Abstract
Background
Trails aimed at raising high density lipoprotein(HDL) cholesterol concentration failed to make better cardiovascular outcomes. HDL particles may be better biomarkers reflecting properties of HDL. This meta-analysis was conducted to evaluate the relation between blood HDL particles level and cardiovascular events.
Methods
PubMed and other databases were searched for eligible studies and NewCastle-Ottawa Quality Assessment Scale(NOS) was used to assess the quality of included studies. A random or fixed-effect model was applied to calculate the pooled hazard ratio(HR).
Results
Twelve studies were finally included. The pooled HR(95%confidence interval) for per standard deviation(SD) increment and top quartile versus bottom quartile were 0.79(0.72,0.86) and 0.65(0.57,0.75), respectively. Subgroup analysis suggested that HR was significantly lower in subjects with a cardiovascular disease(CVD) history than that of people without established CVD. Subclass analysis indicated that HRs for per SD increment of small(0.85) and medium(0.84) HDL particles were significantly lower than that of large HDL particles(0.96).
Conclusions
HDL particle level in blood was inversely related to CVD events, indicating that HDL particles maybe a protective factor in patients with CVD, thus making HDL particles a potential biomarker and therapy target.
Keywords: HDL, Particle, Cardiovascular events, Meta analysis
Background
It is well acknowledged that high density lipoproteins (HDL) play a role in anti-atherosclerosis and protect against the development of cardiovascular diseases (CVD). Serum concentrations of HDL cholesterol (HDL-C), the most widely used biomarker about HDL, are inversely related to risk of atherosclerotic CVD. But trails aimed at increasing HDL-C failed to improve CVD outcomes [1, 2], raising the question whether HDL-C is the best biomarker for assessing the relationship between HDL and CVD risk.
Indeed, HDL is a kind of heterogeneous population of lipoproteins, representing a spectrum of lipoprotein particles ranging 1.063–1.21 g/ml in density and 7–12 nm in size. Since HDL-C may not fully represent the properties of HDL, alternative biomarkers and parameters of HDL, such as the concentration of HDL particles (HDL-P), HDL size and apolipoprotein A1 (apoA1), are getting more attentions. Several studies evaluated the association between HDL-P and CVD outcomes, indicating that HDL-P may be a parameter making more sense in predicting CVD outcomes than HDL-C. In this study, we performed a meta-analysis to explore the relationship between HDL-P and CVD outcomes.
Methods
Selection criteria
Clinical studies were considered eligible if they satisfied the following inclusion criteria: 1) the studies about the association between concentration of HDL-P in plasma or serum and CVD outcomes; 2) Hazard ratio(HR) or odds ratio(OR) adjusted for potential influencing factors with 95% confidence interval(CI) should be available; 3) more than 50 patients should be involved in the study. Studies were excluded on any of the following criteria: 1) review articles or case reports; 2) If studies were published by the same group with overlapping patient populations, the most recent one was selected.
Search strategy
We searched the PubMed, Cochrane Library, China National Knowledge Infrastructure(CNKI) and Weipu(VIP) database using varying combinations of the following keywords: “high density lipoprotein”, “HDL” “subclasses”, “particles”, “subpopulation”, “subfraction”; “CVD”, “cardiovascular diseases”, “cardiovascular events”, “cardiac events”. The results were limited to English and Chinese language. The last search update was performed on November 20,2016. References of all eligible studies were manually searched for additional eligible studies.
Quality assessment and data extraction
The quality of articles was assessed using NewCastle-Ottawa Quality Assessment Scale (NOS) [3, 4], by two investigators independently. Disagreement was resolved by discussion and consulting a third investigator.
Two investigators collected following information from all the included articles independently: first author’s name, publication year, study design, sample size, basic status of subjects(country, age, disease status, treatment), detection method for HDL-P, definition of outcome events, follow-up time, HR/OR for total HDL-P and HDL-P subclasses if applicable.
Statistical methods
STATA 11.0 software was used to perform all statistical analyses. Forest plots were used to assess the pooled HR of HDL-P concentration for CVD events. The I2 test was performed to estimate the heterogeneity between articles. I2 more than 50% indicated significant heterogeneity and a random-effect model was applied. Otherwise, a fixed-effect model was used. Subgroup analysis was conducted to determine the source of heterogeneity. Heterogeneity was also investigated by sensitivity analysis, which was performed by removing each study sequentially to assess the impact of each study. Potential publication bias was assessed with funnel plots and the Begg’s test. P value < 0.05 was considered as statistically significance.
Results
Eligible studies and quality assessment
Flowchart of study selection was illustrated in Fig. 1. Eleven studies [5–15] were finally included in this meta-analysis. The basic characteristics and quality assessment result of the eligible articles were shown in Table 1.
Fig. 1.
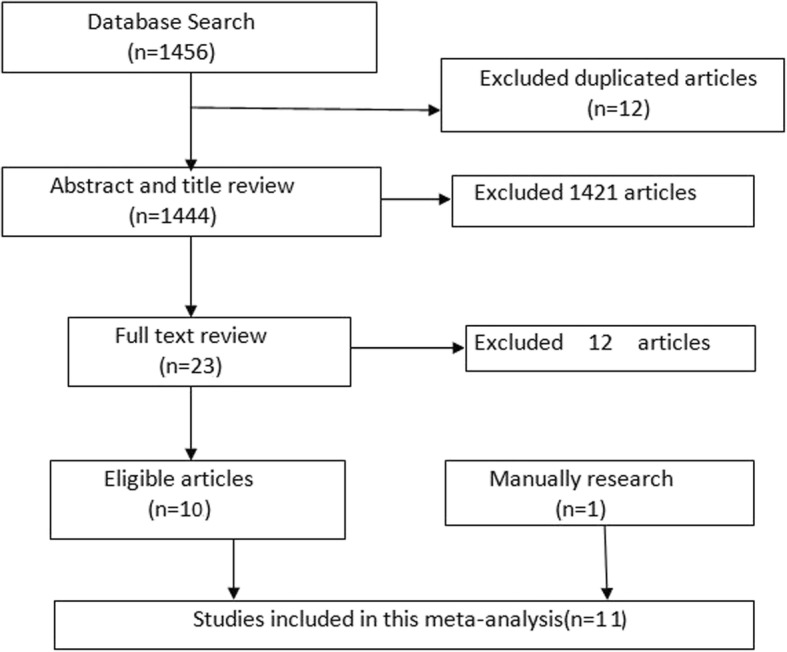
Flowchart of the included studies
Table 1.
Characteristics of the Eligible studies
| First Author | Year of publication | Study design | Sample size(case+control/events+free of events) | Area | Age(years) | Quality score |
|---|---|---|---|---|---|---|
| Mackey | 2012 | cohort study | 5597(227 + 5370) | US | mean of 61.5 | 9 |
| Hsia | 2008 | nested case-control study | group1:404(202 + 202) | US | 50 to 79 | 7 |
| group2:304(152 + 152) | ||||||
| Akinkuolie | 2014 | cohort study | model 1: 25706(947 + 24,759); model 2: 25232(911 + 24,321) | US | Free of events: median of 52.6; Events:median of 57.9 | 9 |
| Berger | 2012 | nested case-control study | 1372(686 + 686) | US | median of 69 | 8 |
| Duprez | 2009 | nested case-control study | 728(248 + 480) | cross-country | median of 49 | 5 |
| Kuller | 2007 | case control study | 428(214 + 214) | US | mean of 51.3 | 8 |
| Otvos | 2006 | nested case-control study | 1061(364 + 697) | US | mean of 64.4 | 7 |
| Chandra | 2015 | cohort study | 1977 | US | not given | 8 |
| Parish | 2012 | cohort study | 20,021(1796 + 18,225) | UK | 40 to 80 | 8 |
| Musunuru | 2009 | cohort study | 4594(337 + 4257) | Sweden | not given | 9 |
| Harchaoui | 2009 | nested case–control study | 2223(822 + 1401) | UK | mean of 65 | 9 |
Since two groups in Hsia’s study [12] used different subjects without overlapping, we treated this study as two independent ones in following analysis. Two models were established in Akinkuolie’s [5] study because of different influencing factors adjusted for. There existed overlapping subjects. The whole sample size was 26,332(data not shown). Eleven articles with 63,064 subjects were involved in this meta-analysis. Seven out of the 11 studies were conducted in America.
We used NOS to evaluate the quality of eligible articles. The full score is nine in NOS system. Duprez’s study [9] scored as five, ranking normal in quality assessment. Others got scores higher than 6, ranking as high quality. And there were four studies getting full marks in NOS quality assessment.
Correlation between total HDL-P concentration and CVD events
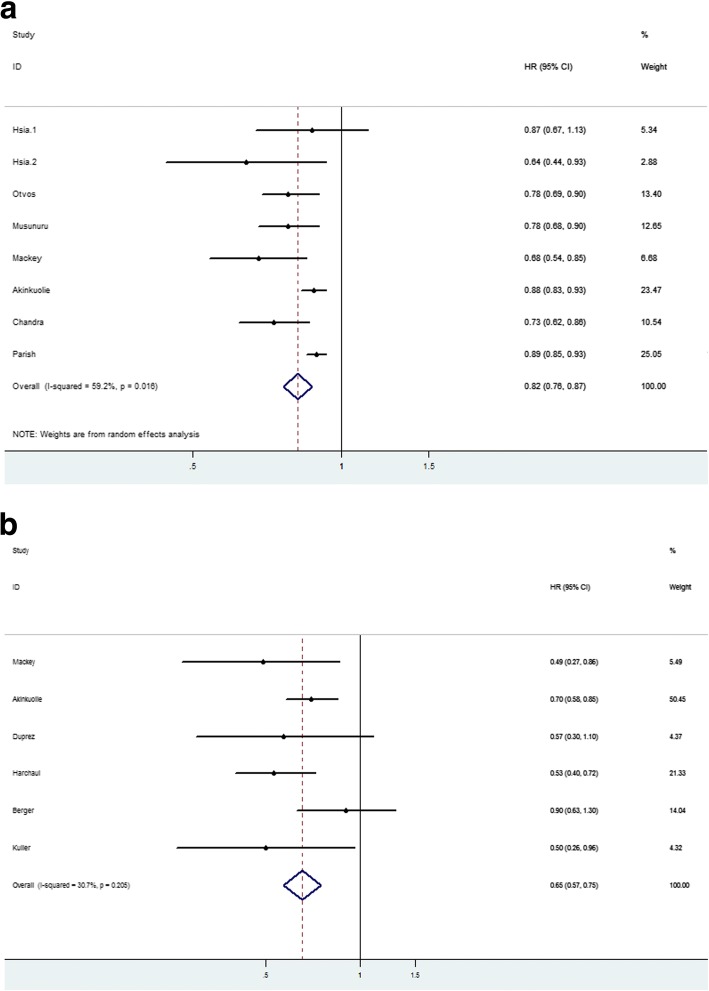
As shown in Table 2, HR or OR was calculated for per standard deviation(SD) increment or top quartile (Q4) versus bottom quartile (Q1) and we calculated pooled HR(OR) for both per SD increment and Q4 vs Q1. The combined HR for per SD increment was 0.82(95%CI:0.76,0.87) using a random-effect model for I2 = 59.2%, P = 0.004(Fig. 2a). The pooled HR for Q4 vs Q1 was 0.65 (95%CI: 0.57,0.75) calculated by a fixed-effect model with I2 = 30.7%, P = 0.205(Fig. 2b). Both indicated that a higher HDL-P level was reversely related with CVD events. The forest plots were drawn in Fig. 2.
Table 2.
Correlation between total HDL-P concentration and CVD events
| Author | Baseline of subjects | Adjusted for | Events | Follow-up time | HR(95%CI) or OR for 1-SD increment | HR(95%CI) or OR for Q4 vs Q1 |
|---|---|---|---|---|---|---|
| Mackey | MESA articipants without self-reported CVD,pregnancy, cancer, cognitive impairment, or weight > 136 kg,lipid-lowering medication use,TG > 400 mg/dl | model1:age,sex,ethnicity,hypertension,smoking; model2: model1 + HDL-C,LDL-P,LDL-C,TG | myocardial infarction, CHD death, resuscitated cardiac arrest, or definite or probable angina (followed by revascularization) | mean of 6.0 years | model1:0.70(0.59–0.82); model2: 0.68(0.54–0.85) | model 1: 0.46(0.30–0.71);model2: 0.49(0.27–0.86) |
| Hsia(group1) | postmenopausal women with intact uterus in Estrogen Plus Progestin Trial | age, treatment arm, smoking, alcohol use, diabetes, hypertension | CHD MI/coronary death | 4 years | 0.87 (0.67–1.13) | – |
| Hsia(group2) | postmenopausal women with prior hysterectomy in Estrogen Alone Trial | age, treatment arm, smoking, alcohol use, diabetes, hypertension | CHD MI/coronary death | 4 years | 0.64 (0.44–0.93) | – |
| Akinkuolie | subjects in the WHS free of self-reported CVD or cancer or lipid-lowering medications | model 1:age, race,blood pressure, smoking, menopausal status,hormone replacement therapy,and treatment assignment.model 2: model 1 + BMI,diabetes,LDL-C, LDL-P,TG and other HDL subclasses. | nonfatal MI, percutaneous coronary,intervention, coronary artery bypass grafting, and CHD death | median of 17 years | model1: 0.91(0.86–0.97); model2: 0.88(0.83–0.93) | model 1:0.77(0.64–0.92); model2:0.70(0.58–0.85) |
| Berger | postmenopausal women from the WHI-OS with no prior history of MI or stroke | smoking status,BMI,systolic blood pressure,use of anti-hypertensive medication,diabetes and physical activity | Ischemic stroke | mean follow-up of 7.9 years | – | 0.90(0.63–1.30) |
| Duprez | HIV-infected patients | model1:age, race, HIV-RNA and ART status, smoking, prior CVD, diabetes, use of BP-lowering drugs, use of lipid-lowering drugs, hepatitis co-infection, CD4+, BMI and major baseline ECG abnormalities;model2:model1 + LDL and triglycerides+D-dimer, IL-6 and hsCRP | non-fatal CHD events (defined as clinical and silent myocardial infarction, coronary revascularization and coronary artery disease requiring drug treatment), non-fatal atherosclerotic non-CHD (defined as stroke and peripheral arterial disease), congestive heart failure and fatal CVD (defines as CVD death and unwitnessed death) | – | – | model1: 0.41(0.2–0.7); model2: 0.57(0.3–1.1) |
| Kuller | men with metabolic syndrome within the MRFIT | white blood cell count,smoking status | CHD death | 18 years | – | 0.50(0.26–0.96) |
| Otvos | men with an established diagnosis of CHD in the VA-HIT | treatment,age, hypertension,smoking,BMI, diabetes | a nonfatal MI or CHD death | median of 5.1 years | 0.78(0.69–0.90) | – |
| Chandra | participants from the Dallas Heart Study not taking any lipid lowering medication or hormone replacement therapy,free from malignancy, connective tissue disease, or HIV | model1:age, sex, ethnicity, hypertension, diabetes, smoking, BMI, non-HDL-C, logTG, any lipid-lowering therapy, hormone replacement therapy, menopause, alcohol intake, and history of CHD at baseline; model2:model1 + HDL-C | non-fatal myocardial infarction, stroke,coronary artery bypass graft (CABG), percutaneous coronary intervention, or cardiovascular death | median of 9.3 years | model1: 0.75(0.65–0.86); model2: 0.73(0.62–0.86) | – |
| Parish | high-risk individuals in the MRC/BHF HPS.A nonfasting blood total cholesterol concentration of at least 3.5 mmol/L (135 mg/dL) and either had a previous diagnosis of CHD, cerebrovascular disease, other occlusive disease of noncoronary arteries, or diabetes mellitus (type I or II) or men 65 years of age undergoing treatment for hypertension | model1:age, sex, simvastatin and vitamin allocation, smoking, prior disease, systolic blood pressure, estimated glomerular filtration rate, medication, and N-terminal pro-B-type natriuretic peptide;model2 = model1 + LDL-P | nonfatal MI or coronary death other than death from heart failure or sudden death | mean of 5.3 years | model1: 0.88(0.83–0.92); model2: 0.89(0.85–0.93) | – |
| Musunuru | healthy people in the MDC-CC. Subjects with prior MI/stroke or on baseline lipid-lowering therapy were excluded | age, gender, systolic blood pressure, use of antihypertensivemedications,diabetes status, and current smoking status | myocardial infarction, stroke, and death from coronary heart disease, a secondary coronary endpoint of myocardial infarction and death from coronary heart disease | mean of 12.2 years | 0.78(0.68–0.90) | – |
| Harchaoui | participants in EPIC-Norfolk cohort.All individuals who reported a history of heart attack or stroke or use of lipid-lowering drugs at the baseline clinic visit were excluded | model1:smoking,myeloperoxidase, paraoxonase 1, and C-reactive protein levels; model2 = smoking+apol poprotein B and logTG | fatal or nonfatal CAD which was defined as codes 410 to 414 according to the International Classification of Diseases, Ninth Revision | average of 6 years | – | model1: 0.78(0.59–1.03); model2: 0.53(0.40–0.72) |
TG triglyeride, CHD coronary heart disease, NMR nuclear magnetic resonance, LDL-P low density lipoprotein particles, LDL-C low density lipoprotein cholesterol, MI myocardial infarction, BMI body mass index, CAD coronary artery disease, HIV Human immunoddficiency virus, ART antiretroviral therapy, ECG electrocardiography, hsCRP high sensitivity C-Reactive Protein, MESA ulti-ethnic study of atherosclerosis, WHS omen’s Health Study, WHI-OS Women’s Health Initiative Observational Study, MRFIT Multiplle Risk Factor Intervention Trial, VA-HIT Veterans Affairs High-Density Lipoprotein Intervantion Trial, HPS Heart Protection Study, MDC-CC Malmö Diet and Cancer Study, EPIC European Prospective Investigation into Cancer and Nutrition
Fig. 2.
Forest plots of HR between total HDL-P concentration and CVD events. a shows the combined HR for per SD increment using a random-effect model for I2 = 59.2%, P = 0.004. b shows the pooled HR for Q4 vs Q1 by a fixed-effect model with I2 = 30.7%, P = 0.205
Assessment of heterogeneity
Evidence of heterogeneity existed in HR for per SD increment. No heterogeneity was detected in HR for Q4 vs Q1. So subgroup meta-analysis was conducted to explore the source of heterogeneity in studies about HR for per SD increment. We divided studies into different subgroups according to study design, baseline status(patients with a history of CVD or not), influencing factors adjusted for(adjusting for lipids or not). There were two models in each of Mackey’s [7], Akinkuolie’s [5], Chandra’s [6] and Parish’s [13] studies. Compared to model 1, model 2 was additionally adjusted for lipids. Table 3 shows the meta-analysis results of subgroups. No grouping basis could explain heterogeneity fully, but case-control studies and studies only about females got I2 < 50% and P > 0.1, indicating insignificant heterogeneity.
Table 3.
Meta-analysis results of subgroups
| Subgroups | I2(%) | P | Pooled HR | 95% CI | |
|---|---|---|---|---|---|
| study design | case-control | 0.0 | 0.418 | 0.78 | 0.70,0.88 |
| cohort | 69.0 | 0.001 | 0.83 | 0.79,0.88 | |
| sex | female only | 18.6 | 0.297 | 0.89 | 0.84,0.93 |
| both | 73.3 | 0.001 | 0.80 | 0.74,0.86 | |
| baseline status | no CVD history | 74.7 | 0.003 | 0.82 | 0.74,0.89 |
| partly with CVD | 60.0 | 0.029 | 0.83 | 0.78,0.89 | |
| influencing factors adjusted for | adjusted for lipids | 70.1 | 0.018 | 0.84 | 0.77,0.91 |
| not adjusted for lipids | 65.2 | 0.005 | 0.81 | 0.76,0.87 | |
Also, sensitivity analysis was conducted by omitting the studies one by one and repeating the meta-analysis. But heterogeneity remained significant with single study removal. Heterogeneity became insignificant with the removal of Akinkuolie’s [5] study together with Parish’s [13] (I2 = 0.0%, P = 0.658). The pooled HR was 0.76(95% CI: 0.71,0.82) with no significant difference from the HR with these two articles included.
Meta-analysis about HDL-P subclasses
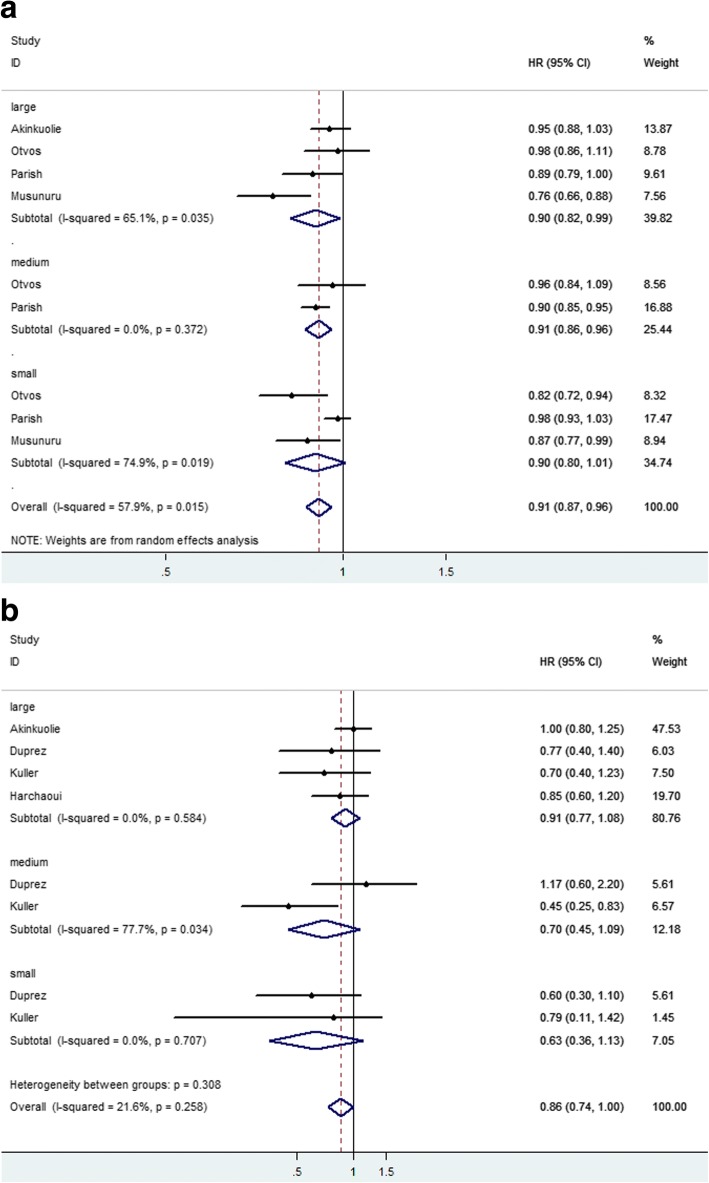
We also collected the data about HR or OR for HDL-P subclasses and CVD events. The diameter ranges of large, medium and small HDL-P were 8.8–14.0 nm, 8.2–8.8 nm, 7.3–8.2 nm, respectively. Seven articles analyzed the relation between levels of large HDL-P and CVD events. Four studied the relation between levels of medium HDL-P and CVD events. Five studied the relation between levels of small HDL-P with CVD events. HRs was calculated for per SD increment and Q4 versus Q1. Forest plots were shown in Fig. 3. In Fig. 3a, HRs were calculated for per SD increment. Subtotal HRs of large, medium and small HDL-P were 0.90(0.82,0.99), 0.91(0.86,0.96) and 0.90(0.80,1.01), respectively. In Fig. 3b, no significant difference was observed in HRs of HDL-P subclasses.
Fig. 3.
Forest plots for small, medium and large HDL-P. a HRs were calculated for per SD increment. b HRs were calculated for Q4 vs Q1
Assessment of publication bias test
As Fig. 4 shows, studies included were symmetrical. As shown in Fig. 4,the Begg’s funnel plots of eight studies about HR calculated for per SD increment(Fig. 4a) and Q4 vs Q1(Fig. 4b) were almost symmetric(P = 0.108, 1.00, respectively),suggesting a low likelihood of publication bias.
Fig. 4.
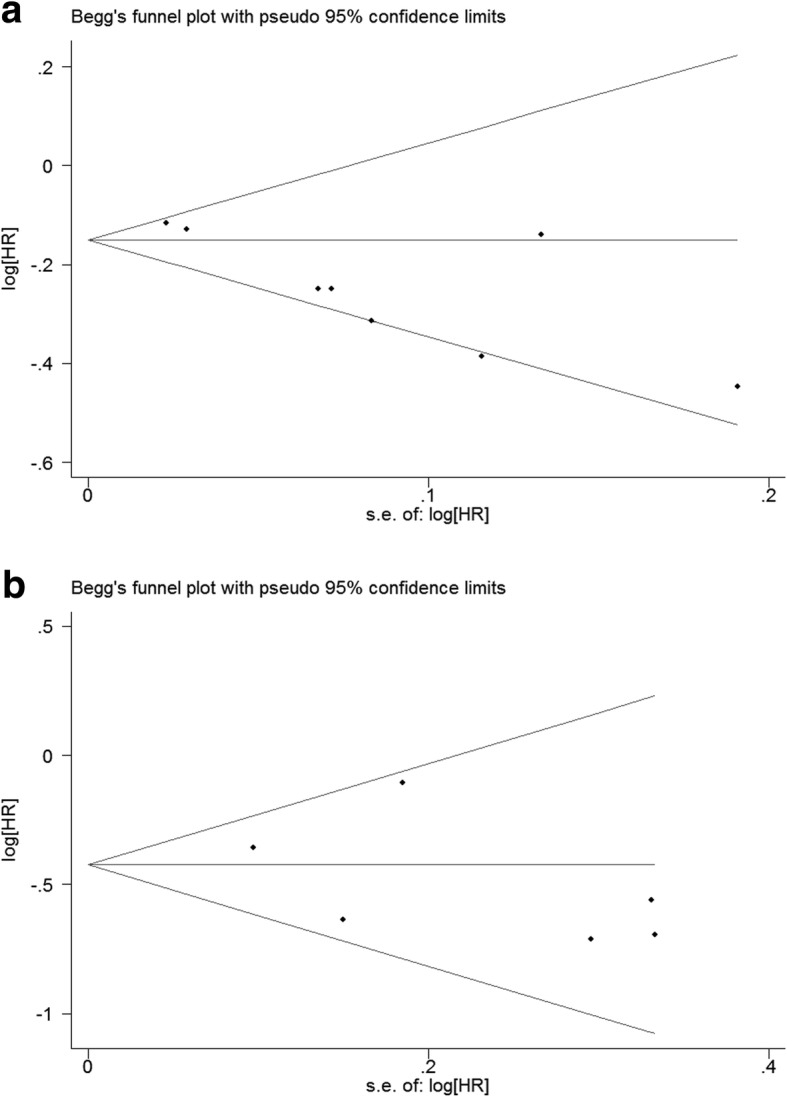
funnel plots of included studies. a shows the Begg's funnel plots of studies about HR calculated for per SD increment. b shows the Begg's funnel plots of studies about HR calculated for Q4 vs Q1
Discussion and conclusions
It is well established that serum HDL takes a cardioprotective role and is reversely related with CVD events risk. Functions of HDL include its role in reverse cholesterol transport (RCT), anti-oxidation and anti-inflammation. In this meta-analysis, we searched and analyzed studies about the value of blood HDL-P levels in predicting incident CVD events.
Results of meta-analysis showed that the pooled HR (or OR) for per SD increment and Q4 versus Q1 of blood HDL-P level was 0.82 and 0.65, 95% CI ranging from 0.76 to 0.87 and 0.57 to 0.75, respectively. Same as established theories, our data indicates that higher HDL-P level maight be a protective factor for CVD events.
We also analyzed clinical value of different HDL-P subfranctions in predicting CVD events. HRs for per SD increment of large, medium and small HDL-P were 0.90, 0.91 and 0.90, with no significant difference. But the results of Kim’s study [16], small and medium HDL-P were significantly and inversely correlated with carotid intima-media thickening measurement results. The possible reason was that the relation between small and medium HDL-P with cardioprotective paraoxonase1 activity may represent the function of HDL. But HRs for Q4 versus Q1 were 0.91, 0.70 and 0.63, respectively. And 95% CI of HRs for Q4vsQ1 cover 1. One possible explanation was the shortage of studies included. Four [5, 8, 9, 14] studies explored the association between large HDL-P levels and CVD events. Only two [8, 9] researches studied medium and small HDL-P.
HDL particles spectrum changes in the whole HDL metabolism. And different HDL-P subclasses have varied functions. Small HDL-P seems to have a stronger anti-inflammatory effect than large HDL-P [17]. Large HDL-P is reported to have the property of inhibiting platelet activation and stimulating the activation of anti-coagulant proteins [18]. Since the structure and ingredient of HDL particle vary in the process of HDL metabolism, amounts of HDL-C and apolipoprotein A1 differ in different HDL particles. Large HDL-P contains more cholesterol inside. So HDL-C may not reflect the whole function and amount of HDL in blood. HDL-P may be a promising biomarker for CVD events and a new target of therapy.
Heterogeneity existed in pooled HR for per SD increment, but not Q4vsQ1. Sensitivity analysis demonstrated removal of Akinkuolie’s [5] and Parish’s [13] studies made heterogeneity insignificant. No publication bias was observed. With 63,064subjects included, total sample size was large in this meta-analysis study. Our study came to a robust result that blood HDL-P concentration is a promising biomarker reversely related to CVD events, regardless of patients’ basic status.
Availability of data and materials
All data analyzed during this study are included in the references.
Abbreviations
- BMI
Body mass index
- CHD
Coronary heart disease
- CI
Confidence interval
- CVD
Cardiovascular disease
- EPIC
European Prospective Investigation into Cancer and Nutrition
- HDL
High density lipoprotein
- HIV
Human immunoddficiency virus
- HPS
Heart Protection Study
- HR
Hazard ratio
- hsCRP
High sensitivity C-Reactive Protein
- MDC-CC
Malmö Diet and Cancer Study
- MESA
Ulti-ethnic study of atherosclerosis
- MI
Myocardial infarction
- MRFIT
Multiplle Risk Factor Intervention Trial
- NMR
Nuclear magnetic resonance
- NOS
NewCastle-Ottawa Quality Assessment Scale
- OR
Odds ratio
- SD
Standard deviation
- TG
Triglyeride
- VA-HIT
Veterans Affairs High-Density Lipoprotein Intervantion Trial
- WHI-OS
Women’s Health Initiative Observational Study
- WHS
Women’s Health Study
Authors’ contributions
YJW designed this study and performed the statistical analysis. ZJF and YQT searched the databases for eligible studies. SL was a major contributor in writing the manuscript. SYL was a major contributor in acquisition of data and analysis of data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
YuJing Wu, Email: yujing_wu5@163.com.
ZhiJuan Fan, Email: 1191040152@qq.com.
YaQiong Tian, Email: 595733855@qq.com.
Shuang Liu, Email: 1430437734@qq.com.
ShuYe Liu, Phone: 86-022-84112332, Email: liushuye@tijmu.edu.cn.
References
- 1.Group HTC HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34(17):1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 3.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. NewCastle-Ottawa quality assessment scale--case control studies[EB/OL] 2011. [Google Scholar]
- 4.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. NewCastle-Ottawa quality assessment scale--cohort studies[EB/OL] 2011. [Google Scholar]
- 5.Akinkuolie AO, Paynter NP, Padmanabhan L, Mora S. High-density lipoprotein particle subclass heterogeneity and incident coronary heart disease. Circ Cardiovasc Qual Outcomes. 2014;7(1):55–63. doi: 10.1161/CIRCOUTCOMES.113.000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra A, Neeland IJ, Das SR, Khera A, Turer AT, Ayers CR, McGuire DK, Rohatgi A. Relation of black race between high density lipoprotein cholesterol content, high density lipoprotein particles and coronary events (from the Dallas heart study) Am J Cardiol. 2015;115(7):890–894. doi: 10.1016/j.amjcard.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackey RH, Greenland P, Goff DJ, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60(6):508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2007;195(1):122–128. doi: 10.1016/j.atherosclerosis.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duprez DA, Kuller LH, Tracy R, Otvos J, Cooper DA, Hoy J, Neuhaus J, Paton NI, Friis-Moller N, Lampe F, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207(2):524–529. doi: 10.1016/j.atherosclerosis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger JS, McGinn AP, Howard BV, Kuller L, Manson JE, Otvos J, Curb JD, Eaton CB, Kaplan RC, Lynch JK, et al. Lipid and lipoprotein biomarkers and the risk of ischemic stroke in postmenopausal women. Stroke. 2012;43(4):958–966. doi: 10.1161/STROKEAHA.111.641324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otvos JD. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the veterans affairs high-density lipoprotein intervention trial. Circulation. 2006;113(12):1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 12.Hsia J, Otvos JD, Rossouw JE, Wu L, Wassertheil-Smoller S, Hendrix SL, Robinson JG, Lund B, Kuller LH. Lipoprotein particle concentrations may explain the absence of coronary protection in the Women's Health Initiative hormone trials. Arterioscler Thromb Vasc Biol. 2008;28(9):1666–1671. doi: 10.1161/ATVBAHA.108.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins R. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF heart protection study. Circulation. 2012;125(20):2469–2478. doi: 10.1161/CIRCULATIONAHA.111.073684. [DOI] [PubMed] [Google Scholar]
- 14.El Harchaoui K, Arsenault BJ, Franssen R, Despre JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150(2):84–93. [DOI] [PubMed]
- 15.Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, Berglund G, Hedblad B, Engstrom G, Williams PT, et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29(11):1975–1980. doi: 10.1161/ATVBAHA.109.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DS, Li YK, Bell GA, Burt AA, Vaisar T, Hutchins PM, Furlong CE, Otvos JD, Polak JF, Arnan MK, et al. Concentration of smaller high-density lipoprotein particle (HDL-P) is inversely correlated with carotid intima media thickening after confounder adjustment: the multi ethnic study of atherosclerosis (MESA). J Am Heart Assoc. 2016;5(5) 10.1161/JAHA.115.002977. [DOI] [PMC free article] [PubMed]
- 17.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17(10):594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Nofer JR, Brodde MF, Kehrel BE. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin Exp Pharmacol Physiol. 2010;37(7):726–735. doi: 10.1111/j.1440-1681.2010.05377.x. [DOI] [PubMed] [Google Scholar]