Abstract
Background
The association between long-term mortality and aortic atheroma in cardiac surgical patients has not been comprehensively investigated. In this study we determine the relationship between grade of atheroma and the risk of long-term mortality in a retrospective cohort of over 20,000 patients undergoing cardiac surgery over a 20 - year period.
Methods
We included 22,304 consecutive intraoperative transesophageal and epiaortic ultrasound examinations performed at Brigham and Women’s Hospital between 1995 and 2014, with long-term follow-up. The extent of atheromatous disease recorded in each examination was used for analysis. Mortality data was obtained from our institution’s data registry. Mortality analyses were done using Cox proportional hazard regression models with follow-up as a time scale. We repeated the analysis in a subgroup of 14,728 patients with more detailed demographics, including postoperative stroke, queried from the institutional Society of Thoracic Surgeons database.
Results
A total of 7,722 mortality events and 872 stroke events occurred. Patients with atheromatous disease demonstrated a significant increase in mortality across all grades of severity, both for the ascending and descending aorta. This relationship remained unchanged after adjusting for additional covariates. Adjustments for postoperative stroke resulted in only minimal attenuation in the risk of postoperative mortality related to aortic atheroma.
Conclusions
Aortic atheromatous disease of any grade in the ascending and descending aorta is a significant long-term risk of long-term, all-cause mortality in cardiac surgery patients. This association remains independent of other conventional risk factors, and is not related to postoperative cerebrovascular accidents.
Thoracic aortic atheroma is associated with increased risk of postoperative complications after cardiac surgery. (1-5) In a study of 189 patients undergoing multivessel coronary artery bypass grafting (CABG) surgery, 45.5% of patients with severe atheromatous disease in the descending aorta sustained strokes in the first week following surgery, compared to patients with no disease or mild disease who experienced no strokes.(4) Furthermore, significant aortic atheroma burden has been associated with a nearly five-fold increase in neuropsychological dysfunction, and is an independent risk factor for renal dysfunction in the first week after cardiac surgery.(1,6)
Patients with a history of stroke and thoracic aortic atheromatous disease have increased mortality and an increased rate of embolic events when compared to those with no atheromatous disease.(7) Ascending aortic atherosclerosis is also associated with a 1.5 fold increase in mortality over a three year period.(2) However, these studies were lacking in patient number and duration of follow-up, were limited to the ascending aorta, or were deficient in the quality of the assessment of aortic disease. Furthermore, the association between long-term mortality and aortic atheroma has not been comprehensively investigated.
Therefore, we examined the relationship between grade of aortic atheromatous disease and long-term mortality in a retrospective cohort of over 20,000 patients undergoing cardiac surgery at a single center over a 19-year period. We hypothesized that given the large number of patients we would be able to identify an association with long-term mortality even at lower grades of atheromatous severity, and that this would be independent of cerebrovascular events or other comorbidities.
Material and Methods
Study Population
In our analysis we included 22,304 consecutive intraoperative cardiac ultrasound examinations performed at the Brigham and Women’s Hospital between 1995 and 2014. The study population consisted of all cardiac surgical patients who had undergone intraoperative epiaortic ultrasound examinations (EPI) and/or transesophageal echocardiography (TEE). The study and the retrospective review of the patients’ medical records were approved by the Partners Healthcare Institutional Review Board.
Data Collection
All patients received standard medical care. Baseline demographics, comorbidities, and imaging data were recorded and stored in an encrypted online database. The extent of ascending and descending thoracic aorta atheromatous disease was graded as none, mild, moderate, or severe by National Board of Echocardiography certified anesthesiologists using a modified version of the Kronzon scale.(8,9) Specifically, mild atheroma was defined as localized atherosclerotic thickening less than 3mm, moderate atheroma as atherosclerotic thickening between 3 and 5 mm, and severe atheroma as atherosclerotic thickening greater than 5 mm or the presence of mobile plaque. Patients without atheroma grading data were excluded from the study. For a discrepancy between the EPI and TEE, the more severe grade was chosen.
Endpoint
The primary endpoint was defined as all-cause mortality. Operative records from our departmental TEE database were matched with the Partners Healthcare System Research Patient Data Registry to obtain mortality data, which is updated from the National Death Index.
Subgroup analysis
We repeated the analysis in a subgroup of 14,728 patients with more detailed demographics queried from the institutional Society of Thoracic Surgeons database. Additional variables include body mass index, race, smoking history, history of diabetes, hypertension, hyperlipidemia, peripheral vascular disease, creatinine, and urgency of operation.
Statistical analysis
Statistical analyses were performed in R 3.1.1 (https://www.r-project.org) using the “survival” package. Kaplan-Meier survival curves were compared using a two-sided non-parametric log-rank test. Univariate analyses were initially performed to examine the association between potential predictors and outcomes, followed by a multivariate model including all potential predictors. Mortality analyses were performed using Cox proportional hazard regression models with follow-up as a time scale. We repeated the analysis in a subgroup of 14,728 patients with more detailed demographics queried form the institutional Society of Thoracic Surgeons database, to further control for variables that were not in our original database but might be associated with an increase in mortality.
In the subgroup analysis, we were also interested in the effect of atheroma on mortality risk that is not related to cerebrovascular accident (CVA). Thus a control direct effect model was performed, including CVA as a variable in the multivariate Cox model under the assumption that after adjusting for other covariates, no unobserved confounding remained for the joint effects of atheroma and CVA on mortality. We also assumed that atheroma did not confound the relationship between CVA and mortality. The proportional hazards assumption was validated by a goodness-of-test based on scaled Schoenfeld residuals.(10) We stratified by variables for which the proportional hazards assumption did not hold. For all analyses, a two-sided p value <0.05 was considered statistically significant.
Results
The average years of follow-up was 6.6 years (a total of 146,767 person years). 7,722 mortality events and 872 CVA events occurred. Of the 22,304 patients with complete information on atheromatous disease and follow-up data, more than 50% had a diseased ascending aorta and over 70% had a diseased descending aorta. The number of patients with moderate and severe disease was much higher in the descending than the ascending aorta (34% versus 14%). Demographic and preoperative data are shown in Table 1.
Table 1.
Demographic Information and Baseline Characteristics
| Characteristics | Total cohort |
|---|---|
| Number of subjects (22,304) | |
| Mean age at the time of surgery (SD) | 66.4 (13.5) |
| Mean BMI, N=14,728, (SD) | 28.3 (6.1) |
| Male | 14333 (64.3%) |
| Past Medical History (N= 14,728) | |
| Past smoker | 6844 (46.5%) |
| Present smoker | 1203 (8.2%) |
| Diabetes | 3844 (26.1%) |
| HLD | 10522 (71.4%) |
| HTN | 10237 (69.5%) |
| PVD | 2031 (13.8%) |
| Creatinine, N= 14,720, (SD) | 1.2 (0.8) |
| Grade of LV Dysfunction (N=18,568) | |
| None | 11488 (61.9%) |
| Mild | 2988(16.1%) |
| Moderate | 2297 (12.4%) |
| Severe | 1795 (9.7%) |
| Grade of RV Dysfunction (N=18,461) | |
| None | 15350 (83.1%) |
| Mild | 2031 (11%) |
| Moderate | 792 (4.3%) |
| Severe | 288 (1.6%) |
| Urgency of Surgery (N= 14,728) | |
| Elective surgery | 9718 (66%) |
| Emergent surgery | 289 (2%) |
| Urgent surgery | 4721 (32.1%) |
| History of Postoperative CVA (N=14,728) | 872 (5.9%) |
| Grade of Atheroma Ascending (N=19,191) | |
| None | 8465 (44.1%) |
| Mild | 7844 (40.9%) |
| Moderate | 2282 (11.9%) |
| Severe | 600 (3.1%) |
| Grade of Atheroma Descending (N=18176) | |
| None | 4446 (24.5%) |
| Mild | 7414 (40.8%) |
| Moderate | 4505 (24.8%) |
| Severe | 1811 (10%) |
LV – left ventricle, RV – right ventricle, HLD- hyperlipidemia, HTN – hypertension, PVD – peripheral vascular disease, BMI – body mass index, CVA – cerebrovascular accident
Univariate analysis
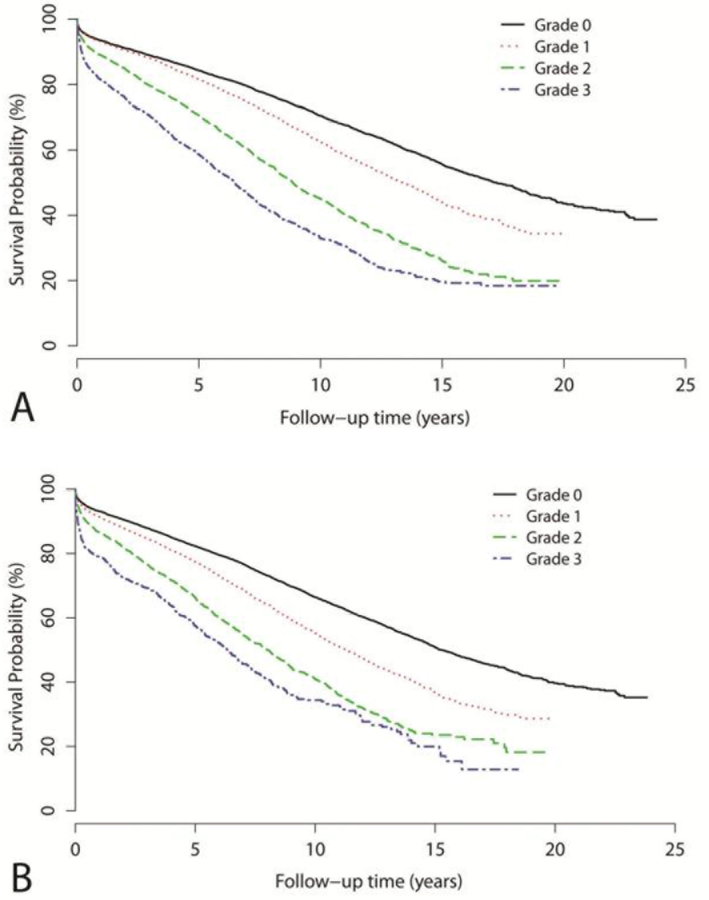
Univariate predictors of long-term, all-cause mortality are listed in Table 2. Patients with atheromatous disease demonstrated a significant increase in mortality across all grades of severity, both for the ascending and descending aorta (Table 2). The Kaplan-Meier curves differentiated between all grades of atheroma in the ascending aorta (Figure 1a) and the descending aorta (Figure 1b) (P<0.001). The significant association was apparent early, with an increase throughout follow-up. In addition, other significant univariate predictors of mortality are age, left and right ventricular function, the type of surgery performed, race, urgent or emergent surgery, past smoking history, diabetes, hyperlipidemia, hypertension, increased creatinine value, peripheral vascular disease and postoperative CVA (table 2).
Table 2.
Univariate Predictors of Long-Term, All-cause Mortality
| COMPLETE DATASET | SUBGROUP DATASET | ||||
|---|---|---|---|---|---|
| VARIABLES | Categories | HR (95% CI) | P-value | HR (95% CI) | P-value |
| MALE SEX | 0.84 (0.81 to 0.88) | <.001 | 0.79 (0.73 to 0.84) | <.001 | |
| LV DYSFUNCTION | |||||
| None | Reference | - | Reference | - | |
| Mild | 1.60 (1.5 to 7.1) | <.001 | 1.67 (1.51 to 1.84) | <.001 | |
| Moderate | 1.94 (1.81 to 2.07) | <.001 | 2.10 (1.89 to 2.34) | <.001 | |
| Severe | 2.14 (1.99 to 2.3) | <.001 | 2.26 (2.02 to 2.35) | <.001 | |
| RV DYSFUNCTION | |||||
| None | Reference | - | Reference | - | |
| Mild | 1.57 (1.46 to 1.68) | <.001 | 2.00(1.8 to 2.21) | <.001 | |
| Moderate | 1.68 (1.51 to 1.86) | <.001 | 2.05 (1.76 to 2.39) | <.001 | |
| Severe | 1.80 (1.53 to 2.13) | <.001 | 2.24 (1.76 to 2.84) | <.001 | |
| SURGERY STATUS | |||||
| Tricuspid Valve | Reference | - | Reference | - | |
| Mitral Valve | 0.64 (0.57 to 0.72) | <.001 | 0.58 (0.49 to 0.67) | <.001 | |
| CABG | 0.71 (0.64 to 0.8) | <.001 | 0.61 (0.53 to 0.7) | <.001 | |
| Aortic Valve | 0.50 (0.45 to 0.57) | <.001 | 0.53 (0.46 to 0.63) | <.001 | |
| Other | 0.81 (0.72 to 0.91) | <.001 | 0.76 (0.64 to 0.9) | <.001 | |
| BMI | - | - | 0.98 (0.98 to 0.99) | <.001 | |
| RACE | |||||
| Caucasian | - | - | Reference | ||
| Asian | - | - | 0.55 (0.36 to 0.85) | <.001 | |
| Black | - | - | 0.90 (0.71 to 1.13) | 0.368 | |
| Hispanic | - | - | 0.73 (0.56 to 0.96) | <.001 | |
| Native American | - | - | 0.52 (0.34 to 0.79) | <.001 | |
| PAST SMOKER | - | - | 1.28 (1.19 to 1.37) | ||
| CURRENT SMOKER | - | - | 0.91 (0.81 to 1.03) | ||
| DIABETES | - | - | 1.7 (1.58 to 1.83) | ||
| HLD | - | - | 1.18 (1.09 to 1.28) | ||
| HTN | - | - | 1.74 (1.61 to 1.89) | ||
| PVD | - | - | 1.97 (1.81 to 2.13) | ||
| CREATININE | 1.36 (1.33 to 1.39) | ||||
| SURGERY STATUS | |||||
| Elective | - | - | Reference | ||
| Urgent | - | - | 1.72 (1.60 to 1.84) | <.001 | |
| Emergency | - | - | 2.18 (1.80 to 2.64) | <.001 | |
| CVA | - | - | 1.78 (1.58 to 2.00) | <.001 | |
| ATHEROMA | |||||
| ASCENDING AORTA | None | Reference | - | Reference | - |
| Mild | 1.59 (1.5 to 1.67) | <.001 | 1.76 (1.61 to 1.92) | <.001 | |
| Moderate | 2.44 (2.28 to 2.62) | <.001 | 2.85 (2.56 to 3.19) | <.001 | |
| Severe | 3.09 (2.76 to 3.45) | <.001 | 4.03 (3.41 to 4.76) | <.001 | |
| DESCENDING AORTA | |||||
| None | Reference | - | Reference | - | |
| Mild | 1.79 (1.67 to 1.93) | <.001 | 2.02 (1.77 to 2.31) | <.001 | |
| Moderate | 3.03 (2.81 to 3.26) | <.001 | 3.75 (3.28 to 4.29) | <.001 | |
| Severe | 4.28 (3.93 to 4.66) | <.001 | 5.2 (4.48 to 6.03) | <.001 | |
LV- left ventricle, RV – right ventricle, BMI – body mass index, HLD – hyperlipidemia, HTN – hypertension, PVD – peripheral vascular disease, CVA – cerebrovascular accident
Figure 1.
Kaplan Meier Curves showing percent survival in relation to aortic atheroma grade in the ascending (A) and descending (B) aorta. (P<0.001), Grade 0 - No atheroma Grade 1 - Mild atheroma Grade 3 - Moderate atheroma Grade 4 - Severe Atheroma
Cox proportional regression
In multivariate Cox Proportional Regression analyses, the association between moderate and severe ascending and descending atheromatous disease and mortality remained strong after adjusting for other predictors (Table 3). We observed an incremental increase in the risk of death beginning with mild disease, compared to normal aorta. Age, left ventricular and right ventricular function, urgent surgery status, past smoking history, increasing creatinine value, diabetes, hypertension, peripheral vascular disease, and postoperative CVA were also significantly and independently associated with all-cause mortality. As a matter of fact, the hazard ratio of severe atheromatous disease (1.89 for the ascending and 2.2 for the descending aorta) was greater than any other included variable, in the complete dataset as well as the subgroup analysis. Thus, in the subgroup the risk of dying as a result of having moderate or severe atheromatous disease is greater than having urgent surgery, diabetes, or peripheral vascular disease.
Table 3.
Grade of Atheroma and Mortality: Cox Proportional Hazard Regression Model of the complete dataset
| ASCENDING AORTA (N=18,515) | DESCENDING AORTA (N= 18,905) | ||||
|---|---|---|---|---|---|
| VARIABLE | Categories | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value |
| GRADE | None | Reference | |||
| Mild | 1.18 (1.11 to 1.26) | <.001 | 1.27 (1.17 to 1.38) | <.001 | |
| Moderate | 1.46 (1.34 to 1.59) | <.001 | 1.67 (1.53 to 1.83) | <.001 | |
| Severe | 1.89 (1.65 to 2.16) | <.001 | 2.20 (1.99 to 2.44) | <.001 | |
| MALE SEX | 0.96 (0.90 to 1.01) | 0.117 | 0.97 (0.91 to 1.02) | 0.24 | |
Model stratified by year of operation, age, left and right ventricular function, and surgical type.
Subgroup analysis
In the subgroup analysis of 14,728 patients, aortic atheroma continued to be an independent risk factor for postoperative mortality with hazard ratios that were very similar to the complete dataset after adjusting for additional covariates (Table 4). The addition of postoperative CVA to the cox regression model as an independent variable resulted in only minimal attenuation in the risk of postoperative mortality related to aortic atheroma (Table 5).
Table 4.
Grade of Atheroma and Mortality: Cox Proportional Hazard Regression Model of the subgroup
| ASCENDING AORTA (N=10849) | DESCENDING AORTA (N=11232) | ||||
|---|---|---|---|---|---|
| VARIABLE | Categories | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value |
| GRADE | |||||
| None | Reference | ||||
| Mild | 1.12 (0.99 to 1.26) | 0.068 | 1.34 (1.12 to 1.61) | .002 | |
| Moderate | 1.36 (1.17 to 1.58) | <.001 | 1.64 (1.36 to 1.99) | <.001 | |
| Severe | 1.81 (1.45 to 2.26) | <.001 | 2 (1.62 to 2.46) | <.001 | |
| MALE SEX | 0.86 (0.77 to 0.95) | 0.004 | 0.86 (0.78 to 0.95) | 0.004 | |
| BMI | 0.99 (0.98 to 1) | 0.013 | 0.99 (0.98 to 1) | 0.011 | |
| RACE | |||||
| Caucasian | Reference | ||||
| Asian | 0.71 (0.37 to 1.34) | 0.288 | 0.7 (0.37 to 1.31) | 0.261 | |
| Black | 0.82 (0.57 to 1.16) | 0.257 | 0.82 (0.57 to 1.18) | 0.277 | |
| Hispanic | 0.64 (0.42 to 0.97) | 0.034 | 0.72 (0.49 to 1.07) | 0.104 | |
| Others | 0.68 (0.4 to 1.17) | 0.165 | 0.74 (0.43 to 1.27) | 0.281 | |
| PAST SMOKER | 1.28 (1.15 to 1.42) | <.001 | 1.28 (1.16 to 1.42) | <.001 | |
| CURRENT SMOKER | 1.12 (0.93 to 1.36) | 0.231 | 1.03 (0.85 to 1.24) | 0.778 | |
| DIABETES | 1.49 (1.33 to 1.66) | <.001 | 1.49 (1.34 to 1.66) | <.001 | |
| HLD | 0.88 (0.78 to 0.99 | 0.030 | 0.89 (0.79 to 1) | 0.052 | |
| HTN | 1.34 (1.18 to 1.53) | <.001 | 1.28 (1.13 to 1.45) | <.001 | |
| PVD | 1.44 (1.28 to 1.62) | <.001 | 1.40 (1.25 to 1.58) | <.001 | |
| CREATININE | 1.47 (1.39 to 1.56) | <.001 | 1.48 (1.39 to 1.56) | <.001 | |
| STATUS | |||||
| Elective | Reference | Reference | |||
| Urgent | 1.32 (1.19 to 1.48) | <.001 | 1.32 (1.19 to 1.46) | <.001 | |
| Emergent | 1.56 (1.13 to 2.14 | .006 | 1.48 (1.08 to 2.05) | 0.016 | |
Model stratified by year of operation, age, left and right ventricular function, and surgical type. BMI – body mass index, HLD – hyperlipidemia, HTN – hypertension, PVD – peripheral vascular disease, CVA – cerebrovascular accident
Table 5.
Grade of atheroma and mortality, further adjusted for cerebrovascular accident
| ASCENDING AORTA | DESCENDING AORTA | ||||
|---|---|---|---|---|---|
| VARIABLE | Categories | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value |
| GRADE | |||||
| Mild | 1.11 (0.99 to 1.25) | 0.08 | 1.34 (1.12 to 1.61) | .002 | |
| Moderate | 1.35 (1.16 to 1.57) | <.001 | 1.63 (1.35 to 1.97) | <.001 | |
| Severe | 1.80 (1.44 to 2.25) | <.001 | 1.98 (1.61 to 2.44) | <.001 | |
Shown are the hazard ratios for atheroma in the ascending and descending aorta from the regression model in Table 4, when further adjusted for CVA. Minimal attenuation is noted.
Comment
The presence of significant aortic atheromatous disease has been associated with collateral cardiovascular disease in ambulatory patients, and short-term adverse postoperative outcomes in cardiac surgical patients. (1-3,11) The results of this investigation now demonstrate a robust relationship between grade of aortic atheroma and long-term mortality in a retrospective cohort of over 20,000 patients at a single center. While patients with high-grade aortic atheromatous disease had the greatest increase in mortality, patients with mild disease also showed a significant increase in mortality. This relationship remained even after controlling for patient demographics, comorbidities, and known confounders. Furthermore, previous studies were often limited to the ascending aorta while we were able to show an equally strong effect in the ascending and descending aorta.
The mechanism by which aortic atheroma confers an increased risk of mortality has not been definitively established. Atheroma is believed to be a rich source of cerebral emboli following surgical manipulation of the atherosclerotic aorta. (3,4) Blauth and colleagues reported that a significant percentage of patients who died following cardiac surgery had evidence at autopsy of atheroembolism of multiple organs.(12) These investigators observed that atheroembolic events occurred in 37% of patients with severe atheromatous disease of the ascending aorta, but in only 2% of patients without significant disease. In our analysis, mortality risk was independent of whether patients had a postoperative stroke, suggesting that other mechanisms may be involved. In addition, as can be seen in the Kaplan-Meier curves, the majority of deaths cannot be attributed to the perioperative period.
Alternatively, the presence of atheroma in the thoracic aorta may be a hallmark of severe systemic cardiovascular disease.(8) Aortic plaque is associated with increased inflammatory markers and elevated levels of homocysteine and prothrombin, and increased aortic wall thickness assessed by multidetector computed tomography predicts coronary artery disease severity; over ninety percent of patients with thoracic aorta calcification also present with coronary artery plaque. (11,13) Hypertension, DM, PVD, and a history of stroke are also correlated with advanced aortic atheromatous disease.(4) It may be that atheromatous burden is a surrogate marker for a multitude of variables both caused by the disease process (HTN, renal disease, PVD, CAD), and also the severity of disease. However, after controlling for comorbidities associated with systemic vascular disease (HTN, HLD, PVD, CVA), aortic atheroma continued to be an independent risk factor for mortality in our patient population.
More comprehensive analysis of aortic atherosclerotic disease may provide insight into the relationship between atheroma grade and cause of death. Previous studies have suggested that the location and characteristics of atherosclerotic disease may impact postoperative stroke and mortality. In a review of 921 patients undergoing CABG surgery, van der Linden et al. found that location of atheroma in the middle-lateral segment of the ascending aorta is an independent predictive factor for postoperative stroke (RR 26.2).(14) Ferrari et al. found that patients with mobile aortic atheroma had increased mortality when compared to patients with non-mobile atheromatous disease.(15) Atherosclerotic load in major vessel beds is associated with an increased risk of death.(16) When comparing atheromatous disease in the coronary arteries, aortic arch, extracranial carotid arteries, and intracranial carotid, Bos. et al found that aortic arch atherosclerosis was found to be associated with the greatest increase in mortality.
The authors postulate that given its central location in the arterial system, aortic arch atherosclerosis represents a better reflection of the total burden of atherosclerosis.(16) Nonetheless, we found an equally strong association between the ascending and descending aortic atheroma and risk of mortality.
It is well established that adjustments in surgical technique, such as minimizing aortic manipulation and adjustment of cross clamp position when atheroma is present, can decrease the incidence of perioperative stroke.(17) Further research is warranted to determine whether patients found to have atheromatous disease on intraoperative TEE may benefit from further modification of surgical plan and surgical technique. In a casematched comparative study of patients with severe atheromatous disease, Sharony et al. found that off-pump coronary artery bypass technique was associated with lower morbidity and mortality when compared to coronary artery bypass.(18) Demaria et al. have found that off-pump coronary artery bypass technique was associated with less operative mortality and strokes in octogenarians then conventional CABG under CBP.(19) Patients with high grade atheroma may benefit from adjustment of surgical plan.
Limitations
Observational, retrospective studies are susceptible to limitations associated with potential inconsistency in data collection, the lack of prospectively delineated definitions of predictors and outcomes, and missing data. Data in our study was collected using departmental and institutional databases that have been validated historically for accuracy and consistency. Furthermore, the data was collected by experienced, National Board of Echocardiography certified cardiac anesthesiologists who specifically adhered to published, guideline recommended criteria for grading aortic atheroma using simple measurements of atheroma thickness that are highly reproducible. [4]
Quality of echo technology, and practice patterns of echocardiography, evolved over the 20 year period of the study. It is possible that the relatively poorer quality of echo in the early period of this study could have affected the grading of atheroma relative to the later part of the study. Patients in the earlier years of our study who only received TEE and not epiaortic ultrasound exams may have had under-reporting of atheroma in the aortic arch and ascending aorta due to limited visualization with the TEE probe. However, multiple studies indicated that there is a good correlation between atheromatous disease in the three aortic segments.(8,20) In addition, despite decreased ability to image the aortic arch and ascending aorta with the TEE probe, the interobserver variability within these segments was similar to that of the better visualized descending aorta.(8)
Another limitation is that we could not adjust for surgical practice patterns with respect to how surgical technique was modified in response to the presence of atheromatous disease found on intraoperative ultrasound exams. It is possible that changes in surgical planning, such as modifying cross clamp location, modified aortic crossclamp time, aortic endarterectomy, and circulatory arrest with retrograde cerebral perfusion, improved outcomes and led to an underestimation of the effect of atherosclerosis on mortality.
Mortality data was obtained through the research patient data registry, which is updated from the national death index. Another limitation is that this database did not provide information about cause of death, or allow for the ability to differentiate between cardiovascular and non-cardiovascular mortality. Finally, the inclusion of over 20,000 patients in this investigation not only contributes to the robust strength of the results, but also represents the largest published series on this topic from a single center.
Conclusions
Aortic atheromatous disease of any grade is a significant risk factor of all-cause, longterm mortality in cardiac surgical patients. This association remains independent of other conventional risk factors, and is not related to postoperative cerebral vascular accidents. Further research is warranted to elucidate whether direct mechanisms are responsible for the observed increase in postoperative mortality, or if atheromatous disease serves as a marker for systemic cardiovascular disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davila-Roman VG, Kouchoukos NT, Schechtman KB, Barzilai B. Atherosclerosis of the ascending aorta is a predictor of renal dysfunction after cardiac operations. J Thorac Cardiovasc Surg 1999;117:111-6. [DOI] [PubMed] [Google Scholar]
- 2.Davila-Roman VG, Murphy SF, Nickerson NJ, Kouchoukos NT, Schechtman KB, Barzilai B. Atherosclerosis of the ascending aorta is an independent predictor of long-term neurologic events and mortality. J Am Coll Cardiol 1999;33:1308-16. [DOI] [PubMed] [Google Scholar]
- 3.Evered LA, Silbert BS, Scott DA. Postoperative cognitive dysfunction and aortic atheroma. Ann Thorac Surg 2010;89:1091-7. [DOI] [PubMed] [Google Scholar]
- 4.Hartman GS, Yao FS, Bruefach M, 3rd et al. Severity of aortic atheromatous disease diagnosed by transesophageal echocardiography predicts stroke and other outcomes associated with coronary artery surgery: a prospective study. Anesth Analg 1996;83:701-8. [DOI] [PubMed] [Google Scholar]
- 5.Hogue CW, Jr, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999;100:642-7. [DOI] [PubMed] [Google Scholar]
- 6.Goto T, Baba T, Yoshitake A, Shibata Y, Ura M, Sakata R. Craniocervical and aortic atherosclerosis as neurologic risk factors in coronary surgery. Ann Thorac Surg 2000;69:834-40. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira RG, Worthington A, Huang CC, Aranki SF, Muehlschlegel JD. Sex differences in the prevalence of diastolic dysfunction in cardiac surgical patients. J Card Surg 2015;30:238-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartman GS, Peterson J, Konstadt SN, et al. High reproducibility in the interpretation of intraoperative transesophageal echocardiographic evaluation of aortic atheromatous disease. Anesth Analg 1996;82:539-43. [DOI] [PubMed] [Google Scholar]
- 9.Katz ES, Tunick PA, Rusinek H, Ribakove G, Spencer FC, Kronzon I. Protruding aortic atheromas predict stroke in elderly patients undergoing cardiopulmonary bypass: experience with intraoperative transesophageal echocardiography. J Am Coll Cardiol 1992;20:70-7. [DOI] [PubMed] [Google Scholar]
- 10.Xue X, Xie X, Gunter M, et al. Testing the proportional hazards assumption in case-cohort analysis. BMC Med Res Methodol 2013;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeltsch M, Klass O, Klein S, et al. Aortic wall thickness assessed by multidetector computed tomography as a predictor of coronary atherosclerosis. Int J Cardiovasc Imaging 2009;25:209-17. [DOI] [PubMed] [Google Scholar]
- 12.Blauth CI, Cosgrove DM, Webb BW, et al. Atheroembolism from the ascending aorta. An emerging problem in cardiac surgery. J Thorac Cardiovasc Surg 1992;103:1104-11; discussion 1111-2. [PubMed] [Google Scholar]
- 13.Kronzon I, Tunick PA. Aortic atherosclerotic disease and stroke. Circulation 2006;114:63-75. [DOI] [PubMed] [Google Scholar]
- 14.van der Linden J, Hadjinikolaou L, Bergman P, Lindblom D. Postoperative stroke in cardiac surgery is related to the location and extent of atherosclerotic disease in the ascending aorta. J Am Coll Cardiol 2001;38:131-5. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari E, Vidal R, Chevallier T, Baudouy M. Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants. J Am Coll Cardiol 1999;33:1317-22. [DOI] [PubMed] [Google Scholar]
- 16.Bos D, Leening MJ, Kavousi M, et al. Comparison of Atherosclerotic Calcification in Major Vessel Beds on the Risk of All-Cause and Cause-Specific Mortality: The Rotterdam Study. Circ Cardiovasc Imaging 2015;8. [DOI] [PubMed] [Google Scholar]
- 17.Moss E, Puskas JD, Thourani VH, et al. Avoiding aortic clamping during coronary artery bypass grafting reduces postoperative stroke. J Thorac Cardiovasc Surg 2015;149:175-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharony R, Bizekis CS, Kanchuger M, et al. Off-pump coronary artery bypass grafting reduces mortality and stroke in patients with atheromatous aortas: a case control study. Circulation 2003;108 Suppl 1:II15-20. [DOI] [PubMed] [Google Scholar]
- 19.Demaria RG, Carrier M, Fortier S, et al. Reduced mortality and strokes with off-pump coronary artery bypass grafting surgery in octogenarians. Circulation 2002;106:I5-10. [PubMed] [Google Scholar]
- 20.Marschall K, Kanchuger M, Kessler K, et al. Superiority of transesophageal echocardiography in detecting aortic arch atheromatous disease: identification of patients at increased risk of stroke during cardiac surgery. J Cardiothorac Vasc Anesth 1994;8:5-13. [DOI] [PubMed] [Google Scholar]