Abstract
Prompt diagnosis of acute ST segment elevation myocardial infarction (STEMI) by the initial ECG is important in order to perform an urgent coronary angiography as soon as possible and achieve successful revascularization, therewith improving mortality and morbidity. Several diseases and conditions can mimic an acute myocardial infarction (AMI) but may not benefit from a (percutaneous) revascularization strategy. This narrative clinical review will discuss the ECG features of some of the causes of non-ischemic ST segment elevation to facilitate early recognition, prevent wrongful diagnosis and improve treatment outcomes.
Keywords: Electrocardiography, Repolarization abnormalities, ST segment elevation, Myocardial infarction, Pericarditis, Myocarditis, Takotsubo cardiomyopathy, J wave, Early repolarization, Brugada syndrome, Left bundle branch block, Hypertrophy, Ventricular pacing, Hyperkalemia, Hypercalcemia
1. Introduction
The diagnosis of STEMI should be made by a 12-lead ECG.1 Presence of ST segment elevation (STE) in a patient with acute chest pain should be considered as STEMI. If uncertainty about the diagnosis exists, repetitive ECG recordings, together with additional information from echocardiography and laboratory testing, can be included as long as it does not cause any delay in reperfusion therapy.
Since only a minority of patients with chest pain and STE seen in the emergency department have a final diagnosis of AMI, also alternative non-ischemic causes may be considered, especially if less typical symptoms are present.2,3 Although these patients would not directly benefit from revascularization therapy, an urgent coronary angiography (CAG) might still be indicated according to the guidelines to rule out obstructive coronary artery disease in the first place, especially when uncertainty exists.
This paper provides an updated comprehensive review of some non-ischemic causes of STE.
2. Electrophysiology of ST elevation
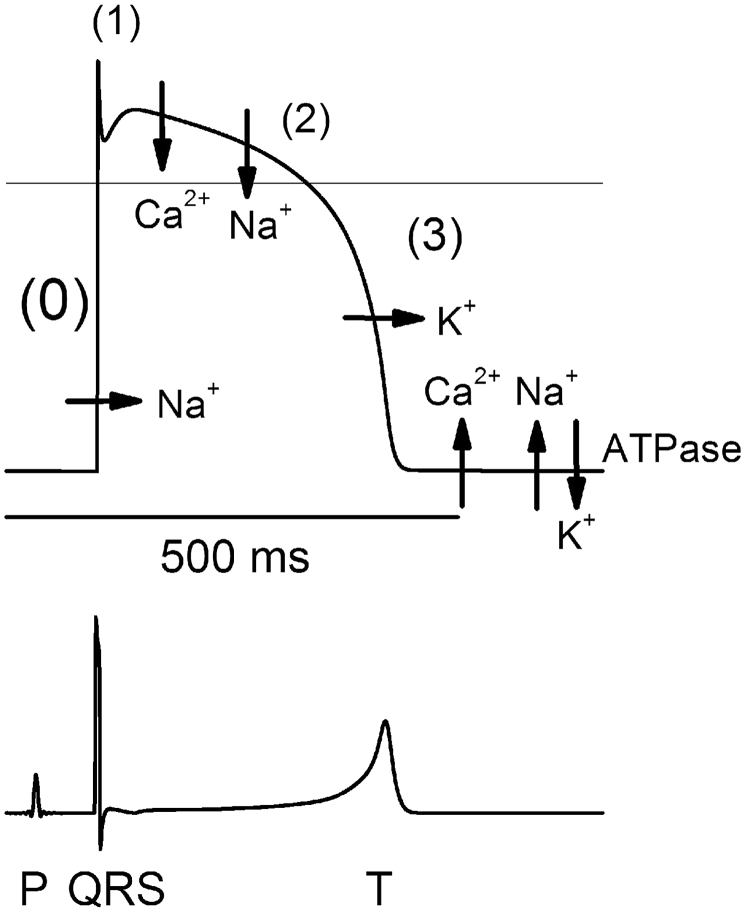
In a typical dome shaped action potential (AP) the ST segment starts with the J point at the onset of the plateau phase (phase 2) when the majority of myocardial cells have gone through rapid depolarization (phase 0) and early repolarization (phase 1). It ends with the T wave when repolarization (phase 3) returns the myocardial cells back into the negative charged resting phase (phase 4) (see Fig. 1). In normal situations the ST segment will be reflected as an isoelectric horizontal line at the ‘baseline’ because all cells have the same membrane potential during plateau phase 2 and no net voltage gradient is present in the myocardium.4, 5, 6
Fig. 1.
Schematic illustration of the action potential on the ECG. How the different electrophysiological phases of the action potential correspond with the surface ECG. With permission from: Timour Q, Frassati D, Descotes J, et al. Sudden death of cardiac origin and psychotropic drugs. Front Pharmacol. 2012;3:76.
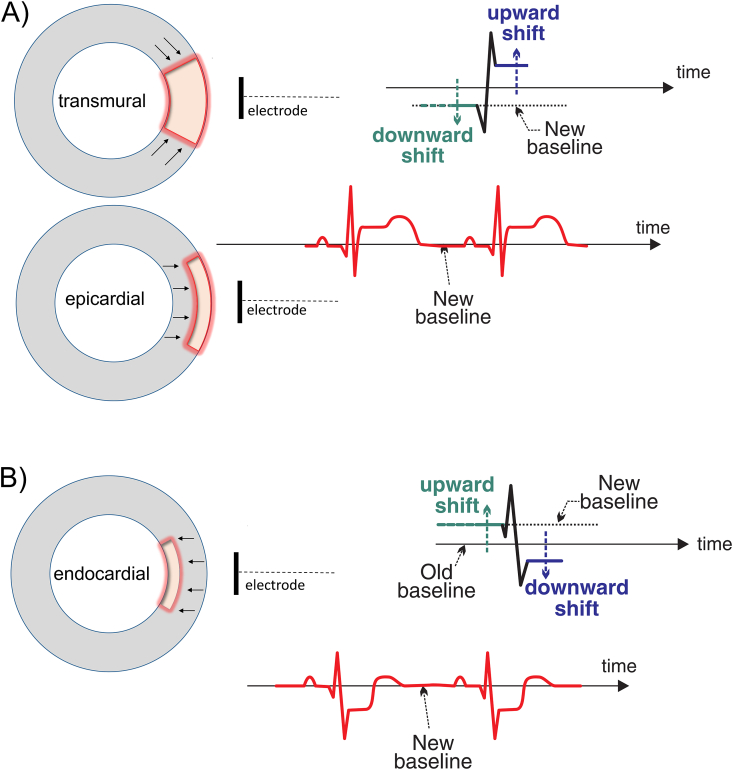
Any (local) delay in activation or distortion in duration, height and/or shape of the AP causes a voltage gradient (‘current of injury’) between injured myocardial cells and surrounding unaffected cells that influences the ST segment. Depending on the timing and location of the lesion, the ST segment can become elevated or depressed with many possible morphologies (eg. strait horizontal or oblique, upsloping or downsloping, concave upward or convex upward, dome shaped, etc.). In general, the ST segment vector points towards a lesion causing STE in transmural or epicardial lesions, and depression of the ST segment if only (sub)endocardial damage is present (see Fig. 2).
Fig. 2.
Schematic illustration of ST elevation. A) ST segment elevation in transmural or (sub)epicardial ischemia/lesion. The current of injury during systole causes an upward shift of the ST segment. The diastolic injury vector causes a further elevation of the ST segment because the downward shift of the TP and PR segment is artificially depicted as an isoelectric line. B) In (sub)endocardial ischemia/lesion the opposite is observed with a downward shift of the ST segment and elevation of the TP and PR segment. Adapted with permission from: Stroobandt RX, Barold SS, Sinnaeve AF. ECG from basics to essentials: step by step. 1st ed. Chichester: Wiley-Blackwell; 2016.
3. ST elevation myocardial infarction
In STEMI transmural ischemia is present due to abrupt blockage of a coronary artery, both organic or functional (see Fig. 3).
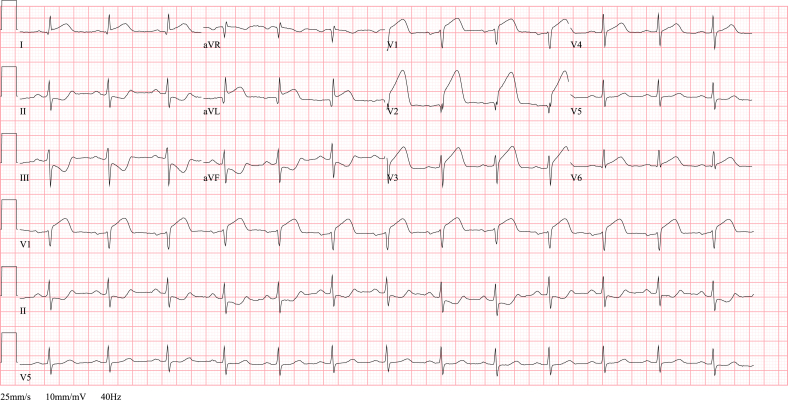
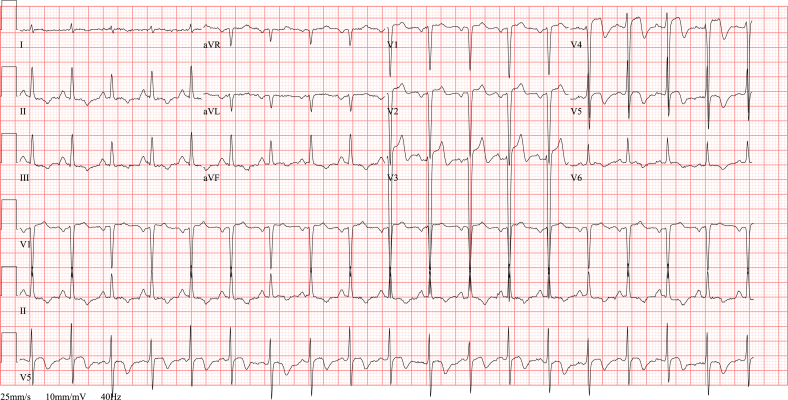
Fig. 3.
ECG STEMI. ECG showing an anterolateral STEMI in a 68 year old man with acute onset chest pain since 2 h. Note the ST segment elevation in anteroseptal and high lateral leads (I, aVL, V1-V3) and reciprocal ST depression in inferior leads (II, III and aVF). The coronary angiography revealed an ostial occlusion of the left anterior descending artery.
Ischemia counteracts the working of ion channels causing a change in AP by loss of voltage gradient, both in repolarization (diastole) and in depolarization (systole).4,7 Leads ‘overseeing’ an ischemic region record the current of injury in systole as an elevation of the ST segment. During diastole this actually would cause a depression of the TP and PR segment, but since the baseline is (artificially) depicted isoelectric all other segments have an upward shift recognized as additional STE (see Fig. 2).
Opposing leads can show STE inverted as reciprocal ST segment depression.4,5,7 The presence of concomitant reciprocal ST-segment depression in opposing leads has a high specificity for a STEMI and is therefore always important to look for, sometimes by using additional recordings of the right precordial leads (V3R and V4R) or posterior leads (V7-V9).
An important remark must be made that simultaneous ST abnormalities can interfere, known as ST segment ‘counterpoise’.8 This can lead to ‘cancellation’ if ST elevation (or depression) is present at the same time in two opposing sides of the heart. Contrary, ST segment abnormalities might also be enhanced in case of both (minor) ST elevation on one side and (major) ST depression on the other side, causing the risk of overlooking STE due to remarkable ST depression in the opposing leads.
Upsloping convex STE during the acute phase of AMI, as described by Harold E.B. Pardee in his original paper in 1920,9 is considered the typical morphology of a STEMI (“Pardee's sign”) and represents a high positive predictive value. Nevertheless, mostly a concave morphology of STE is seen which is less specific for STEMI and can be found as well in many other non-ischemic causes.
Weakening of the electrical activity in ischemic myocardium leads to a decreased R wave amplitude. If myocardial cells are no longer active, as is the case in a more extensive infarction, only the activity of opposing areas appear on the ECG as Q waves. Small Q waves may be present in healthy individuals as the normal left-to-right depolarization of the interventricular septum (‘septal’ Q waves) seen in the left-sided leads (I, aVL, V5 and V6) or in leads III and aVR as a normal variant. Q waves may also (temporarily) exist in circumstances where the vector of depolarization has changed; e.g. intraventricular conduction defect, ventricular overload and myocardial hypertrophy.
Following an infarction the weakened transmural necrotic myocardium may develop an aneurysm, most frequent of the apex and anterior wall. An aneurysm may be recognized as persistent STE, T wave inversion and loss of R wave in the precordial leads. The morphology of the ST segment ranges from subtle concave to ample convex. It must be noted that a more extensive acute myocardial infarction may still develop in or near a former infarcted region, resulting in new or more obvious STE with the pre-existent loss of R wave amplitude. The appearance of distinct Q waves, absence of reciprocal ST depression and ECG abnormalities that remain unchanged over time can help distinguish an aneurysm from STEMI. Of course, and especially in this situation, the ECG has to be interpreted in conjunction with the clinical presentation and possible information from other diagnostic tools (e.g. echocardiography).
4. Pericarditis/Myocarditis
Although the pericardial sac itself has no electrical activity, inflammation of the pericardium (pericarditis) can disrupt the AP in the epicardium.5 As a result of the diffuse process the current of injury may be recognized without correspondence to a specific coronary territory. STE will be present in most leads, except for aVR and V1 where ST segment depression can be seen due to its distant and opposite position of the normal heart axis (see Fig. 4). Involvement of the atria is responsible for depression of the PR segment and is highly suggestive for pericarditis although it may be sporadically seen in cases where myocardial infarction or early repolarization extended to the atria.
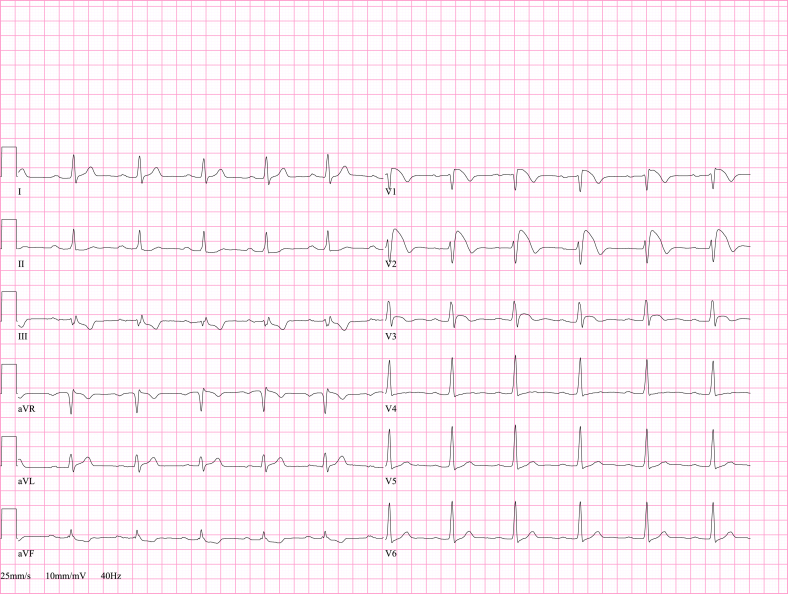
Fig. 4.
ECG pericarditis. ECG abnormalities suggestive of pericarditis in a 40 year old man with elevated inflammatory parameters and chest pain in supine position and deep inspiration. Echocardiography showed normal left ventricular systolic function with absence of pericardial effusion. After initiation of aspirin the symptoms disappeared and the ECG normalized.
The repolarization abnormalities undergo different chronological phases that might not all be seen in every patient.10 In the first phase, up to two weeks after the onset of symptoms, an upward concave STE with positive T wave and PR depression can be appreciated (with opposite findings in lead aVR and V1). After the first days to several weeks, PR and ST segments normalize and the T wave may become flattened. The last phase consists of a symmetrical T wave inversion (mostly in leads with former STE) that gradually will vanish in the next weeks or months, but may be permanent in some cases.
STE in pericarditis/myocarditis has mainly a concave morphology and unaltered R wave amplitude.
5. Takotsubo cardiomyopathy
Takotsubo cardiomyopathy (TCM) is a transient condition with decreased contractility of mid and often apical portions of the left ventricle and compensatory hyperkinesia of the basal segments. Typically it is precipitated by an event of excessive emotional or physical stress and is therefore also known as “stress cardiomyopathy” or “broken-heart syndrome”. Generally, ECG changes do not correspond to a specific coronary territory and usually lack reciprocal abnormalities. The etiology still remains unclear, but is thought to be a combination of factors that include catecholamine-related cardiotoxicity and coronary or microvascular vasospasm.11 Typically, there is no evidence of myocardial necrosis on cardiac imaging afterwards.
The initial finding on the ECG is STE (see Fig. 5), mainly in anteroseptal leads (V2-V4). These disappear in a few days and are replaced by T wave inversion and a prolonged QT interval. In many cases these ECG abnormalities may last for several months before they normalize, even when systolic function has already fully recovered in the meantime. Due to ‘stunning’ of the myocardial cells a decrease in R wave amplitude or appearance of Q waves may occasionally exist, similar to a STEMI.
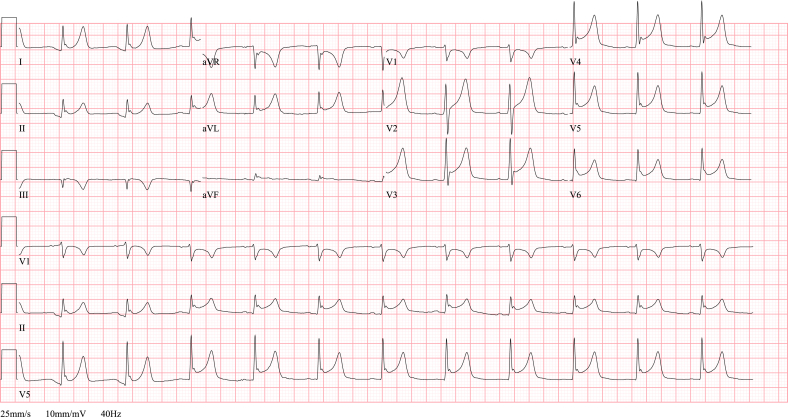
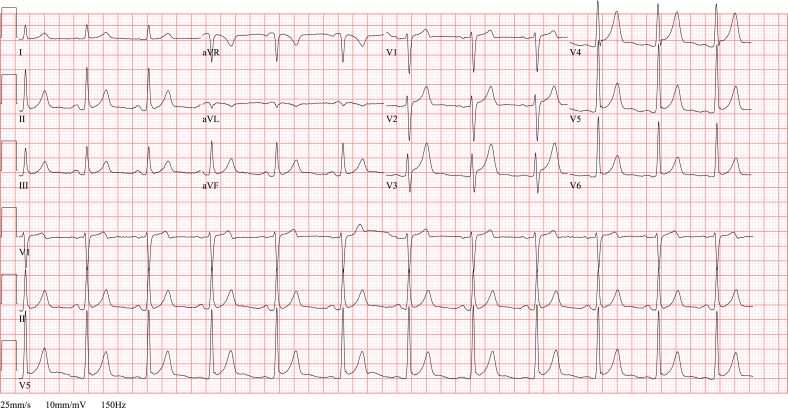
Fig. 5.
ECG TCM. ECG in a 60 year old female with respiratory distress due to acute exacerbation of COPD. An acute anterior myocardial infarction is suspected because of the ST elevation in anteroseptal leads (V1-V4), T wave inversion in inferolateral leads (II, III, aVF, V5-V6) and her worrisome respiratory condition. However, the urgent coronary angiography does not reveal any significant coronary stenosis. The diagnosis of a takotsubo cardiomyopathy is made based on the diffuse akinesia of the mid-ventricular segments, apical dilatation and hyperdynamic contractility of the basal segments, as seen on the echocardiography after the catheterization. After successful treatment of her respiratory problem, left ventricular systolic function has fully recovered.
Since absence of angiographic significant coronary artery stenosis is an important diagnostic element, TCM is actually more a diagnosis of exclusion. This makes it difficult in most cases to avoid a coronary angiography in the diagnostic work-up of acute chest pain and STEMI like ECG abnormalities, especially if cardiac enzymes are elevated.
6. J wave syndromes
The term J wave syndrome is being used to describe both Brugada syndrome (BrS) and early repolarization syndrome (ERS) although they have a different electrocardiographic appearance and clinical phenotype.12, 13, 14 This concept still has a lot of controversy and is under continuous debate.
The similarity consists of the presence of J waves; an elevation of the J point in lead V1-V3 (BrS) or a notch or slur in the second half of the R wave in inferior and/or lateral leads (ERS). Although the J point is not a true distinct point, it defines the transition in the majority of myocardial cells from the end of depolarization (end QRS) to the start of the plateau phase (onset of ST segment).
Prominent J waves sometimes seen in electrolyte disorders (hypocalcemia) or severe hypothermia are generally called ‘Osborn waves’ (after the researcher that extensively described this ECG-abnormality in hypothermic dogs) and are not considered part of the pathophysiologic spectrum of the hereafter discussed syndromes.
6.1. Brugada syndrome
The inherited combination of an increased incidence of ventricular tachyarrhythmia or sudden cardiac death (SCD) and specific electrocardiographic abnormalities are the hallmark of BrS.15
The diagnosis is made when clinical symptoms are present together with the typical type 1 Brugada pattern (BrP).16,17 This type 1 morphology is characterized by a high take-off of QRS-ST ≥ 2 mm elevation in the right precordial leads (V1-V2) without a clear r’ and with an upward convex (‘coved’) or rectilinear slowly progressive descending STE followed by a negative T wave and a slightly longer QRS duration at the right precordial leads (see Fig. 6, Fig. 7).18 The ‘new’ type 2 BrP, that is not diagnostic for BrS, includes both the ‘old’ type 2 and 3 pattern and typically has a distinct r’ wave ≥2 mm in lead V1-V2 with subsequently ≥0,5 mm STE and a positive or biphasic T wave in V2 that creates a ‘saddle back’ morphology (see Fig. 7).
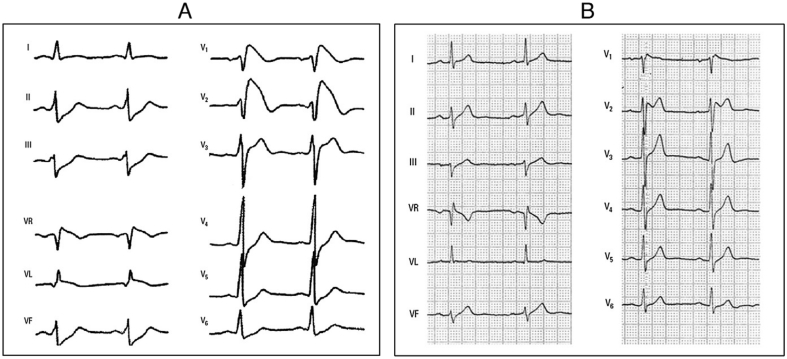
Fig. 6.
ECG type 1 BrP. ECG in an asymptomatic 17 year old boy with type 1 Brugada pattern, seen at screening consultation. In lead V1 the caved ST elevation with termination in a negative T wave is seen.
Fig. 7.
ECG type 1 and 2 BrP. A) Type 1 Brugada pattern with progressively downsloping coved STE with T wave inversion and no clear QRS-ST transition. B) Type 2 Brugada pattern with a distinct r’ followed by a concave ST segment and positive T wave forming a ‘saddle back’ morphology. With permission from: Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433–442.
Type 1 and 2 BrP can sometimes cause some confusion with an incomplete right bundle branch block in which generally the r’ wave is recognizable and narrow, an S wave is present in V6, the QRS duration is the same in all precordial leads and the ST segment is distinct.
The exact pathophysiology is still not yet understood and highly debated. A first theory proposed in 1989 ascribes the syndrome to a conduction disturbance of the right ventricular outflow tract (RVOT),19 and has been confirmed during ablation.20 A second functional theory presumes a difference in action potentials between RVOT epicardium and endocardium, explaining why the current of injury is only seen in right precordial leads (V1-V3) (see Fig. 8). Several gene mutations are identified that affect ion channels and might influence both the excitation and conduction.21,22
Fig. 8.
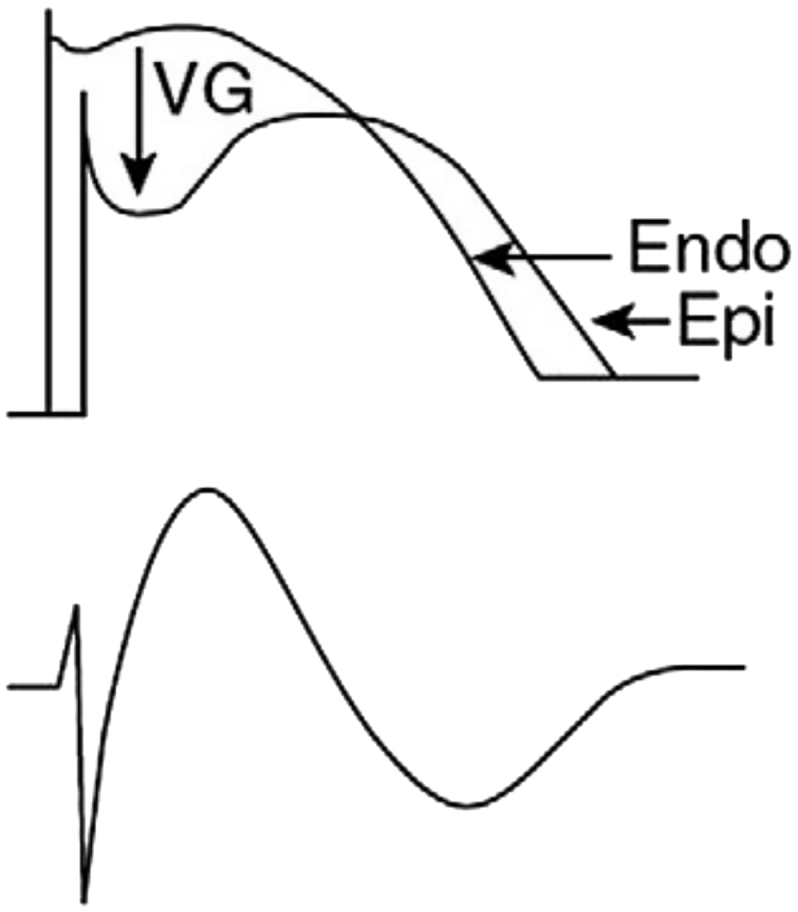
Schematic illustration of type 1 Brugada pattern. “The difference in endocardial [Endo] and epicardial [Epi] action potential (above) due to increased transient outward current of K+ (Ito) in RVOT epicardium at the beginning of phase 2 gives rise to a local voltage gradient [VG] as seen on the ECG in right precordial leads (under).” With permission from: Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433–42.
A type 1 BrP can be provoked (or unmasked) by a sodium channel blocker such as ajmaline, flecainide or procainamide. This can also occur with certain medicationa or specific circumstances like fever, electrolyte disorders, alcohol or cocaine intoxication.
6.2. Early repolarization
Early repolarization (ER) is confusing because it has been used in the past for different ECG abnormalities since real consensus was lacking. Most often it was used to describe the normal concave STE up to 3 mm in left precordial leads and sometimes in inferior leads that are referred to as ‘benign early repolarization pattern’, predominantly seen in healthy young or African males and athletes (see Fig. 9).23,24
Fig. 9.
ECG ER pattern. ECG of early repolarization pattern in a 21 year old African soccer player presenting in the emergency department with right-sided chest pain after physical collision with another player. Cardiac enzymes are not elevated and echocardiography does not reveal any functional or structural abnormalities. Symptoms disappeared within several days without any changes of the ECG at follow up visit 2 weeks later.
According to a new proposed definition the ‘true’ ER pattern consists of a J wave that begins ≥1 mm above the baseline in at least 2 contiguous leads except leads V1-V3 (see Fig. 10).13,24,25 The amplitude of the ST segment is measured at the end of the J wave (Jterminal or Jt) and assessment if it is horizontal, upward or downward sloping is done 100 ms after Jt.
Fig. 10.
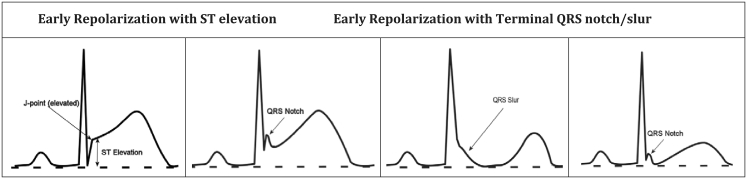
Early repolarization pattern. Different examples of early repolarization pattern with J point and ST segment elevation (first), QRS notch (second and fourth) and QRS slur (third). With permission from: Patton KK, Ellinor PT, Ezekowitz M, et al. Electrocardiographic Early Repolarization: A Scientific Statement From the American Heart Association. Circulation. 2016;133:1520–9.
The exact etiology of the ER pattern is not yet known and still researched. As with BrS, different theories have been proposed that are based on (a combination of) electrical disturbances during depolarization (phase 0 by INa or IK-ATP), early repolarization (phase 1 by Ito) and/or the plateau phase (phase 2 by ICa-L) that causes a loss-of-dome AP and therewith an injury of current in the epicardium that can be arrhythmogenic.14,24,25
The last decade this subject has drawn a lot more attention because a J wave was also rarely found in patients with idiopathic ventricular fibrillation, firstly described by Haïssaguerre and defined as the ERS.26,27 An increased risk of ventricular tachyarrhythmia and SCD was found in a subgroup of patients with a J wave and a horizontal or downsloping ST segment in the inferior and inferolateral leads.
However, the usually observed type of J point elevation – rapid upsloping concave ST segment and high R wave amplitude in precordial leads must be considered a normal pattern in absence of personal or family history of malignant arrhythmia and SCD.28, 29, 30, 31
In case of anterior STEMI the amplitude of the R wave is often smaller, the STE more pronounced and not upsloping and the QT interval longer than in early repolarization.32
7. Secondary repolarization abnormalities
Changes of the ST segment and T wave in conjunction with altered depolarization and QRS morphology, for example with abnormal intraventricular conduction or increased myocardial mass, are called secondary repolarization abnormalities.
7.1. Left bundle branch block
In left bundle branch block (LBBB) the left ventricle is depolarized later due to a non-conducted stimulus in the left His bundle, recognized as a prolonged QRS duration (>120 ms) and left axis deviation. Because the wave front of activation cannot run via the fast-conducting His-Purkinje fibers, it is conducted slowly from cell-to-cell. The delayed activation of the epicardium reverses the direction of repolarization, now starting in (sub)endocardial cells and causing the typical discordant pattern: repolarization (ST segment and T wave) is inverted to depolarization (QRS complex). Therefore, concave STE is present in leads with a negative QRS complex (V1-V3, inferior leads), and vice versa (ST depression and T wave inversion with a positive QRS complex in lateral leads) (see Table 1).
Table 1.
ECG characteristics of left bundle branch block.
|
Differentiating between LBBB and STEMI is not always easy, especially since both conditions can be present at the same time (e.g. dysfunction of the left bundle branch in anteroseptal ischemia). Concordantb STE or excessive discordance is abnormal in LBBB and may be a sign of ischemia, as defined in the original33 and modified34,35 Sgarbossa criteria (see Table 2).
Table 2.
Modified Sgarbossa criteria 34 to recognize acute myocardial infarction in case of left bundle branch block.
|
| Some exceptions that can be considered as normal: |
|
7.2. Ventricular pacing
In pacemaker stimulation of the right ventricle (RV-pacing) repolarization abnormalities are almost the same as in LBBB due to the same delay in depolarization of the left ventricle and epicardial cells.36 The best observed difference with LBBB is a QS pattern mostly seen in leads V5-V6, whereas a clear R wave is present in LBBB (see Table 3). Although the Sgarbossa criteria can principally also be used in RV-pacing (as in LBBB), its applicability is reduced due to the low sensitivity for STEMI.10
Table 3.
ECG characteristics of right ventricular pacing.
| Characteristics of RV-pacing: |
|
7.3. Left ventricular hypertrophy
Depolarization of the epicardium is delayed in hypertrophy due to the thickened muscle wall, leading to an inverted direction of repolarization and discordant repolarization abnormalities (‘strain’ pattern) best seen in leads presenting the highest QRS amplitude (especially lateral leads).
The right and septal precordial leads can resemble concave ST elevation with prominent T waves. Lateral leads often have concave ST depression and an asymmetrical negative T wave with a slow descend and a fast return to the baseline, sometimes with a final small positive deflection what leads to a biphasic T wave (‘overshooting’).
Repolarization abnormalities can be present even without remarkable increase of QRS amplitude.
8. Electrolyte disorders
Disturbances in serum concentration of electrolytes influence the membrane potential of myocytes and cause temporary repolarization abnormalities. Herewith, STE is mostly seen with hyperkalemia and hypercalcemia.
8.1. Hyperkalemia
The intercellular conduction is delayed in hyperkalemia, causing an ample T wave (narrow and peaked: the ‘Eiffel Tower effect’) and a shortened QT interval in precordial leads. Furthermore, there is a decrease of the P wave amplitude, widening of the QRS complex and discordant ST abnormalities with STE in right precordial leads (V1-V3).37 With further increasing concentration of potassium some kind of idioventricular rhythm without clear P waves can be seen. The severely broadened QRS complexes (sometimes >180 ms) are deformed into sinusoid complexes, eventually ending in asystole.
In case of ample T waves taller than the R waves, hyperkalemia must always be ruled out (for example by blood gas analysis). Reciprocal abnormalities, concordant STE or a broad based T wave can be important clues of AMI.
8.2. Hypercalcemia
Severe hypercalcemia will lead to a shorter duration of the AP due to decreased activity of Ca2+-channels during the plateau phase (phase 2) and an earlier onset of repolarization (phase 3). The interval between the onset of the QRS complex and the onset of the T wave (QTonset) will decrease and the T wave will ‘move forward’. This can give the impression of STE, especially in precordial leads. A QTonset < 180 ms has a high specificity for hypercalcemia. Besides this, the T wave will also change shape with a steeper incline and a more flattened decline (the opposite applies for the ‘normal’ T wave morphology).38 Like in hyperkalemia, the diagnosis is easily made with a quick blood gas analysis.
9. Other non-ischemic causes of ST elevation
Another cause of non-ischemic STE is pre-excitation (e.g. Wolff-Parkinson-White syndrome) where (a part of) the left ventricle is abnormally activated via accessory pathways that can give rise to secondary repolarization abnormalities. In conditions that cause right ventricular overload, e.g. pulmonary embolism, mainly T wave inversion is seen and sometimes also STE. In these cases dilatation and pressure overload leads to delayed or absent depolarization of parts of the myocardium (eg. RV or RVOT), causing a current of injury. Furthermore, (transient) STE can be seen in hypothermia (Osborn waves), intracranial pathologies (especially in subarachnoid or intraparenchymal hemorrhage) and post-cardioversion.39,40
10. Conclusion
STE can be present in more situations than only myocardial infarction (see Fig. 11). Good knowledge and awareness of the non-ischemic causes of STE is important to help diagnose these patients that might benefit from other treatment than urgent revascularization. In this paper the different alternative diagnoses of STE are therefore discussed.
Fig. 11.
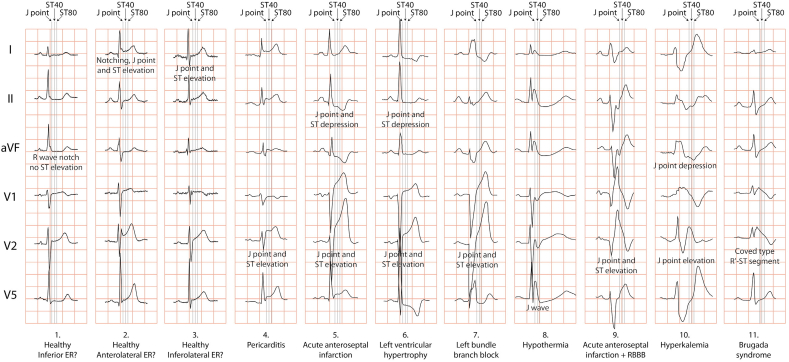
Differentiation of J point elevation and/or ST segment elevation. Some examples of elevated J point or ST segment in early repolarization [ER] (column 1, 2, 3), pericarditis (column 4), acute myocardial infarction [AMI] (column 5), hypertrophy (column 6), left bundle branch block [LBBB] (column 7), hypothermia (column 8), AMI+LBBB (column 9), hyperkalemia (column 10) and Brugada syndrome (column 11). The ER pattern with horizontal ST segment elevation inferior (column 1) is thought to represent a higher risk for malignant arrhythmias. With permission from: Postema PG, Wilde AA. Do J waves constitute a syndrome? J Electrocardiol. 2013;46:461–5.
Disclaimers, conflicts of interest, source of funding
None declared.
Acknowledgements
Thanks to DS and GP for sharing the ECG's in the database.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
See brugadadrugs.org for a comprehensive list of all medication.
Same polarity as the QRS complex.
References
- 1.Ibanez B., James S., Agewall S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2017 Aug 26 [Epub ahead of print]. PMID: 28886621. [Google Scholar]
- 2.Brady W.J., Perron A.D., Martin M.L. Cause of ST segment abnormality in ED chest pain patients. Am J Emerg Med. 2001;19:25–28. doi: 10.1053/ajem.2001.18029. PMID: 11146012. [DOI] [PubMed] [Google Scholar]
- 3.Barbagelata A., Ware D.L. Denying reperfusion or falsely declaring emergency: the dilemma posed by ST-segment elevation. J Electrocardiol. 2006;39:S73–S74. doi: 10.1016/j.jelectrocard.2006.06.006. PMID: 16916518. [DOI] [PubMed] [Google Scholar]
- 4.Klabunde R.E. Cardiac electrophysiology: normal and ischemic ionic currents and the ECG. Adv Physiol Educ. 2017;41:29–37. doi: 10.1152/advan.00105.2016. PMID: 28143820. [DOI] [PubMed] [Google Scholar]
- 5.Stroobandt R.X., Barold S.S., Sinnaeve A.F. first ed. Wiley-Blackwell; Chichester: 2016. ECG from Basics to Essentials: Step by Step. [Google Scholar]
- 6.Rautaharju P.M., Surawicz B., Gettes L.S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the American college of cardiology foundation; and the heart rhythm society. Endorsed by the international society for computerized electrocardiology. J Am Coll Cardiol. 2009;53:982–991. doi: 10.1016/j.jacc.2008.12.014. PMID: 19281931. [DOI] [PubMed] [Google Scholar]
- 7.Di Diego J.M., Antzelevitch C. Acute myocardial ischemia: cellular mechanisms underlying ST segment elevation. J Electrocardiol. 2014;47:486–490. doi: 10.1016/j.jelectrocard.2014.02.005. PMID: 24742586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madias J.E. Discordance of diagnosis of ventricular aneurysm made by the electrocardiogram and myocardial imaging: “ST-segment counterpoise” as a hypothetical mechanism. J Electrocardiol. 2006;39:340–341. doi: 10.1016/j.jelectrocard.2006.02.009. PMID: 16777523. [DOI] [PubMed] [Google Scholar]
- 9.Pardee H.E.B. An electrocardiographic sign of coronary artery obstruction. Arch Intern Med. 1920;26:244–257. [Google Scholar]
- 10.Hanna E.B., Glancy D.L. ST-segment elevation: differential diagnosis, caveats. Cleve Clin J Med. 2015;82:373–384. doi: 10.3949/ccjm.82a.14026. PMID: 26086496. [DOI] [PubMed] [Google Scholar]
- 11.Pelliccia F., Kaski J.C., Crea F. Pathophysiology of takotsubo syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. PMID: 28606950. [DOI] [PubMed] [Google Scholar]
- 12.Postema P.G., Wilde A.A. Do J waves constitute a syndrome? J Electrocardiol. 2013;46:461–465. doi: 10.1016/j.jelectrocard.2013.06.013. PMID: 23866292. [DOI] [PubMed] [Google Scholar]
- 13.Antzelevitch C., Yan G.X. J-wave syndromes: Brugada and early repolarization syndromes. Heart Rhythm. 2015;12:1852–1866. doi: 10.1016/j.hrthm.2015.04.014. PMID: 25869754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antzelevitch C., Yan G.X., Ackerman M.J. J-Wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. J Arrhythm. 2016;32:315–339. doi: 10.1016/j.joa.2016.07.002. PMID: 27761155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. PMID: 1309182. [DOI] [PubMed] [Google Scholar]
- 16.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. PMID: 24011539. [DOI] [PubMed] [Google Scholar]
- 17.Priori S.G., Blomstrom-Lundqvist C., Mazzanti A. ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). Endorsed by: association for European paediatric and congenital cardiology (AEPC) Eur Heart J. 2015;36:2757–2759. doi: 10.1093/eurheartj/ehv445. PMID: 26320108. [DOI] [PubMed] [Google Scholar]
- 18.Bayés de Luna A., Brugada J., Baranchuk A. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433–442. doi: 10.1016/j.jelectrocard.2012.06.004. PMID: 22920782. [DOI] [PubMed] [Google Scholar]
- 19.Martini B., Nava A., Thiene G. Ventricular fibrillation without apparent heart disease: description of six cases. Am Heart J. 1989;118:1203–1209. doi: 10.1016/0002-8703(89)90011-2. PMID: 2589161. [DOI] [PubMed] [Google Scholar]
- 20.Nademanee K., Veerakul G., Chandanamattha P. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. PMID: 21403098. [DOI] [PubMed] [Google Scholar]
- 21.Antzelevitch C., Patocskai B. Brugada syndrome: clinical, genetic, molecular, cellular, and ionic aspects. Curr Probl Cardiol. 2016;41:7–57. doi: 10.1016/j.cpcardiol.2015.06.002. PMID: 26671757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tse G., Liu T., Li K.H. Electrophysiological mechanisms of Brugada syndrome: insights from pre-clinical and clinical studies. Front Physiol. 2016;7:467. doi: 10.3389/fphys.2016.00467. PMID: 27803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muramoto D., Yong C.M., Singh N. Patterns and prognosis of all components of the J-wave pattern in multiethnic athletes and ambulatory patients. Am Heart J. 2014;167:259–266. doi: 10.1016/j.ahj.2013.10.027. PMID: 24439988. [DOI] [PubMed] [Google Scholar]
- 24.Patton K.K., Ellinor P.T., Ezekowitz M. Electrocardiographic early repolarization: a scientific statement from the American heart association. Circulation. 2016;133:1520–1529. doi: 10.1161/CIR.0000000000000388. PMID: 27067089. [DOI] [PubMed] [Google Scholar]
- 25.Macfarlane P.W., Antzelevitch C., Haissaguerre M. The early repolarization pattern: a consensus paper. J Am Coll Cardiol. 2015;66:470–477. doi: 10.1016/j.jacc.2015.05.033. PMID: 26205599. [DOI] [PubMed] [Google Scholar]
- 26.Viskin S. Idiopathic ventricular fibrillation “Le Syndrome d'Haïssaguerre” and the fear of J waves. J Am Coll Cardiol. 2009;53:620–622. doi: 10.1016/j.jacc.2008.11.011. PMID: 19215838. [DOI] [PubMed] [Google Scholar]
- 27.Haïssaguerre M., Derval N., Sacher F. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. PMID: 18463377. [DOI] [PubMed] [Google Scholar]
- 28.Uberoi A., Jain N.A., Perez M. Early repolarization in an ambulatory clinical population. Circulation. 2011;124:2208–2214. doi: 10.1161/CIRCULATIONAHA.111.047191. PMID: 21986288. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Riera A.R., Abreu L.C., Yanowitz F. “Benign” early repolarization versus malignant early abnormalities: clinical-electrocardiographic distinction and genetic basis. Cardiol J. 2012;19:337–346. doi: 10.5603/cj.2012.0063. PMID: 22825893. [DOI] [PubMed] [Google Scholar]
- 30.Tikkanen J.T., Huikuri H.V. Characteristics of “malignant” vs. “benign” electrocardiographic patterns of early repolarization. J Electrocardiol. 2015;48:390–394. doi: 10.1016/j.jelectrocard.2014.12.020. PMID: 25634766. [DOI] [PubMed] [Google Scholar]
- 31.Pargaonkar V.S., Perez M.V., Jindal A. Long-term prognosis of early repolarization with J-wave and QRS slur patterns on the resting electrocardiogram: a cohort study. Ann Intern Med. 2015;163:747–755. doi: 10.7326/M15-0598. PMID: 26501238. [DOI] [PubMed] [Google Scholar]
- 32.Smith S.W., Khalil A., Henry T.D. Electrocardiographic differentiation of early repolarization from subtle anterior ST-segment elevation myocardial infarction. Ann Emerg Med. 2012;60:45–56. doi: 10.1016/j.annemergmed.2012.02.015. e2. PMID: 22520989. [DOI] [PubMed] [Google Scholar]
- 33.Sgarbossa E.B., Pinski S.L., Barbagelata A. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (global utilization of streptokinase and tissue plasminogen activator for occluded coronary arteries) investigators. N Engl J Med. 1996;334:481–487. doi: 10.1056/NEJM199602223340801. PMID: 8559200. [DOI] [PubMed] [Google Scholar]
- 34.Smith S.W., Dodd K.W., Henry T.D. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012;60:766–776. doi: 10.1016/j.annemergmed.2012.07.119. PMID: 22939607. [DOI] [PubMed] [Google Scholar]
- 35.Meyers H.P., Limkakeng A.T., Jr., Jaffa E.J. Validation of the modified Sgarbossa criteria for acute coronary occlusion in the setting of left bundle branch block: a retrospective case-control study. Am Heart J. 2015;170:1255–1264. doi: 10.1016/j.ahj.2015.09.005. PMID: 26678648. [DOI] [PubMed] [Google Scholar]
- 36.Brady W.J., Lentz B., Barlotta K. ECG patterns confounding the ECG diagnosis of acute coronary syndrome: left bundle branch block, right ventricular paced rhythms, and left ventricular hypertrophy. Emerg Med Clin North Am. 2005;23:999–1025. doi: 10.1016/j.emc.2005.07.004. PMID: 16199335. [DOI] [PubMed] [Google Scholar]
- 37.Sims D.B., Sperling L.S. Images in cardiovascular medicine. ST-segment elevation resulting from hyperkalemia. Circulation. 2005;111:e295–e296. doi: 10.1161/01.CIR.0000165127.41028.D1. PMID: 15897351. [DOI] [PubMed] [Google Scholar]
- 38.Durant E., Singh A. ST elevation due to hypercalcemia. Am J Emerg Med. 2017;35 doi: 10.1016/j.ajem.2017.02.005. 1033.e3-e6. PMID: 28209241. [DOI] [PubMed] [Google Scholar]
- 39.Wang K., Asinger R.W., Marriott H.J. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003;349:2128–2135. doi: 10.1056/NEJMra022580. PMID: 14645641. [DOI] [PubMed] [Google Scholar]
- 40.Coppola G., Carità P., Corrado E. ST segment elevations: always a marker of acute myocardial infarction? Indian Heart J. 2013;65:412–423. doi: 10.1016/j.ihj.2013.06.013. PMID: 23993002. [DOI] [PMC free article] [PubMed] [Google Scholar]