Abstract
We here report a case of a 32‐year‐old man with genetically diagnosed myotonic dystrophy and persistent atrial fibrillation (AF) who underwent a low‐voltage zone (LVZ) ablation. His cardiac function was normal except for a prophylactic pacemaker implantation for a high risk of complete atrioventricular block. An LVZ was found in the anteroseptal left atrium during sinus rhythm and was ablated during induced AF after a pulmonary vein antrum isolation, which terminated the AF and rendered it noninducible by rapid pacing and/or isoproterenol. During 20 months of follow‐up, no atrial tachyarrhythmias were observed with pacemaker monitoring of antiarrhythmic drugs.
Keywords: ablation, arrhythmia, atrial fibrillation, low‐voltage zone, myotonic dystrophy
1. INTRODUCTION
Myotonic dystrophy (MD) is an autosomal dominantly inherited neuromuscular disorder. The centers for disease control and prevention have reported that 11 of every 100 000 people are affected in Northern England.1 AF is sometimes found with an occurrence rate of 25%, which is an independent predictor of sudden cardiac death in MD.2 Low‐voltage zones (LVZs) are known to be a substrate of atrial fibrillation (AF), and an LVZ ablation after a pulmonary vein antrum isolation (PVAI) has been shown to improve the outcomes in patients with persistent AF and LVZs.3 We here report a patient with genetically diagnosed MD and persistent AF, in whom an LVZ ablation after a PVI eliminated the AF.
2. CASE
A 32‐year‐old man with genetically diagnosed MD type 1 had prophylactically undergone a dual‐chamber pacemaker implantation for first‐degree atrioventricular block 1 year before an AF ablation because his long HV interval further prolonged from 75 ms at baseline to 100 ms after an injection of the class Ic antiarrhythmic agent, pilsicainide (50 mg), suggesting a high risk of complete atrioventricular block. He was referred to our hospital for catheter ablation of drug‐refractory persistent AF. Repeat DC cardioversions were performed at the outpatient clinic, but the AF soon recurred and became sustained at all times. Transthoracic echocardiography revealed a normal LA diameter of 32 mm and a normal left ventricular function.
At the beginning of the procedure, DC cardioversion failed to restore sinus rhythm and then a pulmonary vein antrum isolation (PVAI) was initially performed during ongoing AF under navigation with a NavX™ system (Abbott, Chicago, Illinois). After completion of the PVAI, a DC shock temporarily restored SR during which an LA voltage map was created using bipolar electrograms obtained from a 20‐pole circular mapping catheter (Reflexion HD™, Abbott). An LVZ, defined as an area with a bipolar peak‐to‐peak voltage amplitude of < 0.5 mV, was identified in the anteroseptal and septal LA where fragmented potentials were observed at some points (Figure 1A,B). An LVZ ablation was performed in a dragging fashion, during which the AF suddenly terminated and converted to SR (Figure 2A,B). It was noted that the AF termination site exhibited fractionated electrograms during sinus rhythm before the ablation. The LVZ at the anteroseptal area of the LVZ was completely ablated, after which AF was no longer induced by rapid atrial pacing before or after an isoproterenol infusion although it had been easily induced by catheter manipulation before the LVZ ablation after the PVAI. During a follow‐up period of 20 months, no atrial tachyarrhythmias were observed of antiarrhythmic drugs. His symptoms markedly improved, and his quality of life returned to the same level as that before suffering from AF.
Figure 1.
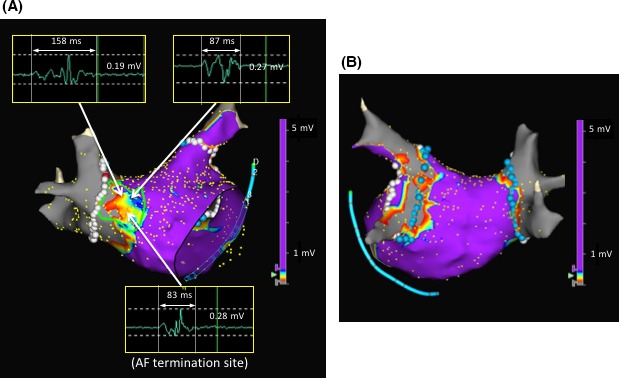
LA voltage maps after the pulmonary vein antrum isolation in the RAO (panel A) and PA (panel B) views. An LVZ was defined as an area with a bipolar peak‐to‐peak electrogram amplitude of <0.5 mV, and one was identified in the anteroseptal and septal LA. The color gradation indicates the serial changes in the bipolar voltage from purple at >0.5 mV to gray at <0.1 mV. Fragmented potentials were recorded in the LVZ, and the numbers in the electrogram recording indicate both the peak‐to‐peak electrogram amplitude and the width. A site where AF termination occurred during the LVZ ablation is shown in the map with fragmented electrograms during sinus rhythm. The area surrounded by the green line indicates the extent of the LVZ ablation. No LVZ was identified in the posterior LA (panel B). AF, atrial fibrillation; LVZ, low‐voltage zone; LA, left atrium; PA, posterior‐anterior; RAO, right anterior oblique
Figure 2.
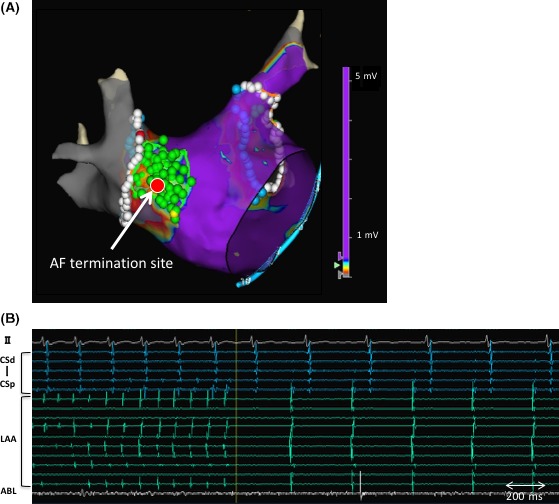
Termination site of atrial fibrillation during the LVZ ablation (the red tag indicated by the arrow) (panel A) and an ECG and electrocardiograms at the time of the AF termination (panel B). AF was terminated and converted to SR during the LVZ ablation. The LVZ ablation was continued until the anteroseptal LVZ was completely ablated. The green tags indicate the LVZ ablation region. The bipolar electrograms recorded in the LA appendage, by the ablation catheter, and in the coronary sinus are shown (panel B). ABL, ablation catheter; AF, atrial fibrillation; CS, coronary sinus; LVZ, low‐voltage zone; LA, left atrium; LAA, left atrial appendage; SR, sinus rhythm
3. DISCUSSION
MD is well known to be associated with atrial and ventricular fibrosis.4 Atrial fibrosis is visualized by electroanatomical mapping as low‐voltage zones (LVZs), which are known as a substrate of AF, and an LVZ ablation after a PVAI has been shown to improve the outcomes in patients with persistent AF and LVZs.3
In general, patients with persistent AF and LVZs are likely older (mean age, 65 years old) and more frequently have a larger LA, underlying heart disease, and comorbidities such as hypertension, diabetes, and renal dysfunction.3, 5 The DR‐FLASH score is advocated to predict the presence of an LVZ by accumulating the number of comorbidities of AF.5 The score in this patient was one, indicating that the odds ratio of the existence of an LVZ was 0.24.5 Therefore, we believed that the LVZ in this patient was primarily caused by the MD rather than a secondary effect of comorbidities related to AF.
The LVZ was considered to be a substrate of the AF in this patient because an LVZ with fragmented potentials was identified in the anteroseptal LA where the AF was terminated during the LVZ ablation, and further LVZ ablation rendered the AF noninducible albeit the AF had been easily induced before the LVZ ablation.
It is still unclear whether maintenance of sinus rhythm can lead to an improved survival in patients with MD; however, to some extent, ablation can contribute to improve their symptoms and quality of life.
In conclusion, we found an LVZ in the LA that functioned as a substrate for AF in a patient with persistent AF and MD. The ablation of that LVZ terminated and eliminated the AF.
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
Otsubo T, Tsuchiya T, Yamaguchi T, Takahashi N. Left atrial low‐voltage zone ablation of persistent atrial fibrillation in a patient with myotonic dystrophy: A case report. J Arrhythmia. 2018;34:302–304. https://doi.org/10.1002/joa3.12059
REFERENCES
- 1. Norwood FL, Harling C, Chinnery PF, Eagle M, Bushby K, Straub V. Prevalence of genetic muscle disease in Northern England: in‐depth analysis of a muscle clinic population. Brain. 2009;132:3175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Groh WJ, Groh MR, Saha C, et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008;19:2688–97. [DOI] [PubMed] [Google Scholar]
- 3. Yamaguchi T, Tsuchiya T, Nakahara S, et al. Efficacy of left atrial voltage‐based catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:1055–63. [DOI] [PubMed] [Google Scholar]
- 4. Petri H, Ahtarovski KA, Vejlstrup N, et al. Myocardial fibrosis in patients with myotonic dystrophy type 1: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2014;16:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kosiuk J, Dinov B, Kornej J, et al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR‐FLASH score. Heart Rhythm. 2015;12:2207–12. [DOI] [PubMed] [Google Scholar]


