Abstract
Studies on the effects of alcohol use on HIV disease progression have been contradictory, with at least one study finding a positive effect of low alcohol consumption on CD4 count. In addition, most such studies have taken place in the developed West. We investigated the association between alcohol use and immune reconstitution through CD4 count response among HIV-infected individuals on antiretroviral therapy (ART) at an urban sub-Saharan African clinic. This was a retrospective cohort study of treatment-naïve HIV-infected adults initiating ART in Nairobi, Kenya and followed for 12 months between January 2009 and December 2012. At enrollment, a standardized questionnaire was used to collect data on sociodemographic variables and alcohol consumption. CD4 count was measured every six months. Linear regression models assessed the association between CD4 count and alcohol consumption, categorized as abstinent, moderate, or hazardous. Overall, 854 participants were included, 522 of which were women, with 85 (25.6%) men and 50 (9.6%) women reporting any alcohol use, and 8 (2.4%) men and 7 (1.3%) women reporting hazardous drinking. At baseline, alcohol use was associated with higher education and socioeconomic status. Median CD4 count was higher among alcohol users compared to those who abstained at baseline and at 6 and 12 months post-ART initiation, although this was only significant at 6 months. There were no differences in adherence between abstainers and drinkers. While overall alcohol use was significantly associated with higher CD4 counts, moderate and hazardous use treated separately were not. We conclude that, while alcohol use was associated with higher CD4 counts at 12 months post-ART, the mechanism for this association is unclear but may reflect unmeasured socioeconomic or nutritional differences. Additional research is required on the specific drinking patterns of this population and the types of alcoholic beverages consumed to clarify this relationship.
Keywords: HIV, alcohol, CD4 count, antiretroviral therapy, Africa
Introduction
The results of studies on the effect of alcohol use on HIV disease progression are limited and contradictory. Several studies have found a correlation between heavy or frequent drinkers and lower CD4 counts and higher viral loads (Baum et al., 2010; Samet et al., 2007) compared to abstainers, while others have found no correlation (Conen et al., 2013; Ghebremichael et al., 2009; Kaslow et al., 1989; Kowalski et al., 2012). In addition, a recent French study reported that, compared with abstinent patients, those with low alcohol consumption were more likely to have significantly higher CD4 counts, while those reporting moderate consumption had CD4 cell counts not significantly different from those of abstinent patients (Carrieri et al., 2014). The influence of alcohol consumption on disease progression is thought to be mediated by poor adherence to antiretroviral therapy (ART) (Braithwaite et al., 2007; Chander, Lau, & Moore, 2006; Cook et al., 2001; Hendershot, Stoner, Pantalone, & Simoni, 2009; Jaquet et al., 2010; Samet, Horton, Meli, Freedberg, & Palepu, 2004), and delay in seeking care based on a perception that abstinence is required for ART initiation (Azar, Springer, Meyer, & Altice, 2010; Braithwaite et al., 2007; Chander et al., 2006; Cook et al., 2001; Haberer et al., 2013; Hendershot et al., 2009; Jaquet et al., 2010; Samet et al., 2004).
While the majority of these studies have been carried out in the developed world, in sub-Saharan Africa (SSA), which has the highest rate of HIV infection (5.9%) in the world (UNAIDS, 2015), patterns of alcohol consumption and even the kinds of alcoholic beverages that are present may vary significantly from the Western patterns (Hargreaves et al., 2002; Parry et al., 2005; Shaffer, Njeri, Justice, Odero, & Tierney, 2004; Willis, 2002). Understanding the role of alcohol on disease progression is crucial in providing appropriate screening and treatment regimens, and interventions among these highly at-risk populations.
This study describes the prevalence of alcohol use, explores sociodemographic characteristics associated with drinking, and examines the relationship between alcohol consumption and CD4 counts among HIV-infected patients at an ART treatment clinic in Nairobi, Kenya.
Methods
This is a retrospective cohort study of HIV-infected adults aged 15 years or older who were ART-naïve and initiated ART within 3 months of enrollment in the Coptic Hope Center for Infectious Diseases in Nairobi, Kenya between January 2009 and December 2012. The Hope Center, established in 2004 in collaboration with the Coptic Orthodox Mission and University of Washington (Chung et al., 2009), offers free ART and palliative care to HIV-infected adults and children, and is supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through a cooperative agreement from the US Centers for Disease Control and Prevention (CDC).
At clinic enrollment, patients are confirmed as HIV-positive and offered ART if they are eligible based on Kenyan national guidelines: WHO stage I or II if CD4 ≤ 350 cells/μl, WHO stage III or IV irrespective of CD4 count, and any HIV-infected patient co-infected with either Tuberculosis (TB), Hepatitis B with evidence of liver disease, and any patient with HIV-associated nephropathy. Patients who report receiving ART prior to enrollment are considered ART-experienced; those who report never having received ART are considered ART-naïve. Baseline sociodemographic variables and information on alcohol consumption are collected using a standardized questionnaire. CD4 counts are regularly obtained at enrollment prior to ART initiation, and every 6 months thereafter; samples are processed at an on-site laboratory (Becton Dickinson FACSCali-bur, San Jose, CA, USA).
Adherence in this study is measured by treatment interruption using a strategy used earlier with similar data (Pyne-Mercier et al., 2011). Clients are required to refill their antiretroviral prescriptions every 30 or 60 days, and refills for missed doses are adjusted accordingly at each pharmacy visit. If, for example, a client received a 30-day supply on June 1, their next scheduled pharmacy refill date would be July 1; treatment interruption would occur if the client did not return until July 3 or later. Interruption is measured as the number of late visits (3+ days) to the pharmacy to obtain prescription refills.
All patients who initiate ART are seen by a social worker at enrollment who routinely inquires about alcohol use. Two questions pertaining to alcohol consumption were used in this analysis: “Do you drink alcohol?” (Y/N) and “If yes, number of drinks per week”. Alcohol consumption is defined based on National Institute of Alcohol Abuse and Alcoholism (NIAAA) criteria and categorized as abstinent, moderate, or hazardous (NIAAA, 2005). Those defined as abstinent reported no alcohol use; heavy drinkers reported ≥14 drinks per week for men ≤66 years of age, or ≥ 7 drinks per week for women of any age and men > 66 years of age; those who reported any alcohol use but not “hazardous” as above were defined as moderate. Due to the low number of patients reporting hazardous drinking, the majority of analyses combined moderate and hazardous drinkers.
Baseline characteristics between those excluded as lost to follow-up and the final study population were examined using either t-tests for continuous or Chi-square tests for categorical variables. Baseline differences based on alcohol use were also evaluated using t-tests or Chi-square tests. CD4 count at 12 months was compared based on alcohol consumption using univariate t-tests and multivariable linear regression. All demographic and baseline clinical variables were used in subsequent regression models, except for highly correlated variables. Past experience with these data has shown that our socioeconomic variables – education level, income, and living in slum conditions – are highly correlated (colli-near) and that data on education are more complete and accurate; therefore, only education level was used in the regression models as an indicator of socioeconomic status.
This study was approved by the Ethical Review Committee of Kenyatta National Hospital (Nairobi, Kenya), the Institutional Review Board of the University of Washington (Seattle, WA, USA), and the US Centers for Disease Control and Prevention Associate Director for Science.
Results
Between January 2009 and December 2012, 4758 patients enrolled at the Hope Center (Figure 1). Of these, 3661 patients were excluded from the analysis due to the following: never on ART (n = 2034), ART-experienced at baseline (n = 968), or initiated ART > 3 months after enrollment (n = 659). Of the remaining 1097 participants, two were not asked about their alcohol use during their baseline interview and 241 were either lost to follow-up during the 12-month study period (n = 184) or did not have a CD4 count at 12 months (n = 57). Thus, the final analysis included 854 ART-naïve patients initiating ART within 3 months of enrollment and followed for 12 months post-ART initiation. Sensitivity analysis did not reveal any significant difference in alcohol consumption between those who were retained 12 months post-ART versus those who were lost to follow up [data not shown].
Figure 1.
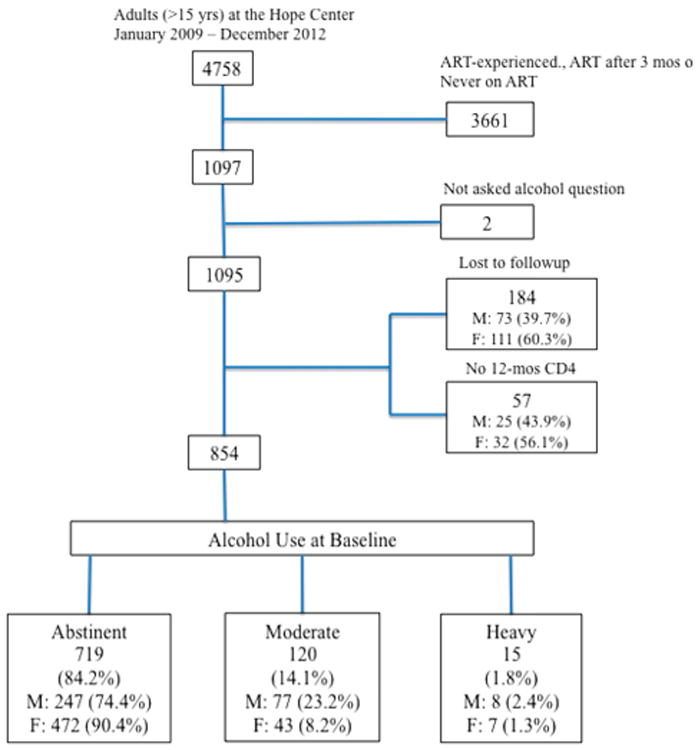
Study flow chart.
Of the 854 HIV-infected patients that formed our Kenyan study population, 719 (84.2%) reported no alcohol consumption, 120 (14.1%) reported moderate alcohol use, and 15 (1.8%) were classified as hazardous drinkers (Figure 1). More than half (61.1%) of the cohort were women, 630 (73.8%) had completed secondary education or higher, and 477 (55.9%) were married or cohabitating (Table 1). A substantial number of participants (37.0%) reported monthly household income of less than or equal to $23 (USD) compared to the average monthly income of $113 (USD) (World Bank, 2016). Compared to men, women reported greater abstinence (90.4% vs. 74.4%; p < 0.001) and lower hazardous drinking (1.3% vs. 2.4%; p < 0.001) (see Figure 1).
Table 1.
Baseline characteristics of adults initiating ART, by alcohol use at enrollment.
| Baseline characteristic | Abstinent | Moderate and hazardous alcohol use | p-Value |
|---|---|---|---|
| N (%) or median (IQR) | N (%) or median (IQR) | ||
| 719 (84.2%) | 135 (15.8%) | ||
| Age | 37.2 (32.5–44.3) | 37.9 (33.0–43.8) | 0.605 |
| Gender | <0.001 | ||
| Male | 247 (74.4%) | 85 (25.6%) | |
| Female | 472 (90.4%) | 50 (9.6%) | |
| Education | 0.045 | ||
| Primary or lower | 198 (88.4%) | 26 (11.6%) | |
| Secondary or higher | 521 (82.7%) | 109 (17.3%) | |
| Marital status | 0.763 | ||
| Alone (Divorced/Separated/Single) | 319 (84.6%) | 58 (15.4%) | |
| Married/Cohabiting | 400 (83.9%) | 77 (16.1%) | |
| WHO stage | 0.024 | ||
| 1/2 | 382 (81.6%) | 86 (18.4%) | |
| 3/4 | 337 (87.3%) | 49 (12.7%) | |
| BMI | 21.8 (19.4–25.4) | 22.6 (19.9–25.5) | 0.132 |
| CD4 | 126.0 (49.8–220.0) | 151.0 (70.0–219.0) | 0.099 |
| Monthly household earnings | <0.001 | ||
| >22.12 (USD) | 340 (78.5%) | 93 (21.5%) | |
| ≤22.12 (USD) (Poverty level) | 289 (91.5%) | 27 (8.5%) | |
| Living in slum conditions | 0.383 | ||
| No | 437 (85.5%) | 74 (14.5%) | |
| Yes | 139 (82.7%) | 29 (17.3%) |
Note: p-values in bold text are significant at the 0.05 level.
Patients with post-primary education and household income above the poverty line were significantly more likely to consume alcohol compared to patients with lower education and household income below the poverty line (p = 0.045 and p < 0.001, respectively) (Table 1). That is, those with lower socioeconomic status were more likely to report abstinence. Although not statistically significant, there was a slight trend for higher baseline BMI and a marginally significant trend toward higher CD4 counts among drinkers versus abstainers.
Post-ART CD4 count increased among both drinkers and abstainers, with drinkers showing consistently higher median CD4 counts throughout the 12-month follow-up period (Table 2) though the difference was only significant at 6 months. BMI also increased steadily post-ART and was slightly higher for drinkers than abstainers but not significantly. Treatment interruption, as measured by the median number of late visits, did not differ between drinkers and abstainers during the 12-month period (p = 0.845).
Table 2.
CD4, BMI, and treatment interruption of adults initiating ART, by alcohol use at enrollment.
| Time post-ART initiation | Abstinent (719) | Mod + Hazard (135) | p-Value |
|---|---|---|---|
|
| |||
| Median CD4 cells/uL (IQR) | Median CD4 cells/uL (IQR) | ||
| Baseline | 126.0 (49.8–220.0) | 151.0 (70.0–219.0) | 0.605 |
| 6 months | 222.0 (145.0–348.0) | 270.0 (166.0–369.5) | 0.034 |
| 12 months | 257.0 (171.2–385.0) | 299.0 (190.5–432.0) | 0.086 |
| BMI | BMI | p-Value | |
|
| |||
| Baseline | 21.8 (19.4–25.4) | 22.6 (19.9–25.5) | 0.132 |
| 6 months | 22.5 (20.3–25.5) | 22.8 (20.6–26.0) | 0.370 |
| 12 months | 23.6 (21.0–26.6) | 23.8 (21.2–26.3) | 0.798 |
| # Late visits | # Late visits | ||
|
|
|||
| 12 months | 5.0 (3.0–7.0) | 5.0 (3.0–7.0) | 0.845 |
Note: p-values in bold text are significant at the 0.05 level.
Results of the multivariable regression models are shown in Table 3. CD4 count increase was associated with younger age (p = 0.003), higher baseline CD4 counts (p < 0.001), and alcohol use (p = 0.051). Using categorical variables for Moderate and Hazardous alcohol consumption separately does not change the basic structure of the model although in these models neither Moderate (p = 0.122) nor Hazardous (p = 0.093) consumption reach statistical significance.
Table 3.
Multivariable regression models of CD4 at 12 months.
| Alcohol use | Estimatea | 95% CI | p |
|---|---|---|---|
| Abstinent | Reference | NA | NA |
| Moderate/hazardous | 33.24 | (−0.1, 66.6) | 0.051 |
| Age (years) | −2.14 | (−3.5, −0.7) | 0.003 |
| Gender | |||
| Male | Reference | NA | NA |
| Female | 8.58 | (−19.8, 36.9) | 0.552 |
| Education level | |||
| None/primary | Reference | NA | NA |
| Secondary+ | 17.07 | (−10.0, 44.2) | 0.216 |
| Residence status | |||
| Aloneb | Reference | NA | NA |
| Married/cohabitating | 11.21 | (−14.1, 36.6) | 0.386 |
| WHO stage | |||
| 1/2 | Reference | NA | NA |
| 3/4 | −8.77 | (−34.8, 17.3) | 0.509 |
| Baseline BMI | 1.09 | (−1.8, 4.1) | 0.723 |
| Baseline CD4 | 0.59 | (0.5, 0.7) | <0.001 |
| # Late pharmacy visits | −1.46 | (−5.4, 2.5) | 0.471 |
Note: p-values in bold text are significant at the 0.05 level.
Estimate reflects CD4 count compared with referent.
Divorced/widowed/separated/single.
Discussion
Our results indicate that within a population of HIV-infected clients initiating ART at an urban SSA clinic, more than 15% consume alcohol with less than 2% reporting hazardous alcohol use. We also found differences in reported alcohol use between genders with women reporting higher rates of abstinence (90.4% vs. 74.4%) and lower rates of hazardous drinking (1.3% vs. 2.4%). Alcohol use was also associated with higher socio-economic indicators. Regression models show that any alcohol use is associated with increased CD4 counts, but neither Moderate nor Hazardous are by themselves significantly associated with increased CD4 counts.
The prevalence of alcohol consumption in our population of HIV-infected adults was similar to an estimate from Kenya of 14.6% among the adult population (WHO, 2011). However, other estimates of alcohol use are considerably higher in some subpopulations and regions in Kenya (e.g., Abu-Sáad & Mburu, 2001; Hargreaves et al., 2002; Ndetei et al., 2009; Shaffer et al., 2004).
Our results are in contrast to several earlier studies that found a negative or no impact of alcohol use on CD4 counts in that we found a positive association between alcohol consumption and post-ART CD4 counts even after controlling for other sociodemographic factors including overall health and treatment interruption. Our outcome is, however, similar to that from an earlier French study that found that those with low alcohol consumption (<10 g daily) were more likely to have significantly higher CD4 cell counts over time compared to abstainers (Carrieri et al., 2014). That study also found that those who consumed more than 10 g daily had CD4 counts not significantly different from abstainers. Carrieri et al. (2014) suggest that low alcohol consumption may be acting as a proxy for other healthy behaviors, such as higher-quality food consumption, exercise, and perhaps higher social status rather than as a true “protective effect” much like alcohol's apparent risk-reductive effect on cardiovascular morbidity and mortality.
While we obtained similar results to the French study, it is difficult to assess the comparability between a developed European population and that in a resource-limited African context. Education/socioeconomic status among drinkers in our study was significantly higher at baseline suggesting that consuming alcohol may be an indicator of better nutrition, better overall health status, or higher socioeconomic status. Indeed, a cross-sectional population-based study of alcohol and nutrition in South Africa (Serfontein, Venter, Kruger, MacIntyre, & Pisa, 2010) found that increased alcohol use in urban contexts increased total dietary energy without a significant diminution of most macro- and micronutrients. Serfontein et al. (2010) suggest that those who can afford to buy alcohol may also have access to abundant and more nutritious foods. Our data suggest that, since there were no differences in treatment interruption, alcohol consumption is likely a confounding factor signaling higher socioeconomic status and/or access to more and better food much like the French study.
Kenyans also have a different mix of alcoholic beverages available to them, specifically “unrecorded” beverages, those alcoholic products – typically non-commercially produced beverages, such as the maize-based beer busaa and the distilled changaa – that are not counted in official estimates of drinking rates and may not be considered as alcohol consumption by some patients (Parry et al., 2005). In Kenya, these homebrews have been estimated to be consumed at almost three times the rate (by volume) of recorded beverages (Willis, 2002). Since these products tend to vary widely in ingredients, alcohol content, and standard serving size (Papas et al., 2010), the kind and degree to which these unrecorded products are consumed by our sample population represent a largely unknown confounding factor.
This study has several limitations. First, questions pertaining to alcohol use were asked only at baseline, so there are no data on alcohol use over time and whether being on ART affected consumption of alcohol. Second, only two basic questions were asked concerning alcohol use, rather than the more detailed Alcohol Use Disorders Identification Test (AUDIT) questionnaire which limited our analyses in that we could not adequately differentiate moderate alcohol use from alcohol abuse and dependence. Third, although our rates of alcohol consumption accord well with WHO survey data, they are much lower than that reported by other researchers in SSA generally and Kenya specifically. Finally, our data do not include information on the types of unrecorded beverages (busaa and changaa) that are likely consumed by some of our study population, nor do the social workers clarify with patients what quantity of alcohol constitutes “a drink”.
Nevertheless, this study adds important data to the debate regarding the effects of alcohol consumption on HIV-infected patients in SSA. Additional data are needed on the extent of alcohol consumption, alcohol use over time, and the types of alcohol being consumed and in what relative quantities such that effective, culturally appropriate interventions can be developed.
Acknowledgments
A. Cagle implemented and designed the study, and participated in all data analysis. M. Chung, G. John-Stewart, and C. McGrath helped to design the study, interpret the data, and write the paper. B. Richardson assisted with statistical analyses and helped to design the study and write the paper. D. Donovan assisted on issues of substance abuse and helped write the paper. S. Sakr, N. Yatich, and R. Ngomoa helped to conduct the study in the field. Agnes Chepngeno Langat provided CDC guidance and editorial support. We would like to acknowledge the contributions of the research personnel and data management teams in Nairobi, Kenya, and Seattle, WA, USA; and the staff and clinicians at the Coptic Hope Center in Nairobi, Kenya, for providing a conducive environment for HIV care and research. The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention/Government of Kenya.
Funding: The Coptic Hope Center for Infectious Diseases is supported by the U.S. President's Emergency Plan for AIDS Relief (PEP-FAR) through a cooperative agreement [U62/CCU024512] from the Centers for Disease Control and Prevention. During development of this manuscript, Dr. McGrath was supported by the University of Washington STD/AIDS Research Training Fellowship [NIH NRSA T32AI007140] and a research career development award [K12HD052023: Building Interdisciplinary Research Careers in Women's Health Program-BIRCWH] supported by ORWH, NIAID, and NICHD of the National Institutes of Health.
Footnotes
Disclosure statement: No potential conflict of interest was reported by the authors.
References
- Abu-Sáad I, Mburu J. The influence of settlement on substance use and abuse among nomadic population in Israel and Kenya. Amsterdam: Royal Tropical Institute, KIT; 2001. [Google Scholar]
- Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug and Alcohol Dependence. 2010;112(3):178–193. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Research and Human Retroviruses. 2010;26(5):511–518. doi: 10.1089/aid.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite R, Conigliaro J, Roberts M, Shechter S, Schaefer A, McGinnis K, et al. Justice A. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19(4):459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri MP, Protopopescu C, Raffi F, March L, Reboud P, Spire B, et al. Group, A. C. A.-C. S. Low alcohol consumption as a predictor of higher CD4+ cell count in HIV-treated patients. Journal of Acquired Immune Deficiency Syndromes. 2014;65(4):e148–e150. doi: 10.1097/QAI.0000000000000087. [DOI] [PubMed] [Google Scholar]
- Chander G, Lau B, Moore RD. Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2006;43(4):411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MH, Drake AL, Richardson BA, Reddy A, Thiga J, Sakr SR, et al. John-Stewart GC. Impact of prior HAART use on clinical outcomes in a large Kenyan HIV treatment program. Current HIV Research. 2009;7(4):441–446. doi: 10.2174/157016209788680552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conen A, Wang Q, Glass TR, Fux CA, Thurnheer MC, Orasch C, et al. Weber R. Association of alcohol consumption and HIV surrogate markers in participants of the Swiss HIV Cohort Study. Journal of Acquired Immune Deficiency Syndromes. 2013;64(5):472–478. doi: 10.1097/QAI.0b013e3182a61ea9. [DOI] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. Journal of General Internal Medicine. 2001;16(2):83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebremichael M, Paintsil E, Ickovics JR, Vlahov D, Schuman P, Boland R, et al. Zhang H. Longitudinal association of alcohol use with HIV disease progression and psychological health of women with HIV. AIDS Care. 2009;21(7):834–841. doi: 10.1080/09540120802537864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer JE, Baeten JM, Campbell J, Wangisi J, Katabira E, Ronald A, et al. Ware NC. Adherence to antiretroviral prophylaxis for HIV prevention: A substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Medicine. 2013;10(9):e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves JR, Morison LA, Chege J, Rutenburg N, Kahindo M, Weiss HA, et al. Buve A. Socioeconomic status and risk of HIV infection in an urban population in Kenya. Tropical Medicine & International Health. 2002;7(9):793–802. doi: 10.1046/j.1365-3156.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: Review and meta-analysis. Journal of Acquired Immune Deficiency Syndromes. 2009;52(2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet A, Ekouevi DK, Bashi J, Aboubakrine M, Messou E, Maiga M, et al. Ba-Gomis FO. Alcohol use and non-adherence to antiretroviral therapy in HIV-infected patients in West Africa. Addiction. 2010;105(8):1416–1421. doi: 10.1111/j.1360-0443.2010.02978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow RA, Blackwelder WC, Ostrow DG, Yerg D, Palenicek J, Coulson AH, Valdiserri RO. No evidence for a role of alcohol or other psychoactive drugs in accelerating immunodeficiency in HIV-1—positive individuals: A report from the multicenter AIDS cohort study. JAMA. 1989;261(23):3424–3429. [PubMed] [Google Scholar]
- Kowalski S, Colantuoni E, Lau B, Keruly J, McCaul ME, Hutton HE, et al. Chander G. Alcohol consumption and CD4 T-cell count response among persons initiating antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2012;61(4):455–461. doi: 10.1097/QAI.0b013e3182712d39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism. The physician's guide to helping patients with alcohol problems. Washington, DC: National Institutes of Health; 2005. [Google Scholar]
- Ndetei DM, Khasakhala LI, Ongecha-Owuor FA, Kuria MW, Mutiso V, Kokonya DA. Prevalence of substance abuse among patients in general medical facilities in Kenya. Substance Abuse. 2009;30(2):182–190. doi: 10.1080/08897070902802125. [DOI] [PubMed] [Google Scholar]
- Papas RK, Sidle JE, Wamalwa ES, Okumu TO, Bryant KL, Goulet JL, et al. Justice AC. Estimating alcohol content of traditional brew in Western Kenya using culturally relevant methods: the case for cost over volume. AIDS and Behavior. 2010;14(4):836–844. doi: 10.1007/s10461-008-9492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry CD, Plüddemann A, Steyn K, Bradshaw D, Norman R, Laubscher R. Alcohol use in South Africa: Findings from the first demographic and health survey (1998) Journal of Studies on Alcohol. 2005;66(1):91–97. doi: 10.15288/jsa.2005.66.91. [DOI] [PubMed] [Google Scholar]
- Pyne-Mercier LD, John-Stewart GC, Richardson BA, Kagondu NL, Thiga J, Noshy H, et al. Chung MH. The consequences of post-election violence on antiretroviral HIV therapy in Kenya. AIDS care. 2011;23(5):562–568. doi: 10.1080/09540121.2010.525615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. Journal of Acquired Immune Deficiency Syndromes. 2007;46(2):194–199. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcoholism: Clinical and Experimental Research. 2004;28(4):572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Serfontein M, Venter C, Kruger A, MacIntyre U, Pisa P. Alcohol intake and micronutrient density in a population in transition: The transition and health during urbanisation in South Africa (THUSA) study: Original research. South African Journal of Clinical Nutrition: Alcohol Consumption in South Africa: From Molecules To Society. 2010;23(2):S22–S28. [Google Scholar]
- Shaffer D, Njeri R, Justice A, Odero W, Tierney W. Alcohol abuse among patients with and without HIV infection attending public clinics in western Kenya. East African Medical Journal. 2004;81(11):594–598. [PubMed] [Google Scholar]
- UNAIDS. Fact sheet, Kenya. 2015 Retrieved from http://www.unaids.org/en/regionscountries/countries/kenya/
- WHO. Global Status Report on Alcohol and Health 2011, Africa Region 2011 [Google Scholar]
- Willis J. Potent brews: A social history of alcohol in East Africa 1850-1999. Oxford: James Currey; 2002. [Google Scholar]
- The World Bank. GDP per capita (current US$) Retrieved March 22, 2016, from http://data.worldbank.org/indicator/NY.GDP.PCAP.CD.


