Abstract
Exposure to second-hand smoke (SHS) is a major public health problem and a risk factor for morbidity and mortality. The objective of this randomized trial was to estimate the impact of a culturally-sensitive intervention to reduce SHS exposure in Mexican-American households.
Method
A total of 91 households (with a child under 18 years of age and two adults, one of whom was a smoker) were recruited from a population-based cohort of Mexican-American households and randomized to receive the experimental intervention (EI; n=47) or standard care (SC; n=44). Of these, 74 households (83%) provided baseline, 6-month, and 12-month survey and nicotine monitor data (EI, n=39; SC, n=35). The EI materials, designed to increase the participants' likelihood of adopting a smoke-free indoor home air policy, included one culturally-appropriate bilingual comic book for children and two fotonovelas for adults.
Results
Ambient nicotine levels significantly decreased over the 12 study months (F=13.6, DF=147; p<0.001); with a significantly greater decrease in the EI households compared to the SC households (F=4.1, DF=72; p<0.05). At 12 months, 73% of EI households had banned smoking vs. 56% of SC households. Ambient nicotine levels, measured using nicotine air sampling monitors, were significantly associated with self-reported SHS exposure at the 12-month follow-up. Knowledge of the health effects of SHS increased from baseline to 6 and 12 months in the EI condition but not in the SC condition (F=6.0, DF=238; p<0.01), and smokers and quitters in the EI group reported an increased perception of health vulnerability compared to those in the SC group.
Conclusion
Our low-cost intervention impacted SHS-related knowledge and exposure among Mexican Americans. This culturally-appropriate intervention has the potential to decrease SHS-related health problems in the target population substantially.
Keywords: Smoking, Second-hand smoke, Prevention, Cessation, Exposure
1. Introduction
Exposure to second-hand smoke (SHS) is a major public health problem and a risk factor for morbidity and mortality (USDHHS, 2010). SHS contains at least 250 toxic chemicals (including more than 50 carcinogens), and exposure among nonsmokers is associated with a 20–30% increased risk of lung cancer (Samet et al., 2009). Furthermore, SHS is causally linked to stroke, heart disease, and respiratory disorders (Borrelli, McQuaid, Novak, Hammond, & Becker, 2010; Iribarren, Darbinian, Klatsky, & Friedman, 2004; O'Toole, Conklin, & Bhatnagar, 2008; U.S. Department of Health and Human Services, 2006).
The first global study on effects of SHS, conducted in 192 countries, determined that SHS exposure was responsible for 603,000 deaths in 2004 from coronary heart disease, lower respiratory infections, asthma, and lung cancer. In addition, disability-adjusted life-years lost due to SHS exposure amounted to 10.9 million. An estimated 40% of nonsmoking children, 33% of male nonsmokers, and 35% of female nonsmokers were exposed to SHS in 2004 (Oberg, Jaakkola, Woodward, Peruga, & Pruss-Ustun, 2011).
Children are particularly vulnerable to the negative consequences of SHS exposure, yet in the United States, 40% of children ≤5 years of age live with a smoker. In infants and children, SHS is associated with severe and frequent asthma attacks, respiratory infections, ear infections, and sudden infant death syndrome (Treyster & Gitterman, 2011). A leading cause of morbidity and mortality among low-income children is passive smoke (Emmons et al., 2001). In 2006, the Surgeon General recommended that for children to be fully protected from SHS, indoor smoking must be eliminated entirely (U.S. Department of Health and Human Services, 2006). A randomized controlled trial, conducted by Emmons et al., determined that motivational interventions are capable of reducing young children's passive smoke exposure through a randomized control trial.
In Texas, 18.5% of all adults and 17.1% of Hispanics are current smokers (CDC, 2010). Among a cohort of Mexican-American adults in Houston Texas, we have found that the prevalence of current smoking varies by sex: 28.7% of men and 9.5% of women (Wilkinson et al., 2005). Of great importance, approximately 3000 deaths each year in Texas are attributable to SHS exposure (Alo & Huang, 2003). Thirty-eight percent of the diverse population of Texas is Hispanic (U.S. Census Bureau, 2010). Mexican Americans account for the majority of Texas Hispanics (88%), and Hispanics represent the most rapidly growing population segment in Texas (U.S. Census Bureau, 2010). Approximately 38% of Mexican Americans participating in the 1999–2002 National Health and Nutrition Examination Survey reported exposure to SHS at home or at work (Pickett, Schober, Brody, Curtin, & Giovino, 2006). Mexican Americans are a high-risk group for developing SHS-related illnesses and have limited access to healthcare. Among Hispanics in the United States, heart disease is the leading cause of death, and cancer is the second leading cause (National Center for Health Statistics, 2010), with lung cancer the leading cause of malignant neoplasm-related death among Hispanics (U.S. Cancer Statistics Working Group, 2010). In a recent qualitative/quantitative investigation of SHS exposure among Hispanic/Latino Californians living in multiunit housing, high levels of SHS were reported along with little ability to protect nonsmokers and their families from this health-compromising exposure (Baezconde-Garbanati et al., 2011).
In light of these facts, we designed the Project Clean Air–Safe Air (Project CASA), a culturally-appropriate intervention to reduce and eliminate SHS exposure in Mexican-American households in the Houston, Texas area. The primary aim of the study was to estimate the impact of an intervention that aimed to reduce SHS exposure in the target households. Secondary aims were to estimate the effect of the intervention in: (1) helping smokers move through the stages of change for quitting, (2) improving smokers' and nonsmokers' knowledge and attitudes about SHS, (3) enhancing perceived health vulnerability among smokers and quitters, and (4) facilitating smoking cessation.
2. Methods
2.1. Overview of study design and intervention
Potential participants were drawn from a population-based cohort of Mexican origin households recruited from the Houston-area. This cohort, known as Mano a Mano, is maintained by the Department of Epidemiology at MD Anderson Cancer Center under direction of one of the authors (MLB). The cohort recruitment methodology has been described previously (Wilkinson et al., 2005). The study was a randomized, controlled trial with assessments at baseline, 6-month, and 12-month post-intervention. Households were pair-matched based on intensity of smoking as reflected by the number of cigarettes smoked daily and were randomized to receive either: (1) the experimental intervention (EI), which consisted of two culturally-appropriate fotonovelas (illustrated storybooks) and one comic book, designed to promote a tobacco-free indoor-air environment, or (2) standard care (SC), which consisted of giving family members an American Cancer Society (1999) booklet entitled Set Yourself Free: Deciding to Quit: A Smoker's Guide.
A power calculation for assessing group differences was based on the primary outcome variable, level of nicotine measured at 12 months post-intervention. Effect sizes were based on a 12-month difference in mean nicotine levels between intervention groups of 0.5 to 0.6 μg/m3 and assuming a common standard deviation of 0.8. Using a two-sample t-test, a sample size of 40 in each group with an estimated attrition of 5 households per group provides 73% to 87% power to detect group differences of 0.5 to 0.6 at p<.05.
2.2. Recruitment and enrollment
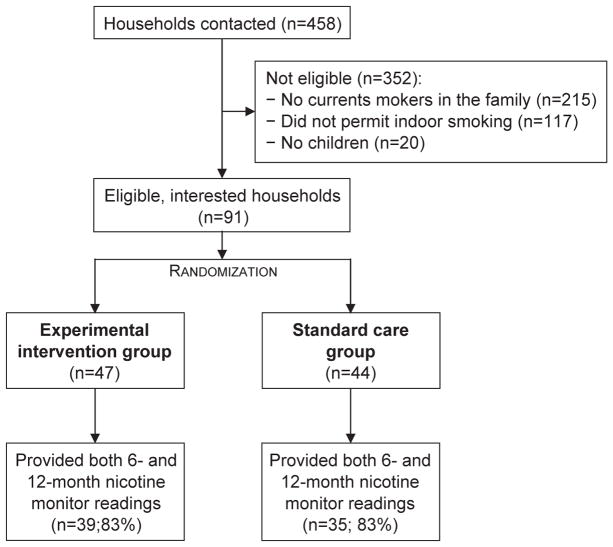
Households with a child under the age of 18 years and two adults, one of whom was a smoker, were identified from the cohort database and invited to participate in the study. Adult members of the aforementioned households were contacted by telephone, explained the purpose of the study and the study procedures, and invited to meet with study personnel to receive further information and consider enrolling into Project CASA. Initially 458 households were contacted to participate in the study; of these, 352 did not meet the eligibility criteria, and 15 eligible households refused to participate. Thus, a total of 91 households (85.8% of those eligible) were enrolled in the study at baseline (Fig. 1).
Fig. 1.
Household recruitment and retention, through a 12-month nicotine monitoring assessment.
All participating households were visited by a pair of bilingual interviewers at baseline, at which time informed consent was obtained, baseline survey data were collected from one or two participating adults, and the fotonovelas and comic book (EI group) or Set Yourself Free booklet (SC group) was provided. With assistance from the research interviewers as needed, surveys were administered using hand-held computers. In addition, an ambient air monitor, for assessing levels of nicotine due to second-hand smoke, was installed in each participating residence at baseline. The 6-month and 12-month assessments were conducted using the same methodology. Seven days after placement (at baseline, 6-month, and 12-month assessments), the air monitors were removed (at the time of the visit for survey administration). At the completion of the study, each participating household received a $25 gift card to a local grocery store. All study procedures were approved by The University of Texas MD Anderson Cancer Center Institutional Review Board.
2.3. Intervention conditions
2.3.1. Experimental intervention
The EI materials, which were designed to increase participants' likelihood of adopting a smoke-free indoor home air policy, included one culturally-appropriate bilingual comic book for children and two fotonovelas for adults. The EI materials detailed the many benefits of a smoke-free household. Specifically, the comic book displayed a story of a child who excelled in sports after his family members decided to eliminate SHS from their home. One fotonovela told a story about a family with a smoking father; the other told a story about a single mother who smoked. Each story was abundantly illustrated with drawings (comic book) and photographs (fotonovelas) addressing SHS-related issues and was designed to increase awareness of the negative impact of SHS on various aspects of life (e.g., children's health, children's education, physical performance, family budget, etc.). In addition, all printed materials included basic facts about SHS and quit-smoking tips and resources. The comic book and fotonovelas, which were designed to be appropriate for low-literacy users and displayed individuals with distinctly Hispanic appearance, addressed the needs of the smokers in the family and the needs of the nonsmoking adults and youth in the family. Each fotonovela integrated strategies such as preparing the smoker to set a quit date (modeling of skilled behavior) and contained examples of multiple benefits related to smoke-free lifestyles. PDF files of these materials can be accessed on the page of MD Anderson's Tobacco Outreach Education Program at http://www.mdanderson.org/toep.
2.3.2. Standard care
Each household in the SC condition received one copy of the American Cancer Society's (1999) self-help booklet titled, “Set Yourself Free: Deciding to Quit: A Smoker's Guide,” which is a resource designed to help cigarette smokers quit smoking. Households in this intervention group were not provided with culturally-appropriate information regarding SHS and the benefits of a smoke-free indoor home air policy.
2.4. Measures
2.4.1. Objective measures of household SHS exposure
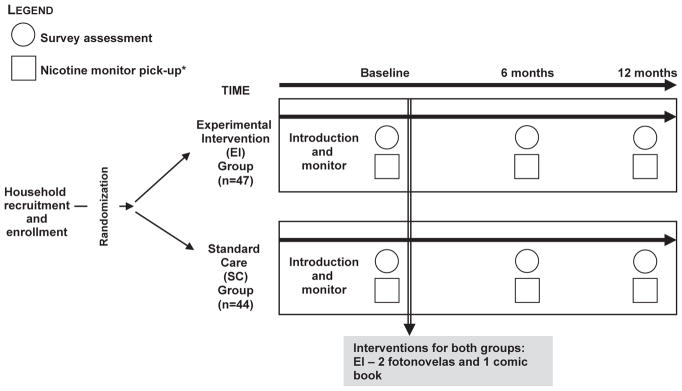
To objectively estimate SHS exposure in the target households, the research interviewers placed and activated two nicotine air sampling monitors in each household at the baseline, 6-month and 12-month follow-up visits (Fig. 2). The small, lightweight monitors, provided by Dr. S. Katharine Hammond from the University of California, Berkley, passively measure ambient nicotine, a marker for SHS exposure (Hammond & Leaderer, 1987). Each monitor was placed in a different high-traffic room within the home and had an identifying barcode to designate its location. Seven days after placement, each monitor was removed by the research interviewers and sent for analysis to Dr. Hammond's laboratory. Ambient nicotine concentrations were provided in units of μg/m3 that represented an average weekly concentration. Most of the field blanks sent between the MD Anderson Cancer Center and University of California, Berkeley have contained less than detectable levels of nicotine.
Fig. 2.
Study design and measurement components.
2.4.2. Self-administered surveys
For the majority of households there were two adult participants, a non-smoker and a smoker, or two smokers. A primary informant in each household was selected. Initially our plan was to select only non-smokers as primary informants, but many households had either only two adults who were both smokers or single-parent households in which the mother was a smoker. Owing to the high risk of SHS exposure among children in these households, we decided to include this household demographic. Of 91 households recruited, 17 had two smokers (primary informant was someone who was able to provide the required information), 57 had one smoker and one non-smoker (primary informant was the non-smoker) and 17 had one smoker and no non-smokers (primary informant was the smoker). At baseline, adults in the participating households, both smokers, and nonsmokers, were asked to complete a baseline survey assessing sociodemographics (age, sex), smoking history, and SHS exposure in the home. The current report also focuses on the secondary outcomes of knowledge and attitudes of SHS hazards, perceived health vulnerability, and self-reported smoking status following intervention. The SHS knowledge items (Table 2) were composed based on consultations with fellow researchers and Mexican-American experts and rated using a 5-point Likert scale ranging from strongly agree to strongly disagree. Respondents who failed to select strongly agree (or strongly disagree, wherever appropriate) were considered to exhibit inadequate knowledge. Health vulnerability was measured with one item related to the effect of continued smoking or relapse among smokers or quitters by asking, “How much would your continuing to smoke hurt your health?” (response options=a lot, some or a little, not at all). Surveys were administered at baseline, 6 months, and 12 months (Fig. 2).
Table 2.
Survey items for assessing second-hand smoke (SHS) knowledge.a
|
Response options: 1=strongly agree, 2=agree, 3=disagree, 4=strongly disagree.
2.5. Data analyses
To evaluate the primary aim, the impact of the intervention on SHS exposure was estimated using mixed model regression of mean ambient nicotine levels (in the two rooms) over time. Separate analyses were run for the high exposure and low exposure rooms. To examine differences in trends over time between the EI and SC, a conditional model was fitted to the data. In addition to the main effects of intervention condition and time, the time-by-condition interactions were included as fixed effects in the model. The estimated slopes were compared between the groups with type III tests of fixed effects. Fixed effect coefficients, standard errors, and p-values were used to summarize the results. The coefficients of time-by-condition terms provided estimates of the average rate of change from baseline to 12 months for the EI and SC groups. The analysis was conducted using Proc Mixed in SAS (SAS, Inc., 2012), which allows for unbalanced designs, missing data, and different covariance structures. The Kenward–Roger method adjusted for small sample correction (Kenward & Roger, 1997).
Subjectively-measured SHS exposure among nonsmokers was based on the SHS index, computed from the responses of the primary informants representing the family to the question “Is smoking allowed inside your home?” (yes/no). Mixed model regression for binary outcomes was used to model the average trend over time and to analyze the differences in patterns of change from baseline to 6-month and 12-month assessments between the EI and SC groups.
Nicotine values recorded from the nicotine monitors were used to validate the subjectively-derived SHS index at 12 months. The extent of agreement between the subjective and objective measurements was computed using independent-sample t-tests. Secondary aims considered intervention effects on self-reported measures of knowledge and attitudes about SHS exposure (baseline smokers and non-smokers), perceived health vulnerability (smokers), and smoking cessation (baseline smokers only). Similar to the analytic approach used for the primary analysis, a mixed model regression was conducted using Proc Mixed in SAS to assess differences in trends over time between the EI and SC intervention groups for knowledge and attitudes and perceived health vulnerability. In addition to the main effects of intervention condition and time, the time-by-condition interactions were included as fixed effects in the model. The estimated slopes were compared between the groups with type III tests of fixed effects. Fixed effect coefficients, standard errors, and p-values were used to summarize the results. The coefficients of time-by-condition terms provided estimates of the average rate of change from baseline to 12 months for the EI and SC groups. Because several households had only one participant at 12-month follow-up, we report unadjusted statistics and p-values associated with these analyses. Correlation of responses within households was accounted for in the mixed model regression, and we also report statistics and p-values adjusted for household correlation. A logistic model was fitted to evaluate the effect of intervention on smoking behavior.
3. Results
3.1. Study participation
Of 91 households that agreed to participate in the study and completed a baseline home visit, 47 were randomly assigned to the EI and 44 to SC. Eighty-nine households provided baseline nicotine-monitor data (EI, n=47; SC, n=42). Of these 89 households, 74 (or 83%) provided baseline, 6-month, and 12-month nicotine monitor data (EI, n=39; SC, n=35) (Table 1). Participants (n=167) from these 91 households completed the baseline surveys (EI, n=85; SC, n=82); of these, 119 (71%), representing 76 households, completed all three assessments (EI, n=60; SC, n=59).
Table 1.
Survey and nicotine monitoring assessments.a
| Assessment time point | Experimental intervention | Standard care | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Households completing survey assessment | Households completing nicotine monitoring | Smokers/non-smokers completing survey assessment | Households completing assessment | Households completing nicotine monitoring | Smokers/non-smokers completing survey assessment | |
| Baseline | 47 | 47 | 55/30 | 44 | 42 | 53/29 |
| 6 months | 41 | 41 | 39/23 | 36 | 36 | 42/22 |
| 12 months | 40 | 39 | 39/20 | 36 | 35 | 37/23 |
Reported as absolute numbers.
3.2. Differences between completers and dropouts
Completers were defined as participants who completed the baseline, 6-month, and 12-month survey assessments, and dropouts were defined as participants who did not complete all three survey assessments. Survey dropout rates in the two groups were as follows: EI, 29%; SC, 28%. Completers and dropouts were compared with respect to their baseline socio-demographic characteristics and smoking-related variables and did not differ with respect to age, baseline smoking status, pros or cons of smoking, temptations to smoke or stage of change. The proportion of males was lower in the completer group (61% vs. 77%, p<0.05).
3.3. Characteristics of participants enrolled at baseline
Of the 119 participants completing the baseline, 6-month, and 12-month surveys, the mean age was 39 years (SD, 12 years), and 70% were females. Seventy-nine (66.4%) were smokers at baseline. No baseline group differences (EI vs SC) were observed in the subsample that completed the 6-month and 12-month surveys with respect to age (37.1 years vs 38.7 years, p=.38), sex (67% vs 62% females, p=.51), intensity of smoking (48.4 vs 47.9 cigarettes per week, p=.93), baseline smoking status (65% vs 65% smokers, p=.99), pros (3.5 vs 3.5, p=.98) and cons (5.6 vs 5.4, p=.68) of smoking, temptations to smoke (18.4 vs 17.1, p=.43), or knowledge of SHS hazards (2.5 vs 2.6, p=.23).
3.4. Smoking status at 12 months
Of the 119 participants who completed the 12-month survey, 79 (66.4%) were smokers at baseline (EI, n=40; SC, n=39). At 12 months, 90% of the baseline smokers in each group (EI, n=36; SC, n=35) were still using tobacco.
3.5. SHS exposure among nonsmokers
Impact of the EI on nonsmokers' objectively measured SHS exposure was based on the 74 households that provided baseline as well as 6-month and 12-month nicotine monitor data. Baseline nicotine levels did not differ between conditions (high exposure room): 1.14 μg/m3 [SD, 2.5] in EI vs. 0.55 μg/m3 [SD, 0.84] in SC. Although mean nicotine level was higher in EI, there was no significant difference using both the independent sample t-test (p=.16) and non-parametric Mann–Whitney test (p=.77). In the low exposure room the corresponding means were: 0.72 μg/m3 [SD, 1.5] in EI versus 0.40 μg/m3 [SD, 0.66] in SC. Similarly there was no significant difference using both the independent sample t-test (p=.25) and non-parametric Mann–Whitney test (p=.83). In the high exposure room, longitudinal comparisons revealed a significant main effect for time: 12-month nicotine levels were significantly lower than baseline levels (F=13.6; DF=147; p<0.001; Fig. 3). There was also a significant time-by-condition effect (F=4.1; DF=72; p<0.05): the mean ambient nicotine level decreased from baseline to 6 months and 12 months in the EI condition (1.14 μg/m3 to 0.37 μg/m3 to 0.20 μg/m3, p<0.01). There is a decrease in the SC condition as well due to content of standard care intervention (0.55 μg/m3 to 0.15 μg/m3 to 0.17 μg/m3, p=.99); however, this decrease was not statistically significant. Estimated slopes showed a significant difference in the average rate of change between the EI and SC households (−0.9 [SE, 0.3] vs. −0.4 [SE, 0.3]; p<0.05) (Fig. 3). In the low exposure room, longitudinal comparisons revealed a significant main effect for time: 12-month nicotine levels were significantly lower than baseline levels (F=19.5; DF=147; p<0.001). There was no significant time-by-condition effect; there were similar reductions in the EI and SC households.
Fig. 3.
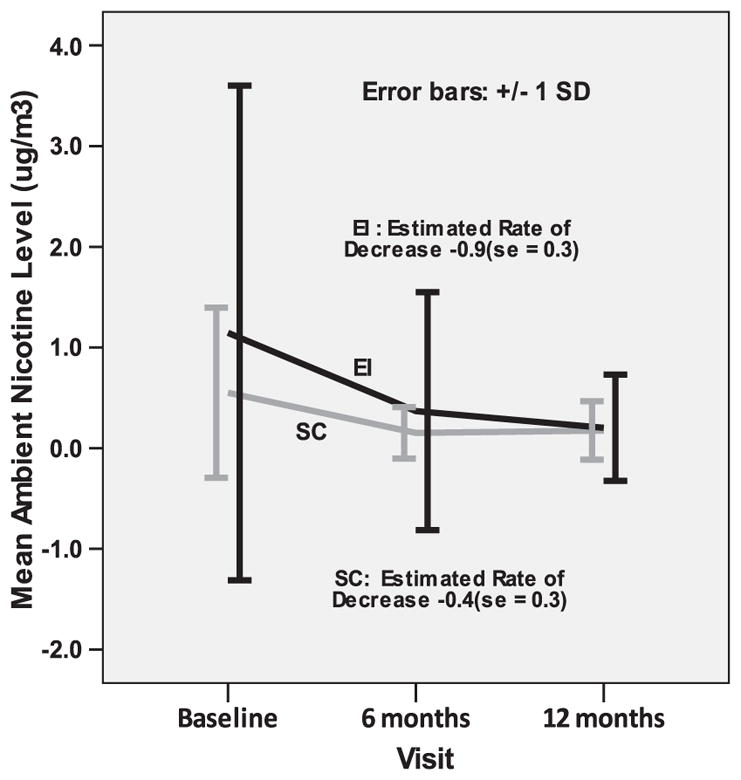
Mean ambient nicotine level in high-exposure room by intervention condition.
Our analysis of the impact of the EI on subjectively measured SHS exposure of nonsmokers was based on the 76 households that completed the baseline as well as 6-month and 12-month follow-up assessments. At baseline, all recruited households allowed smoking indoors (Fig. 4). Longitudinal comparisons revealed a significant main effect for time (F=53.1; DF=72; p<0.001): the percentage of households that banned smoking was significantly higher at 12 months than at baseline, and the proportion of households that banned smoking was higher in the EI group than in the SC group. At 6 months, 57% of EI households had banned smoking, vs. 47% of SC households. At 12 months, 73% of EI households had banned smoking, vs. 56% of SC households.
Fig. 4.
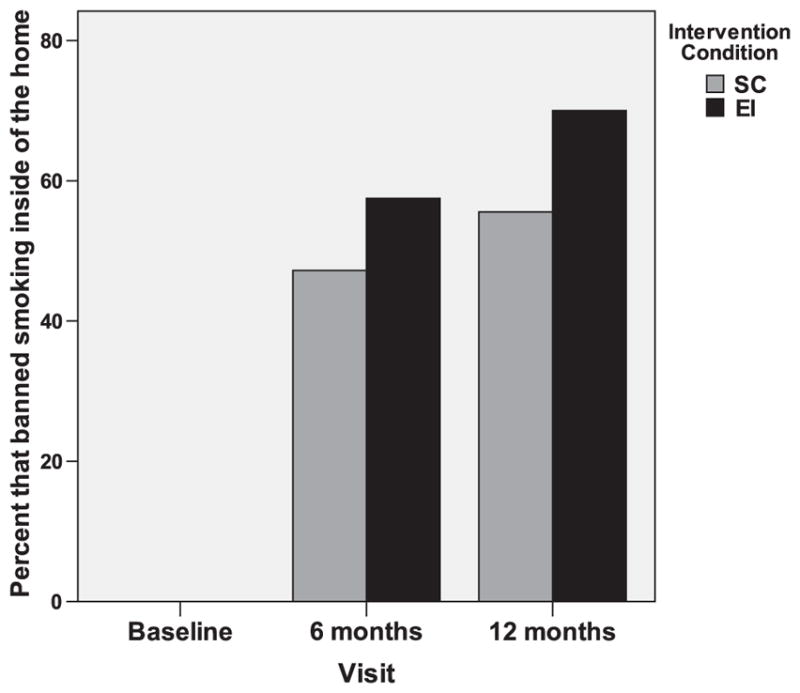
Percentage of households that banned smoking by intervention condition.
Nicotine values recorded from the passive diffusion nicotine monitors were used to validate the subjectively derived SHS index at 6 and 12 months. The degree of association between the subjective and objective measures was computed using independent sample t-tests. Home nicotine concentrations measured using monitors were significantly associated with self-reported nicotine exposure at the 12-month follow-up. In the maximum exposure room, the mean nicotine level was 0.47 μg/m3 [SD=0.66] in households that did not ban smoking indoors vs. 0.04 μg/m3 [SD=0.04] in households that did ban smoking indoors (p<0.01). In the minimum exposure room, the mean nicotine level was 0.22 μg/m3 [SD=0.27] in households that did not ban smoking indoors vs. 0.02 μg/m3 [SD=0.01] in households that did ban smoking indoors (p<0.01).
3.6. Knowledge of SHS health effects
Analysis of knowledge of the health effects of SHS was based on the 119 participants who completed the baseline, 6-month, and 12-month assessments. Overall knowledge was computed as an average score over 24 knowledge items (Table 2) with scores ranging from 0 to 4, with 4 representing the highest score and 0 representing the lowest score. Negatively worded items were reverse coded before scoring. At baseline, no differences were seen between the EI and SC groups in overall knowledge scores (Fig. 5). There was a significant time-by-condition effect (F=6.0; DF=238; p<0.01): the mean overall knowledge score increased from baseline to 6 months and 12 months in the EI condition but did not increase in the SC condition. The corresponding statistics after adjusting for correlation within households are (F=6.0; DF=238; p<0.01). Post hoc comparisons revealed a significant increase in overall knowledge scores from baseline to 6 months and 12 months in the EI condition but no such increase in the SC condition.
Fig. 5.
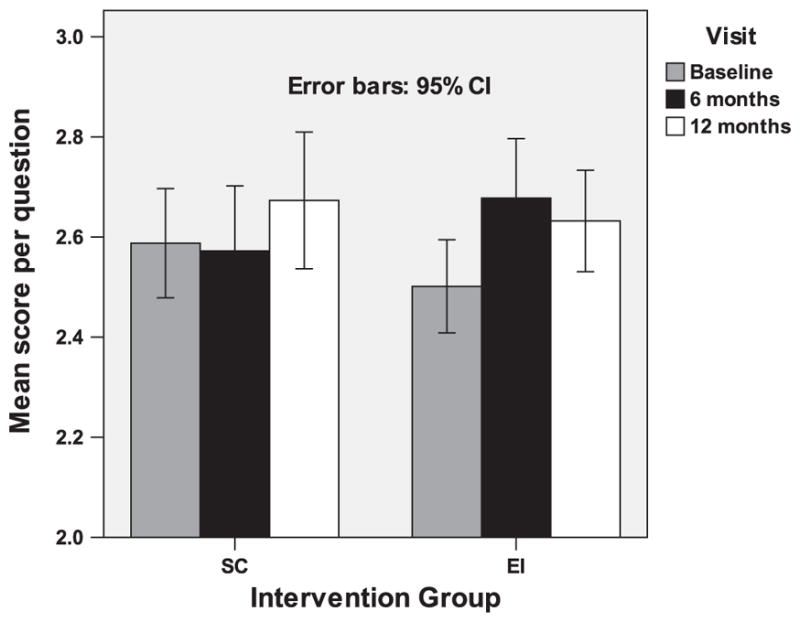
Overall knowledge about SHS by condition.
3.7. Perceived health vulnerability among baseline smokers
We hypothesized that baseline smokers randomly assigned to the EI would have an increased perception of health vulnerability compared to those assigned to SC. We observed a significant time-by-condition effect (F=5.4; DF=87; p<0.01). The corresponding statistics after adjusting for correlation within households are (F=5.8; DF=93; p<0.01). In the EI condition, there was a significant decrease in the mean scale score, implying an increase in perceived vulnerability from baseline to 6 months and 12 months (0.38 to 0.28 to 0.21), whereas in the SC condition, an increase was seen from baseline to 6 months and 12 months (0.26 to 0.76 to 0.46) (Fig. 6).
Fig. 6.
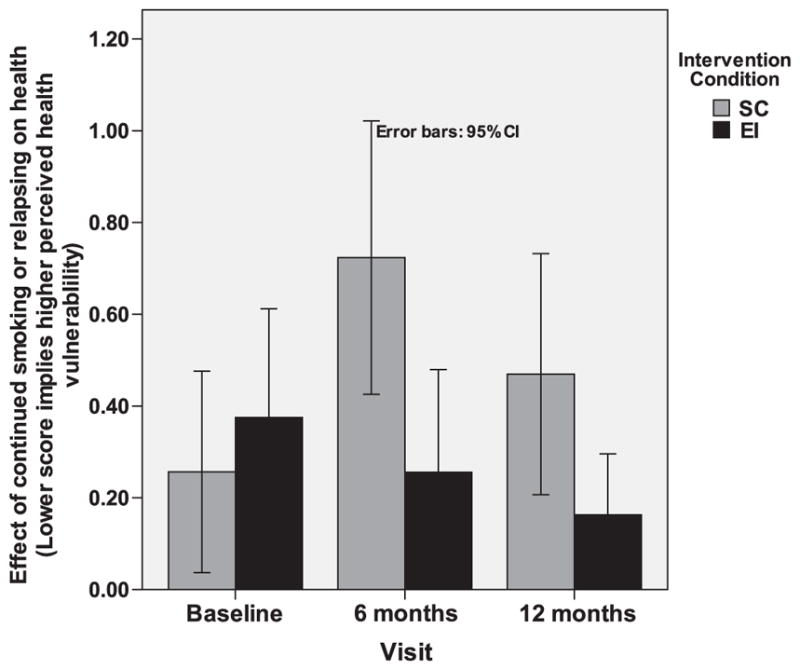
Perception of impact of continued smoking or relapse on health.
4. Discussion
Our findings indicate that our culturally relevant intervention has the potential to substantially decrease SHS-related health problems in Mexican-origin households. Ninety-eight percent of the participating households (89/91) had analyzable results on the baseline nicotine monitoring. A considerable number of participants exhibited inadequate knowledge with respect to SHS and health risks. Results at 6-month follow-up revealed a strong initial impact of our program in terms of objectively and subjectively measured SHS exposure, knowledge of the health risks of SHS, and health vulnerability perception. At 12 months, intervention households continued to be favorably influenced by the customized comic book and fotonovelas, resulting in greater numbers of households that were completely free of SHS. Results from this project will help in optimizing culturally sensitive interventions and eliminating SHS from Mexican-American households.
Our team designed a tailored intervention to address the major health issue of exposure to SHS among the Mexican-American population in the Houston area. In this paper, we provided the concept of a culturally sensitive intervention approach as well as practical strategies and our learning objectives for the target population. Scientific evidence supports a causal relationship between SHS and disease. Previous studies have provided substantial evidence that SHS is associated with respiratory tract infections, asthma, chronic ear infections, dental caries, and sudden infant death syndrome in children (Treyster & Gitterman, 2011; USDHHS, 2006). In adults, lung cancer, stroke, heart disease, and respiratory infections have been causally linked to SHS (Iribarren et al., 2004; O'Toole et al., 2008; Samet et al., 2009; USDHHS, 2006).
Levels of acculturation vary tremendously in the Mexican origin population in Texas (Page, 2006). These differences impact causal beliefs about disease causation (Palmquist, Wilkinson, Sandoval, & Koehly, 2012) and also influence lifestyles and health behaviors (Larkey, Hecht, Miller, & Alatorre, 2001; Wilkinson et al., 2005). Accordingly, when we were designing this intervention, it was important to consider culturally important factors such as familismo (importance of family), personalismo (relationships between individuals), and respeto (respect of authority) (Antshel, 2002; Kreuter, Lukwago, Bucholtz, Clark, & Sanders-Thompson, 2003). This intervention was designed to educate smokers about the impact of their indoor smoking behavior on the health and well-being of their family members in an effort to motivate families to create a smoke free household. Future data analyses from Project CASA will investigate the impact of intervention on perceived health vulnerability of household members and familial social influence processes.
Previous reports have indicated that Hispanic populations, specifically Mexican-American populations, have a lower smoking prevalence than Caucasian populations. However, these reports have also indicated a higher risk of exposure to SHS among Mexican Americans (Lloyd-Jones et al., 2009). In 2000, Hispanics were almost twice as likely as Caucasians to work in the service industry or labor sector, and according to the National Restaurant Association, the service industries are least likely to be covered by smoke-free policies (U.S. Census Bureau, 2000).
The California Tobacco Control Program surveyed Californians regarding knowledge of SHS. Results indicated that 76.2% of smokers, compared to 92.3% of nonsmokers, believed that SHS causes lung cancer (California Department of Public Health, 2008). In 2008, California had a population of approximately 37 million people, approximately 35% of whom were Hispanic. California is a leader in legalizing smoke-free environments — by 1995, the state had banned smoking in indoor workplaces, including bars, clubs, and restaurants. The findings of the Tobacco Control Program survey found that by 2005, 94.8% of the people surveyed indicated that they worked in a completely smoke-free environment. Nevertheless, recently published data from the 2005 and 2007 California Health Interview Surveys revealed that among all racial/ethnic groups, Hispanics had the highest rates of SHS exposure at work (19.5%) (Max, Sung, & Shi, 2012). And even though of all racial/ethnic groups Hispanics had the lowest SHS exposure at home, efforts are needed to eliminate this problem completely.
Comparatively, Texas does not have a statewide smoke-free work-place law. It is estimated that only 36% of the citizens of the State of Texas work in a smoke-free environment. In 2009, the statewide smoking ban failed to be approved by the state legislature; however, 30 cities in Texas, including Austin, Dallas, El Paso, Houston Laredo, and San Antonio, have citywide smoke-free policies (TX Smoke-Free Ordinance Database, 2011). Texas and California have similar proportions of Hispanic residents (Texas, 36%; California, 35%) and share similar demographic make-up of their populations and yet have completely opposite approaches to smoking bans.
Among the study limitations that ought to be mentioned are the relatively small number of participating households and a possibility that our study participants grew better at smoking outside for the week of monitoring. However, the monitors were placed in households randomized in both groups, so the effect would not affect the difference seen by group.
In conclusion, in the contemporary society with more and more states and communities adopting smoke-free environment regulations in public places, smokers are likely to use tobacco at home at increasing rates. In 2006/2007, only half of households in the United States with both children and smokers had complete smoking bans (Mills, White, Pierce, & Messer, 2011). Importantly, such bans appear to be less common in households with older children in minority communities, including Hispanics (Mills et al., 2011). Therefore, it is imperative to have methodologically-strong home-based interventions, and our study has demonstrated the ability of a low-cost intervention to favorably impact SHS-related knowledge and exposure in Mexican-American households.
HIGHLIGHTS.
A randomized trial to reduce and eliminate exposure of nonsmokers to secondhand smoke
Increase participants' likelihood of adopting a smoke-free indoor home air policy
Ambient nicotine levels decreased at a 12-month follow-up.
Acknowledgments
Role of funding sources
Flight Attendant Medical Research Institute ($300K; Dr. Prokhorov, Principal Investigator; FAMRI #052374).
This study was partially supported by the Intramural Research Program of the National Human Genome Research Institute at the NIH (Z01HG200335; Dr. Koehly, Principal Investigator).
The Mano a Mano cohort receives funds collected pursuant to the Comprehensive Tobacco Settlement of 1998 and appropriated by the 76th legislature to The University of Texas MD Anderson Cancer Center; by the Caroline W. Law Fund for Cancer Prevention, and by the Dan Duncan Family Institute for Risk Assessment and Cancer Prevention.
The funders did not contribute to the design and conduct of the study, the data collection, analysis, and interpretation of the data, the preparation, review, or approval of the manuscript.
The authors would like to thank Gloria Cortez and Mariquita Sanchez for their tireless data collection efforts on this project, as well as the Mano a Mano cohort staff who assisted with recruitment.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Contributors
Alexander V. Prokhorov: the principal investigator on this project. He conceptualized the experimental intervention and the overall study design. Led all the key aspects of the study. Took the leading role in writing this paper.
Karen Suchanek Hudmon: assisted in conceptualizing the study design and edited the paper.
Salma K. Marani: provided biostatistical expertise and assisted in writing the paper. Melissa L. Bondy: the leader of the Mexican-American cohort in the Houston area.
Helped in recruitment strategies and procedures.
Leticia A. Gatus: acted as the project director on Project CASA. Coordinated the complex field activities.
Margaret R. Spitz: as then-Chair of the Department of Epidemiology, provided important guidance with respect to various scientific aspects of Project CASA.
Anna V. Wilkinson: as the study collaborator, helped with multiple scientific and logistical issues. Assisted in writing the paper.
S. Katharine Hammond: Provided nicotine monitors and ambient nicotine analysis throughout the study.
Laura M. Koehly: as the study collaborator and expert in social networks analysis, assisted with selection of the eligibility criteria and provided valuable support in statistical analysis.
All authors have reviewed and approved the final manuscript.
References
- Alo C, Huang P. Secondhand smoke exposure among middle and high school students—Texas, 2001. Morbidity and Mortality Weekly Report. 2003;52:152–154. [PubMed] [Google Scholar]
- Antshel KM. Integrating culture as a means of improving treatment adherence in the Latino population. Psychology, Health & Medicine. 2002;7:435–449. [Google Scholar]
- Baezconde-Garbanati LA, Weich-Reushe K, Espinoza L, Portugal C, Barahona R, Garbanati J, et al. Secondhand smoke exposure among Hispanics/Latinos living in multiunit housing: Exploring barriers to new policies. American Journal of Health Promotion. 2011;25:S82–S90. doi: 10.4278/ajhp.100628-QUAL-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B, McQuaid EL, Novak SP, Hammond SK, Becker B. Motivating Latino caregivers of children with asthma to quit smoking: A randomized trial. Journal of Consulting and Clinical Psychology. 2010;78:34–43. doi: 10.1037/a0016932. [DOI] [PubMed] [Google Scholar]
- Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108:18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- Hammond SK, Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environmental Science & Technology. 1987;21:494–497. doi: 10.1021/es00159a012. [DOI] [PubMed] [Google Scholar]
- Iribarren C, Darbinian J, Klatsky AL, Friedman GD. Cohort study of exposure to environmental tobacco smoke and risk of first ischemic stroke and transient ischemic attack. Neuroepidemiology. 2004;23:38–44. doi: 10.1159/000073973. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Kreuter MW, Lukwago SN, Bucholtz RD, Clark EM, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: Targeted and tailored approaches. Health Education & Behavior. 2003;30:133–146. doi: 10.1177/1090198102251021. [DOI] [PubMed] [Google Scholar]
- Larkey LK, Hecht ML, Miller K, Alatorre C. Hispanic cultural norms for health-seeking behaviors in the face of symptoms. Health Education & Behavior. 2001;28:65–80. doi: 10.1177/109019810102800107. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Max W, Sung HY, Shi Y. Exposure to secondhand smoke at home and at work in California. Public Health Reports. 2012;127:81–88. doi: 10.1177/003335491212700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AL, White MM, Pierce JP, Messer K. Home smoking bans among U.S. households with children and smokers. Opportunities for intervention. American Journal of Preventive Medicine. 2011;41:559–565. doi: 10.1016/j.amepre.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet. 2011;377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- O'Toole TE, Conklin DJ, Bhatnagar A. Environmental risk factors for heart disease. Reviews on Environmental Health. 2008;23:167–202. doi: 10.1515/reveh.2008.23.3.167. [DOI] [PubMed] [Google Scholar]
- Page RL. Acculturation in Mexican immigrants: A concept analysis. Journal of Holistic Nursing. 2006;24:270–278. doi: 10.1177/0898010106289839. quiz 279–281. [DOI] [PubMed] [Google Scholar]
- Palmquist AE, Wilkinson AV, Sandoval JM, Koehly LM. Age-related differences in biomedical and folk beliefs as causes for diabetes and heart disease among mexican origin adults. Journal of Immigrant and Minority Health. 2012;14:596–601. doi: 10.1007/s10903-011-9522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett MS, Schober SE, Brody DJ, Curtin LR, Giovino GA. Smoke-free laws and secondhand smoke exposure in US non-smoking adults, 1999–2002. Tobacco Control. 2006;15:302–307. doi: 10.1136/tc.2005.015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, et al. Lung cancer in never smokers: Clinical epidemiology and environmental risk factors. Clinical Cancer Research. 2009;15:5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treyster Z, Gitterman B. Second hand smoke exposure in children: Environmental factors, physiological effects, and interventions within pediatrics. Reviews on Environmental Health. 2011;26:187–195. doi: 10.1515/reveh.2011.026. [DOI] [PubMed] [Google Scholar]
- Wilkinson AV, Spitz MR, Strom SS, Prokhorov AV, Barcenas CH, Cao Y, et al. Effects of nativity, age at migration, and acculturation on smoking among adult Houston residents of Mexican descent. American Journal of Public Health. 2005;95:1043–1049. doi: 10.2105/AJPH.2004.055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Web References
- American Cancer Society. Set Yourself Free Booklet. [Date the reference was last accessed: 06/05/2012];Deciding to Quit: A Smoker's Guide. 1999 No.2054-Rev.08/05. http://www.cancer.org/acs/groups/content/@highplains/documents/webcontent/setyourselffreepdf.pdf.
- California Department of Public Health. Secondhand Smoke in California. California Department of Public Health; 2008. [Date the reference was last accessed: 06/05/2012]. http://www.cdph.ca.gov/programs/tobacco/Documents/CTCPFactShSHSinCA2008.pdf. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Tobacco Control State Highlights 2010. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Date the reference was last accessed: 06/05/2012]. http://www.cdc.gov/tobacco/data_statistics/state_data/state_highlights/2010/pdfs/highlights2010.pdf. [Google Scholar]
- National Center for Health Statistics. Health, United States, 2009: With Special Feature on Medical Technology. Hyattsville, MD: 2010. [Date the reference was last accessed: 06/05/2012]. http://www.cdc.gov/nchs/data/hus/hus09.pdf. [PubMed] [Google Scholar]
- SAS Version 9.2; SAS Institute Inc. (SAS, Inc. Cary, NC: 2012. [Date the reference was last accessed: 06/05/2012]. http://www.sas.com/company/ [Google Scholar]
- TX Smoke-Free Ordinance Database. [Date the reference was last accessed: 06/05/2012];Most Protected Municipalities Report. 2011 http://www.utmb.edu/shsordinances/createReports.aspx.
- U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2010. [Date the reference was last accessed: 06/05/2012]. http://www.cdc.gov/cancer/npcr/uscs/download_data.htm. [Google Scholar]
- U.S. Census Bureau. [Date the reference was last accessed: 06/05/2012];Current Population Survey, Table 10.1: Occupation of the Employed Civilian Population 16 Years and Over by Sex, Hispanic Origin, and Race: March 2000. 2000 http://www.census.gov/populatiokn/socdemo/hispanic/ppl-171/tab10-1.pdf.
- U.S. Census Bureau. [Date the reference was last accessed: 06/05/2012];State and County Quick Facts, 2010. 2010 http://quickfacts.census.gov/qfd/states/48000.html.
- U.S. Department of Health and Human Services (USDHHS) The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Date the reference was last accessed: 06/05/2012]. http://www.surgeongeneral.gov/library/reports/secondhandsmoke/fullreport.pdf. [Google Scholar]
- U.S. Department of Health and Human Services (USDHHS) How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Date the reference was last accessed: 06/05/2012]. http://www.surgeongeneral.gov/library/reports/tobaccosmoke/full_report.pdf. [PubMed] [Google Scholar]