Abstract
A 10‐month‐old infant experienced cardiac arrest caused by ventricular fibrillation (VF). His electrocardiogram (ECG) at rest was within the normal range. Amiodarone was indispensable due to its refractoriness to defibrillation. After implantable cardioverter defibrillator (ICD) implantation, ICD shock was delivered. ICD recordings documented VF and ventricular tachycardia (VT) triggered by premature ventricular contractions with an extremely short coupling interval (240 ms), which were controlled by verapamil. To the best of our knowledge, our case is the first infant with ScTdP. As the electrical storm with ScTdP occurs unpredictably, it can be a cause of sudden infant death syndrome.
Keywords: short‐coupled variant of torsade de pointes, sudden infant death syndrome, ventricular fibrillation, ventricular tachycardia, verapamil
1. CASE PRESENTATION
A 10‐month‐old boy suffered an out‐of‐hospital cardiac arrest while he was being carried quietly by his mother without crying or moving. He had no family history of cardiac disease or sudden death. Ventricular fibrillation (VF) was recorded when an ambulance arrived, and one time of defibrillation shock by automated external defibrillator (AED) restored sinus rhythm (Figure 1A). However, the restored status easily degenerated into pulseless polymorphic ventricular tachycardia (VT) in the ambulance (Figure 1B). Arriving at our hospital, he was in pulseless VT. Defibrillation was performed three times, but sinus rhythm could not be sustained. Amiodarone (AMD) infusion (5 mg/kg) successfully suppressed the consecutive VTs, and once in the intensive care unit, the patient was cooled to 34°C to protect the central nervous system, after which he made an intact neurological recovery. A 12‐lead electrocardiogram (ECG) under sedation was within the normal range and showed no sign of long QT syndrome, Brugada syndrome, or early repolarization syndrome (Figure 1C). Electrolyte and metabolic abnormalities were excluded, and no structural heart disease was detected by echocardiography. Torsade de pointes (TdP) recorded by AED was triggered by premature ventricular contractions (PVCs) with extremely short coupling intervals (200‐240 ms) (Figure 1B). After AMD infusion (5 μg/kg/min) for five days, we added oral AMD administration (45 mg/kg/day) to AMD infusion. AMD infusion was gradually reduced and was stopped four days after overlapping. The concentrations of AMD and mono‐N‐desethylamiodarone (MDEA) under only AMD infusion were 736.9 ng/mL and 153.4 ng/mL, respectively. Immediately after stopping AMD infusion, those were 376.1 ng/mL and 386.7 ng/mL. One month later, we placed an implantable cardioverter defibrillator (ICD). The concentrations before the ICD implantation were 354.7 ng/mL and 210.5 ng/mL, respectively. Although we should have considered dose‐up of AMD, we were concerned about adverse effects and we did not. The ICD was implanted via median sternotomy for this small infant. A unipolar epicardial oval patch lead for cardioversion and defibrillation was placed on the left outer surface of the pericardium as a left ventricular lead, and bipolar epicardial leads were placed on the inferior surface of the right ventricle for sensing and pacing. The ICD was placed in the large pocket, which was created by dissecting the right half of the rectus abdominis between the internal abdominal oblique and the transversus abdominis muscle (Figure S1).
Figure 1.
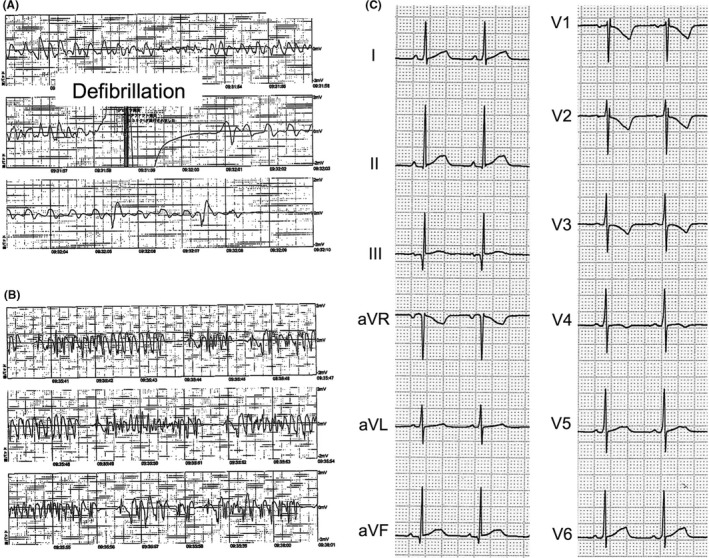
A, Defibrillation for ventricular fibrillation by automated external defibrillator. B, Torsade de pointes triggered by premature ventricular contraction with a short coupling interval of 240 ms. C, Twelve‐lead electrocardiographic recording at rest showing sinus bradycardia (heart rate = 83 beats per minute) due to deep sedation, a normal QT interval (QTc = 380 ms) calculated with Fridericia formula, with no sign of Brugada syndrome or early repolarization syndrome
Repeated 24‐hour Holter ECGs documented no sustained VT or VF, and 2 weeks after ICD implantation, he was discharged. Eight days after discharge, he was readmitted because of ICD shock. ICD recordings documented VF and VT triggered by short‐coupled PVCs (coupling interval: 240 ms) (Figure 2A,B). Moreover, 24‐hour Holter ECGs after ICD shock documented isolated PVCs with a short coupling interval (240 ms) and nonsustained VTs triggered by the short‐coupled PVCs at rest (Figure 2C). Based on these ECG findings, a diagnosis of short‐coupled variant of TdP (ScTdP) was established. As verapamil was reported to be effective for ScTdP,1 we started low‐dose verapamil administration (3 mg/kg/day) in addition to the amiodarone under close monitoring of his cardiac function and gradually increase the dose up to 45 mg: 5 mg/kg/day. Sustained VT and VF resulting in ICD shock were being controlled by the combination of verapamil (45 mg: 5 mg/kg/day) and amiodarone (45 mg: 5 mg/kg/day) at the most recent follow‐up, while nonsustained VTs within 2 s were often documented. Drug administration tests were not performed because of parents’ refusal. Uncommon cardiomyopathies inducing lethal arrhythmia or myocarditis could not be excluded because cardiac magnetic resonance imaging and myocardial biopsy were not performed due to the small body size. However, it was unlikely that the etiology of the lethal arrhythmia in our patient was due to cardiomyopathy or myocarditis because he had no symptoms of common cold, and echocardiography demonstrated normal cardiac function without structural heart disease.
Figure 2.
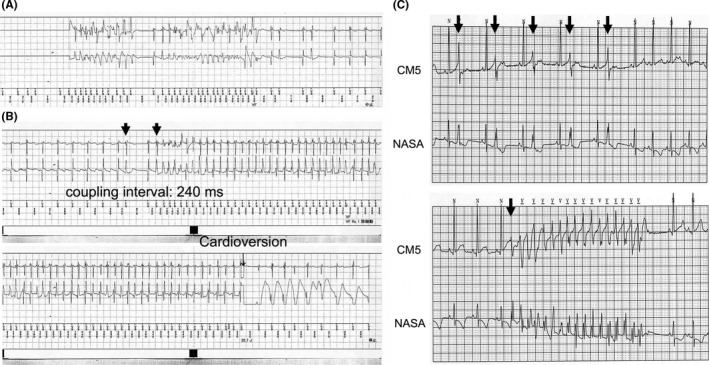
A and B, Electrocardiogram recorded in implantable cardioverter defibrillator (ICD). Upper electrogram recordings were from the v‐sense/pace leads on right ventricle, and lower electrogram recordings were from epicardial coil lead for cardioversion and defibrillation on left ventricle to ICD can. A, Ventricular fibrillation, which was spontaneously restored to sinus rhythm. B, Defibrillation for ventricular tachycardia, which was triggered by premature ventricular contraction with a short coupling interval of 240 ms. The corrected QT interval before short‐long‐short sequence was not prolonged (QTc = 363 ms), which was calculated with Fridericia formula. C, Holter electrocardiogram recording before verapamil administration, showing isolated premature ventricular contractions with a short coupling interval (240 ms) and the nonsustained polymorphic ventricular tachycardia triggered by short‐coupled premature ventricular contraction at rest. Short‐long‐short sequences were not found in this electrogram
Certain gene mutations in this type of arrhythmia have been recently reported among familial cases.2, 3 His parents submitted written informed consent for genetic analysis in accordance with the guideline approved by review board in Shiga University of Medical Science. To clarify the underlying molecular pathogenesis of his arrhythmia, we performed genetic analysis for 52 genes related to lethal arrhythmias including KCNQ1, KCNH2, SCN5A, CACNA1C and RyR2. However, we identified no genetic mutations in our patient.
2. DISCUSSION
Leenhardt et al. first reported the lethal arrhythmia, TdP triggered by PVCs with an extremely short coupling interval (<300 ms), termed ScTdP.1 The typical finding of ScTdP is a closely coupled PVC initiating TdP without structural heart disease or QT prolongation. As far as the diagnosis of ScTdP is concerned, it is difficult to judge whether the coupling interval is short because a coupling interval in an infant might be shorter than in an adult, and there is no validated normal coupling interval in infant. Regarding this patient, we considered the coupling interval less than 300 ms an appropriate coupling interval to diagnose ScTdP.
Until recently, the onset of ScTdP was considered to occur in adulthood because previously reported cases were all adults.1, 3 To the best of our knowledge, our patient is the first infant with ScTdP. The primary concern in patients with ScTdP is that TdP and its electrical storm occur unpredictably at rest without any sign. Although the prevalence of this lethal arrhythmia remains unknown, it can be a cause of sudden infant death syndrome.
Regarding the mechanisms of ScTdP, the possibility of calcium channel leakage resulting from RyR2 gene mutations and an abnormal His‐Purkinje system has been discussed. Mutations in the RyR2 gene, coding for catecholaminergic polymorphic ventricular tachycardia (CPVT), have been recently reported among familial cases of ScTdP.2, 3 Contrasting with CPVT, ScTdP occurs at rest, and calcium channel leakage during nonstressful conditions may contribute to the occurrence of ScTdP. Haïssaguerre et al. showed that the triggering PVC could arise commonly from either the right or left Purkinje system and that ablation of the trigger resulted in a very high cure rate of 89%.4 Scheinman speculated that in patients with short‐coupled PVCs, calcium waves result in delayed after‐depolarizations, which lead to reentrant arrhythmias due to defective gate function, explaining the short coupling interval as well as the focal nature of the disease process.5 Considering the proposed causes, verapamil and catheter ablation could be therapeutic options. However, verapamil is basically contraindicated in infants due to its negative inotropic and chronotropic effects. Although his cardiac function was normal at the most recent follow‐up, ongoing evaluation is mandatory. The PVCs initiating TdP had the same morphology, right bundle branch block and left axis deviation, which were speculated by Holter ECG, while we could not detect PVCs triggering ScTdP in 12‐lead ECG because PVCs were not frequent; therefore, catheter ablation could be a curative option when this patient grows up.4
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
ETHICAL APPROVAL
Genetic analysis for the ScTdP was approved by the ethical committee in Shiga University of Medical Science. The approval number was 23‐128‐3.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study (provided by the patient's parents).
Supporting information
Kise H, Ohno S, Kono Y, et al. Electrical storm in an infant with short‐coupled variant of torsade de pointes. J Arrhythmia. 2018;34:315–318. https://doi.org/10.1002/joa3.12071
REFERENCES
- 1. Leenhardt A, Glaser E, Burguera M, Nürnberg M, Maison‐Blanche P, Coumel P. Short‐coupled variant of torsade de pointes. A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994;89:206–15. [DOI] [PubMed] [Google Scholar]
- 2. Ohno S, Zankov DP, Ding WG, et al. KCNE5 (KCNE1L) variants are novel modulators of Brugada syndrome and idiopathic ventricular fibrillation. Circ Arrhythm Electrophysiol. 2011;4:352–61. [DOI] [PubMed] [Google Scholar]
- 3. Fujii Y, Itoh H, Ohno S, et al. A type 2 ryanodine receptor variant associated with reduced Ca2+ release and short‐coupled torsades de pointes ventricular arrhythmia. Heart Rhythm. 2017;14:98–107. [DOI] [PubMed] [Google Scholar]
- 4. Haïssaguerre M, Shoda M, Jaïs P, et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962–7. [DOI] [PubMed] [Google Scholar]
- 5. Scheinman MM. Role of the His‐Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm. 2009;6:1050–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


