattern: median 30% (top tertile) versus 0% (lower 2 tertiles) for the HIV− men (P = .03 by Wilcoxon rank-sum test), and 0% versus 11%, respectively (P = .16), for the HIV+ men. Interestingly, the HIV+ nonfrail men in the lowest tertile of the CD4 IL2 response exhibited the greatest development of frailty, but this was not statistically significant.
Table 4.
Development of Frailty in Human Immunodeficiency Virus HIV-Uninfected (HIV−) and HIV-Infected (HIV+) Nonfrail Men Followed After Measurement of Cytomegalovirus (CMV)-Induced IL-2-Producing CD4 T Cells
| IDa | HIV Status | Total CD4 IL-2 Response (% of CD4 T Cells) | Number of Follow-up Visitsb | Follow-up Visits with Fried Frailty Phenotype | |
|---|---|---|---|---|---|
| Number | Percent | ||||
| 1 | Negative | 0.05 | 12 | 1 | 8.3% |
| 2 | 0.20 | 12 | 1 | 8.3% | |
| 3 | 0.43 | 10 | 0 | 0% | |
| 4 | 0.96 | 9 | 0 | 0% | |
| 5 | 1.30 | 10 | 0 | 0% | |
| 6 | 2.27 | 14 | 4 | 28.6% | |
| 7 | 2.50 | 12 | 0 | 0% | |
| 8 | 3.62 | 13 | 4 | 30.8% | |
| 9 | 4.65 | 13 | 2 | 15.4% | |
| 10 | 5.15 | 12 | 4 | 33.3% | |
| 11 | Positive | 0.27 | 9 | 5 | 55.6% |
| 12 | 0.56 | 13 | 0 | 0% | |
| 13 | 0.64 | 13 | 3 | 23.1% | |
| 14 | 0.90 | 12 | 2 | 16.7% | |
| 15 | 0.93 | 10 | 0 | 0% | |
| 16 | 1.23 | 9 | 1 | 11.1% | |
| 17 | 1.30 | 12 | 1 | 8.3% | |
| 18 | 2.72 | 14 | 1 | 7.1% | |
| 19 | 3.95 | 13 | 0 | 0% | |
| 20 | 4.23 | 3 | 0 | 0% | |
aIdentifiers (IDs) are ranked in ascending order of the percentage of CD4 T cells that produced IL-2 in response to stimulation with CMV antigens.
Figure 1.
Unadjusted Kaplan-Meier survival curves showing the proportions remaining frailty-free among individuals with summed CD4 T-cell IL-2 response in the upper tertile (>2.5%, dashed lines) and in the bottom two tertiles (≤2.5%, solid lines). Data are presented separately for HIV-uninfected (HIV-) nonfrail men (left) and HIV-infected (HIV+) nonfrail men (right). The P-values obtained from log-rank tests are shown. Hash marks and the numbers above indicate censoring times and the numbers of individuals lost, respectively.
DISCUSSION
This study investigated the relationship between chronic immune activation and T-cell responses to CMV, using a panel of CMV antigens that captures most of the large and complex T-cell response to CMV. We studied this broad panel for 2 reasons. First, CMV-specific T-cell responses vary greatly depending on the antigens used in both healthy donors [8, 25] and HIV+ men [25]. Secondly, although many studies have measured responses to CMV pp65 and/or IE-1, most CMV-responsive T cells recognize other CMV components [8, 25].
For the most part, strong correlations between CMV responses and systemic inflammatory markers were not present in groups stratified by HIV status alone or frailty status alone, but rather in groups stratified by both variables, especially in nonfrail men and HIV− frail men. Significant correlations were observed with both CD4 and CD8 T-cell responses. Correlations with inflammatory markers such as IFN-γ, IL-6, TARC, and activated T cells (both CD4 and CD8) were generally positive, while those with chemokines were generally negative. The correlations in the nonfrail men were not affected by HCV because only 1 nonfrail man in this study had HCV infection. It is possible that HCV affected immune activation in the HIV+ men in this study, but that hypothesis requires analysis of many more donors.
We found that nonfrail HIV− men in the highest tertile of CD4 IL-2 responses to CMV were significantly more likely to develop frailty during a median of 6 years of follow-up than similar men in the lower 2 tertiles. This finding is clearly preliminary, being based on only 10 men, but it resembles the higher incidence of frailty in people in the highest quartile of serum IL-6 reported by Harris et al [1] and supports the testable hypothesis that high CMV-induced CD4 T-cell production of IL-2 predicts onset of frailty in HIV− nonfrail men. This study did not support such a relationship in HIV+ men. In both HIV− and HIV+ men, serum CRP was much higher in frail than nonfrail men, and the same was true for IL-6, though to a lesser extent. These data are consistent with previous studies in the MACS [28, 29] and in the HIV− older adult population [30–33].
Relationships between CMV T-cell responses and chronic immune activation were scarcer in HIV+ frail men than in the other groups of men, with only 2 strong and significant correlations, both involving the TNF-α response. This may reflect immunological dysfunction or dysregulation that has occurred with the onset of frailty in HIV+ men.
This study has several limitations. We did not test T-cell responses to all CMV ORFs, which number in the hundreds [34]. Although the panel used was the most extensive tested to date for correlation with chronic immune activation, and correlated highly (r > 0.9) with the total response to all known CMV antigens in healthy donors aged 18–55 [8], such correlation could be different in older, HIV+, and/or frail donors. Similarly, we did not test all known markers of chronic immune activation. However, the markers studied include many of the most extensively studied soluble inflammatory mediators, as well as cellular activation markers. The sample size was relatively small, due to the complexity and expense of the studies conducted, but even so strong correlations were present, and about one-third of these remained significant after adjustment for multiple comparisons. Clearly, studies with larger sample size and longer follow-up would be beneficial. The men studied were not formally confirmed as CMV-seropositive, but almost certainly were, because they all responded immunologically to CMV antigens. We did not assess CMV DNA in plasma, because this is rarely present in people who are not immunocompromised ([35], and unpublished MACS data). Because no CMV-seronegative people were studied, the study sheds no light on development of frailty in such people.
Another possible limitation of the study is in the correction for multiple comparisons. Correcting for multiple comparisons may increase the likelihood of false-negative results [27], which would be undesirable given the high cost and labor-intensiveness of the tests we conducted. Our count of the number of independent tests was conservative, because many of the T-cell responses measured were strongly correlated with each other, as were the inflammatory markers. To take this into account, we allowed a somewhat liberal false discovery proportion of 0.2 in adjusting for the number of comparisons among these variables. It is difficult to determine if we have balanced these conflicting tendencies accurately. However, it is likely that comparisons that were significant after the adjustments performed are biologically meaningful, at least as a basis for hypothesis generation. It should also be noted that many of the correlations observed, in addition to being strong (≥0.6 in absolute magnitude), exhibited patterns of correlation that were consistent across many inflammatory biomarkers, for example positive correlations with proinflammatory markers and activated T cells, and negative correlations with chemokines. This suggests that these correlations are not random false-positive findings. Nevertheless, the possibility of false-positive findings has not been excluded, and, again, the present findings require confirmation.
Because of its cross-sectional design, the present study cannot address whether the correlations observed between CMV responses and immune activation reflect a causative role for CMV responses. An interpretation of these correlations, which has not been excluded, is that larger T-cell responses to CMV could be a result, rather than a cause, of immune activation. In this connection, there is evidence that inflammation can foster reactivation of latent CMV [36] and that CMV replicates better in activated than in resting myeloid cells [37, 38]. Longitudinal studies will be needed to address this question.
Given the many diseases that have been linked to chronic immune activation, including frailty and other manifestations of aging, understanding the etiology and mechanisms of age-related inflammation increases in importance as both the HIV− and HIV+ populations age and the prevalence of frailty increases in both populations. The results from this study suggest that both CMV and frailty status should be taken into account in studies of age-related inflammation. One study of short-term suppression of CMV infection with valganciclovir in men with advanced HIV infection led to significant reductions in activated T cells [39]; however, this is not necessarily generalizable to the larger population of virologically suppressed HIV+ people treated at earlier stages of HIV infection, as is now common, or to the general population.
Much of what we know about chronic CMV infection and the immune response to it is very narrow in scope, with sparse data on responses to most CMV antigens and the in vivo distribution and form of CMV infection in different organ or tissue compartments [40] and at different ages [41]. Studies that adequately address the complexity of chronic CMV infection in both HIV− populations and populations of HIV+ people treated effectively at early stages of HIV infection are therefore needed to understand the role of chronic CMV infection in the inflammation of aging and its health consequences.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Louis Picker and Andrew Sylwester for providing peptide pools and assistance in their use, Jacquett Johnson for help with data management, SHARE staff for specimen management and clinic assessments, and SHARE participants for their commitment to this longitudinal study.
Financial support. This work was supported by grants U01-AI35042, UM1-AI35043, UM1-AI068613, UL1-TR001079 (Institute for Clinical and Translational Research [ICTR]), R01-AI108907, R01-AI108907, and R21-AG-043874 from the National Institutes of Health, and funding from the Milstein Medical Asian American Partnership Foundation (www.mmaapf.org).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: New York Academy of Sciences-Gerosciences Interest Group Meeting, New York, NY, April, 2016, Abstract #25; International Conference on Frailty and Sarcopenia, Philadelphia, PA, April, 2016, Abstract #P109; 7th International Workshop on HIV and Aging, Washington DC, September, 2016, Abstract #21; 8th International Workshop on HIV and Aging, New York, NY, October, 2017, Abstract #12.
References
- 1. Harris TB, Ferrucci L, Tracy RP et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 1999; 106:506–12. [DOI] [PubMed] [Google Scholar]
- 2. Regidor DL, Detels R, Breen EC et al. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS 2011; 25:303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wada NI, Jacobson LP, Margolick JB et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2014; 20:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Althoff KN, Jacobson LP, Cranston RD et al. ; Multicenter AIDS Cohort Study (MACS) Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014; 69:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desquilbet L, Jacobson LP, Fried LP et al. ; Multicenter AIDS Cohort Study HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007; 62:1279–86. [DOI] [PubMed] [Google Scholar]
- 6. Piggott DA, Muzaale AD, Mehta SH et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 2013; 8:e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McElhaney JE, Zhou X, Talbot HK et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012; 30:2060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sylwester AW, Mitchell BL, Edgar JB et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202:673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vescovini R, Biasini C, Fagnoni FF et al. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J Immunol 2007; 179:4283–91. [DOI] [PubMed] [Google Scholar]
- 10. Weinberger B, Lazuardi L, Weiskirchner I et al. Healthy aging and latent infection with CMV lead to distinct changes in CD8+ and CD4+ T-cell subsets in the elderly. Hum Immunol 2007; 68:86–90. [DOI] [PubMed] [Google Scholar]
- 11. Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol 2007; 81:7759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hadrup SR, Strindhall J, Køllgaard T et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol 2006; 176:2645–53. [DOI] [PubMed] [Google Scholar]
- 13. Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc 2005; 53:747–54. [DOI] [PubMed] [Google Scholar]
- 14. Strandberg TE, Pitkala KH, Tilvis RS. Cytomegalovirus antibody level and mortality among community-dwelling older adults with stable cardiovascular disease. JAMA 2009; 301:380–2. [DOI] [PubMed] [Google Scholar]
- 15. Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc 2006; 54:1046–54. [DOI] [PubMed] [Google Scholar]
- 16. Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol 2010; 172:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Savva GM, Pachnio A, Kaul B et al. ; Medical Research Council Cognitive Function and Ageing Study Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell 2013; 12:381–7. [DOI] [PubMed] [Google Scholar]
- 18. Wang GC, Kao WH, Murakami P et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol 2010; 171:1144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naeger DM, Martin JN, Sinclair E et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 2010; 5:e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–8. [DOI] [PubMed] [Google Scholar]
- 21. Detels R, Jacobson L, Margolick J et al. The Multicenter AIDS Cohort Study, 1983 to … Public Health 2012; 126:196–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hultin LE, Menendez FA, Hultin PM et al. Assessing immunophenotyping performance: proficiency-validation for adopting improved flow cytometry methods. Cytometry B Clin Cytom 2007; 72:249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hultin LE, Chow M, Jamieson BD et al. Comparison of interlaboratory variation in absolute T-cell counts by single-platform and optimized dual-platform methods. Cytometry B Clin Cytom 2010; 78:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fried LP, Tangen CM, Walston J et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–56. [DOI] [PubMed] [Google Scholar]
- 25. Li H, Margolick JB, Bream JH et al. Heterogeneity of CD4+ and CD8+ T-cell responses to cytomegalovirus in HIV-infected and HIV-uninfected men who have sex with men. J Infect Dis 2014; 210:400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKay HS, Margolick JB, Martínez-Maza O et al. Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine 2017; 90:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc 1995; 57:289–300. [Google Scholar]
- 28. Margolick JB, Bream JH, Martiñez-Maza O et al. Frailty and circulating markers of inflammation in HIV+ and HIV- men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 2017; 74:407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erlandson KM, Ng D, Jacobson LP et al. Inflammation, immune activation, immunosenescence, and hormonal biomarkers in the frailty-related phenotype of men with or at risk for HIV. J Infect Dis 2017; 215:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc 2002; 50:1268–71. [DOI] [PubMed] [Google Scholar]
- 31. Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc 2007; 55:864–71. [DOI] [PubMed] [Google Scholar]
- 32. Walston J, McBurnie MA, Newman A et al. ; Cardiovascular Health Study Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002; 162:2333–41. [DOI] [PubMed] [Google Scholar]
- 33. Fried LP, Xue QL, Cappola AR et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci 2009; 64:1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stern-Ginossar N, Weisburd B, Michalski A et al. Decoding human cytomegalovirus. Science 2012; 338:1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Para MF, Kalish LA, Collier AC, Murphy EL, Drew WL; Viral Activation Transfusion Study Group Correlates of change in cytomegalovirus viremia in patients with advanced human immunodeficiency virus infection who require transfusion. J Infect Dis 2001; 183:1673–7. [DOI] [PubMed] [Google Scholar]
- 36. Dupont L, Reeves MB. Cytomegalovirus latency and reactivation: recent insights into an age old problem. Rev Med Virol 2016; 26:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol 1991; 72(Pt 9):2059–64. [DOI] [PubMed] [Google Scholar]
- 38. Sissons JG, Bain M, Wills MR. Latency and reactivation of human cytomegalovirus. J Infect 2002; 44:73–7. [DOI] [PubMed] [Google Scholar]
- 39. Hunt PW, Martin JN, Sinclair E et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gordon CL, Miron M, Thome JJ et al. Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J Exp Med 2017; 214:651–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parry HM, Zuo J, Frumento G et al. Cytomegalovirus viral load within blood increases markedly in healthy people over the age of 70 years. Immun Ageing 2016; 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.