Abstract
Background & Aims
Gastric Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) cells exert important functions during injury and homeostasis. Bone morphogenetic protein (BMP) signaling regulates gastric inflammation and epithelial homeostasis. We investigated if BMP signaling controls the fate of Lgr5+ve cells during inflammation.
Methods
The H+/K+-adenosine triphosphatase β-subunit promoter was used to express the BMP inhibitor noggin (Nog) in the stomach (H+/K+-Nog mice). Inhibition of BMP signaling in Lgr5 cells was achieved by crossing Lgr5-EGFP-ires-CreERT2 (Lgr5-Cre) mice to mice with floxed alleles of BMP receptor 1A (Lgr5-Cre;Bmpr1aflox/flox mice). Lgr5/GFP+ve cells were isolated using flow cytometry. Lineage tracing studies were conducted by crossing Lgr5-Cre mice to mice that express Nog and tdTomato (Lgr5-Cre;H+/K+-Nog;Rosa26-tdTom). Infection with Helicobacter felis was used to induce inflammation. Morphology of the mucosa was analyzed by H&E staining. Distribution of H+/K+-adenosine triphosphatase-, IF-, Ki67-, CD44-, CD44v9-, and bromodeoxyuridine-positive cells was analyzed by immunostaining. Expression of neck and pit cell mucins was determined by staining with the lectins Griffonia (Bandeiraea) simplicifolia lectin II and Ulex europaeus agglutinin 1, respectively. Id1, Bmpr1a, Lgr5, c-Myc, and Cd44 messenger RNAs were measured by quantitative reverse-transcription polymerase chain reaction.
Results
Lgr5-Cre;Bmpr1aflox/flox mice showed diminished expression of Bmpr1a in Lgr5/GFP+ve cells. Infection of Lgr5-Cre;Bmpr1aflox/flox mice with H felis led to enhanced inflammation, increased cell proliferation, parietal cell loss, and to the development of metaplasia and dysplasia. Infected Lgr5-Cre;H+/K+-Nog;Rosa26-tdTom mice, but not control mice, showed the presence of tomato+ve glands lining the lesser curvature that stained positively with Griffonia (Bandeiraea) simplicifolia lectin II and Ulex europaeus agglutinin 1, and with anti-IF, -CD44, -CD44v9, and -bromodeoxyuridine antibodies.
Conclusions
Inflammation and inhibition of BMP signaling activate Lgr5+ve cells, which give rise to metaplastic, dysplastic, proliferating lineages that express markers of mucus neck and zymogenic cell differentiation.
Keywords: Chief Cells, Dysplasia, Differentiation, Metaplasia
Abbreviations used in this paper: ATPase, adenosine triphosphatase; BMP, bone morphogenetic protein; BrdU, bromodeoxyuridine; EGFP, enhanced green fluorescent protein; ERK, extracellular signal–regulated kinase; GFP, green fluorescent protein; GSII, Griffonia (Bandeiraea) simplicifolia lectin II; HBSS, Hank's balanced salt solution; H/K-nog, H/K-noggin; IF, intrinsic factor; mRNA, messenger RNA; QRT-PCR, quantitative reverse-transcription polymerase chain reaction; SPEM, spasmolytic polypeptide expressing metaplasia; TFF2, Trefoil factor 2
Summary.
This study reports the novel observation that inflammation and inhibition of bone morphogenetic protein signaling activate Leucine-rich repeat-containing G-protein-coupled receptor 5 +ve cells located on the lesser curvature of the oxyntic mucosa that give rise to spasmolytic polypeptide expressing metaplasia as well as dysplastic, proliferating lineages. These findings offer new insights into the mechanisms that lead to gastric metaplasia and dysplasia.
Chronic inflammation has been recognized as an important causative factor for the development of gastric metaplasia, dysplasia, and neoplasia. Numerous experimental models have confirmed that exposure of the gastric epithelium to chronic inflammatory stimuli such as infection with Helicobacter organisms can lead to significant aberrations in gastric epithelial homeostasis.1, 2, 3, 4, 5
The mechanisms involved in the pathogenesis of metaplasia, dysplasia, and neoplasia in the context of chronic inflammation have been only partially characterized. One current hypothesis is that chronic inflammation and mucosal injury can cause aberrations in the normal biological functions of gastric epithelial cells, leading to the development of metaplastic and dysplastic changes of the gastric mucosa and, ultimately, to neoplasias.1, 2, 3, 4, 5, 6 Indeed, both intestinal metaplasia and spasmolytic polypeptide expressing metaplasia (SPEM), which is characterized by the aberrant expression of Trefoil factor 2 (TFF2), and of mucins that bind the lectin Griffonia (Bandeiraea) simplicifolia lectin II (GSII) at the base of glands of the oxyntic mucosa, have been associated with inflammation-induced gastric neoplasms.2, 5, 7, 8, 9 It has been suggested that SPEM might derive from reprograming of zymogenic cells during situations of inflammation and injury such as those triggered by infection with Helicobacters.10
Several studies have identified, on the basis of expression of molecular markers, such as the Wnt target gene Lgr5,11 populations of gastric epithelial cells that can self-renew and that can show multilineage differentiation capacity. In addition to Lgr5, other potential markers for gastric stem/progenitor cells have been investigated recently, including villin, Prom1/Cd133, Cd44, Dckl1/ Dcamkl1, Troy, Mist-1, Sox-2, Tff2, and Runx1.11, 12, 13, 14, 15, 16, 17 It has been suggested that genetic and epigenetic alterations of these cells might lead to their transformation and to the initiation of tumor growth.14
Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) cells, in particular, have been shown to play an important role in gastrointestinal tissue homeostasis.11, 13, 14 In the stomach, cells with Lgr5 transcriptional activity initially were identified at the base of antral glands and, to a lesser degree, along the lesser curvature of the oxyntic mucosa, an area known to be one of the most common locations for the development of gastric carcinomas.11, 18, 19 Recent studies, however, which have taken advantage of a different mouse model, have shown that Lgr5 cells are widely distributed in glands of the corpus where they represent a subpopulation of chief cells.20 It also has been suggested that in the corpus these cells do not show stem cell characteristics during homeostasis but that after injury they acquire stem cell functions to promote epithelial repair.20
An increased number of Lgr5-expressing cells has been detected in patients with gastritis and intestinal metaplasia and in gastric carcinomas.20, 21, 22 In support of these findings, a recent study showed that Lgr5-expressing chief cells can be a major cell-of origin of gastric neoplasms.20 Reports also have shown that inflammatory stimuli such as Helicobacter organisms can adhere to Lgr5-expressing cells, leading to their proliferation and activation.23
The bone morphogenetic proteins (BMPs) are regulatory peptides that exert important effects on the growth and differentiation of gastrointestinal tissues. The actions of these proteins can be blocked by secreted inhibitory molecules, such as noggin, gremlin, and chordin, which are expressed in vivo to modulate the actions of the BMPs.24, 25 It has been shown that loss of BMP signaling in the stomach leads to perturbations of the normal homeostatic mechanisms of the gastric mucosa, leading to the development of metaplasia, dysplasia, and neoplasia.25, 26, 27 In support of these observations studies have shown that BMP receptors such as BMPR1A and the BMP signal transducing proteins Smad1, 5, and 8 are widely expressed in the glandular stomach.26, 28
In a series of published studies26 we showed that transgenic expression in the corpus of the mouse stomach of the BMP inhibitor noggin leads to the development of SPEM, parietal cell loss, and to increased cell proliferation. We also reported that expression of noggin enhances H felis– and Helicobacter pylori–induced inflammation and gastric epithelial cell proliferation, leading to the accelerated development of dysplasia, and to increased expression and activation of the oncogenic proteins activation-induced cytidine deaminase and signal transducer and activator of transcription (STAT)3.27
The mechanisms that regulate the number and function of Lgr5+ve cells during gastric inflammation, and those involved in the development of gastric metaplasia and dysplasia, have been only partially characterized. Accordingly, we sought to investigate the role of BMP signaling in the regulation of Lgr5 cell homeostasis during gastric inflammation. In particular, we tested the hypothesis that inflammation and inhibition/loss of BMP signaling induce the activation of Lgr5+ve cells, located in the oxyntic mucosa of the lesser curvature, that lead to the development of metaplastic and dysplastic epithelial cell lineages.
Materials and Methods
Mice
The H/K-noggin (H/K-nog) transgenic mice were generated in our laboratory and they were described previously.26 Pathogen-free C57BL/6 mice, Lgr5-enhanced green fluorescent protein (EGFP)-ires-CreERT2 (Lgr5-Cre) mice, in which the expression of both Cre and green fluorescent protein (GFP) is driven by endogenous Lgr5 regulatory sequences,11 and Rosa26-tdTom (Rosa26-Tom) mice aged 4–10 weeks were purchased from Jackson Laboratory (Bar Harbor, ME). Bmpr1aflox/flox29 mice were received from the laboratory of Dr Yuji Mishina (University of Michigan). Mouse genotyping was described elsewhere.26
Specific inhibition of BMP signaling in Lgr5 cells was achieved by crossing Lgr5-Cre mice to Bmpr1aflox-flox mice to generate Lgr5-Cre;Bmpr1aflox-flox mice. To conduct lineage tracing in the presence of Noggin, H+/K+-nog mice were crossed to Rosa26-Tom reporter mice to generate H+/K+-nog;Rosa26-Tom mice. Lgr5-Cre mice then were crossed to both H+/K+-Nog;Rosa26-Tom and Rosa26-Tom mice to generate Lgr5-Cre;H+/K+-Nog;Rosa26-Tom and Lgr5-Cre;Rosa26-Tom mice. Cre was activated by 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight). All mice, including Lgr5-Cre, Lgr5-Cre;Bmpr1aflox-flox, Lgr5-Cre;H+/K+-Nog;Rosa26-Tom, and Lgr5-Cre;Rosa26-Tom mice received tamoxifen injections. The mice were maintained on a C57BL/6 background. In some experiments, mice were injected with 200 μL of a 10 mg/mL solution of bromodeoxyuridine (BrdU, BD Biosciences, San Jose, CA) 2 hours before tissue collection. In all experiments, animals were fasted overnight with free access to water before tissue collection. Mice were housed under specific pathogen-free conditions in automated watered and ventilated cages on a 12-hour light/dark cycle in the animal maintenance facility at the University of Michigan. All animal experiments were approved by the University of Michigan Animal Care and Use Committee.
H felis Culture and Infection
Two- to 3-month-old Lgr5-Cre mice, Lgr5-Cre;Bmpr1aflox/floxmice, Lgr5-Cre;Rosa26-Tom mice, and Lgr5-Cre;H+/K+-Nog;Rosa26-Tom mice were inoculated orally with overnight broth cultures of approximately 108 cfu of H felis organisms.27, 30 Control animals were inoculated with broth without organisms. Both infected and noninfected animals were killed 12 weeks after inoculation. Similar numbers of male and female mice were used for the studies.
RNA Isolation and Quantitative Reverse-Transcription Polymerase Chain Reaction Analysis
RNA was isolated from full-thickness samples of the corpus of mice using TRIzol reagent (Invitrogen, Carlsbad, CA), followed by DNase treatment and purification with the RNeasy Mini kit (Qiagen, Valencia, CA).26 Quantitative reverse-transcription polymerase chain reaction (QRT-PCR) was performed according to previously published methods26 using primer sequences and protocols that were obtained from commercially available sources. Gapdh, Cd44, and Bmpr1a primers were obtained from Integrated DNA Technologies (Coralville, IA). Bmpr1a QRT-PCR primers span exons 2–3 of the gene. The sequences are as follows: primer 5’-CTTGGCAATGACTTTCACCTG-3’ binds in exon 3 (284-304 in Genebank NM-009758.4), primer 5’-TCGCTTTGATACTGTCTTGGAA-3’ binds in exon 2 (170-191 in Genebank NM-009758.4). The deletion in the Bmpr1a floxed mice from the laboratory of Y. Mishina deletes a portion of exon 2. Accordingly, these primers will not amplify a product in the recombined mice. The Id1, c-Myc, and Lgr5 primers were obtained from Qiagen.
Gastric Epithelial Cell Isolation and Flow Cytometry
Fluorescence-activated cell sorting isolation of Lgr5/GFP+ cells from Lgr5-Cre and Lgr5-Cre;Bmpr1aflox/flox mice was performed as previously described,31 with modifications. Both Lgr5-Cre and Lgr5-Cre;Bmpr1aflox/flox mice were injected with 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight). The mice were killed 15 days after tamoxifen injection. Samples of gastric mucosa were placed in Mg2+-, Ca2+-free Hank’s balanced salt solution (HBSS), 5% fetal bovine serum, 2 mmol/L EDTA, shaken for 20 minutes at 37°C, and filtered through a 100-μm cell strainer. After filtration the samples were pelleted and resuspended in HBSS containing 0.1 U/mL Liberase (Roche, Basel, Switzerland) and 40 μg/mL DNase I (Sigma, St. Louis, MO). The solutions were shaken for 10 minutes at 37°C, filtered through a 100-μm cell strainer, and spun at 1500 rpm for 5 minutes. After 1 wash with HBSS, the pellets were resuspended in HBSS containing aqua viability dye (Live/Dead Fixable Aqua dead cell stain kit; Invitrogen) according to the manufacturer’s instructions. After 1 wash the cells were resuspended in HBSS containing 1% fetal bovine serum, filtered through a 50-μm filter (Partec/Sysmex, Lincolnshire, IL), and used for analysis. GFP+ve cells were isolated using fluorescence-activated cell sorting. Analyses were performed using an iCyte Synergy (Sony Biotechnology, San Jose, CA) with WINLIST V.8 software (VertySoftware House, available: http://www.vsh.com). The laser emission wavelengths and filter used were 488-nm laser with a 525/50-nm filter. Aqua viability dye was excited with a 405-nm laser and detected with a 577/25-nm filter. The sorted cells were collected into sterile 1.5-mL tubes containing 300 μL RNA lysis buffer and used for RNA extraction (Zymo Research, Irvine, CA).
Histochemical Analysis and Image Acquisition
Tissue fixation, generation of paraffin sections, H&E staining, and immunostaining were performed according to previously published methods.26, 27 Sections were stained with the following primary antibodies: anti-H+/K+-adenosine triphosphatase (ATPase) α-subunit (1:500, catalog number: DO31-3; Medical and Biological Laboratories, Nagoya, Japan), anti-Ki67 (1:500, catalog number: MA5-14520; Thermo Scientific, Waltham, MA), anti-BrdU (1:200, catalog number: MO744; Dako Corporation, Carpinteria, CA), anti-intrinsic factor (anti-IF) (1:1000; gift from Dr Jason Mills, Washington University, St. Louis, MO), anti-red fluorescent protein (1:200, catalog number: 600-401-379; Rockland/Biomol, Hamburg, Germany), anti-TFF2 (1:200, catalog number: ab49536; Abcam, Cambridge, UK), anti-CD44 (1:30, catalog number: 550538; BD Biosciences), anti-CD44v9 (1:200, catalog number CAC-LKG-M002; Cosmo Bio, Carlsbad, CA), and with Alexa 488–conjugated anti-GFP (1:200, catalog number A21311; Invitrogen). In some experiments the slides were stained for 1.5 hours at 37°C with Alexa 488–conjugated GSII (1:1000, catalog number: FL-1211; Vector Laboratories, Burlingame, CA) and fluorescein isothiocyanate–conjugated Ulex europaeus agglutinin 1 lectin (1:200, catalog number: FL-1061; Vector Laboratories). For immunohistochemistry with detection with diaminobenzidine as substrate, slides were rinsed and subsequently treated with biotin-conjugated secondary antibodies (1:200; Vector Laboratories) for 30 minutes at room temperature. To visualize biotin staining, the Vectastain Elite ABC kit was used (Vector Laboratories). For immunofluorescence analysis, Alexa 555–donkey anti-rabbit (1:500), Alexa 594–donkey anti-rat (1:500), and Alexa 488–donkey anti-rat and anti-mouse (1:500) secondary antibodies were used (Molecular Probes, Eugene, OR). ProLong Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen) was used for nuclear counterstain and mounting medium. Control experiments were performed by incubating the slides in the presence of the secondary antibodies without the primary antibodies (data not shown). Visualization of slides was performed with a Nikon (Tokyo, Japan) Eclipse E 800 fluorescence microscope for all other studies.
For histologic scoring of gastritis, sections from the stomachs of 3–4 separate animals from each of the 4 treatment groups were examined and scored largely as previously described.32 Slides containing sections of well-oriented gastric glandular mucosa were examined in a blinded fashion. Only fields that contained full-thickness gastric mucosa that was oriented perpendicularly were analyzed. All well-oriented fields were scored. Each field was scored separately for the presence or absence and severity of neutrophilic cell infiltration, gastritis, and epithelial metaplasia/dysplasia.32 The severity of the changes was graded using a scale from 1 (mild) to 3 (severe), as follows. For neutrophilic infiltration: a score of 1 was defined as 2–3 clusters of neutrophils in the lamina propria, a score of 2 was defined as many clusters of neutrophils, and a score of 3 was defined as neutrophils present throughout the lamina propria. For gastritis: a score of 1 was defined as focal displacement of glands by inflammatory infiltrate, a score of 2 was defined as inflammatory infiltrate displacing less than 50% of glands in the field, and a score of 3 was defined as more than 50% of glands displaced by inflammatory infiltrate. For dysplasia: a score of 1 (epithelial metaplasia) was defined as loss of parietal cells with replacement by mucus-type cells or SPEM; a score of 2 (cellular dysplasia) was defined as anisocytosis/karyosis, loss of polarity, abnormal shape, and/or multiple epithelial layers; and a score of 3 (glandular dysplasia) was defined as dilation, irregularity, and disorganization of glands, with cellular dysplasia. Each field was scored, and the final score was calculated and expressed as a weighted average of all fields with that lesion as follows: sum of the number of fields for each score × the score, divided by the total number of fields.
The number of GFP-, H+K+-ATPase α-subunit– and Ki67-positive cells was determined by counting the number of stained cells in at least 3–4 fields in slides derived from 3 to 6 animals from each treatment group, using the Fiji imaging program (ImageJ, National Institutes of Health, Bethesda, MD).33
Data Analysis
Data are expressed as means ± SE. Statistical analysis was performed using the Student t test. P values less than .05 were considered significant.
Results
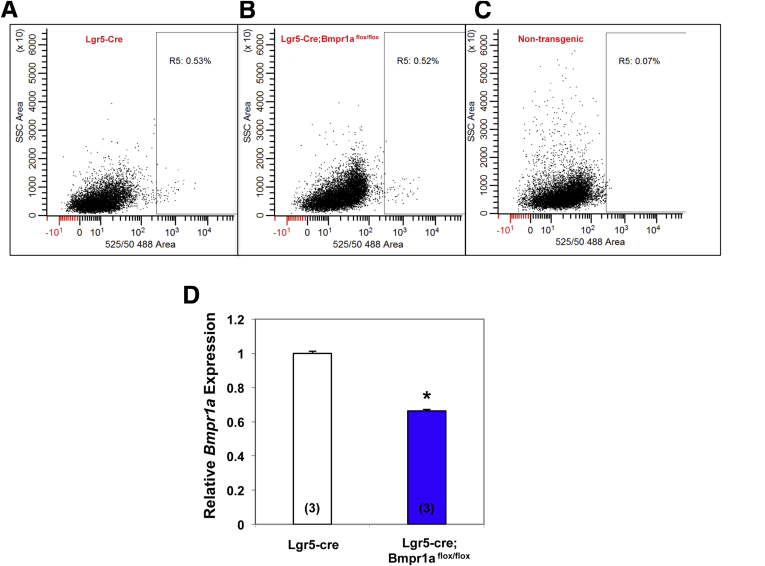
We sought to test the hypothesis that BMP signaling regulates the census and fate of Lgr5 cells in the oxyntic mucosa. Accordingly, we deleted BMP receptor 1A (Bmpr1a) in cells with Lgr5 transcriptional activity. For these studies we took advantage of Lgr5-Cre mice, which express Cre and EGFP in cells with Lgr5 transcriptional activity located only in glands of the antrum and of the lesser curvature, but not in those of the greater curvature.11, 18 To show diminished expression of Bmpr1a in Lgr5 cells from Lgr5-Cre;Bmpr1aflox-flox mice, we isolated GFP+ve cells from the gastric mucosa of both Lgr5-Cre and Lgr5-Cre;Bmpr1aflox-flox mice using flow cytometry. As shown in Figure 1, GFP+ve cells from Lgr5-Cre;Bmpr1aflox-flox mice showed diminished expression of Bmpr1a messenger RNA (mRNA) when compared with cells isolated from control Lgr5-Cre mice.
Figure 1.
Deletion of Bmpr1a in Lgr5 cells. GFP+ve cells from 1- to 2-month-old Lgr5-EGFP-ires-CreERT2 mice (Lgr5-Cre mice) and Lgr5-EGFP-ires-CreERT2;Bmpr1aflox-flox mice (Lgr5-Cre;Bmpr1aflox-flox mice) were isolated by flow cytometry. Mice were treated with 1 intraperitoneal. injection of tamoxifen (0.1 mg/g body weight) and killed 15 days after tamoxifen. Flow cytometry plots of cells isolated from (A) Lgr5-Cre mice and (B) Lgr5-Cre;Bmpr1aflox-flox mice. (C) Flow cytometry plot of cells isolated from nontransgenic negative control mice. (D) Bmpr1a mRNA signals in cells from Lgr5-Cre;Bmpr1aflox-flox mice were compared with those detected in cells from control mice using QRT-PCR. Values are shown as means ± SE. Numbers in parenthesis indicate the number of animals used in each group. *P < .05.
The finding that inhibition of Bmpr1a mRNA expression in Lgr5-Cre;Bmpr1aflox-flox mice was not complete likely reflects variable levels of efficiency of Cre-mediated recombination in Lgr5 cells. It is conceivable that not all Cre-expressing Lgr5 cells could effectively and equally delete the target (Bmpr1a) after tamoxifen activation.
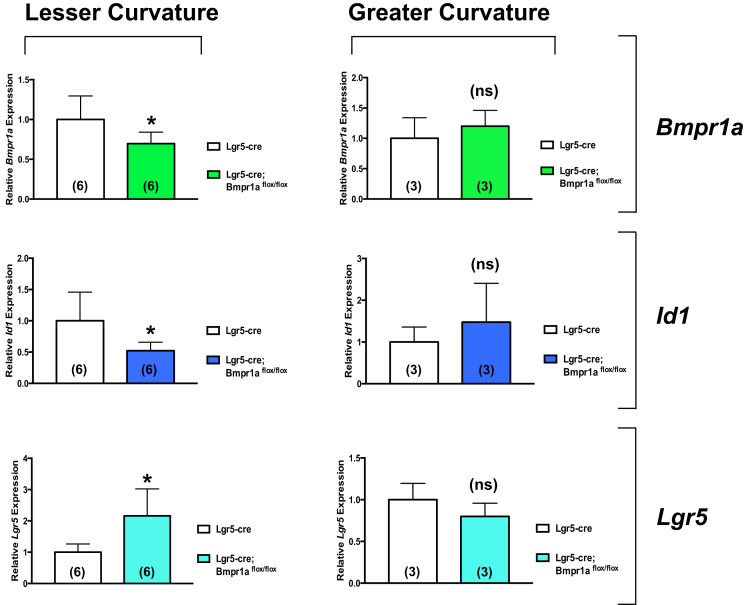
The specificity of this effect and the presence of effective inhibition of BMP signaling were confirmed by QRT-PCR assays that showed a significant decrease in the expression of Bmpr1a and Id1, a well-characterized BMP-responsive gene,34, 35, 36 together with an increase in that of Lgr5 in samples obtained from the mucosa of the lesser, but not the greater curvature of Lgr5-Cre;Bmpr1aflox-flox mice (Figure 2).
Figure 2.
Expression of Bmpr1a, Id1, and Lgr5 in the lesser and greater curvatures of Lgr5-Cre and Lgr5-Cre;Bmpr1aflox-floxmice. One- to 2-month-old Lgr5-Cre mice and Lgr5-Cre;Bmpr1aflox-flox mice were treated with 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight) and killed 5 months after tamoxifen. Bmpr1a, Id1, and Lgr5 mRNA signals in the lesser and greater curvatures of Lgr5-Cre mice were compared with those detected in Lgr5-Cre;Bmpr1aflox-flox mice using QRT-PCR. Values are shown as means ± SE. Numbers in parenthesis indicate the number of animals used in each group. *P < .05.
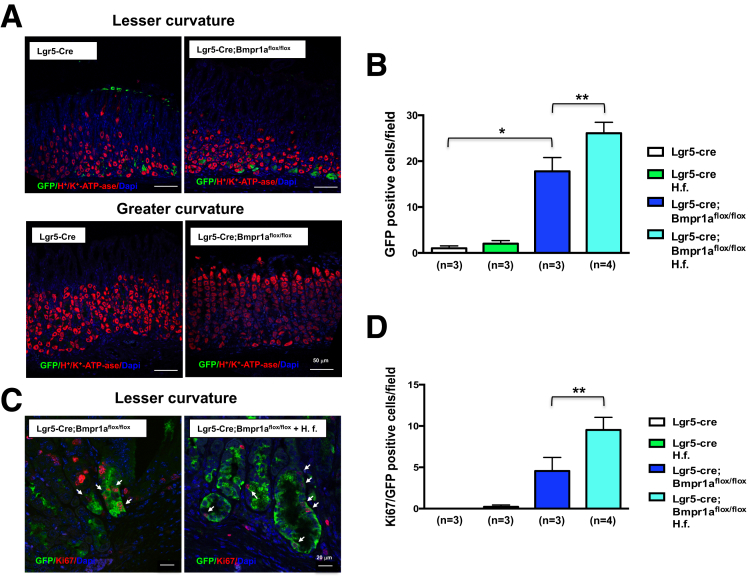
We examined the distribution of cells with Lgr5 transcriptional activity in the oxyntic mucosa of the lesser and greater curvatures of Lgr5-Cre mice.11 Staining of sections of these areas of the stomach with anti-GFP and anti–H+K+-ATPase antibodies showed, in agreement with previously published reports,11, 18 that GFP+ve cells are located in the mucosa of the lesser but not the greater curvature of Lgr5-Cre;Bmpr1aflox-flox mice (Figure 3A).
Figure 3.
Regulation of cells with Lgr5 transcriptional activity by BMP signaling. One- to 2-month-old Lgr5-Cre mice and Lgr5-Cre;Bmpr1aflox-flox mice were treated with 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight) and inoculated with H felis for 2 months after tamoxifen. Animals were analyzed 3 months after inoculation and 5 months after tamoxifen injection. (A) Paraffin sections of the lesser and greater curvatures of Lgr5-Cre and Lgr5-Cre;Bmpr1aflox-flox mice were stained with Alexa 488–conjugated anti-GFP antibodies (green) together with an anti-H+,K+-ATPase α-subunit primary antibody and an Alexa 555–conjugated secondary antibody (red). Scalebar: 50 μm. (C) Paraffin sections of the lesser curvature of Lgr5-Cre and Lgr5-Cre;Bmpr1aflox-flox mice were stained with Alexa 488–conjugated anti-GFP antibodies (green) together with an anti-Ki67 antibody and an Alexa 555–conjugated secondary antibody (red). Scale bar: 20 μm. Arrows point to GFP/Ki67-positive cells. Bars represent the number of (B) GFP- and of (D) GFP/Ki67-positive cells that were detected in the mucosa of the lesser curvature of Lgr5-Cre mice and of Lgr5-Cre;Bmpr1aflox-flox mice in the presence and absence of H felis (H. f.). Values are shown as means ± SE. Numbers in parenthesis indicate the number of animals used in each group. *P < .05 vs Lgr5-Cre mice. **P < .05 vs Lgr5-Cre;Bmpr1aflox-flox mice. DAPI, 4'6-diamidino-2-phenylindole.
To investigate the role of BMP signaling in the regulation of Lgr5 cells during inflammation, we infected both Lgr5-Cre and Lgr5-Cre;Bmpr1aflox-flox mice with H felis, which is known to induce an inflammatory response in the gastric mucosa of mice.37 The mice were studied 3 months after inoculation, and 5 months after tamoxifen injection. First, we examined if inhibition of BMP signaling in Lgr5 cells and the presence of H felis affect the number of cells with Lgr5 transcriptional activity. Accordingly, we counted the number of GFP+ve cells in sections obtained from the lesser curvature of both Lgr5-Cre and Lgr5-Cre;Bmpr1aflox-flox mice in the presence and absence of H felis. As shown in Figure 3A and B, Lgr5-Cre;Bmpr1aflox-flox mice showed a statistically significant increase in the number of GFP+ve cells along the lesser but not the greater curvature. Infection with H felis further enhanced this effect (Figure 3B and C). We assessed if inhibition of BMP signaling and infection with H felis modulated the number of proliferating GFP+ve cells. For these experiments we stained the sections with antibodies directed against both GFP and Ki67, a mitotic marker. As shown in Figures 3C and D, although both noninfected and H felis–infected Lgr5-Cre mice did not show the presence of a significant number of Ki67+ve/GFP+ve cells, Lgr5-Cre;Bmpr1aflox-flox mice showed a significant increase in the number of proliferating GFP+ve cells. The observation that this effect was more pronounced in the presence of H felis supports the notion that both BMP signaling and inflammatory stimuli regulate the census of Lgr5+ve cells.
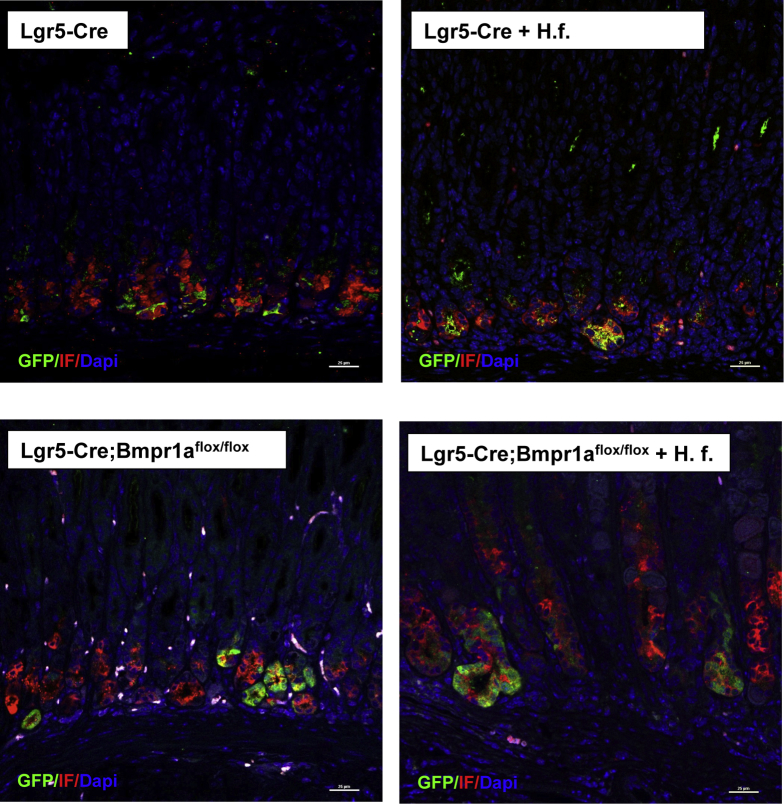
A recent report has indicated that in the oxyntic mucosa Lgr5 cells represent a subset of chief cells.20 Accordingly, we investigated if in our model, cells with Lgr5 transcriptional activity express IF, a chief cell marker. Staining of sections of the lesser curvature of Lgr-Cre and Lgr5-Cre;Bmpr1aflox-flox mice in the presence or absence of H felis, with both anti-GFP and anti-IF antibodies, showed that chief cells express GFP, confirming the notion that Lgr5 cells represent a subpopulation of chief cells (Figure 4).
Figure 4.
Expression of IF in cells with Lgr5 transcriptional activity. One- to 2-month-old Lgr5-Cre mice and Lgr5-Cre;Bmpr1aflox-flox mice were treated with 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight) and inoculated with H felis 2 months after tamoxifen. Animals were analyzed 3 months after inoculation and 5 months after tamoxifen injection. Paraffin sections of the lesser curvature of Lgr5-Cre and of Lgr5-Cre;Bmpr1aflox-flox mice in the presence and absence of H felis (H. f.) were stained with anti-IF primary antibodies and an Alexa 555–conjugated secondary antibody (red) together with Alexa 488–conjugated anti-GFP antibodies (green). Scale bar: 25 μm. Similar results were observed in at least 1 other mouse and in 6 other mice in the H felis–infected Lgr5-Cre;Bmpr1aflox-flox group. DAPI, 4'6-diamidino-2-phenylindole.
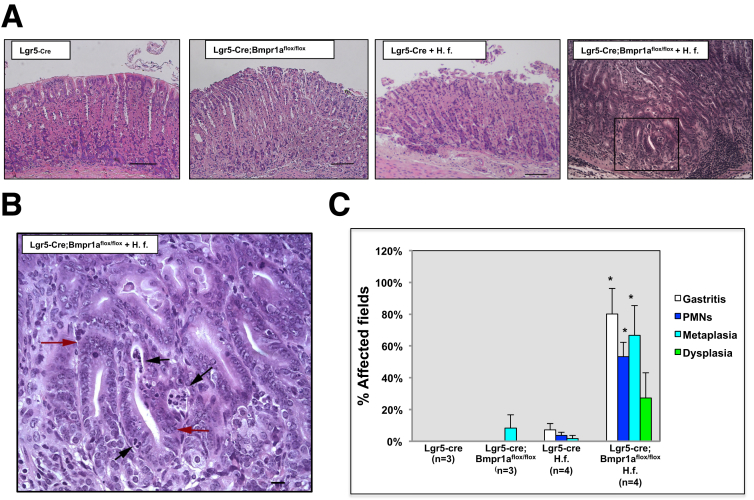
We examined the morphologic consequences of diminished expression of Bmpr1a in Lgr5 cells in the presence and absence of inflammatory stimuli. Microscopic and morphometric analysis of H&E-stained sections of the lesser curvature of uninfected Lgr5-Cre mice did not show any morphologic alterations, whereas infection with H felis induced only mild inflammation. In addition, uninfected Lgr5-Cre;Bmpr1aflox-flox mice showed the presence of minor metaplastic changes. In contrast, the mucosa of the lesser curvature of H felis–infected, Lgr5-Cre;Bmpr1aflox-flox mice showed robust morphologic abnormalities characterized by a reduction in the number of cells that had the morphologic appearance of parietal cells, a significant increase in the severity of the inflammatory infiltrates, and the presence of areas of metaplastic and dysplastic mucosa (Figure 5).
Figure 5.
Enhanced inflammatory changes, metaplasia, and dysplasia in the gastric epithelium of H felis–infected Lgr5-Cre;Bmpr1aflox-floxmice. One- to 2-month-old Lgr5-Cre mice and Lgr5-Cre;Bmpr1aflox-flox mice were treated with 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight) and inoculated with H felis 2 months after tamoxifen. Animals were analyzed 3 months after inoculation and 5 months after tamoxifen injection. (A) Representative H&E-stained paraffin sections of the lesser curvature of Lgr5-Cre and of Lgr5-Cre;Bmpr1aflox-flox mice in the presence and absence of H felis (H. f.). The magnified window depicts dysplastic and inflammatory changes in H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice. Scale bars: (A) 100 μm, (B) 25 μm. (B) Red arrows point to areas of dysplastic epithelium, black arrows indicate clusters of polymorphonuclear cells. (C) Graph bars represent the percentage of fields affected by gastritis, neutrophilic infiltrates, metaplasia, and dysplasia calculated in both Lgr5-Cre and Lgr5-Cre;Bmpr1aflox-flox mice in the presence and absence of H felis. Values are shown as means ± SE. Numbers in parenthesis indicate the number of animals used in each group. *P < .05 vs H felis–infected Lgr5-Cre mice.
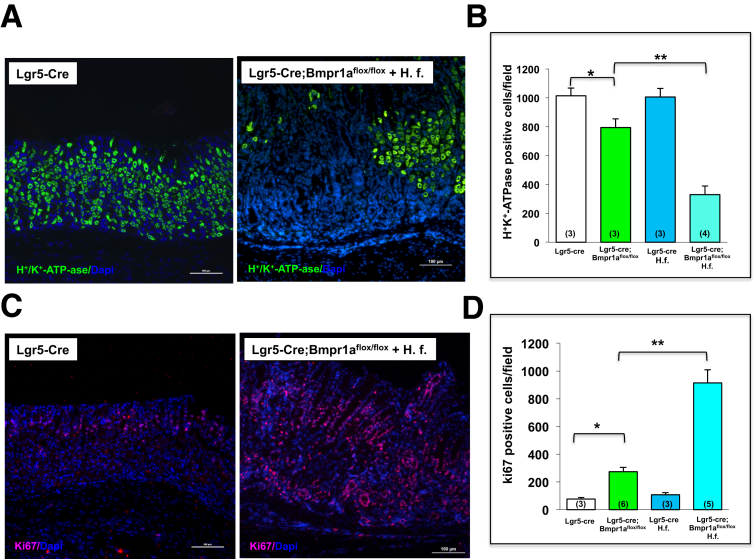
To confirm that inhibition of BMP signaling in Lgr5 cells and infection with H felis cause alterations in parietal cell homeostasis and induction of cell proliferation in the mucosa of the lesser curvature, we analyzed the number of parietal and proliferating cells by immunostaining for H+,K+-ATPase α-subunit and Ki67. As shown in the histologic sections and in the bar graphs shown in Figure 6 representing the number of positively stained cells, H felis–infected Lgr5-Cre; Bmpr1aflox-flox mice showed a marked decrease in the number of parietal cells (Figure 6A and B), and a robust increase in the number of Ki67-positive nuclei (Figure 6C and D). In contrast, uninfected Bmpr1aflox-flox mice showed only a mild decrease in parietal cell number and a small increase in the number of Ki67-positive cells. No significant changes were seen in both uninfected and infected control Lgr5-cre mice (Figure 6B and D).
Figure 6.
Decreased parietal cells and increased cell proliferation in Helicobacter-infected Lgr5-Cre;Bmpr1aflox-floxmice. One- to 2-month-old Lgr5-Cre mice and Lgr5-Cre;Bmpr1aflox-flox mice were treated with 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight) and inoculated with H felis 2 months after tamoxifen. Animals were analyzed 3 months after inoculation and 5 months after tamoxifen injection. Paraffin sections of the lesser curvature of Lgr5-Cre mice and of Lgr5-Cre;Bmpr1aflox-flox mice, in the presence and absence of H felis (H. f.), were stained with an (A) anti-H+,K+-ATPase α-subunit primary antibody and an Alexa 488–conjugated secondary antibody (green), and with an (C) anti-Ki67 primary antibody and an Alexa 555–conjugated secondary antibody (red). Scale bars: 100 μm. Bars represent the number of (B) H+,K+-ATPase α-subunit and of (D) Ki67-positive cells detected in Lgr5-Cre mice and in Lgr5-Cre;Bmpr1aflox-flox mice in the presence and absence of H felis. Values are shown as means ± SE. Numbers in parenthesis indicate the number of animals used in each group. *P < .05 vs Lgr5-Cre mice. **P < .05 vs Lgr5-Cre;Bmpr1aflox-flox mice. DAPI, 4'6-diamidino-2-phenylindole.
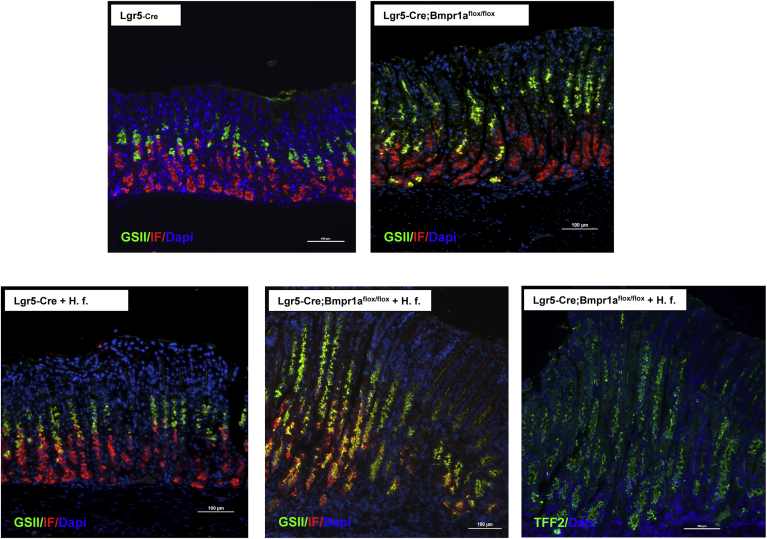
We sought to investigate if infection with H felis and diminished expression of Bmpr1a in the gastric epithelium lead to the emergence of SPEM. For these studies we stained sections of the mucosa of the lesser curvature of both control noninfected and H felis–infected Lgr5-Cre and Lgr5-Cre; Bmpr1aflox-flox mice with specific anti-IF antibodies and with the lectin GSII, which is known to bind neck-cell mucins. As shown in the overlay images depicted in Figure 7, both infected and noninfected Lgr5-Cre mice did not show any significant abnormalities, whereas noninfected Lgr5-Cre;Bmpr1aflox-flox mice showed a few foci of cells expressing GSII-positive mucins at the base of the glands. In contrast, H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice showed robust co-expression of mucus neck-cell mucins and IF in cells located at the base of the fundic glands of the mucosa of the lesser curvature. Identical results were observed when the slides were stained with antibodies against TFF2 (Figure 7).
Figure 7.
Development of SPEM in H felis–infected Lgr5-Cre;Bmpr1aflox-floxmice. One- to 2-month-old Lgr5-Cre mice and Lgr5-Cre;Bmpr1aflox-flox mice were treated with 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight) and inoculated with H felis 2 months after tamoxifen. Animals were analyzed 3 months after inoculation and 5 months after tamoxifen injection. Gastric paraffin sections of the lesser curvature of Lgr5-Cre and of Lgr5-Cre;Bmpr1aflox-flox mice in the presence and absence of H felis (H. f.) were stained with anti-IF primary antibodies and Alexa 555–conjugated secondary antibodies (red) together with Alexa 488–conjugated GS II (green). Gastric paraffin sections of the lesser curvature of H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice were stained with anti-TFF2 primary antibodies and an Alexa 488–conjugated secondary antibody. Scale bar: 100 μm. Similar results were observed in at least 3 other mice in each group. DAPI, 4′,6-diamidino-2-phenylindole.
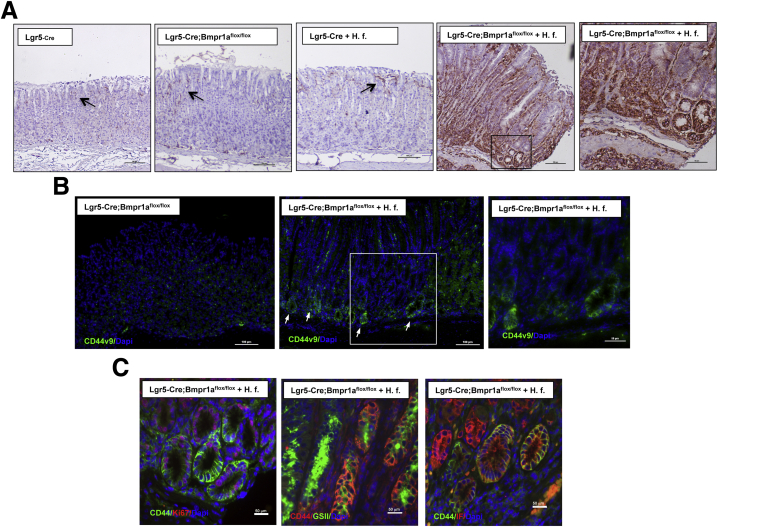
The adhesion molecule and Wnt target CD44 participates in the response of the gastric epithelium to inflammatory stimuli. In addition, CD44 appears to be involved in the pathogenesis of SPEM and to label putative gastric cancer stem cells, suggesting that it might play an important role in the process of inflammation/injury-induced carcinogenesis.38, 39, 40, 41, 42, 43 It also has been shown that alternative mRNA splicing produces Cd44 variant isoforms, such as Cd44v9, which are more specifically expressed in H pylori gastritis, SPEM, and in gastric carcinomas.40, 41 Thus, we examined if H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice show perturbations in the expression of both CD44 and of CD44v9. Staining of sections of the lesser curvature of H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice with anti-CD44 antibodies showed robust expression of CD44 in the mucosa of the lesser curvature (Figure 8A), with intense staining noted in nests of epithelial cells located at the base of the glands. In contrast, staining of the sections of the lesser curvature of noninfected Lgr5-Cre;Bmpr1aflox-flox mice and of both infected and noninfected Lgr5-Cre mice showed the presence of only a few rare CD44+ve cells at the level of the isthmus and the pits of the gastric glands (Figure 8A). Similarly, incubation of sections of the lesser curvature of H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice, but not of noninfected mice, with anti-CD44v9 antibodies showed the presence of extensive staining of cells located at the base of the glands, suggesting that both inflammation and loss of BMP signaling modulate the expression of this splice variant of CD44 (Figure 8B). To better define the phenotypic characteristics of CD44-expressing cells in the mucosa of the lesser curvature of H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice, we co-stained the sections with anti-CD44 antibodies together with the lectin GSII, IF, and Ki67. As shown in Figure 8C, CD44+ve cells, located at the base of the glands, expressed markers of cell proliferation, mucus neck-cell mucins, and IF, suggesting that SPEM can arise in proliferating CD44+ve cells.
Figure 8.
Expression of CD44 in H felis–infected Lgr5-Cre;Bmpr1aflox-floxmice. One- to 2-month-old Lgr5-Cre mice and Lgr5-Cre;Bmpr1aflox-flox mice were treated with 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight) and inoculated with H felis 2 months after tamoxifen. Animals were analyzed 3 months after inoculation and 5 months after tamoxifen injection. (A) Paraffin sections of the lesser curvature of Lgr5-Cre and Lgr5-Cre;Bmpr1aflox-flox mice in the presence and absence of H felis (H. f.) stained with anti-CD44 primary antibodies and a biotin-conjugated secondary antibody. The magnified window shows intense CD44 staining of epithelial cells in H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice. Scale bar: 100 μm, 50 μm in the magnified window. Arrows point to CD44+ve cells. (B) Paraffin sections of the lesser curvature of noninfected and H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice stained with anti-CD44v9 primary antibodies and Alexa 488–conjugated secondary antibodies. The magnified window shows CD44v9 staining of epithelial cells at the base of glands in H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice. Scale bar: 100 μm, 50 μm in the magnified window. Arrows point at CD44v9+ve cells. (C) Paraffin sections of the lesser curvature of H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice stained with anti-CD44 primary antibodies and Alexa 594-conjugated secondary antibodies (red) together with Alexa 488–conjugated GSII (green), anti-CD44 primary antibodies and Alexa 488–conjugated secondary antibodies (green) together with IF antibodies and Alexa 555–conjugated secondary antibodies (red) and anti-CD44 primary antibodies and Alexa 488–conjugated secondary antibodies (green) together with anti-Ki67 antibodies and Alexa 555–conjugated secondary antibodies (red). Similar results were observed in at least 2 other mice in each group. Scale bar: 50 μm. DAPI, 4′,6-diamidino-2-phenylindole.
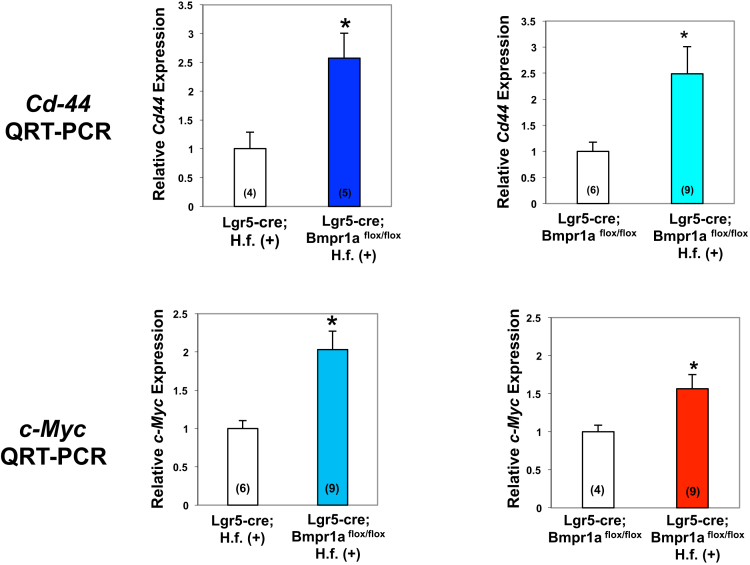
By using QRT-PCR assays we also observed increased expression of CD44 mRNA in the gastric mucosa of the lesser curvature of H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice when compared with both infected Lgr5-Cre mice and noninfected Lgr5-Cre;Bmpr1aflox-flox mice, confirming the notion that inhibition of BMP signaling in Lgr5 cells and the presence of inflammatory stimuli lead to increased CD44 mRNA expression (Figure 9).
Figure 9.
Expression of Cd44 and c-Myc mRNAs in the lesser curvature of Lgr5-Cre;Bmpr1aflox-floxmice. One- to 2-month-old Lgr5-Cre mice and Lgr5-Cre;Bmpr1aflox-flox mice were treated with 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight) and inoculated with H felis 2 months after tamoxifen. Animals were analyzed 3 months after inoculation and 5 months after tamoxifen injection. Cd44 and c-Myc mRNA signals in samples of the lesser curvature of Lgr5-Cre mice and of Lgr5-Cre;Bmpr1aflox-flox mice in the presence and absence of H felis (H. f.) were measured using QRT-PCR. Numbers in parenthesis indicate the number of animals used in each group. *P < .05.
In addition to CD44, the oncoprotein c-myc, another Wnt target, has been implicated in gastric carcinogenesis and it has been shown to cooperate with CD44 to regulate gastric cancer growth.44, 45 Accordingly, we performed QRT-PCR assays in which we measured the expression of c-Myc mRNA. As shown in Figure 9, H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice showed a significant increase in the expression of c-Myc when compared with both infected Lgr5-Cre mice and noninfected Lgr5-Cre;Bmpr1aflox-flox mice, suggesting that inhibition of BMP signaling in Lgr5 cells leads to the development of a pro-oncogenic environment characterized by increased expression of multiple targets of Wnt signaling.
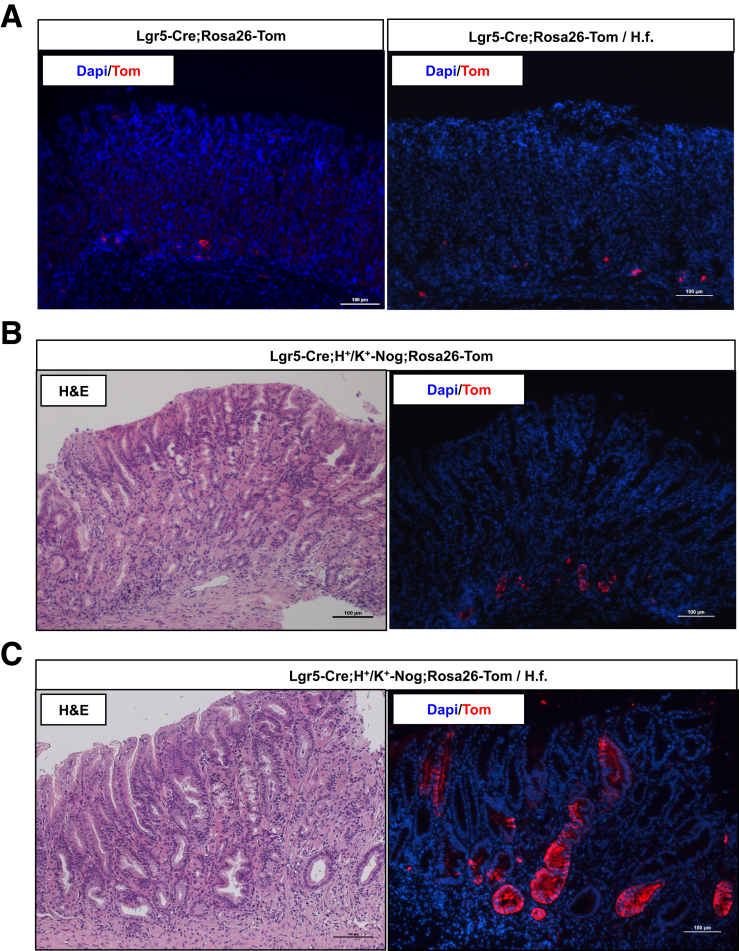
To perform lineage tracing studies in the presence of the BMP inhibitor noggin, we took advantage of Lgr5-Cre;H+/K+-Nog;Rosa26-Tom mice that express both noggin and tomato in the oxyntic mucosa. As shown in Figure 10A a few rare tomato+ve cells could be seen along the lesser curvature of the oxyntic mucosa of both H felis–infected and noninfected Lgr5-Cre control mice. As shown in the H&E-stained sections, expression of noggin caused significant morphologic changes of the gastric mucosa in agreement with previously published reports,26, 27 but only a modest increase in the number of tomato+ve cells (Figure 10B), whereas infection with H felis enhanced this effect of noggin, leading to the appearance of robust tomato expression in glandular structures that were located along the lesser curvature of the oxyntic mucosa (Figure 10C).
Figure 10.
Distribution of tomato-positive cells in H felis–infected Lgr5-Cre;H+/K+-Nog;Rosa26-Tom mice. One- to 2-month-old Lgr5-Cre;Rosa26-Tom mice and Lgr5-Cre;H+/K+-Nog;Rosa26-tdTom mice were treated with 1 intraperitoneal injection of tamoxifen (0.1 mg/g body weight) and inoculated with H felis 2 months after tamoxifen. Animals were analyzed 3 months after inoculation and 5 months after tamoxifen injection. Gastric paraffin sections of the lesser curvature of Lgr5-Cre;Rosa26-Tom and Lgr5-Cre;H+/K+-Nog;Rosa26-tdTom in the presence and absence of H felis (H. f.) were stained with anti-tomato (red fluorescent protein) primary antibodies and Alexa 555–conjugated secondary antibodies. Matching sections of the gastric mucosa of both noninfected and H felis–infected Lgr5-Cre;H+/K+-Nog;Rosa26-tdTom mice were stained with H&E. Similar results were observed in at least 3 other mice in each group. DAPI, 4′,6-diamidino-2-phenylindole.
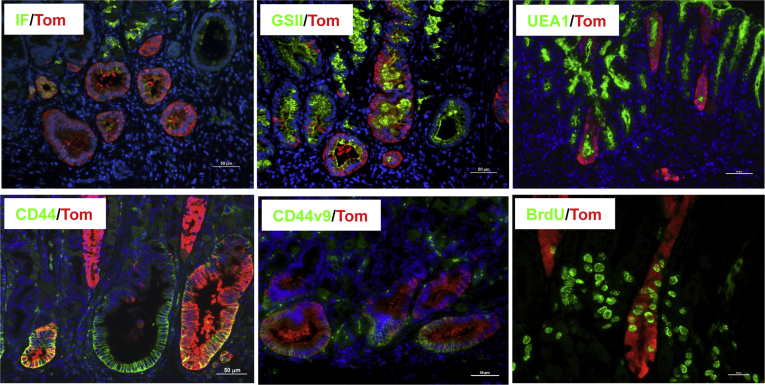
We investigated the cellular lineages that derive from cells with Lgr5 transcriptional activity in the H felis–infected Lgr5-Cre;H+/K+-Nog;Rosa26-Tom mice. As shown in Figure 11, staining of sections with the lectins Ulex europaeus agglutinin 1 and GSII, molecules that label pit and mucus neck-cell mucins, respectively,5 and with anti-CD44, anti-CD44v9, and anti-IF antibodies, showed expression of pit and mucus neck-cell markers, CD44, CD44v9, and IF in the tomato-positive cells. These data suggest that pit cell lineages, SPEM, CD44+ve, and CD44v9+ve cells can arise from cells with Lgr5 transcriptional activity in the presence of inflammatory stimuli and inhibition of BMP signaling. Moreover, as shown in Figure 11, we observed that some of the tomato+ve cells were proliferating because they could be positively stained with anti-BrdU antibodies.
Figure 11.
Lineage tracing of cells with Lgr5 transcriptional activity in H felis–infected Lgr5-Cre;H+/K+-Nog;Rosa26-Tom mice. 1-2-month old Lgr5-Cre;H+/K+-Nog;Rosa26-tdTom mice were treated with one i.p. injection of tamoxifen (0.1 mg/g body weight) and inoculated with H. felis two months post tamoxifen. Animals were analyzed 3 months after inoculation, and 5 months after tamoxifen injection. Gastric paraffin sections of the lesser curvature of H felis–infected Lgr5-Cre;H+/K+-Nog;Rosa26-tdTom mice were stained with anti-tomato (red fluorescent protein) primary antibodies and Alexa 555–conjugated secondary antibodies together with anti-IF antibodies and Alexa 488–conjugated secondary antibodies (IF/Tom), Alexa 488–conjugated GSII (GSII/Tom), Alexa 488–conjugated Ulex europaeus agglutinin 1 (UEA1), anti-CD44 and anti-CD44v9 primary antibodies and Alexa 488–conjugated secondary antibodies (CD44/Tom and CD44v9/Tom), and anti-BrdU primary antibodies and Alexa 488–conjugated secondary antibodies (BrdU/Tom). Scale bars: 50 μm. Similar results were observed in at least 5 other H felis–infected Lgr5-Cre;H+/K+-Nog;Rosa26-tdTom mice except for the experiments with the anti-CD44v9 antibodies that were repeated in 1 other mouse.
Discussion
In this article, we report a series of novel observations that underscore the importance of BMP signaling in the regulation of gastric epithelial homeostasis. In particular, we present evidence that inhibition of BMP signaling and infection with H felis lead to the activation and expansion of Lgr5+ve cells that give rise to metaplastic proliferating lineages that express markers of SPEM.
Although the level of deletion of Bmpr1a in Lgr5 cells was not complete, H felis–infected Lgr5-Cre;Bmpr1aflox-flox mice showed robust phenotypic changes, suggesting that even partial inhibition of BMP signaling in Lgr5 cells can induce significant aberrations in the normal homeostatic mechanisms of the gastric mucosa. A possible explanation for this finding lies in the observation that Lgr5 cells can express growth-promoting and proinflammatory peptides,10, 38, 39, 40, 41, 42, 43 factors known to play an important role in the regulation of cell proliferation and in the response of the gastric mucosa to inflammatory stimuli. It therefore is conceivable that diminished BMP signaling in Lgr5 cells could induce the expression of these molecules leading to broader and more widespread responses that might affect the biological functions not only of Lgr5 cells, but also of other adjacent cell types.
Numerous studies have shown that inflammation, loss of parietal cells, and gastric mucosal injury lead to the reprogramming of a subset of chief cells that undergo profound biological modifications, characterized by the activation of proliferative mechanisms and by the expression of markers of mucus neck-cell differentiation at the base of the glands of the oxyntic mucosa.5, 10, 20 These events have been associated with the development of dysplastic changes and gastric carcinogenesis.5, 7, 8, 9, 20
Recent reports have shown that in the corpus Lgr5+ve cells express IF, and that these cells represent a subpopulation of chief cells with Lgr5 transcriptional activity.18, 20 Interestingly, elegant lineage tracing studies conducted in Lgr5-EGFP-IRES-CreERT2/+;Rosa26R mice that were treated with either the proinflammatory compound L365 or the protonophore DMP777, which causes ablation of parietal cells without any significant inflammation, did not detect the presence of markers of SPEM in lineages derived from Lgr5 cells located in the lesser curvature.18 These observations suggest that inflammation and loss of parietal cells, stimuli that can effectively induce SPEM,5 are not sufficient by themselves to support the development of metaplastic lineages in Lgr5+ve cells.
In our study we observed that either infection with H felis or inhibition of BMP signaling by themselves caused only small changes in the number and fate of Lgr5+ve cells. However, infection with H felis in the presence of inhibition of BMP signaling led to robust aberrations in the normal homeostatic mechanisms of the gastric epithelium characterized by the emergence of SPEM in glands derived from cells with Lgr5 transcriptional activity located along the lesser curvature of the oxyntic mucosa. It is possible that some of these changes could have been caused by the heightened level of inflammation caused by H felis in the presence of loss of BMP signaling, a mechanism that we previously showed has anti-inflammatory effects.27 However, the observation that neither L365 nor H felis by themselves were able to induce the emergence of SPEM in Lgr5 progenitors18 suggests that BMP signaling has additional, specific actions on the physiological mechanisms that regulate the proliferation and differentiation of gastric epithelial cells. In support of this hypothesis, transgenic mice that express the BMP signaling inhibitor noggin in the gastric epithelium develop epithelial hyperproliferation and extensive SPEM in the context of only minimal inflammatory changes.26, 27
An important factor that should be considered in the interpretation of our results is that in this study we used the Lgr5-EGFP-ires-CreERT2 (Lgr5-Cre) mouse model in which EGFP and Cre were expressed only in the antrum and along the lesser curvature of the oxyntic mucosa.11 It is therefore conceivable that the changes observed in our studies could have been detected in more extensive areas of the corpus if we had used the Lgr5-2A-CreERT2 mouse, a biological system20 that allows an unrestricted expression of the transgene in all segments of the gastric mucosa.
The intracellular mechanisms responsible for the development of SPEM have been only partially characterized. Recent reports have indicated that activation of the Ras/extracellular signal–regulated kinase (ERK) kinase signal transduction pathway in chief cells leads to SPEM, suggesting that this signaling mechanism plays an important role in the biological processes that cause the emergence of this type of metaplasia.20, 46 Interestingly, in a series of previously published studies we observed that inhibition of BMP signaling leads to enhanced activation of the ERKs and to increased growth factor gene expression in the gastric mucosa.26 We also reported that BMP-4, a member of the BMP family of regulatory peptides, inhibits growth factor–stimulated ERK2 activation in primary cultures of canine gastric epithelial cells,47 confirming the notion that the BMP signal transduction pathway restrains the induction of growth factor signaling in the stomach. On the basis of these observations it is conceivable to speculate that inhibition of BMP signaling in Lgr5 cells could remove an inhibitory break on ERK activation, leading to the induction of a series of intracellular events that ultimately could lead to the development of SPEM and to the stimulation of proliferative mechanisms. In support of this observation recent studies have shown that induction of Ras activation in Lgr5-expressing chief cells can lead to the development of metaplasia and neoplasia.20, 46
We reported that diminished expression of Bmpr1a and noggin cause increased expression of CD44 in the gastric epithelium of the lesser curvature and in epithelial cells derived from Lgr5+ve cells. CD44 has been implicated in the pathogenesis of inflammation, SPEM, and in the process of gastric carcinogenesis because Cd44-expressing cells isolated from gastric tumors behave as gastric cancer stem cells.38, 39, 40, 41, 42, 43 Studies also have shown that induction of mitogen-activated protein kinase/ERK signaling leads to increased expression of CD44,38 and to the activation of STAT3, a pro-oncogenic molecule that has been linked to gastric carcinogenesis.48, 49 Elegant biochemical experiments have shown that CD44 physically interacts with phosphorylated STAT3 and that the CD44/phosphorylated STAT3 complex is necessary for induction of cell proliferation.38 Because we previously reported that H pylori–infected noggin transgenic mice show enhanced activation of STAT3,27 it is possible that inhibition of BMP signaling in Lgr5 cells could activate a biochemical cascade that links the ERKs to CD44 and STAT3, leading to stimulation of cell proliferation and to the development of metaplastic changes of the gastric mucosa.
The expression of CD44 in pathologic states of the stomach is complex, and there are some isoforms of CD44, such as CD44v6 and CD44v9, that have been more specifically linked to gastric inflammation and neoplasia.40, 41 Our experiments confirm the involvement of CD44v9 in the phenotypic changes observed in Lgr5-Cre;Bmpr1aflox-flox mice. Future studies will be needed to analyze in more detail the CD44 isoforms that specifically are linked to altered BMP signaling and to Lgr5 cell fate during Helicobacter-induced inflammation.
In our study we also observed a significant increase in the expression of c-Myc, a Wnt target that has been implicated in gastric carcinogenesis and that appears to cooperate with CD44 in the regulation of gastric cancer growth.44, 45 Thus, inhibition of BMP signaling and activation of inflammatory mechanisms appear to lead to the induction of multiple Wnt targets and to the development of a pro-oncogenic environment.
In conclusion, we have shown that inflammation and inhibition of BMP signaling lead to the activation of cells with Lgr5 transcriptional activity that give rise to dysplastic, proliferating lineages that express markers of both mucus neck and zymogenic cell differentiation. These findings underscore the importance of BMP signaling in the regulation of gastric inflammation and epithelial homeostasis, providing new clues for a better understanding of the pathophysiological mechanisms that lead to the development of both dysplastic and neoplastic lesions in the stomach.
Acknowledgments
The authors thank Kathy McClinchey for technical assistance and the University of Michigan Flow Cytometry Core for technical assistance with cell sorting.
Footnotes
Author contributions Wei Ye and Hidehiko Takabayashi were responsible for the study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript, and statistical analysis; Maria Mao and Yitian Yang were responsible for the acquisition of data, analysis and interpretation of data, technical support, and statistical analysis; Elise S. Hibdon was responsible for the acquisition of data, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content; Linda C. Samuelson was responsible for the analysis and interpretation of data and critical revision of the manuscript for important intellectual content; Kathryn A. Eaton was responsible for the acquisition, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content; and Andrea Todisco was responsible for the study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis, obtained funding, study supervision, and writing the manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants RO1DK083373 and R56DK116617 (A.T.), by the University of Michigan Gastrointestinal Peptide Research Center (P30-DK-34933), by the University of Michigan Comprehensive Cancer Center John S. and Suzanne C. Munn Cancer Fund (A.T.), and by the Funderburg Award in Gastric Biology Related to Cancer from the Foundation for Digestive Health and Nutrition (A.T.).
References
- 1.Peek R.M., Jr. IV. Helicobacter pylori strain-specific activation of signal transduction cascades related to gastric inflammation. Am J Physiol Gastrointest Liver Physiol. 2001;280:G525–G530. doi: 10.1152/ajpgi.2001.280.4.G525. [DOI] [PubMed] [Google Scholar]
- 2.Nomura S., Baxter T., Yamaguchi H., Leys C., Vartapetian A.B., Fox J.G., Lee J.R., Wang T.C., Goldenring J.R. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–594. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Zavros Y., Eaton K.A., Kang W., Rathinavelu S., Katukuri V., Kao J.Y., Samuelson L.C., Merchant J.L. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–2366. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 4.Peek R.M., Fiske C., Wilson K.T. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen C.P., Mills J.C., Goldenring J.R. Murine models of gastric corpus preneoplasia. Cell Mol Gastroenterol Hepatol. 2017;3:11–26. doi: 10.1016/j.jcmgh.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi E., Sochacki P., Khoury N., Patel B.B., Majumdar A.P. Cancer stem cells in Helicobacter pylori infection and aging: implications for gastric carcinogenesis. World J Gastrointest Pathophysiol. 2014;5:366–372. doi: 10.4291/wjgp.v5.i3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halldorsdottir A.M., Sigurdardottir M., Jonasson J.G., Oddsdóttir M., Magnússon J., Lee J.R., Goldenring J.R. Spasmolytic polypeptide expressing metaplasia (SPEM) associated with gastric cancer in island. Dig Dis Sci. 2003;48:431–441. doi: 10.1023/a:1022564027468. [DOI] [PubMed] [Google Scholar]
- 8.Goldenring J.R., Nam K.T., Wang T.C., Mills J.C., Wright N.A. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noto J.M., Peek R.M., Jr. Gastric-to-intestinal transdifferentiation and cancer. Proc Natl Acad Sci U S A. 2012;109:20173–20174. doi: 10.1073/pnas.1218345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam K.T., Lee H.-J., Sousa J.F., Weis V.G., O'Neal R.L., Finke P.E., Romero-Gallo J., Shi G., Mills J.C., Peek R.M., Jr., Konieczny S.F., Goldenring J.R. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker N., Meritexell H., Kujala P., van de Wetering M., Snippert H.J., van Es J.H., Sato T., Stange D.E., Begthel H., van den Born M., Danenberg E., van den Brink S., Korving J., Abo A., Peters P.J., Wright N., Poulsom R., Clevers H. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Arnold K., Sarkar A., Myram M.A., Polo J.M., Bronson R., Sengupta S., Seandel M., Geijsen N., Hochedlinger K. Sox2+ adult stem/progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills J.C., Shivdasani R.A. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–424. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao X.T., Gumucio D.L. Current molecular markers for gastric progenitor cells and gastric cancer stem cells. J Gastroenterol. 2011;46:855–865. doi: 10.1007/s00535-011-0413-y. [DOI] [PubMed] [Google Scholar]
- 15.Stange D.E., Koo B.K., Huch M., Sibbel G., Basak O., Lyubimova A., Kujala P., Bartfeld S., Koster J., Geahlen J.H., Peters P.J., van Es J.H., van de Wetering M., Mills J.C., Clevers H. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa Y., Ariyama H., Stancikova J., Sakitani K., Asfaha S., Renz B.W., Dubeykovskaya Z.A., Shibata W., Wang H., Westphalen C.B., Chen X., Takemoto Y., Kim W., Khurana S.S., Tailor Y., Nagar K., Tomita H., Hara A., Sepulveda A.R., Setlik W., Gershon M.D., Saha S., Ding L., Shen Z., Fox J.G., Friedman R.A., Konieczny S.F., Worthley D.L., Korinek V., Wang T.C. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo J., Kimura S., Yamamura A., Koh C.P., Hossain M.Z., Heng D.L., Kohu K., Voon D.C., Hiai H., Unno M., So J.B., Zhu F., Srivastava S., Teh M., Yeoh K.G., Osato M., Ito Y. Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology. 2017;152:218–223. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Nam K.T., O'Neal R.L., Coffey R.J., Finke P.E., Barker N., Goldenring J.R. Spasmolytic polypeptide-expressing metaplasia (SPEM) in the gastric oxyntic mucosa does not arise from Lgr5-expressing cells. Gut. 2012;61:1678–1685. doi: 10.1136/gutjnl-2011-301193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajani J.A., Barthel J.S., Bekaii-Saab T., Bentrem D.J., D'Amico T.A., Das P., Denlinger C., Fuchs C.S., Gerdes H., Hayman J.A., Hazard L., Hofstetter W.L., Ilson D.H., Keswani R.N., Kleinberg L.R., Korn M., Meredith K., Mulcahy M.F., Orringer M.B., Osarogiagbon R.U., Posey J.A., Sasson A.R., Scott W.J., Shibata S., Strong V.E., Washington M.K., Willett C., Wood D.E., Wright C.D., Yang G. NCCN Gastric Cancer Panel. J Natl Compr Canc Netw. 2010;8:378–409. doi: 10.6004/jnccn.2010.0030. [DOI] [PubMed] [Google Scholar]
- 20.Leushacke M., Tan S.H., Wong A., Swathi Y., Hajamohideen A., Tan L.T., Goh J., Wong E., Denil S.L.I.J., Murakami K., Barker N. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol. 2017;19:774–786. doi: 10.1038/ncb3541. [DOI] [PubMed] [Google Scholar]
- 21.Jang B.G., Lee B.L., Kim W.H. Distribution of LGR5+ cells and associated implications during the early stage of gastric tumorigenesis. PLoS One. 2013;8:e82390. doi: 10.1371/journal.pone.0082390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X.-B., Yang G., Zhu L., Tang Y.L., Zhang C., Ju Z., Yang X., Teng Y. Gastric Lgr5+stem cells are the cellular origin of invasive intestinal-type gastric cancer in mice. Cell Res. 2016;26:838–849. doi: 10.1038/cr.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigal M., Rothenberg M.E., Logan C.Y., Lee J.Y., Honaker R.W., Cooper R.L., Passarelli B., Camorlinga M., Bouley D.M., Alvarez G., Nusse R., Torres J., Amieva M.R. Helicobacter pylori activates and expands Lgr5(+) stem cells through direct colonization of the gastric glands. Gastroenterology. 2015;148:1392–1404. doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 24.Brazil D.P., Church R.H., Surae S., Godson C., Martin F. BMP signaling: agony and antagony in the family. Trends Cell Biol. 2015;25:249–264. doi: 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Todisco A. Regulation of gastric metaplasia, dysplasia, and neoplasia by bone morphogenetic protein signaling. Cell Mol Gastroenterol Hepatol. 2017;3:339–347. doi: 10.1016/j.jcmgh.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinohara M., Mao M., Keeley T.M., El-Zaatari M., Lee H.J., Eaton K.A., Samuelson L.C., Merchant J.L., Goldenring J.R., Todisco A. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060. doi: 10.1053/j.gastro.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takabayashi H., Shinohara M., Mao M., Phaosawasdi P., El-Zaatari M., Zhang M., Ji T., Eaton K.A., Dang D., Kao J., Todisco A. Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology. 2014;147:396–406. doi: 10.1053/j.gastro.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C.A., Odeberg J., Djureinovic D., Takanen J.O., Hober S., Alm T., Edqvist P.H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J.M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Pontén F. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 29.Mishina Y., Hanks M.C., Miura S., Tallquist M.D., Behringer R.R. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- 30.Eaton K.A., Benson L.H., Haeger J., Gray B.M. Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice. Infect Immun. 2006;74:4673–4684. doi: 10.1128/IAI.01887-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demitrack E.S., Gifford G.B., Keeley T.M., Carulli A.J., VanDussen K.L., Thomas D., Giordano T.J., Liu Z., Kopan R., Samuelson L.C. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34:2522–2536. doi: 10.15252/embj.201490583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eaton K.A., Danon S.J., Krakowka S., Weisbrode S.E. A reproducible scoring system for quantification of histologic lesions of inflammatory disease in mouse gastric epithelium. Comp Med. 2007;57:57–65. 32. [PubMed] [Google Scholar]
- 33.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;28;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locklin R.M., Riggs B.L., Hicok K.C., Horton H.F., Byrne M.C., Khosla S. Assessment of gene regulation by bone morphogenetic protein 2 in human marrow stromal cells using gene array technology. J Bone Miner Res. 2001;16:2192–2204. doi: 10.1359/jbmr.2001.16.12.2192. [DOI] [PubMed] [Google Scholar]
- 35.Miyazono K., Maeda S., Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Bonilla-Claudio M., Wang J., Bai Y., Klysik E., Selever J., Martin J.F. Bmp signaling regulates a dose-dependent transcriptional program to control facial skeletal development. Development. 2012;139:709–719. doi: 10.1242/dev.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S., Moss S.F. Rodent models of Helicobacter infection, inflammation and disease. Methods Mol Biol. 2012;921:89–98. doi: 10.1007/978-1-62703-005-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khurana S.S., Riehl T.E., Moore B.D., Fassan M., Rugge M., Romero-Gallo J., Noto J., Peek R.M., Jr., Stenson W.F., Mills J.C. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013;288:16085–16097. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishimoto T., Izumi D., Watanabe M., Yoshida N., Hidaka K., Miyake K., Sugihara H., Sawayama H., Imamura Y., Iwatsuki M., Iwagami S., Baba Y., Horlad H., Komohara Y., Takeya M., Baba H. Chronic inflammation with Helicobacter pylori infection is implicated in CD44 overexpression through miR-328 suppression in the gastric mucosa. J Gastroenterol. 2015;50:751–757. doi: 10.1007/s00535-014-1019-y. [DOI] [PubMed] [Google Scholar]
- 40.Wada T., Ishimoto T., Seishima R., Tsuchihashi K., Yoshikawa M., Oshima H., Oshima M., Masuko T., Wright N.A., Furuhashi S., Hirashima K., Baba H., Kitagawa Y., Saya H., Nagano O. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci. 2013;104:1323–1329. doi: 10.1111/cas.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zavros Y. Initiation and maintenance of gastric cancer: a focus on CD44 variant isoforms and cancer stem cells. Cell Mol Gastroenterol Hepatol. 2017;4:55–63. doi: 10.1016/j.jcmgh.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seishima R., Wada T., Tsuchihashi K., Okazaki S., Yoshikawa M., Oshima H., Oshima M., Sato T., Hasegawa H., Kitagawa Y., Goldenring J.R., Saya H., Nagano O. Ink4a/Arf-dependent loss of parietal cells induced by oxidative stress promotes CD44-dependent gastric tumorigenesis. Cancer Prev Res (Phila) 2015;8:492–501. doi: 10.1158/1940-6207.CAPR-15-0025-T. [DOI] [PubMed] [Google Scholar]
- 43.Takaishi S., Okumura T., Tu S., Wang S.S., Shibata W., Vigneshwaran R., Gordon S.A., Shimada Y., Wang T.C. Identification of gastric cancer stem cells using cell surface makers CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkins J.A., Sansom O.J. Is a critical mediator of the phenotypes of Apc loss in the intestine. Cancer Res. 2008;68:4963–4966. doi: 10.1158/0008-5472.CAN-07-5558. [DOI] [PubMed] [Google Scholar]
- 45.Park J., Kim S.Y., Kim H.J., Kim K.M., Choi E.Y., Kang M.S. A reciprocal regulatory circuit between CD44 and FGFR2 via c-myc controls gastric cancer cell growth. Oncotarget. 2016;7:28670–28683. doi: 10.18632/oncotarget.8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi E., Hendley A.M., Bailey J.M., Leach S.D., Goldenring J.R. Expression of activated Ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology. 2016;150:918–930. doi: 10.1053/j.gastro.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nitsche H., Ramamoorthy S., Sareban M., Pausawasdi N., Todisco A. Functional role of bone morphogenetic protein-4 in isolated canine parietal cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G607–G614. doi: 10.1152/ajpgi.00194.2006. [DOI] [PubMed] [Google Scholar]
- 48.Ernst M., Najdovska M., Grail D., Lundgren-May T., Buchert M., Tye H., Matthews V.B., Armes J., Bhathal P.S., Hughes N.R., Marcusson E.G., Karras J.G., Na S., Sedgwick J.D., Hertzog P.J., Jenkins B.J. STAT3 and STAT1 mediate IL-11 dependent and inflammation-associated gastric tumurogenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–1738. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li N., Grivennikov S.I., Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19:429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]