Abstract
Background/Objectives
Surgery is commonly performed in frail older individuals seeking care for urologic conditions. While it is known that major urologic surgery is associated with increased mortality and loss of function among frail older adults, outcomes of minor urologic surgery in this population remain unknown.
Design
Retrospective cohort study.
Setting
U.S. nursing homes (NHs).
Participants
NH residents ages 65 and older undergoing minor inpatient urologic surgery (i.e., cystoscopy, bladder biopsy, transurethral resection of bladder tumor, prostate biopsy, transurethral resection of prostate, removal of ureteral obstruction, and suprapubic tube placement) in the United States between 2004 and 2012 (N=34,605).
Measurements
One-year mortality and changes in functional status before and after surgery using the Minimum Data Set-Activities of Daily Living (MDS-ADL) summary scale.
Results
Overall 1-year mortality was 50% and, on average, residents experienced a 1.9-point worsening in their MDS-ADL score at 1 year, whereas the most highly functional residents [baseline quartile of MDS-ADL scores (0–12)] experienced a 4.7-point worsening in their MDS-ADL scores at 1 year. Functional decline among residents 1 year after surgery was associated with a decline in function in the 6 months prior to surgery (AHR 2.39, 95% CI 2.29–2.49), emergent procedures (AHR 1.37, 95% CI 1.31–1.43), older age (age 85+ v. age 65–74, AHR 1.17, 95% CI 1.11–1.23) and cognitive impairment at baseline (AHR 1.15, 95% CI 1.11–1.20).
Conclusion
Despite the lower complexity of minor urologic procedures, nursing home residents experience high mortality and many demonstrate sustained functional decline up to 1-year post operatively.
Keywords: Medicare, frail, minimum data set (MDS), elderly
Introduction
Almost two thirds of all urologic surgery is performed in the elderly.1 The number of older individuals undergoing urologic surgery will increase dramatically in the near future, as the older population is expected to double between 2010 and 2050.2,3 Nursing home residents represent the frailest of older individuals undergoing surgery and they embody several factors contributing to vulnerability including older age, increased comorbidity, polypharmacy, cognitive deficits, limited social support and poor functional status.4 The benefits of surgery in the nursing home population, however minor that surgery may be, are uncertain and the extent to which surgery extends life and promotes functionality remains unknown.
Functional status is defined as everyday behaviors necessary to maintain daily life such as the ability to walk, bathe, dress, get out of bed, and use the toilet. Poor functional status has been associated with decreases in quality of life, limited survival, increased hospitalization and is also a determinant of caregiver needs and health care costs.5–7 In nursing home residents who have limited life expectancy, 43% 1-year mortality in men and 32% 1-year mortality in women,8 the goal of surgery is often to improve functionality in the final years of life. However, the trajectory of functional status following common, minor urologic surgery has not yet been investigated.
In order to better define the functional outcomes resulting from minor elective urologic surgery in the frail elderly, we performed a retrospective cohort study among nursing home residents undergoing inpatient cystoscopy, bladder biopsy, transurethral resection of bladder tumor (TURBT), transurethral resection of the prostate (TURP), prostate biopsy, removal of ureteral obstruction, and suprapubic tube (SPT) placement using national Medicare claims linked to the Minimum Data Set for Nursing Homes. We expected to find poor outcomes, both in terms of function and mortality, one year after surgery despite the relatively “minor” nature of these surgical procedures. Findings from this study will provide a better understanding of the functional risks associated with these minor procedures in this growing and vulnerable patient population and will be instrumental in counseling patients undergoing these procedures in the future to set realistic expectations.
Methods
Subjects and Databases
We identified a cohort of nursing home residents undergoing minor urologic surgery by linking Medicare Inpatient Files (2004–2012) with the Minimum Data Set for Nursing Homes (2003–2013). The Medicare Inpatient File contains data on all fee-for-service inpatient hospitalizations for Medicare beneficiaries. The Minimum Data Set is a mandatory assessment of all nursing home residents receiving Medicare or Medicaid. These assessments include functional, cognitive and health data on residents and are performed by nursing staff on admission, readmission, quarterly, and when there is a change in clinical status.
Nursing home residents undergoing minor urologic procedures were identified in the Medicare Inpatient File using International Classification of Diseases, Ninth Revision codes. The following codes were identified: transurethral removal of obstruction from ureter and renal pelvis (56.0), percutaneous cystostomy (57.17), other suprapubic cystotomy (57.18), other cystoscopy (57.32), closed [transurethral] biopsy of bladder (57.33), other transurethral excision or destruction of lesion or tissue of bladder (57.49), closed [percutaneous] [needle] biopsy of prostate (60.11), transurethral (ultrasound) guided laser induced prostatectomy (TULIP) (60.21), and other transurethral prostatectomy (60.29). Nursing home residents were classified as long-term and included in the cohort if they had completed at least two or more consecutive full or quarterly assessments more than 90 days apart during the 6 months prior to their procedure and had a nursing home length of stay at least 90 days after the procedure. This study was approved by our Institutional Review Board.
Outcome Measures
Our primary outcome measures were functional status and mortality. Functional status was measured using the Minimum Data Set-Activities of Daily Living (MDS-ADL) score. This is a validated measure of function in the minimum data set that measures seven domains including: dressing, personal hygiene, toilet use, transfer, locomotion, bed mobility and eating. Summary scores range from 0 (total independence in all seven domains) to 28 (total dependence on all seven domains)9 and scores have been validated against other standardized measures of functional independence.10,11 We defined baseline function using the MDS-ADL summary score reported on the most recent assessment prior to surgery. Changes in functional status were defined as a change of ≥ 2 points in the MDS-ADL summary score in either direction, where an increase of ≥ 2 points indicated a decline in functional status and a decrease of ≥ 2 points indicated an improvement in functional status. Changes of < 2 points on the MDS-ADL summary score indicated no change in functional status, consistent with existing literature.12–14 MDS-ADL measurements were made in three month increments starting 6 months prior to surgery and up to 12 months after surgery. Mortality was measured using the date of death information from the Medicare Denominator file.
Covariates
Demographic data on age, gender, race, and type of admission (elective versus emergent) were obtained from the Medicare Inpatient Files. Information on comorbidity was collected both from the Medicare Inpatient Files and from the Minimum Data Set and used to calculate a Charlson Comorbidity Index score.15 ADL decline at baseline was determined based on a ≥ 2 point increase in MDS-ADL summary score in the 6 months prior to surgery and ADL groups at baseline were determined based on quartiles.
Cognitive impairment was calculated using a combination of measures. An individual was determined to have cognitive impairment if they had a disease diagnosis of Alzheimer’s Dementia or Dementia in either the Medicare Inpatient File or the Minimum Data set, a score of ≥ 3 on the Minimum Data Set Cognitive Performance Scale (MDS-CPS) measured in the Minimum Data Set 2.0 up to year 201016,17 or a score of 0–7 on the Brief Interview for Mental Status (BIMS) score measured in the Minimum Data Set 3.0 starting in 2010.18
Statistical Analysis
We fit multivariable Cox regression models to estimate the hazards ratios for mortality at 1 year after surgery and for functional decline among survivors at 1 year after surgery. Both models were adjusted for the following covariates: age group (65–74, 75–84 and ≥ 85 years), gender, race (white, black, other), admission status (elective versus emergent), ADL decline at baseline, MDS-ADL score at baseline (0–12, 13–17, 18–21, 22–28), cognitive impairment at baseline, Charlson score, and calendar year that the procedure was performed in.
Dynamic trends of MDS-ADL scores in 3-month increments from baseline were calculated by tabulating the proportion of subjects at 3, 6, 9, and 12 months after surgery with increases of more than 2 points from baseline. In order to accommodate missing outcome data, we used multiple imputation to fill-in missing ADL scores. In order to make the assumption of missing at random more plausible, imputation models also used age, gender, race, type of admission (elective versus emergent), and Charlson score. Results are presented as percentages in stacked bar graphs.
We used mixed-effects spline models to explore MDS-ADL functional trajectories before and after surgery. We chose the cubic spline model to represent the non-linear nature of our data over time, allowing the data to fit separate curves for each segment of time.19 This method allowed for us to incorporate multiple measurements for each subject spanning from 6 months prior to surgery to 12 months after surgery. More specifically, these were cubic spline models with knots placed at −1, 0, 3, 6 and 9 months, where 0 months represents baseline. Models included fixed and random effects for the coefficients of the spline with each subject’s measurements scattered around a subject-specific smooth curve. Fixed effects included age, gender, race and comorbidity, allowing for the population trajectory to shift based on the baseline characteristics. Mortality was graphed using the Kaplan-Meier method to estimate cumulative 1-year mortality from the date of surgery.
In order to contextualize our findings, we compared the outcomes of mortality and functional trajectories between our nursing home resident cohort undergoing minor urologic surgery with a propensity matched cohort of nursing home residents not undergoing these procedures. These cohorts were matched based on age, gender, race, baseline MDS-ADL score, baseline cognitive impairment and baseline Charlson score. Propensity matching was performed based on caliper matching without replacement (caliper width 0.2 standard deviations of the logit propensity) to derive a matched sample.20 To ensure effectiveness of the propensity score balancing, we compared standardized differences in each predictor before and after matching. Once an adequate level of matching was achieved, we assessed the association of the outcomes (mortality and functional status) with the predictor of nursing home residents undergoing and not undergoing minor urologic surgery.
Results
Resident characteristics
A total of 36,405 residents underwent minor urologic surgery during the study period. Residents had a mean age of 81.5 ± 7.4 years and the majority were male (63.4%) and white (81.2%) (Table 1). The mean MDS-ADL summary score prior to surgery was 17.4 ± 7.2, reflective of a poorly functioning cohort, and 29.3% of individuals experienced decline in MDS-ADL score (≥ 2 point increase in MDS-ADL score) in the 6 months prior to surgery. The majority of residents (60.9%) demonstrated cognitive impairment at baseline.
Table 1.
Characteristics of nursing home residents undergoing minor urologic surgery
| Characteristic | Total Obs: N (%) |
|---|---|
| Total N (%) | 36405 (100.0) |
| Age (year): | |
| Mean ± SD | 81.5 ± 7.4 |
| Age Group: N (%) | |
| 65 to 74 | 7331 (20.1) |
| 75 to 84 | 15619 (42.9) |
| 85 over | 13455 (37.0) |
| Gender: N (%) | |
| Male | 23089 (63.4) |
| Female | 13316 (36.6) |
| Race: N (%) | |
| White | 29547 (81.2) |
| Black | 5913 (16.2) |
| Others | 945 (2.6) |
| Admission Status: N (%) | |
| Elective | 7753 (21.3) |
| Emergent | 28652 (78.7)) |
| Baseline ADL score: | |
| Mean ± SD | 17.4 ± 7.2 |
| ADL groups at baseline: N (%) | |
| 0 to 12 | 8393 (23.1) |
| 13 to 17 | 7204 (19.8) |
| 18 to 21 | 10059 (27.6) |
| 22 to 28 | 10749 (29.5) |
| ADL decline at baseline: N (%) | |
| No | 25741 (70.7) |
| Yes | 10664 (29.3) |
| Cognitive impairment at baseline: N (%) | |
| No | 14220 (39.1) |
| Yes | 22185 (60.9) |
| Baseline Charlson score: | |
| Mean ± SD | 1.3 ± 1.1 |
| Charlson score groups: N (%) | |
| 0 | 4408 (12.1) |
| 1 | 7137 (19.6) |
| 2 | 7504 (20.6) |
| 3,4 | 11446 (31.4) |
| >=5 | 5910 (16.2) |
| Procedure year: N (%) | |
| 2004 | 4952 (13.6) |
| 2005 | 4266 (11.7) |
| 2006 | 3957 (10.9) |
| 2007 | 3680 (10.1) |
| 2008 | 3896 (10.7) |
| 2009 | 3700 (10.2) |
| 2010 | 3696 (10.2) |
| 2011 | 4231 (11.6) |
| 2012 | 4027 (11.1) |
| Mortality after procedure: N (%) | |
| Death within one month | 4073 (11.2) |
| Death within one year | 18019 (49.5) |
Mortality
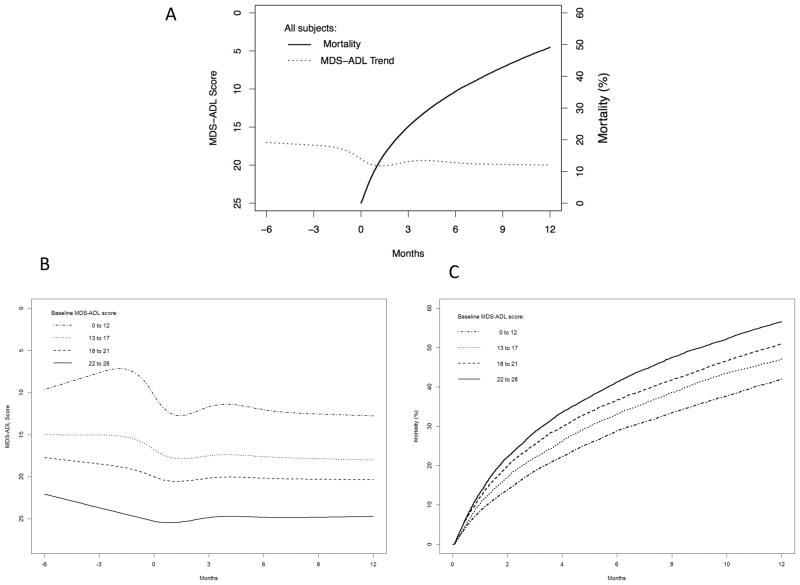
Mortality following the procedure was high, at 11.2% and 49.5% at 1 and 12 months after surgery, respectively. (Figure 1A). Survival analysis stratified by quartile of baseline MDS-ADL score demonstrated that one-year mortality increased with increasing functional impairment (Figure 1B). In multivariate analysis (Table 2), there were many factors associated with 1-year mortality including older age (AHR 1.65, 05% CI 1.58–1.72 for oldest age of ≥85 compared to ages 65–70), functional decline in the 6 months prior to baseline (AHR 1.23, 95% CI 1.19–1.26), worse baseline functional status (AHR 1.14, 95% CI 1.35–1.47 for MDS-ADL score 22–28 compared to score 0–12), higher/worse Charlson score at baseline (AHR 1.42, 95% CI 1.34–1.50 for Charlson score ≥5 compared to Charlson score 0) and procedures that were performed emergently (AHR 1.41, 95% CI 1.36–1.47). Factors that were protective against 1-year mortality included female gender (AHR 0.88, 95% CI 0.85–0.91) and non-white race (AHR 0.94, 95% CI 0.90–0.98 for black and AHR 0.83, 95% CI 0.76–0.92 for other races). Cognitive impairment was not statistically significantly associated with 1-year mortality.
Figure 1.
1-year mortality and functional trajectories before and after surgery, both overall (A) and stratified by baseline MDS-ADL score (B,C). Figures are adjusted for age, gender, race and Charlson score.
Table 2.
Resident characteristics associated with 1-year mortality. Values are adjusted for calendar year.
| Basic Statistics | Univariate Cox Model | Multivariate Cox Model | |||||
|---|---|---|---|---|---|---|---|
| Variable Name | Observed N (%) | Event # (%) | P value | HR & 95% CI | P value | HR & 95% CI | P value |
| Total N (%) | 36405 (100.0) | 18019 (49.5) | |||||
| Age Group | |||||||
| 65 to 74 | 7331 (20.1) | 2967 (40.5) | <.0001 | Ref. | Ref. | ||
| 75 to 84 | 15619 (42.9) | 7440 (47.6) | 1.25 (1.20 – 1.31) | <.0001 | 1.26 (1.21 – 1.32) | <.0001 | |
| 85 over | 13455 (37.0) | 7612 (56.6) | 1.62 (1.55 – 1.69) | <.0001 | 1.65 (1.58 – 1.72) | <.0001 | |
| Gender | |||||||
| Male | 23089 (63.4) | 11567 (50.1) | 0.0025 | Ref. | Ref. | ||
| Female | 13316 (36.6) | 6452 (48.5) | 0.95 (0.92 – 0.98) | 0.0014 | 0.88 (0.85 – 0.91) | <.0001 | |
| Race | |||||||
| White | 29547 (81.2) | 14686 (49.7) | 0.0200 | Ref. | Ref. | ||
| Black | 5913 (16.2) | 2906 (49.1) | 0.98 (0.94 – 1.02) | 0.3583 | 0.94 (0.90 – 0.98) | 0.0034 | |
| Others | 945 (2.6) | 427 (45.2) | 0.86 (0.79 – 0.95) | 0.0031 | 0.83 (0.76 – 0.92) | 0.0002 | |
| Admission Status | |||||||
| Elective | 7753 (21.3) | 3069 (39.6) | <.0001 | Ref. | Ref. | ||
| Emergent | 28652 (78.7) | 14950 (52.2) | 1.49 (1.43 – 1.55) | <.0001 | 1.41 (1.36 – 1.47) | <.0001 | |
| ADL decline at baseline | |||||||
| No | 25741 (70.7) | 12076 (46.9) | <.0001 | Ref. | Ref. | ||
| Yes | 10664 (29.3) | 5943 (55.7) | 1.32 (1.27 – 1.36) | <.0001 | 1.23 (1.19 – 1.26) | <.0001 | |
| ADL groups at baseline | |||||||
| 0 to 12 | 8393 (23.1) | 3504 (41.7) | <.0001 | Ref. | Ref. | ||
| 13 to 17 | 7204 (19.8) | 3370 (46.8) | 1.18 (1.12 – 1.23) | <.0001 | 1.09 (1.04 – 1.15) | 0.0003 | |
| 18 to 21 | 10059 (27.6) | 5096 (50.7) | 1.33 (1.27 – 1.38) | <.0001 | 1.19 (1.14 – 1.24) | <.0001 | |
| 22 to 28 | 10749 (29.5) | 6049 (56.3) | 1.54 (1.48 – 1.61) | <.0001 | 1.41 (1.35 – 1.47) | <.0001 | |
| Cognitive impairment at baseline | |||||||
| 0 | 14220 (39.1) | 6605 (46.4) | <.0001 | Ref. | Ref. | ||
| 1 | 22185 (60.9) | 11414 (51.4) | 1.15 (1.12 – 1.19) | <.0001 | 1.02 (0.98 – 1.05) | 0.3320 | |
| Charlson score groups | |||||||
| 0 | 4408 (12.1) | 1884 (42.7) | <.0001 | Ref. | Ref. | ||
| 1 | 7137 (19.6) | 3322 (46.5) | 1.12 (1.06 – 1.19) | <.0001 | 1.12 (1.06 – 1.19) | <.0001 | |
| 2 | 7504 (20.6) | 3685 (49.1) | 1.23 (1.16 – 1.30) | <.0001 | 1.22 (1.16 – 1.29) | <.0001 | |
| 3,4 | 11446 (31.4) | 5889 (51.5) | 1.31 (1.24 – 1.38) | <.0001 | 1.30 (1.23 – 1.37) | <.0001 | |
| >=5 | 5910 (16.2) | 3239 (54.8) | 1.44 (1.36 – 1.52) | <.0001 | 1.42 (1.34 – 1.50) | <.0001 | |
Functional status
MDS-ADL scores declined from 6 months prior to surgery to 12 months after surgery (Figure 1A). Average MDS-ADL score was 17.0 (95% CI 16.9–17.1) at baseline, 19.4 (95% CI 19.3–19.5) 3 months after surgery and 18.9 (95% CI 18.7–19.0) at 12 months after surgery, with all values adjusted for age, gender, race, admission status (emergent vs. elective) and Charlson score. Functional trajectories stratified by baseline MDS-ADL score are demonstrated in Figure 1B. Residents in the best quartile of baseline MDS-ADL scores (0–12) experienced the most profound decrease in MDS-ADL score, measured as 7.3 (95% CI 7.0–7.7) at baseline and 12.0 (95% CI 11.7–12.3) measured at 12 months after surgery, representing a decrease of 4.7 points.
The proportion of residents who experienced functional decline was greatest in the 3 months after surgery (29.8%), representing 39.2% of surviving residents (Supplemental Figure 1). The proportion of residents who died rose from 24.0% at 3 months to 49.5% at 12 months after surgery, while the proportion of residents who maintained functional status dropped from 38.4% to 21.1% during the same time period. Only 7.8% and 8.5% of residents demonstrated an improvement in functional status at 3 and 12 months after surgery.
Multivariate analysis demonstrated that functional decline in the 6 months prior to surgery was the strongest predictor of functional decline among survivors at 1 year after surgery (AHR 2.39, 95% CI 2.29–2.49) (Table 3). Other predictors of functional decline among survivors 1 year after surgery include older age (age 85+ vs. age 65–74, AHR 1.17, 95% CI 1.11–1.23), female gender (AHR 1.05, 95% CI 1.01–1.10), procedures that were performed emergently (AHR 1.37, 95% CI 1.31–1.43) and baseline cognitive impairment (AHR 1.15, 95% CI 1.11–1.20). Charlson score did not statistically significantly affect the outcome.
Table 3.
Resident characteristics associated with functional decline among 1-year survivors. Values are adjusted for calendar year.
| Basic Statistics | Univariate Cox Model | Multivariate Cox Model | |||||
|---|---|---|---|---|---|---|---|
| Variable Name | Observed N (%) | Event N (%) | P value | HR & 95% CI | P value | HR & 95% CI | P value |
| Total N (%) | 18386 (100.0) | 11405 (62.0) | |||||
| Age Group | |||||||
| 65 to 74 | 4364 (23.7) | 2541 (58.2) | <.0001 | Ref. | Ref. | ||
| 75 to 84 | 8179 (44.5) | 5013 (61.3) | 1.09 (1.04 – 1.15) | 0.0002 | 1.07 (1.02 – 1.12) | 0.0058 | |
| 85 over | 5843 (31.8) | 3851 (65.9) | 1.25 (1.19 – 1.31) | <.0001 | 1.17 (1.11 – 1.23) | <.0001 | |
| Gender | |||||||
| Male | 11522 (62.7) | 7167 (62.2) | 0.5339 | Ref. | Ref. | ||
| Female | 6864 (37.3) | 4238 (61.7) | 0.99 (0.95 – 1.03) | 0.5972 | 1.05 (1.01 – 1.10) | 0.0084 | |
| Race | |||||||
| White | 14861 (80.8) | 9359 (63.0) | <.0001 | Ref. | Ref. | ||
| Black | 3007 (16.4) | 1737 (57.8) | 0.87 (0.82 – 0.91) | <.0001 | 0.94 (0.89 – 0.99) | 0.0149 | |
| Others | 518 (2.8) | 309 (59.7) | 0.93 (0.83 – 1.04) | 0.1977 | 1.07 (0.95 – 1.20) | 0.2588 | |
| Admission Status | |||||||
| Elective | 4684 (25.5) | 2706 (57.8) | <.0001 | Ref. | Ref. | ||
| Emergent | 13702 (74.5) | 8699 (63.5) | 1.21 (1.16 – 1.27) | <.0001 | 1.37 (1.31 – 1.43) | <.0001 | |
| ADL decline at baseline | |||||||
| No | 13665 (74.3) | 7655 (56.0) | <.0001 | Ref. | Ref. | ||
| Yes | 4721 (25.7) | 3750 (79.4) | 2.14 (2.05 – 2.22) | <.0001 | 2.39 (2.29 – 2.49) | <.0001 | |
| ADL groups at baseline | |||||||
| 0 to 12 | 4889 (26.6) | 3594 (73.5) | <.0001 | Ref. | Ref. | ||
| 13 to 17 | 3834 (20.9) | 2655 (69.2) | 0.89 (0.85 – 0.94) | <.0001 | 0.79 (0.75 – 0.84) | <.0001 | |
| 18 to 21 | 4963 (27.0) | 2980 (60.0) | 0.71 (0.68 – 0.74) | <.0001 | 0.57 (0.55 – 0.60) | <.0001 | |
| 22 to 28 | 4700 (25.6) | 2176 (46.3) | 0.50 (0.47 – 0.52) | <.0001 | 0.38 (0.36 – 0.41) | <.0001 | |
| Cognitive impairment at baseline | |||||||
| 0 | 7615 (41.4) | 4703 (61.8) | 0.5240 | Ref. | Ref. | ||
| 1 | 10771 (58.6) | 6702 (62.2) | 1.02 (0.99 – 1.06) | 0.2388 | 1.15 (1.11 – 1.20) | <.0001 | |
| Charlson score groups | |||||||
| 0 | 2524 (13.7) | 1553 (61.5) | 0.1678 | Ref. | Ref. | ||
| 1 | 3815 (20.7) | 2384 (62.5) | 1.03 (0.96 – 1.10) | 0.4084 | 1.02 (0.96 – 1.09) | 0.5650 | |
| 2 | 3819 (20.8) | 2408 (63.1) | 1.05 (0.99 – 1.12) | 0.1263 | 1.01 (0.95 – 1.08) | 0.7131 | |
| 3,4 | 5557 (30.2) | 3454 (62.2) | 1.02 (0.96 – 1.08) | 0.5844 | 1.01 (0.95 – 1.08) | 0.6923 | |
| >=5 | 2671 (14.5) | 1606 (60.1) | 0.96 (0.89 – 1.03) | 0.2311 | 0.96 (0.90 – 1.03) | 0.2770 | |
Propensity matched analysis
Mortality and functional outcomes of propensity matched nursing home residents undergoing and not undergoing minor urologic surgery are shown in Supplemental Figure 2. Residents undergoing the procedures of interest demonstrated higher mortality compared to residents not undergoing these procedures, while functional trajectories were similar between cohorts.
Discussion
Despite the relatively minor nature of the inpatient procedures examined, nursing home residents demonstrated a high mortality rate (50%) and sustained functional decline in the year following surgery. Residents with better functional status at baseline experienced greater functional decline and predictors of this decline included older age, functional decline in the 6 months prior to surgery, procedures being performed on an emergent basis and cognitive impairment at baseline.
This is one of the first studies to evaluate long-term functional outcomes of minor surgery among nursing home residents. For reference, annual mortality rates among all nursing home residents are high – 43% for men and 32% for women.8 Prior research among nursing home residents undergoing a much larger surgery for colon cancer demonstrated a cumulative 1-year mortality of 53% and an average worsening of 3.9 points in their MDS-ADL score.12 These results do not dramatically differ from our findings among patients undergoing procedures of presumed much lower complexity and morbidity, with a 50% 1-year mortality and an average of 1.9 and 4.7 point worsening in MDS-ADL scores among patients from all quartiles and the best quartile of MDS-ADL scores, respectively. Previously, we have published on functional outcomes related specifically to TURP surgery and found that the majority of individuals gained little, if any, improvement in functional status in the year following surgery.14 We further know that function is an important treatment outcome among older adults with limited life expectancy, as prior research has found that 74% of such individuals would forgo burdens of treatment (such as extended length of hospital stay, extent of testing and invasive interventions) if it resulted in an outcome of survival with severe functional impairment.7 These findings underscore the importance of and potential for functional decline and mortality among nursing home residents undergoing any type of procedure, large or small, complex or minor, high or low risk.
Our study found that the magnitude of functional decline was highest among residents with the best quartile of baseline functional status (MDS-ADL 0–12). This could represent “floor effects” related to the outcome measure, whereby the instrument itself has a lower limit to the data values that it can specifically discern. Individuals with better baseline function have more function to lose than their more poorly functioning counterparts, placing them at proportionally higher risk for functional decline. This finding highlights the importance of the potential long-term risks in the more highly functioning nursing home population.
In order to contextualize our findings, we compared 1-year mortality and functional outcomes between nursing home residents undergoing the minor urologic surgery to propensity matched residents not undergoing this type of surgery. We found higher mortality and similar functional trajectories between the two groups. This comparison suggests two important implications: (1) mortality among residents undergoing these procedures is higher than that in the general nursing home population and (2) these procedures, which are often done to improve functionality, may not be fulfilling their intended purpose in this regard. It is important to consider, however, that the comparison group represents a heterogeneous population that is likely experiencing other non-urologic medical and surgical issues that are not accounted for in these analyses.
This study should be interpreted with certain limitations in mind. First, based on the limitations of using Medicare claims data, we are limited in our understanding of the indications that contributed to each surgery. Further research investigating the patient, physician, and system-based factors that influence surgical decision-making among this population is necessary to get a better understanding of the drivers behind this problem. Likewise, important data pertaining to quality of life are lacking in these analyses and may explain some of our findings. For example, if a patient experienced hematuria from a bladder tumor and underwent a TURBT to address this problem, the procedure may have improved their quality of life, but not necessarily their function or longevity. Further, expected improvement in quality of life resulting from surgery may be a strong motivator to perform surgery, however, there are no existing data to either support or refute this claim. Second, it is important to understand that these data only look at nursing home residents undergoing the surgical procedures of interest, and hence, they represent only the numerator and not the denominator of the entire patient population with similar diseases who did not undergo surgery. Furthermore, these findings represent an association, but not causation, between patients undergoing minor urologic surgery and post-operative function and mortality. Third, the high mortality rate in our population makes it somewhat difficult to assess survivors without a survivor bias, whereby those who did not die during the year after surgery may have been healthier than those who did. This bias, however, would only serve to underestimate our findings of functional decline among survivors and further serve to strengthen the study.
The rates of mortality and functional decline after minor urologic surgery are profound among nursing home residents. This is important information to consider when balancing the risks and benefits of any surgical procedure, major or minor, in this vulnerable patient population. Initiatives aimed at improving surgical decision-making and improving functional recovery after surgery in this population are necessary to ensure the best use of surgery in the final years of life.
Supplementary Material
Supplemental Figure 1. Proportion of residents who experienced ADL improvement, decline, maintenance of ADL and death up to 1-year after surgery.
Supplemental Figure 2. 1-year mortality (A) and functional decline (B) for propensity matched residents undergoing and not undergoing minor urology surgery.
Acknowledgments
Funding: Dr. Suskind is funded by NIH-NIA Grants for Early Medical/Surgical Specialists’ Transition to Aging Research (GEMSSTAR) (R03) 1R03AG050872-01 and by NIDDK K12 DK83021-09 K12 Urologic Research (KURe) Career Development Program. Dr. Louise Walter is supported by the National Institute on Aging at the National Institutes of Health (grant number K24AG041180), and this work was also supported by the UCSF CTSI Scholars Program and the UCSF Claude D. Pepper Older Americans Independence Center.
Footnotes
We certify that the work herein is novel and that this research specifically adds to the literature.
Conflict of Interest: None. Dr. Suskind is a consultant for Acoustic Wave Cell Therapy, Inc. This is not related to the work presented here in.
Author Contributions: Suskind: concept and design, interpretation of data, preparation of manuscript. Walter, Boscardin and Finlayson: concept and design, interpretation of data. Zhao and Boscardin: data analysis and interpretation.
Sponsor’s Role: None.
References
- 1.Drach GW, Griebling TL. Geriatric urology. Journal of the American Geriatrics Society. 2003;51(7 Suppl):S355–358. doi: 10.1046/j.1365-2389.2003.51348.x. [DOI] [PubMed] [Google Scholar]
- 2.Werner CA. The Older Population: 2010. 2011. [Google Scholar]
- 3.Mohanty S, Rosenthal RA, Russell MM, Neuman MD, Ko CY, Esnaola NF. Optimal Perioperative Management of the Geriatric Patient: A Best Practices Guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. Journal of the American College of Surgeons. 2016;222(5):930–947. doi: 10.1016/j.jamcollsurg.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Gazala S, Tul Y, Wagg A, et al. Quality of life and long-term outcomes of octo- and nonagenarians following acute care surgery: a cross sectional study. World journal of emergency surgery : WJES. 2013;8(1):23. doi: 10.1186/1749-7922-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. Journal of general internal medicine. 2004;19(10):1027–1033. doi: 10.1111/j.1525-1497.2004.40016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA : the journal of the American Medical Association. 1998;279(15):1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 7.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. The New England journal of medicine. 2002;346(14):1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 8.Sund-Levander M, Grodzinsky E, Wahren LK. Gender differences in predictors of survival in elderly nursing-home residents: a 3-year follow up. Scandinavian journal of caring sciences. 2007;21(1):18–24. doi: 10.1111/j.1471-6712.2007.00431.x. [DOI] [PubMed] [Google Scholar]
- 9.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54(11):M546–553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter GI, Hastie CL, Morris JN, Fries BE, Ankri J. Measuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairment. BMC geriatrics. 2006;6:7. doi: 10.1186/1471-2318-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawes C, Morris JN, Phillips CD, Mor V, Fries BE, Nonemaker S. Reliability estimates for the Minimum Data Set for nursing home resident assessment and care screening (MDS) The Gerontologist. 1995;35(2):172–178. doi: 10.1093/geront/35.2.172. [DOI] [PubMed] [Google Scholar]
- 12.Finlayson E, Zhao S, Boscardin WJ, Fries BE, Landefeld CS, Dudley RA. Functional status after colon cancer surgery in elderly nursing home residents. Journal of the American Geriatrics Society. 2012;60(5):967–973. doi: 10.1111/j.1532-5415.2012.03915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. The New England journal of medicine. 2009;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suskind AM, Walter LC, Zhao S, Finlayson E. Functional Outcomes After Transurethral Resection of the Prostate in Nursing Home Residents. Journal of the American Geriatrics Society. 2016 doi: 10.1111/jgs.14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. Journal of gerontology. 1994;49(4):M174–182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 17.van Dijk PT, Mehr DR, Ooms ME, et al. Comorbidity and 1-year mortality risks in nursing home residents. Journal of the American Geriatrics Society. 2005;53(4):660–665. doi: 10.1111/j.1532-5415.2005.53216.x. [DOI] [PubMed] [Google Scholar]
- 18.Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Medical care. 2015 doi: 10.1097/MLR.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell F. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 20.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Statistics in medicine. 2014;33(6):1057–1069. doi: 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Proportion of residents who experienced ADL improvement, decline, maintenance of ADL and death up to 1-year after surgery.
Supplemental Figure 2. 1-year mortality (A) and functional decline (B) for propensity matched residents undergoing and not undergoing minor urology surgery.