Root respiratory burst oxidase homologue-dependent H2O2 production serves as an early signal to confer salt tolerance on a grafted cucumber through regulation of Na+ exclusion and stomatal closure.
Keywords: Grafting, H+-ATPase, Na+ exclusion, ROS, salinity, signaling, stomatal closure
Abstract
Plant salt tolerance can be improved by grafting onto salt-tolerant rootstocks. However, the underlying signaling mechanisms behind this phenomenon remain largely unknown. To address this issue, we used a range of physiological and molecular techniques to study responses of self-grafted and pumpkin-grafted cucumber plants exposed to 75 mM NaCl stress. Pumpkin grafting significantly increased the salt tolerance of cucumber plants, as revealed by higher plant dry weight, chlorophyll content and photochemical efficiency (Fv/Fm), and lower leaf Na+ content. Salinity stress resulted in a sharp increase in H2O2 production, reaching a peak 3 h after salt treatment in the pumpkin-grafted cucumber. This enhancement was accompanied by elevated relative expression of respiratory burst oxidase homologue (RBOH) genes RbohD and RbohF and a higher NADPH oxidase activity. However, this increase was much delayed in the self-grafted plants, and the difference between the two grafting combinations disappeared after 24 h. The decreased leaf Na+ content of pumpkin-grafted plants was achieved by higher Na+ exclusion in roots, which was driven by the Na+/H+ antiporter energized by the plasma membrane H+-ATPase, as evidenced by the higher plasma membrane H+-ATPase activity and higher transcript levels for PMA and SOS1. In addition, early stomatal closure was also observed in the pumpkin-grafted cucumber plants, reducing water loss and maintaining the plant’s hydration status. When pumpkin-grafted plants were pretreated with an NADPH oxidase inhibitor, diphenylene iodonium (DPI), the H2O2 level decreased significantly, to the level found in self-grafted plants, resulting in the loss of the salt tolerance. Inhibition of the NADPH oxidase-mediated H2O2 signaling in the root also abolished a rapid stomatal closure in the pumpkin-grafted plants. We concluded that the pumpkin-grafted cucumber plants increase their salt tolerance via a mechanism involving the root-sourced respiratory burst oxidase homologue-dependent H2O2 production, which enhances Na+ exclusion from the root and promotes an early stomatal closure.
Introduction
Soil salinity is a global challenge affecting agricultural production worldwide. More than 800 million hectares of agricultural land suffers from soil salinity (Rengasamy, 2010). Among all types of salinity, the most soluble and widespread salt is NaCl, and Na+ toxicity therefore prevails in most natural habitats restricting plant growth. For most glycophytes, the ability of a plant to minimize accumulation of the toxic Na+ in the sensitive shoot is a crucial feature of salinity tolerance. However, as most of the Na+ delivered to the shoot remains in the shoot, and only a small portion can be recirculated back to the root via the phloem (Munns and Tester, 2008), the salt tolerance largely depends on the capacity of plants to limit the net transport of Na+ from the root to the shoot. This process relies on several key mechanisms; one of them is the efficient Na+ efflux from the root to the external medium.
Active Na+ extrusion is mediated by the plasma membrane (PM) Na+/H+ antiporter (Shi et al., 2002). Here, energy-dependent Na+ transport is coupled to the H+ electrochemical potential difference established by H+ translocation pumps (Hasegawa et al., 2003). The NaCl-induced activation of the PM Na+/H+ antiporter has been reported in various plant species, such as tomato (Wilson and Shannon, 1995), Arabidopsis (Qiu et al., 2002) and rice (Martínez-Atienza and Quintero, 2007).
Salt stress also induces the production of reactive oxygen species (ROS) (Zhen et al., 2011). When accumulated in excessive quantities, ROS may react with various cellular targets such as nucleic acids, proteins, lipids and chlorophyll, causing serious damage (Niu and Liao, 2016). At the same time, besides their harmful effects, ROS can also act as signaling molecules that regulate plant development, and biotic and abiotic stress responses (Mittler et al., 2004; Bose et al., 2014; Li et al., 2014; Li et al., 2016). More and more evidence has accumulated suggesting that ROS play an important role in plant salinity tolerance (Wang et al., 2013; Hossain and Dietz, 2016). For example, Arabidopsis AtrbohF knockout mutants, which lack the respiratory burst oxidase proteins (NADPH oxidases that catalyse the production of ROS in the apoplast), showed an increased salt sensitivity and impaired Na+/K+ homeostasis (Ma et al., 2012; Jiang et al., 2013). Among all the ROS, H2O2 has a comparatively long lifespan and a small size, which permits it to traverse the cellular membranes to different cellular compartments. Some recent findings led to speculation that H2O2 may act as a stress signal that regulates the PM Na+/H+ antiport system under saline conditions and alters SOS1 mRNA stability in Arabidopsis which is fundamental to maintaining cellular K+/Na+ homeostasis (Zhang et al., 2007).
H2O2 has also been demonstrated to mediate rapid systemic signaling stimulated by a root-derived ABA triggered by high temperature stress (Li et al., 2014). However, the role and specific mechanisms of H2O2-induced root-to-shoot communication are largely unknown for salinity stress. Several papers demonstrated that H2O2 functions in the regulation of stomatal aperture (Desikan et al., 2005; Danquah et al., 2014; Niu and Liao, 2016). Silencing RBOH1 led to an impaired capacity for stomatal closure in tomato (Zhou et al., 2014; Yi et al., 2015). However, all these reports dealt with H2O2 produced in (or applied to) the shoot and, to the best of our knowledge, no reports are available linking root-originating ROS signals with stomatal operation in salt-grown plants.
Grafting is a widely used agronomic practice that improves a plant’s salt tolerance by replacing the sensitive root with one taken from a more tolerant genotype or species. Plants of the Cucurbitaceae such as melon, watermelon, and cucumber are glycophytes of high economic importance, but all of them are sensitive to Na+ (Zhu et al., 2008). At the same time pumpkin, which belongs to the same family, is considerably more tolerant under saline conditions (Lee et al., 2010; Rouphael et al., 2012). Grafting cucumber scion onto pumpkin rootstock can therefore potentially lead to higher salt tolerance in this species. Previous studies have suggested that pumpkin exhibited a higher capacity in limiting the transport of Na+ from root to shoot than melon and cucumber (Edelstein et al., 2011; Huang et al., 2013). Electrophysiological studies have also demonstrated that pumpkin roots exhibited a high efficiency in extruding Na+ (Lei et al., 2014). As this increased extrusion was concurrent with an increased H+ influx under NaCl stress, this suggested that Na+ exclusion in salt stressed pumpkin roots was the result of an active Na+/H+ antiporter across the PM fueled by the plasma membrane H+-ATPase encoded by PMA (Li et al., 2015). Na+/H+ exchange in the root was inhibited by amiloride (a Na+/H+ antiporter inhibitor) and vanadate (a PM H+-ATPase inhibitor) indicating that the H+-ATPase-driven Na+/H+ antiport plays an important role in dealing with salt stress (Sun et al., 2009).
In the present study, we have compared the accumulation patterns of H2O2 and Na+ between two grafted combinations (self-grafted and pumpkin-grafted cucumber seedlings). The ion fluxes in root and hypocotyl were evaluated by the non-invasive micro-test technology (NMT). Linked with pharmacological experiments, these results demonstrate that root respiratory burst oxidase homologue (RBOH)-dependent H2O2 production confers salt tolerance on grafted cucumber by controlling Na+ exclusion and stomatal closure, thus optimizing plant ionic and water balance under hostile saline conditions.
Materials and methods
Grafting method and growth conditions
The experiment was carried out in the growth chambers at Huazhong Agricultural University, Central China. A salt-sensitive cucumber (Cucumis sativus L.) cv. Jinchun No. 2 (abbreviated here as ‘C’) was used, either as a scion or a rootstock, and a salt-tolerant pumpkin (Cucurbita moschata Duch.) cv. Chaojiquanwang (abbreviated as ‘P’) was used as a rootstock. Two grafted combinations were used in this study: cucumber self-grafted plants (C/C) and pumpkin-grafted plants (C/P). We did not use ungrafted plants as additional controls, since our previous studies showed that the response of ungrafted and self-grafted cucumber/pumpkin to salt stress was similar (Huang et al., 2013); this included plant growth reduction, Na+ concentration, and stomatal conductance under salt stress. Thus, it was concluded that the advantage of grafted cucumber plants is attributable to the rootstock, not the grafting process itself (Lei et al., 2014).
The seeds were soaked in tap water for 6 h and incubated in the dark at 30 °C until germination. Rootstocks were sown 4 d earlier than cucumber scions. When the rootstock seedlings had developed one true leaf, the cucumber seedlings were grafted onto them by using the ‘hole insertion grafting’ method described by Lee (1994). Briefly, the first true leaf of the rootstock was removed and the apex of the rootstock was perforated. The scion was prepared with two cuts giving a sharp edge of about 10 mm of hypocotyl. The scion was then inserted into the rootstock hole from the top. After grafting, the seedlings were placed in a ‘healing chamber’ in which the relative humidity was kept at ≥95% for the first 3 d and then gradually decreased to 75%. The air temperature was kept 28–30 °C, and plants were kept in the darkness for the first 48 h, and then exposed to a 14/10 h light/dark cycle, 28/18 °C, with photosynthetic photon flux density 600 μmol m–2 s–1. After 7 d the grafted plants were transferred to plastic containers (six seedlings per container) containing 8 liters of full-strength Hoagland’s solution. The nutrient solution was refreshed at 3 d intervals and continuously aerated. At the four-leaf stage, grafted combinations were used for subsequent experiments.
Salt treatment and NADPH oxidase inhibitor application
To study the Na+ and H2O2 accumulation patterns in two grafted combinations, NaCl was added into the growth media to obtain a final concentration of 75 mM. The choice of this specific concentration was determined by the fact that we aimed to investigate the signaling role of H2O2 and thus tried to select the concentration that was strong enough to reveal the phenotypic difference but could be considered ‘safe’ in terms of damage to the root. The time courses of malonyldialdehyde (MDA), relative electrical conductivity (REC), Na+ and H2O2 contents were monitored by plant sampling at 0, 1, 3, 12, 24, 48, and 120 h after commencement of salt treatment. The biomass, relative chlorophyll content (measured with a SPAD meter) and chlorophyll fluorescence (Fv/Fm) were measured 120 h after salt treatment.
It was true that 100 mM NaCl treatment led to a more obvious difference of the phenotype (see Supplementary Fig. S1 at JXB online), but high concentrations of NaCl (100 mM or higher) inevitably caused serious damage in the root of C/C with an enhanced H2O2 level (Supplementary Fig. S2). This increased H2O2 level was detected after 5 d of NaCl treatment, which might be a result of an impaired redox system rather than a signal. The purpose of this study was to evaluate the function of root-sourced H2O2 as a molecular signal, so we use 75 mM NaCl to distinguish the salt tolerance between two grafted combinations.
In some experiments, the NADPH oxidase inhibitor diphenylene iodonium (DPI) was added to the medium to a final concentration of 20 μM. The plants were pretreated with DPI for 6 h, and then transferred to Hoagland’s solution containing 75 mM NaCl. The treatments without DPI or NaCl were set as controls. H2O2 content, transpiration rate, stomatal conductance, NADPH oxidase activity, H+-ATPase activity, and related gene (RbohD, RbohF, PMA, SOS1) expression levels were determined 3 h after salt treatment. The tissue Na+ content and Na+ and H+ fluxes in roots and hypocotyls were determined 24 h after salt treatment.
Relative chlorophyll content (SPAD) and chlorophyll fluorescence measurements
Relative chlorophyll content was measured with a chlorophyll meter (SPAD-502, Minolta Corp., Ltd, Osaka, Japan) from the fully expanded functional leaves (the third from the apex). Measurements were made at a central point on the leaflet between the midrib and the leaf margin. Chlorophyll fluorescence was determined with an imaging-PAM chlorophyll fluorometer (Heinz Walz, GmbH, Effeltrich, Germany). Plants were dark-adapted for 30 min to measure the maximum photochemical efficiency of PSII (Fv/Fm) at the same position as chlorophyll content.
Analysis of lipid peroxidation and membrane permeability in leaves
The level of lipid peroxidation in leaves was assessed by measuring the content of malondialdehyde (MDA) using the thiobarbituric acid reaction (Heath and Packer, 1968). Membrane permeability of the leaf was measured as the relative electrical conductivity according to the method described by Zhou and Leul (1998). The washed leaves (0.1 g) were cut into 1 cm2 pieces and placed in a 50 ml test tube containing 30 ml deionized water. The leaf samples were immersed and vibrated for 3 h, and then the conductivity of the solution was measured using a conductivity meter (SG78, Mettler Toledo). After boiling the samples for 15 min, their conductivity was measured again when the solution was cooled to room temperature. The relative electrical conductivity (REC) was calculated as follows:
where C1 and C2 are the electrolyte conductivities measured before and after boiling, respectively.
Determination of H2O2 concentration in roots and leaves
H2O2 was extracted from 0.5 g fresh leaf or root samples ground in 3 ml of 1 M HClO4. After centrifugation, the supernatant was adjusted to pH 6.0–7.0 and filtered through a Sep-Pak C18 cartridge (Millipore, Milford, MA, USA). After elution with 4 ml distilled water, an aliquot of the sample (800 μl) was mixed with 400 μl reaction buffer containing 4 mM 2,2′-azino-di(3-ethylbenzthiazoline-6-sulfonic acid) and 100 mM potassium acetate at pH 4.4, and 400 μl deionized water. The reaction was started by the addition of 3 μl (0.5 U) of horseradish peroxidase. H2O2 content was measured spectrophotometrically at the optical density at 412 nm (Willekens et al., 1997).
Determination of Na+ content in roots and leaves
Dried roots and leaves of two grafted combinations were ground using a mortar and pestle; 0.1 g of powder was then digested with 5 ml of nitric acid for 3 h, and then Na+ concentrations were analysed using an atomic absorption spectrophotometer (Varian spectra AA 220, Varian, Palo Alto, CA, USA).
Measurement of ion fluxes in roots and hypocotyls with NMT
A so-called ‘recovery protocol’ (Cuin et al., 2011) was used to quantify the activity of the Na+ efflux system in plant root and hypocotyls. For this, net Na+ and H+ fluxes were measured using the non-invasive micro-test technology (NMT) technique (YoungerUSA LLC, Amherst, MA, USA) and ASET 2.0 (Sciencewares, Falmouth, MA, USA) and iFluxes 1.0 (YoungerUSA) software (Kochian et al., 1992). Grafted plants were treated with 75 mM NaCl for 24 h, leading to significant accumulation of Na+ in roots and hypocotyls and activation of the Na+ efflux system. The roots and hypocotyls from control and salt-treated plants were then rinsed with distilled water and transferred to the measuring solution containing very little salt (0.1 mM KCl, 0.1 mM CaCl2, 0.1 mM MgSO4, 0.1 mM NaCl, 0.3 mM MES, pH 6.0). Plant specimens were immobilized in the middle of poly-L-lysine-coated coverslips (2 cm×2 cm) in the measuring chamber. Net fluxes were measured after 30 min (for roots) and 15 min (for hypocotyls) equilibration in low-Na+ solution. The measuring sites in hypocotyl were 1 cm above or below the grafting union. Before testing, the upper part of the seedling was removed by a razor blade to expose the xylem vessel (deep colored area indicated in Supplementary Fig. S3). The measuring site in root was 400 μm from the root tip (see Supplementary Fig. S3), which corresponds to the elongation zone and in which a vigorous efflux of Na+ has been observed in our previous study (Lei et al., 2014). The magnitude of steady-state ion fluxes was calculated by data recorded over a 240 s period (Supplementary Fig. S4). The glass micropipettes and measuring solutions were prepared as previously described (Lei et al., 2014).
Determination of transpiration rate and stomatal conductance
The second recently expanded leaves were selected for the determination of transpiration rate and stomatal conductance with an open gas exchange system (Li-6400, Li-Cor, Inc., Lincoln, NE, USA). The assimilatory chamber was controlled to maintain the leaf temperature at 28 °C, CO2 concentration at 360 μmol mol−1, and photosynthetic photon-flux density at 600 μmol m−2 s−1. Five replicate plants per treatment were measured between 8:30 and 11:30 AM.
Determination of relative water content
The relative water content (RWC) of leaves and roots was calculated as described by Weatherley (1950).
Visualization of H2O2 in root using fluorescent dye
Confocal laser scanning microscopy (Leica TCS-SP2, Leica Microsystems GmbH, Wetzlar, Germany) was used to visualize H2O2 accumulation in plant roots in vivo. Roots from two grafted combinations were incubated in the reaction buffer containing 10 mM Hepes–NaOH (pH 7.5) and 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; Invitrogen) for 20 min at 30 °C. Thereafter, the roots were washed with the HEPES–NaOH buffer (pH 7.5) and fluorescence measurements conducted. The dye excitation was at 488 nm; emitted light was detected at 522 nm.
Isolation of the plasma membrane vesicles and determination of NADPH oxidase and H+-ATPase activities
Root plasma membrane vesicles were isolated using a two-phase aqueous polymer partition system (Xia et al., 2009). The NADPH-dependent O2--generating activity in isolated plasma membrane vesicles was determined by the protocol described previously (Zhou et al., 2014). The H+-ATPase activity was determined by measuring the release of inorganic phosphate (Pi) (Kłobus and Janicka-Russak, 2004) and expressed as the difference between the activities measured in the absence and presence of Na3VO4.
Total RNA extraction and gene expression analysis
Total RNA was isolated from the seedling roots using TransZol reagent (TransGen Biotech, Inc., Beijing, China) in accordance with the manufacturer’s protocol. After extraction, the total RNA was dissolved in the diethylpyrocarbonate-treated water. The cDNA template for the quantitative real-time PCR (qRT-PCR) was synthesized from 1 μg of total RNA using HiScript II Q Select RT SuperMix for qPCR (Vazyme, Piscataway, NJ, USA).
For qRT-PCR analysis, we amplified the PCR products in triplicate by using 1×Top Green qPCR SuperMix (TransGen Biotech, Inc., Beijing, China) in 10 μl qRT-PCR assays. The PCR was performed using the ABI 7000 machine (Applied Biosystems), and the cycling conditions consisted of denaturation at 94 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at 55 °C for 15 s, and extension at 72 °C for 15 s. The specific primers (Table 1) were designed based on published mRNA of Cucurbita moschata on the Cucurbit Genomics Database (http://cucurbitgenomics.org) using Primer 5 software. The relative gene expression was determined as previously described by Livak and Schmittgen (2001).
Table 1.
Gene-specific primers designed for qRT-PCR
| Grafted combinations | Gene | Forward primer | Reverse primer | Genomics Database accession |
|---|---|---|---|---|
| C/C | PMA | GGCTGGTGTAGTTTGGA | CATAGTCTTTCTTGGTCGTA | Csa1G423270 |
| SOS1 | CCAACGGAGTGGTAAA | AACAACGGAATCTGTAATC | Csa5G098980 | |
| RbohD | AACAACATCAAGGACCAG | TCACCCAGTAGAAGTAAGC | Csa3G845500 | |
| RbohF | AGCCAGAACATACAGGG | TTAGCCGTTAGGAGACAG | Csa4G050170 | |
| EF1a | ACTGTGCTGTCCTCATTATTG | AGGGTGAAAGCAAGAAGAGC | Csa2G139820 | |
| C/P | PMA | TAGAGTGAAGCCATCTCC | CAAGCATAACGCCAGT | CmoCh11G003690 |
| SOS1 | GGAGCCATTGGTTCGTC | GGTGCCTCGCAGTAAGT | CmoCh04G022490 | |
| RbohD | ATGCCGAATACGAACC | ATTAGCACCACCATCACA | CmoCh14G010850 | |
| RbohF | GTCATCTAACGAAACCTACA | TCCCATCCCTTAACCA | CmoCh04G007610 | |
| EF1a | GCCTCAAACTCCAAGGATGA | GGCTCCTTCTCGAGTTCCTT | CmoCh08G009890 |
All primers were designed based on a published mRNA of Cucurbita moschata on the Cucurbit Genomics Database (http://cucurbitgenomics.org) using Primer 5 software. EF1a is the reference gene.
Results
Pumpkin-grafted cucumber was more tolerant than self-grafted cucumber
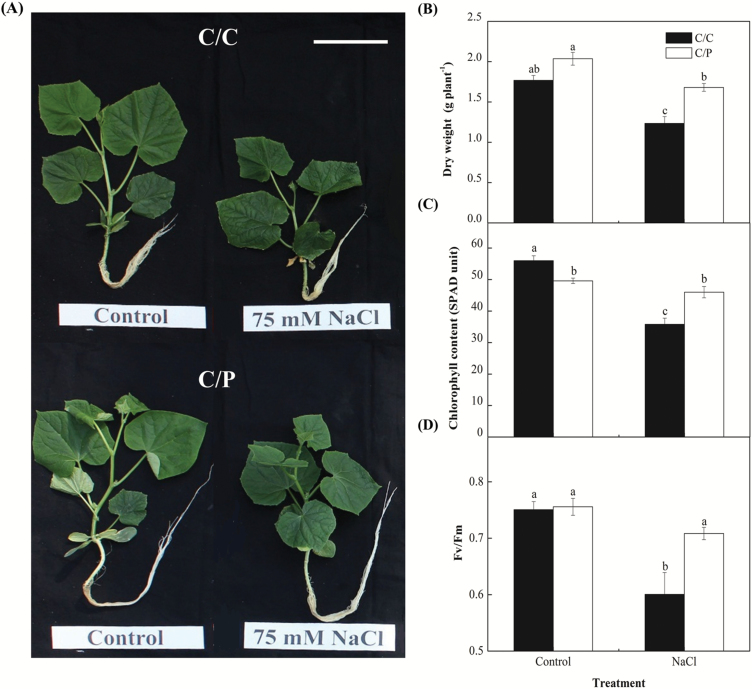
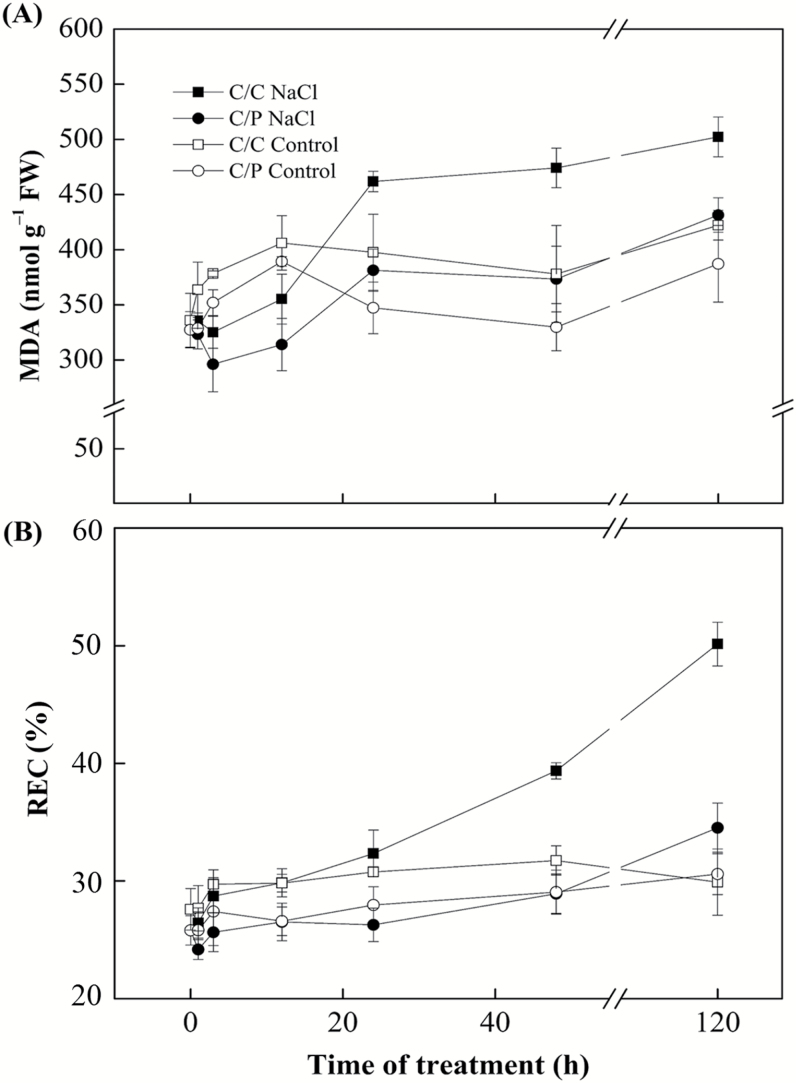
Salt-induced biomass reduction was significantly stronger in the self-grafted (C/C) than in pumpkin-grafted (C/P) cucumber (Fig. 1A, B) after 5 d of salt treatment. Salt treatment had also caused a significant reduction in relative chlorophyll content (SPAD) and chlorophyll fluorescence in leaves of C/C (Fig. 1C, D). To confirm that the salt tolerance of C/P was higher than C/C, the level of MDA and relative electrical conductivity (REC) were measured in C/C and C/P. Salt stress increased MDA content and REC in C/C after 48 h, whereas in C/P plants, the increase in MDA content and REC was only observed after a prolonged treatment until 120 h (Fig. 2B), suggesting that C/P is indeed more tolerant of salt stress than C/C.
Fig. 1.
Effects of NaCl on the growth (A), dry weight (B), chlorophyll content (C) and photochemical efficiency (Fv/Fm) (D) of two grafted combinations, namely pumpkin-grafted cucumber (C/P) and self-grafted cucumber (C/C). Data are mean±SE (n=5). Columns with different letters are significantly different at P<0.05. Scale bar: 10 cm.
Fig. 2.
Effects of 75 mM NaCl treatments on malondialdehyde (MDA) content (A) and relative electric conductivity (REC; B) in the leaves of pumpkin-grafted cucumber (C/P) and self-grafted cucumber (C/C). Data are mean±SE (n=3).
Time-dependent kinetics of Na+ and H2O2 accumulation in salt-treated plants
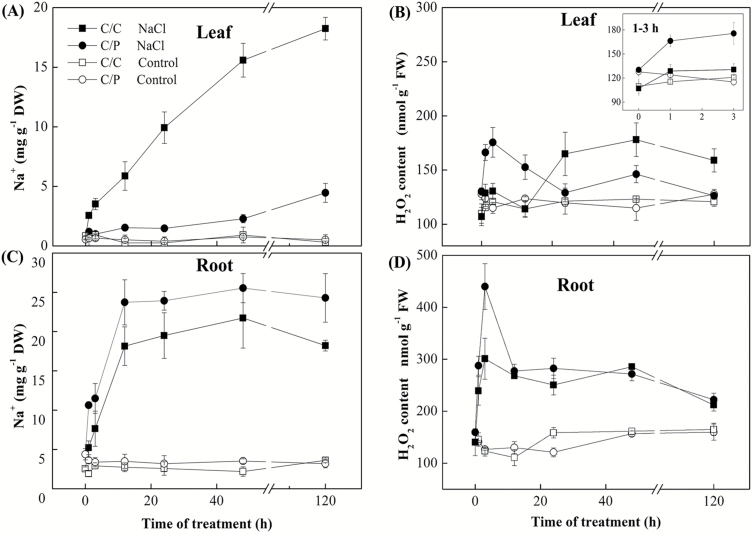
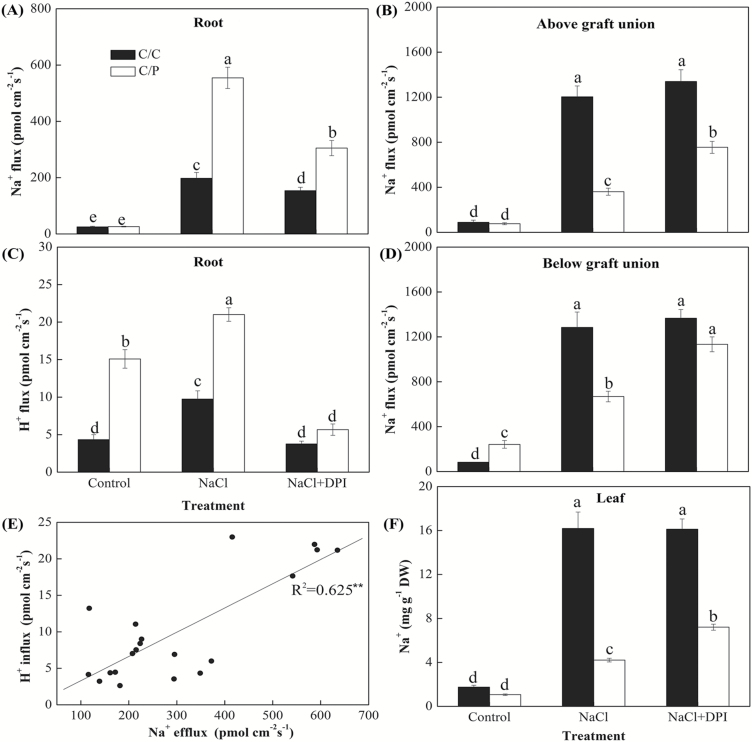
Na+ content in roots of both grafted combinations reached a plateau after about 12 h and then remained more or less constant (Fig. 3A, C), with C/P roots accumulating more Na+ compared with C/C roots (significant at P<0.05). In shoots, Na+ increased sharply in the self-grafted cucumber while in the pumpkin-grafted cucumber Na+ accumulation in the shoot became noticeable only after 48 h of salinity treatment. At the end of experiment, the Na+ concentration in leaves of C/C plants reached 18.2 mg g−1 DW, which was nearly 4 times higher than in the leaves of C/P.
Fig. 3.
Effects of 75 mM NaCl treatments on accumulation patterns of Na+ and H2O2 in pumpkin-grafted cucumber (C/P) and self-grafted cucumber (C/C). Na+ and H2O2 contents were detected in leaves (A, B) and in roots (C, D). Data are mean±SE (n=3–5).
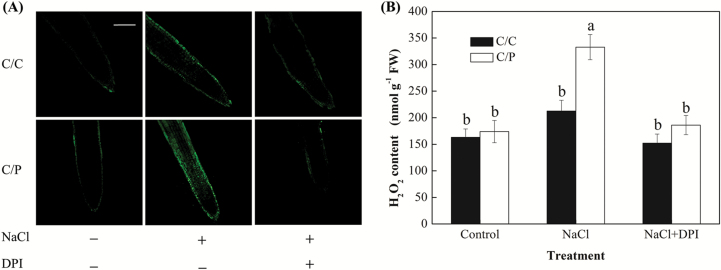
To determine the possible involvement of the H2O2 signal in stress tolerance, the levels of H2O2 in the self-grafted cucumber and the pumpkin-grafted cucumber were measured. The result indicated that the H2O2 content was rapidly elevated in roots of both grafted combinations and reached a peak at 3 h. Then H2O2 levels decreased during the period between 3 and 12 h but remained elevated for at least 48 h after commencement of the treatment (Fig. 3D). While the final H2O2 concentrations were not different between two grafting combinations, the NaCl-induced peak in H2O2 production was twice as high in C/P roots compared with their C/C counterparts (Fig. 3D). Similar results were reported when H2O2 content in root was visualized using the H2DCF-DA fluorescence probe (Fig. 4). Here, NaCl treatment caused a rapid increase in H2DCF-DA-dependent fluorescence in the roots of C/P, but not in C/C (Fig. 4). In leaves, stress-induced H2O2 increase was observed in C/P after 3 h, whereas in C/C plants, H2O2 increase was only observed after a prolonged treatment of 24 h (Fig. 3B).
Fig. 4.
Effects of NaCl and DPI on the endogenous H2O2 level in the roots of pumpkin-grafted cucumber (C/P) and self-grafted cucumber (C/C). The plants were pretreated with DPI for 6 h and then transferred to Hoagland’s solution without DPI for 75 mM NaCl treatment. H2O2 levels were measured by using confocal fluorescence imaging from roots stained with H2DCF-DA. Scale bar: 100 μm. Data are mean±SE (n=3–5). Columns labeled with different letters are significantly different at P<0.05.
Effects of DPI on Na+ transport and accumulation in grafted plants
An NADPH oxidase inhibitor, DPI, was used to investigate the potential role of the plasmalemma-based H2O2 production in regulating Na+ transport and its accumulation in roots and shoots. At the whole-plant level, the DPI pretreatment increased Na+ concentration in leaves of C/P by 71% (Fig. 5F) after 120 h of salinity treatment, compared with non-inhibitor treatment. At the same time, inhibition of the NADPH oxidase resulted in no significant change in Na+ accumulation in leaves of C/C plants (Fig. 5F).
Fig. 5.
Net Na+ and H+ fluxes measured from root and shoot tissues of grafted plants using the non-invasive micro-test technology (NMT). (A, C) Net Na+ and H+ fluxes measured from the apical region of plant roots. (B, D) Net Na+ fluxes measured from the position 1 cm above (B) or below (D) graft union. Data are mean±SE (n=5 biological replicates). (F) Na+ content in leaf of two grafted combinations. Columns with different letters are significantly different at P<0.05. (E) The correlation between Na+ flux and H+ fluxes in roots. Each point represents an individual root measured under salinity conditions.
We next assayed the role of NADPH oxidase-produced H2O2 in regulation of ionic relations in root (Fig. 5A, C) and stem (Fig. 5B, D) at the cellular level, by measuring effect of DPI on net ion fluxes in these tissues using the NMT technique. In the apical regions of the roots, a massive efflux of Na+ from roots was recorded in two grafting combinations following the transfer of salt-treated roots to low-Na+ (0.1 mM) solution (Fig. 5A). The mean rates were 555 and 198 pmol cm–2 s–1 for C/C and C/P, respectively. Notably, the DPI-pretreated C/P displayed 45% lower flux than treatment without DPI while the reduction was only 22% in roots of C/C (Fig. 5A). Salt-treated roots also displayed a net H+ influx in both grafting combinations (Fig. 5C). Higher H+ influxes have been found in C/P compared with C/C, regardless of salinity treatment. DPI pretreatment decreased net H+ fluxes by 61% and 73% in roots of C/C and C/P, respectively. A significant positive correlation (R2=0.625; P<0.05) was found between Na+ efflux and H+ influx in salt-treated roots (Fig. 5E), suggesting the possibility of Na+/H+ antiport.
As mentioned above, the higher salt tolerance in pumpkin-grafted cucumber was correlated to the restricted transport of Na+ from root to shoot. To clarify the process of Na+ transport from root to shoot, we have used the NMT technique to measure ion flux at two positions along the hypocotyl: above (Fig. 5B) and bellow (Fig. 5D) the graft union. In the tissue below the graft union, net Na+ efflux of 1284 pmol cm–2 s–1 was detected in the C/C combination while in C/P it was half (only 669 pmol cm–2 s–1) indicating a reduced Na+ flux by rootstock of pumpkin. When fluxes were measured above the graft union, the values were 1204 pmol cm–2 s–1 for C/C but only 361 pmol cm–2 s–1 for C/P. Notably, root pretreatment with DPI caused much more Na+ to be translocated from the root to the shoot in the positions of above and below graft union in C/P. By contrast, the same treatment did not result in any detectable changes in Na+ translocation to the shoot in C/C.
Effects of DPI on the generation of H2O2 in roots
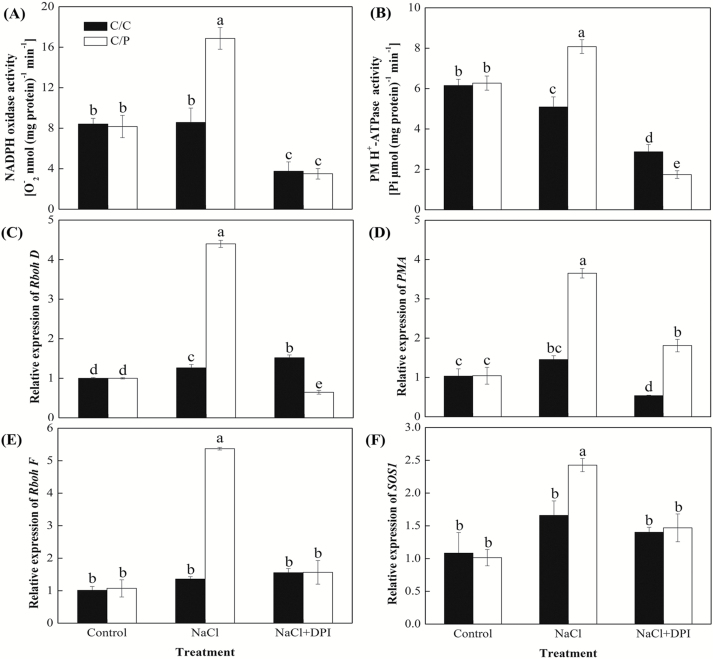
To determine the relationship between the NADPH oxidase activity and salt-induced H2O2 signaling, we have measured endogenous H2O2 levels in salinized roots from the two grafted combinations. A analysis of the H2O2 content supported observation made by the confocal imaging (Fig. 4A). Pretreatment with DPI abolished the NaCl-induced H2O2 accumulations. Importantly, NaCl-induced NADPH oxidase activity was reduced in the DPI-pretreated plants at all time points measured (e.g. after both 3 h (Fig. 6A) and 24 h (see Supplementary Fig. S5) of salt treatment. The transcript level of RbohD and RbohF involved in the generation of NADPH oxidase were both rapidly elevated at 3 h in C/P after salt treatment, but only a small change was observed in C/C (Fig. 6C, E).
Fig. 6.
Effects of NaCl and DPI on the NADPH oxidase-based H2O2 generation (A, C, E) and Na+/H+ antiport system (B, D, F) in roots of pumpkin-grafted cucumber (C/P) and self-grafted cucumber (C/C) after 3 h of treatment. Data are mean±SE (n=3–5). Columns with different letters are significantly different at P<0.05.
DPI reduced Na+/H+ antiporter operation via depressing H+-ATPase activity
As DPI application had a concurrent effect on Na+ and H+ fluxes in roots, we hypothesized that a Na+/H+ antiport system has been a downstream target of the salt-induced H2O2 signal in roots. The change in the elevated PM H+-ATPase activities matched the change in the up-regulated expression of PMA in C/P, which might contribute to the H+-driven Na+ exclusion pathway (Fig. 5B, D, F). The inhibition of NADPH oxidase activity by DPI markedly decreased the PM H+-ATPase activities in C/C and C/P (Fig. 6B).
Effects of DPI on shoot transpiration and H2O2 level in leaves
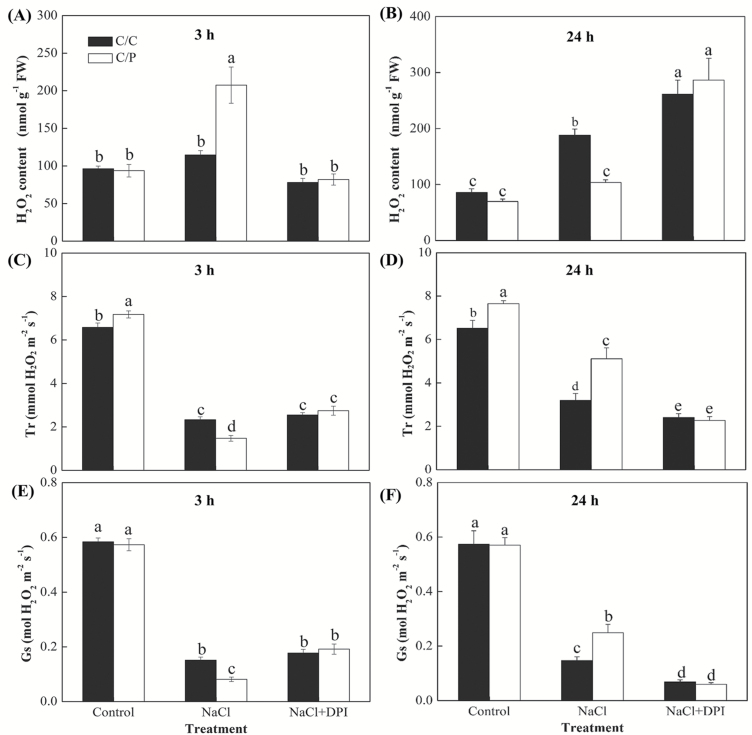
The root-to-shoot delivery of Na+ is affected not only by the rate of xylem Na+ loading but also by the transpiration lift that is conferred by the opening of stomata. In this study, NaCl treatment resulted in a rapid stomatal closure in both grafted combinations in the first 3 h. This closure was more pronounced in C/P than in C/C (80% versus 65% reduction, respectively). The observed trend for the transpiration rate was similar to the trend for the stomatal conductance (Fig. 7C, E). After 24 h of salt treatment, stomatal conductance (Gs) and leaf transpiration rate (Tr) recovered in both grafting combinations but were still lower than those in control (Fig. 7D, F), and the C/P maintained higher Gs and Tr values than C/C. After 3 h of salt treatment, significantly elevated H2O2 levels were only found in the leaves of C/P, and this increase in the leaf H2O2 could be inhibited by DPI pretreatment in the roots (Fig. 7A). In contrast, a significant increase in the H2O2 level was observed in the leaves of C/C only after 24 h of salt stress (Fig. 7B). Interestingly, compared with non-DPI treatment, pretreatment with DPI caused higher H2O2 levels in both grafted combinations after 24 h of salt treatment (Fig. 7B).
Fig. 7.
Effects of NaCl and DPI on the leaf H2O2 contents, leaf transpiration rate (Tr) and stomatal conductance (Gs) in pumpkin-grafted cucumber (C/P) and self-grafted cucumber (C/C) after 3 h (A, C, E) or 24 h (B, D, F) of treatment. Data are mean±SE (n=3–5). Columns with different letters are significantly different at P<0.05.
Discussion
Grafting is an effective way to increase salt tolerance of plants. While previous studies investigated the underlying mechanisms behind the beneficial effects of grafting from the point of view of altered ion homeostasis (Edelstein et al., 2011), root-derived hormones (Albacete et al., 2009) and the antioxidant system (He et al., 2009), the signal transduction aspects of grafting (and, specifically, the role of root-derived H2O2 signals) have never been put in the spotlight. Hydrogen peroxide signaling is known to be important for the acclimation to salt stress conditions (Wang et al., 2013; Hossain and Dietz, 2016). In addition, hydrogen peroxide is one of the known signaling molecules that has an ability to travel long distances (Baxter et al., 2014; Gilroy et al., 2014) and may potentially enable communication between remote plant tissues and organs (Mittler and Blumwald, 2015). Therefore, the grafted plant is a good model for understanding ROS function between root and shoot. Here, two grafted combinations were used to clarify the role of the root-sourced H2O2 in plant response to salt stress. The results indicate that NADPH-generated root H2O2 signals control at least two processes that are essential for plants to handle the salt load. One of them is regulation of Na+ exclusion from roots and the other is rapid stomatal closure upon stress onset (Figs 5 and 7).
H2O2 signal involved in the Na+ exclusion process in pumpkin-grafted plants
As only a small proportion of Na+ can be retrieved from the shoot and moved back to the root via the phloem in plants (Munns and Tester, 2008; Lei et al., 2014), the key factor that determine the Na+ accumulated in scion is the restrictive ability of rootstock to load Na+, as evident from the comparison of different grafting combinations (Zhu et al., 2008; Huang et al., 2010; Edelstein et al., 2011). This notion was further supported in this study. Exposure to salt stress results in increased expression of both SOS1 transcripts (Shi et al., 2000) and SOS1-mediated Na+/H+ exchanger activity in root epidermis (Sun et al., 2009). Here we showed that C/P grafted plants were more efficient in effluxing Na+ from roots compared with C/C plants (Fig. 5A). A strong correlation between H+ influx and Na+ efflux (Fig. 5E) and a reported sensitivity of the measured Na+ efflux to amiloride (Cuin et al., 2011) suggest that the Na+ exclusion in the salt-stressed plants roots is likely the result of an active Na+/H+ antiport across the PM. This result is consistent with the earlier findings from non-grafted cucumber and pumpkin (Lei et al., 2014) that indicated higher Na+ exclusion capacity in pumpkin roots.
Many reports have demonstrated that H2O2 is a key signaling molecule involved in regulation of Na+ transport under salt stress. Among other sources, H2O2 is generated by the plasma membrane-located NADPH oxidase that is encoded by RbohD and RbohF (Xie et al., 2011; Hossain and Dietz, 2016). DPI blocked salinity-induced H2O2 production and reduced salinity tolerance in Arabidopsis (Leshem and Seri, 2007), Oryza sativa (Wang et al., 2013) and Populus euphratica (Sun et al., 2010). Here we found a pronouncedly decrease in Na+ efflux that has mirrored a reduced H2O2 content and NADPH oxidase activity in C/P roots when pretreated with DPI (Fig. 6A, C, E).
The relationship between the SOS pathway and NADPH oxidase-mediated H2O2 signaling has remained elusive until now. It has been suggested that the NADPH oxidase may operate as a salt sensor in plants in tandem with Ca2+-permeable channels (Shabala et al., 2015). According to this model, the plant plasma membranes harbor various non-selective cation channels (NSCCs), which are permeable to Ca2+ and may be activated by both ROS and membrane depolarization (Demidchik and Maathuis, 2007). As a second messenger, Ca2+ could bind to SOS3, which functions in the sensing the Ca2+ signal and contributes to PM Na+/H+ antiporter (SOS1) activation and regulation of cellular Na+ homeostasis. NADPH oxidase-mediated H2O2 accumulation is also critical to SOS1 mRNA stability (Chung et al., 2008). Sun et al. (2010) found that reduction in H2O2 production caused by DPI led to decreased Na+ efflux and H+ influx, and the Ca2+ concentration in the cytoplasm also decreased. Our work reported here suggests that another factor contributing to the stronger Na+ efflux in C/P roots is the higher activity of PM H+-ATPase, which sustains an H+ gradient to drive Na+/H+ antiport across the PM (Fig. 6B, D and Supplementary Fig. S6). This is consistent with the previous observations in non-grafted pumpkin roots, where vanadate (a PM H+-ATPase inhibitor) treatment concurrently decreased both Na+ efflux and H+ influx (Lei et al., 2014). The possible reason for this may be found in the fact that PM H+-ATPase activation may be also mediated by Ca2+. It has been reported that the activity of PM H+-ATPase was dependent on the Ca2+ concentration in the cytosol (Zhang et al., 2007) and the interaction of [Ca2+]cyt and a calcium-dependent protein kinase regulated the PM H+-ATPase in response to fungal elicitors (Lino et al., 1998). As DPI pretreatment decreased both the NADPH oxidase and H+-ATPase activity in roots of both grafting combinations, it appears that the root-derived H2O2 signaling pathway is shared by both plants (i.e. cucumber and pumpkin), but with a different efficiency.
It is obvious that the Na+ exclusion mechanism is just one of many strategies employed by plants to deal with the salt load. Other mechanisms such as storage of excess Na+ in vacuoles and restrictions on Na+ loading into the root stele have also been reported in pumpkin roots (Lei et al., 2014). These might be the reason why C/P plants possessed a higher Na+ efflux but still had more Na+ accumulated in their roots compared with C/C (Fig. 3C). Our previous study also found that some pumpkin genotypes stored a vast amount of Na+ in the stems (Niu et al., 2017) and the genes involved in the Na+ compartmentation process (HKT, NHX) exhibited even higher expression levels in the hypocotyl than in the root (data not published). Using the xylem saps collected from below or above the grafting union, a significant decrease in Na+ concentration has been found in pumpkin-grafted cucumber but nearly no difference in the self-grafted cucumber, suggesting that the grafting union is a barrier for Na+ transport when pumpkin is used as a rootstock (Huang et al., 2013). In the present study, the NMT data are consistent with our previous result for the xylem sap (Fig. 5B, D).
Root-sourced H2O2 signals trigger rapid stomatal closure in the shoot
In recent years, H2O2 has firmly established itself as an important second messenger mediating the broad range of adaptive plant responses (Mittler et al., 2011; Baxter et al., 2014). H2O2 production is highly tissue-specific and possesses a rather complex kinetics. In Cakile maritima, the H2O2 concentration reached a peak within 4 h of salt stress and rapidly declined afterwards, while H2O2 continued to rise in Arabidopsis during the first 72 h after salt treatment (Ellouzi et al., 2011). Xie et al. (2011) also found that mild salt stress causes a rapid and transient accumulation of ROS in Arabidopsis peaking after 1 h followed by a second oxidative burst after about 6 h. These and similar findings have led to the suggestion that H2O2 ‘signatures’ may operate in plant signaling networks (Bose et al., 2014), in addition to well-known cytosolic calcium ‘signatures’ (Dodd et al., 2010). The current work supports this hypothesis and demonstrates that early root-derived H2O2 signals are essential for early stomata closure in grafted C/P combination but is lacking in C/C plants.
In a longer period, accumulation of large amounts of Na+ in leaves of C/C (Fig. 3A) forced the plant to close the stomata to reduce the amount of Na+ delivered from root to shoot via the transpiration flow. In contrast, the pumpkin-grafted plants, C/P, gain advantage in restricting a large proportion of Na+ to roots and hypocotyls and accumulating less Na+ in the leaves (Fig. 3A), making such stomatal closure less essential later on (Fig. 7D, F). DPI-treated C/P impaired the Na+/H+ antiport system located in C/P roots (Fig. 6), which led to an overaccumulation of Na+ in shoots (Fig. 4) and required a more restricted transpiration rate in C/P (Fig. 7D), to protect the photosynthetic system from the ionic stress. However, in the first several hours of salinity, the Na+ in leaves did not reach a toxic level in either of the two combinations (less than 5 mg g−1; Fig. 3A). Thus, a rapid closure of stomata in the early period in C/P plants is unlikely to be driven by the need to restrict Na+ delivery to the shoot but instead may be related to an early signal induced by roots to deal with osmotic stress, reducing water loss and maintaining the plant’s hydration status (see Supplementary Figs S7B and S8).
The obvious question arising from this data is, why are C/P plants able to sense and signal salt stress faster than C/C plants? Given that such signaling was causally related to the root RBOH-dependent H2O2 production, this points to NADPH acting as a tentative sodium sensor, and its more efficient operation (higher sensitivity) in pumpkin roots.
Our current knowledge of how salt stress is sensed by plant tissues is severely limited (Maathuis, 2014; Shabala et al., 2015), and it is highly likely that more than one of the sensory mechanisms may operate in the same cell at the same time, encoding specific information on stress severity, and sharing some common downstream signaling pathway(s). NADPH oxidase has been suggested to be one of these (Shabala et al., 2015). NADPH oxidases are activated by salt stress, at both the transcriptional and the functional level (Xie et al., 2011), and plants lacking functional AtrbohD and AtrbohF genes showed increased hypersensitivity to salinity (Ma et al., 2012), suggested that the NADPH oxidase may also operate as a salt sensor in plants. The model also assumes that NSCCs are located in the immediate proximity of the NADPH oxidase, forming a microdomain in a lipid raft. The onset of the salt stress will lead to a rapid (within seconds) membrane depolarization by 50–80 mV (Shabala et al., 2005; Jayakannan et al., 2015; Chakraborty et al., 2016), resulting in the instantaneous activation of NSCCs and causing a rapid elevation in the cytosolic Ca2+. This elevation will result in a rapid activation of NADPH oxidase and a concurrent increase in ROS accumulation in the apoplastic space. These ROS will further activate NSCCs and amplify stress-induced Ca2+ and ROS transients via self-amplification loops. This self-amplification loop seems to be more efficient in C/P than in C/C grafted plants. Future studies should reveal the molecular mechanisms behind this regulation, as well as interaction of the root RBOH-derived H2O2 signals with other signals propagating between roots and shoots in salt-stressed plants (Baxter et al., 2014; Gilroy et al., 2014; Shabala et al., 2016).
Conclusion
In summary, we have discovered that the root RBOH-dependent H2O2 production operates as an early signal to regulate salt tolerance of grafted cucumber through Na+ exclusion and stomatal closure. This mechanism can alleviate the salt-induced damage in the rootstock-grafted cucumber plants.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Effects of various NaCl concentrations on the growth of the pumpkin-grafted cucumber (C/P) and a self-grafted cucumber (C/C).
Fig. S2. Effects of various NaCl concentrations on H2O2 accumulation in roots of two grafted combinations, namely the pumpkin-grafted cucumber (C/P) and a self-grafted cucumber (C/C).
Fig. S3. The sampling positions for ion fluxes test by the non-invasive micro-test technology (NMT).
Fig. S4. A four-minute continuous flux recording was conducted using NMT from roots of plants from two grafted combinations, namely the pumpkin-grafted cucumber (C/P) and a self-grafted cucumber (C/C).
Fig. S5. Effects of NaCl and DPI on the NADPH oxidase-based H2O2 generation and Na+/H+ antiport system in roots of the pumpkin-grafted cucumber (C/P) and a self-grafted cucumber (C/C) after 24 h of treatment.
Fig. S6. Effect of 75 mM NaCl treatment on the time dependence of SOS1 (A) and PMA (B) transcriptions in roots of the pumpkin-grafted cucumber (C/P) and a self-grafted cucumber (C/C).
Fig. S7. Effect of grafting on kinetics of plant wilting and relative water content for the pumpkin-grafted cucumber (C/P) and a self-grafted cucumber (C/C) treated with 75 mM NaCl.
Fig. S8. Effect of NaCl and DPI on the stomatal aperture in the detached abaxial epidermal strips from the pumpkin-grafted cucumber (C/P) and a self-grafted cucumber (C/C) after 3 h of treatment.
Supplementary Material
Acknowledgements
This work was supported by National Natural Science Foundation of China (31572168, 31372110, 31772357) and the International Science and Technology Cooperation Program of China (2015DFG32310) to ZL.B., China Scholarship Council (CSC) (Grant number 201606765073), the Youth Chenguang Project of Science and Technology of Hubei Province of China to Y.H. and the Australian Research Council and Grain Research and Development Corporation to S.S.
References
- Albacete A, Martínez-Andújar C, Ghanem ME, et al. 2009. Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato. Plant, Cell & Environment 32, 928–938. [DOI] [PubMed] [Google Scholar]
- Baxter A, Mittler R, Suzuki N. 2014. ROS as key players in plant stress signalling. Journal of Experimental Botany 65, 1229–1240. [DOI] [PubMed] [Google Scholar]
- Bose J, Rodrigo-Moreno A, Shabala S. 2014. ROS homeostasis in halophytes in the context of salinity stress tolerance. Journal of Experimental Botany 65, 1241–1257. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Bose J, Shabala L, Shabala S. 2016. Difference in root K+ retention ability and reduced sensitivity of K+-permeable channels to reactive oxygen species confer differential salt tolerance in three Brassica species. Journal of Experimental Botany 67, 4611–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Zhu JK, Bressan RA, Hasegawa PM, Shi H. 2008. Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. The Plant Journal 53, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuin TA, Bose J, Stefano G, Jha D, Tester M, Mancuso S, Shabala S. 2011. Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: in planta quantification methods. Plant, Cell & Environment 34, 947–961. [DOI] [PubMed] [Google Scholar]
- Danquah A, De ZA, Colcombet J, Hirt H. 2014. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnology Advances 32, 40–52. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJ. 2007. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytologist 175, 387–404. [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Harrison J, Hooley R, Neill SJ. 2005. A Role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiology 137, 831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. 2010. The language of calcium signaling. Annual Review of Plant Biology 61, 593–620. [DOI] [PubMed] [Google Scholar]
- Edelstein M, Plaut Z, Ben-Hur M. 2011. Sodium and chloride exclusion and retention by non-grafted and grafted melon and Cucurbita plants. Journal of Experimental Botany 62, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouzi H, Hamed KB, Cela J, Munné-Bosch S, Abdelly C. 2011. Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiologia Plantarum 142, 128–143. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R. 2014. A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends in Plant Science 19, 623–630. [DOI] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. 2003. Plant cellular and molecular responses to high salinity. Annual Review of Plant Biology 51, 463–499. [DOI] [PubMed] [Google Scholar]
- He Y, Zhu ZJ, Yang J, Ni XL, Zhu B. 2009. Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity. Environmental and Experimental Botany 66, 270–278. [Google Scholar]
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts. Archives of Biochemistry and Biophysics 125, 189–198. [DOI] [PubMed] [Google Scholar]
- Hossain MS, Dietz KJ. 2016. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Frontiers in Plant Science 7, 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Bie ZL, He SP, Hua B, Zhen A, Liu ZX. 2010. Improving cucumber tolerance to major nutrients induced salinity by grafting onto Cucurbita ficifolia. Environmental and Experimental Botany 69, 32–38. [Google Scholar]
- Huang Y, Bie ZL, Liu PY, et al. 2013. Reciprocal grafting between cucumber and pumpkin demonstrates the roles of the rootstock in the determination of cucumber salt tolerance and sodium accumulation. Scientia Horticulturae 149, 47–54. [Google Scholar]
- Jayakannan M, Bose J, Babourina O, Shabala S, Massart A, Poschenrieder C, Rengel Z. 2015. The NPR1-dependent salicylic acid signalling pathway is pivotal for enhanced salt and oxidative stress tolerance in Arabidopsis. Journal of Experimental Botany 66, 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CF, Belfield EJ, Mithani A, Visscher A, Ragoussis J, Mott R, Smith JAC, Harberd NP. 2013. ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. The EMBO Journal 32, 4359–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kłobus G, Janicka-Russak M. 2004. Modulation by cytosolic components of proton pump activities in plasma membrane and tonoplast from Cucumis sativus roots during salt stress. Physiologia Plantarum 121, 84–92. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Shaff JE, Kühtreiber WM, Jaffe LF, Lucas WJ. 1992. Use of an extracellular, ion-selective, vibrating microelectrode system for the quantification of K+, H+, and Ca2+ fluxes in maize roots and maize suspension cells. Planta 188, 601–610. [DOI] [PubMed] [Google Scholar]
- Lee JM. 1994. Cultivation of grafted vegetables I. Current status, grafting methods, and benefits. Hortscience 29, 235–239. [Google Scholar]
- Lee JM, Kubota C, Tsao SJ, Bie ZL, Echevarria PH, Morra L, Oda M. 2010. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Scientia Horticulturae 127, 93–105. [Google Scholar]
- Lei B, Huang Y, Sun JY, Xie JJ, Niu ML, Liu ZX, Fan ML, Bie ZL. 2014. Scanning ion-selective electrode technique and X-ray microanalysis provide direct evidence of contrasting Na+ transport ability from root to shoot in salt-sensitive cucumber and salt-tolerant pumpkin under NaCl stress. Physiologia Plantarum 152, 738–748. [DOI] [PubMed] [Google Scholar]
- Leshem Y, Seri LA. 2007. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. The Plant Journal 51, 185–197. [DOI] [PubMed] [Google Scholar]
- Li H, Liu SS, Yi CY, Wang F, Zhou J, Xia XJ, Shi K, Zhou YH, Yu JQ. 2014. Hydrogen peroxide mediates abscisic acid-induced HSP70 accumulation and heat tolerance in grafted cucumber plants. Plant, Cell & Environment 37, 2768–2780. [DOI] [PubMed] [Google Scholar]
- Li M, Ahammed GJ, Li C, Bao X, Yu J, Huang C, Yin H, Zhou J. 2016. Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Frontiers in Plant Science 7, 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Pan XX, Berry JO, et al. 2015. OsSEC24, a functional SEC24-like protein in rice, improves tolerance to iron deficiency and high pH by enhancing H+ secretion mediated by PM-H+-ATPase. Plant Science 233, 61–71. [DOI] [PubMed] [Google Scholar]
- Lino B, Baizabal-Aguirre VM, González de la Vara LE. 1998. The plasma-membrane H+-ATPase from beet root is inhibited by a calcium-dependent phosphorylation. Planta 204, 352–359. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F. 2012. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na⁺/K⁺ homeostasis in Arabidopsis under salt stress. Journal of Experimental Botany 63, 305–317. [DOI] [PubMed] [Google Scholar]
- Maathuis FJ. 2014. Sodium in plants: perception, signalling, and regulation of sodium fluxes. Journal of Experimental Botany 65, 849–858. [DOI] [PubMed] [Google Scholar]
- Martínez-Atienza J, Quintero FJ. 2007. Conservation of the salt overly sensitive pathway in rice. Plant Physiology 143, 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Blumwald E. 2015. The roles of ROS and ABA in systemic acquired acclimation. The Plant Cell 27, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van BF. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9, 490–498. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. 2011. ROS signaling: the new wave?Trends in Plant Science 16, 300–309. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681. [DOI] [PubMed] [Google Scholar]
- Niu L, Liao W. 2016. Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Frontiers in Plant Science 7, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu ML, Xie JJ, Sun JY, Huang Y, Kong QS, Nawaz MA, Bie ZL. 2017. A shoot based Na+ tolerance mechanism observed in pumpkin—An important consideration for screening salt tolerant rootstocks. Scientia Horticulturae 218, 38–47. [Google Scholar]
- Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. 2002. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proceedings of the National Academy of Sciences, USA 99, 8436–8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy P. 2010. Soil processes affecting crop production in salt-affected soils. Functional Plant Biology 37, 613–620. [Google Scholar]
- Rouphael Y, Cardarelli M, Rea E, Colla G. 2012. Improving melon and cucumber photosynthetic activity, mineral composition, and growth performance under salinity stress by grafting onto Cucurbita hybrid rootstocks. Photosynthetica 50, 180–188. [Google Scholar]
- Shabala L, Cuin TA, Newman IA, Shabala S. 2005. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 222, 1041–1050. [DOI] [PubMed] [Google Scholar]
- Shabala S, White RG, Djordjevic MA, Ruan YL, Mathesius U. 2016. Root-to-shoot signalling: integration of diverse molecules, pathways and functions. Functional Plant Biology 43, 87–104. [DOI] [PubMed] [Google Scholar]
- Shabala S, Wu H, Bose J. 2015. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Science 241, 109–119. [DOI] [PubMed] [Google Scholar]
- Shi H, Ishitani M, Kim C, Zhu JK. 2000. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences, USA 97, 6896–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Quintero FJ, Pardo JM, Zhu JK. 2002. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. The Plant Cell 14, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Chen S, Dai S, et al. 2009. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiology 149, 1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Wang MJ, Ding MQ, et al. 2010. H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant, Cell & Environment 33, 943–958. [DOI] [PubMed] [Google Scholar]
- Wang XM, Hou C, Liu J, He WL, Nan WB, Gong HL, Bi YR. 2013. Hydrogen peroxide is involved in the regulation of rice (Oryza sativa L.) tolerance to salt stress. Acta Physiologiae Plantarum 35, 891–900. [Google Scholar]
- Weatherley PE. 1950. Studies in water relations of cotton plants. I. The field measurement of water deficit in leaves. New Phytologist 49, 81–87. [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W. 1997. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. The EMBO Journal 16, 4806–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Shannon MC. 1995. Salt-induced Na+/H+ antiport in root plasma membrane of a glycophytic and halophytic species of tomato. Plant Science 107, 147–157. [Google Scholar]
- Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ. 2009. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiology 150, 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YJ, Xu S, Han B, et al. 2011. Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. The Plant Journal 66, 280–292. [DOI] [PubMed] [Google Scholar]
- Yi C, Yao K, Cai S, et al. 2015. High atmospheric carbon dioxide-dependent alleviation of salt stress is linked to RESPIRATORY BURST OXIDASE 1 (RBOH1)-dependent H2O2 production in tomato (Solanum lycopersicum). Journal of Experimental Botany 66, 7391–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang Y, Yang Y, Wu H, Wang D, Liu J. 2007. Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant, Cell & Environment 30, 775–785. [DOI] [PubMed] [Google Scholar]
- Zhen A, Bie ZL, Huang Y, Liu ZX, Lei B. 2011. Effects of salt-tolerant rootstock grafting on ultrastructure, photosynthetic capacity, and H2O2-scavenging system in chloroplasts of cucumber seedlings under NaCl stress. Acta Physiologiae Plantarum 33, 2311–2319. [Google Scholar]
- Zhou J, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ. 2014. RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. Journal of Experimental Botany 65, 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WJ, Leul M. 1998. Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. Plant Growth Regulation 26, 41–47. [Google Scholar]
- Zhu J, Bie ZL, Li YN. 2008. Physiological and growth responses of two different salt-sensitive cucumber cultivars to NaCl stress. Soil Science and Plant Nutrition 54, 400–407. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.