Abstract
It was found that the expression level of miR-147a was significantly increased and the pathway of PI3K/AKT was dramatically inhibited after radiation. In view of the relationship between miRNA and target genes, we put forward the question, what is the relationship between PI3K/AKT and miR-147a? In order to find the answer to the question, we used bioinformatics techniques to analyze the relationship between miR- 147 (a or b) and PI3K/AKT signaling pathway. miR-147a overexpression plasmid and PDPK1 3′UTR luciferase reporter gene plasmid were constructed. Dual luciferase reporter gene system validation experiments were carried out on miR-147a and PDPK1 relationship. The verification experiments were also carried out. Bioinformatics analysis showed that there is a miR-147a binding site in the non-coding region (3′UTR) of PDPK1. In the experimental groups transfected with wild type PDPK1 gene of 3′UTR plasmid, the luciferase activity decreased (or increased) significantly in miR-147a (or inhibitor) group compared with miR-NC (or anti-miR-NC); There was no significant difference between the miR-147a group (or inhibitor) and the miR-NC group (or anti- miR-NC) in the transfection of PDPK1–3′UTR-Mut gene vector. PDPK1 was a target gene for direct regulation of miR-147a downstream. Verifying test results showed that the expression of PDPK1 mRNA and protein was reduced after overexpression of miR-147a, which was up-regulated after silencing miR-147a in TC, and V79 cells. These results suggest that miR-147a could be involved in the regulation of PDPK1 transcription by binding to the target site in PDPK1 mRNA 3′UTR, and then regulated AKT.
Keywords: miRNA-147a, PDPK1, plasmid, radiation, target
1 | INTRODUCTION
In the medical field, IR is usually an available therapy for cancer therapy, but it also damages normal cells, and tissues1,2 through generating intermediate ions and reactive free radicals. After exposure to IR, such as γ-or X-rays, which are the most common damage factors, organisms instantly stimulate a series of biochemical pathways to keep genetic integrity for promoting autologous cell survival.
MiRNA are a class of non-coding, small, endogenous RNA with 18–25 nucleotides (nt) in length which can regulate gene expression at the post-transcriptional level by binding to the 3′-untranslated regions (UTR) of target miRNA.3–6 A miRNA can target up to 100–200 mRNAs.7 miRNA may play an important role in the regulation of life and activities, and is closely related to the development of animal and plant organs, cell differentiation and apoptosis, and fat metabolism.8,9 Recent studies have found that many miRNA have high or low expression in many models of ionizing radiation injury, which indicates that miRNA is closely related to the occurrence and development of radiation damage.10,11
AKT is the downstream target of phosphatidylinositol-3- kinase (PI3K). P13K contains a subunit with protein molecular weight of 110 kD, which could catalyze PIP2 phosphorylation to generate PIP3. PIP3 enhances the activity of 3-phosphoinositide-dependent protein kinase-l (PDPK1), further activating AKT.12 PDPK1 plays an important role in cell proliferation and survival.13,14 Gene knockout PDPK1 —/— mouse embryos died at 9.5 days. The PDPK1−/+ mice were 40–50% smaller than normal mice, and the inhibition of cell proliferation was associated with low expression of PDPK1.15 In PDPK1 gene knockout cells or mice, AKT kinases were inactivated, indicating that PDPK1 was an important upstream kinase of AKT, and the loss of AKT activity was related to restriction of phosphorylation of itself.15,16 MiR-147 significantly increased after radiation,17 but the PI3K/AKT signaling pathway was inhibited.18,19 MiR-147 inhibits the PI3K/AKT/mTOR signaling pathway to prevented proliferation and migration of breast cancer cells, but its direct target was not elucidated.20,21
In this study, we focuses on the establishment and validation of miR-147 and PDPK1 target relationship.
2 | MATERIALS AND METHODS
2.1 | Cell culture
Thymus cells of KM mice and Chinese hamster lung fibroblasts V79 were cultured in suspension in RPMI-1640 medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2.
2.2 | Prediction of miR-147a targets
Log on to the TargetScanHuman database (http://www.targetscan.org/vert_71/), entering the name miR-147a to get the target genes.
2.3 | DNA, miRNA constructs
To generate luciferase reporter constructs, PDPK1 3'-UTR (or PDPK1 3′-UTRmut) was produced by chemical synthesis. It is loaded into the vector without expression to prepare plasmid. Then the Xbal/Xbal enzyme digestion reaction was used to obtain the target gene fragments which were cloned into the Dual-Luciferase Reporter Gene Expression vector of GV272 (SV40-Luc-MCS). To generate mir-147a constructs, the mir- 147a was produced by PCR using human genomic DNA library. Following primers were used: mir-147a (309 bp), forward primer: 5′- ACGGGCCCTCTAGACTCGAGATAA- CAGCAGCCCACCAAAAAGC- 3′-reverse primer5′-GTTTAAACTTAAGCTTGGTACCCAGGAAAACTTATTTTT GAATTATG- 3′.The resulting PCR products were cloned into the Vector of GV268 (CMV-MCS-SV40-Neomycin). miR- 147a inhibitor plasmid was obtained from Shanghai Jikai Genechem Co., Ltd. Shanghai, China.
2.4| PCR identification of the colony
Single colony was put into 20 μL identification system, which was mixed evenly, placed in a PCR apparatus for reaction, and then subjected to 1% agarose gel electrophoresis. Identification primer were as follows: GV272-PDPK1 3′-UTR Luc-C- F:GAGGAGTTGTGTTTGTGGAC and RVprimer4: GAC- GATAGTCATGCCCCGCG;GV268-miR-147a CMV-F: CGCAAATGGGCGGTAGGCGTG and pcDNA-SEQR: TTATTAGGAAAGGACAGTGGG.The cycling parameters were as follows: 94°C for 3 min, 40 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, another cycle 72°C for 5 min using the ABI real-time PCR detection system.
2.5 | Luciferase assay
293T cells were transfected with the miR-147a (control miRNA) and PDPK1 3′UTR(mut) in conjunction with the luciferase reporter constructs. Forty-eight hours after they were transfected, the cells were lysed and subjected to luciferase assays using the Dual Luciferase Reporter Gene Assay System (Promega, Madison, WI) according to the manufacturer's protocol.
2.6| RNA isolation and real-time polymerase chain reaction (PCR)
2.6.1| PDPK1
Total RNAwas extracted using RNAiso Plus reagent (TAKARA BIO INC., Shiga, Japan). The extraction procedure was then followed by cDNA synthesis using ReverTra Ace qPCR RT MasterMixkit (ToyoboCo.,Ltd.Life ScienceDepartment ScienceDepartmentOsaka Japan).Quantitative PCR was performed using the ABI5500 RTPCR system (Strata-gene) with the SYBR® Green Real-time PCRMasterMix kit (Toyobo Co., Ltd. Life ScienceDepartment) with the following conditions: 95°C for 1min, 40 cycles of 95°C for 15 s, 60°C for 30 s, 72°C for 45 s. Primer sequence of various genes were designed as follows: PDPK1 forward primer 5′-GCACATCCAGATCACAGAT-3′ and reverse primer 5′ -TTACACGCCGACTTCTCT-3′; GAPDH forward prime: 5′-AGGTCGGTGTGAACGGATTTG- 3′ and reverse primer: 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
2.6.2 | MiR-147a
Total RNA was extracted using RNAiso Plus reagent (TAKARA BIO INC.). The extraction procedure was then followed by cDNA synthesis using miRNA cDNA Synthesis Kit (Beijing ComWin Biotech Co., Ltd. Beijing, China). Quantitative PCR was performed using the ABI5500 RT-PCR system with miRNA qPCR Assay Kit (Beijing ComWin Biotech Co., Ltd. China) with the following conditions: 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 1 min. Primer sequences of various genes were designed as follows: mir147 forward primer: GTGTGCGGAAATGCTTCTGCTA and reverse primer: provided by the kit without sequence; U6 forward primer: CTCGCTTCGGCAGCACA and reverse primer AACGCTTCACGAATTTGCGT.
2.6.3 | Immunoblotting
Thymus cells and V79 were collected and RIPA lysis buffer and 1 mM phenyl methyl sulfonyl fluoride (PMSF) (99:1) were added according to the amount of cells. The mixed liquid induced complete lysis and was subsequently centrifuged (4°C, 12 000 r/min, 5 min). The protein concentration was determined using the bicinchoninic acid assay (BCA) assay. The resulting supernatant was preserved at —80°C. The blocked blots were incubated with primary antibodies overnight at 4°C using PDPK1 antibody dilutions (1:1500) recommended by the manufacturer (Wanleibio Co., Ltd. China) and GAPDH (1:2500 Abcam, Cambridge, UK). Subsequent incubation was performed with horseradish peroxidase (HRP)-conjugated Goat-anti-rabbit antibody (1:3000, Jackson ImmunoResearch, Inc., West Grove, PA). The signal was detected using enhanced chemiluminescence (ECL) reagents.
3 | RESULTS
3.1 | The expression of miR-147a and PDPK1 was opposite after irradiation
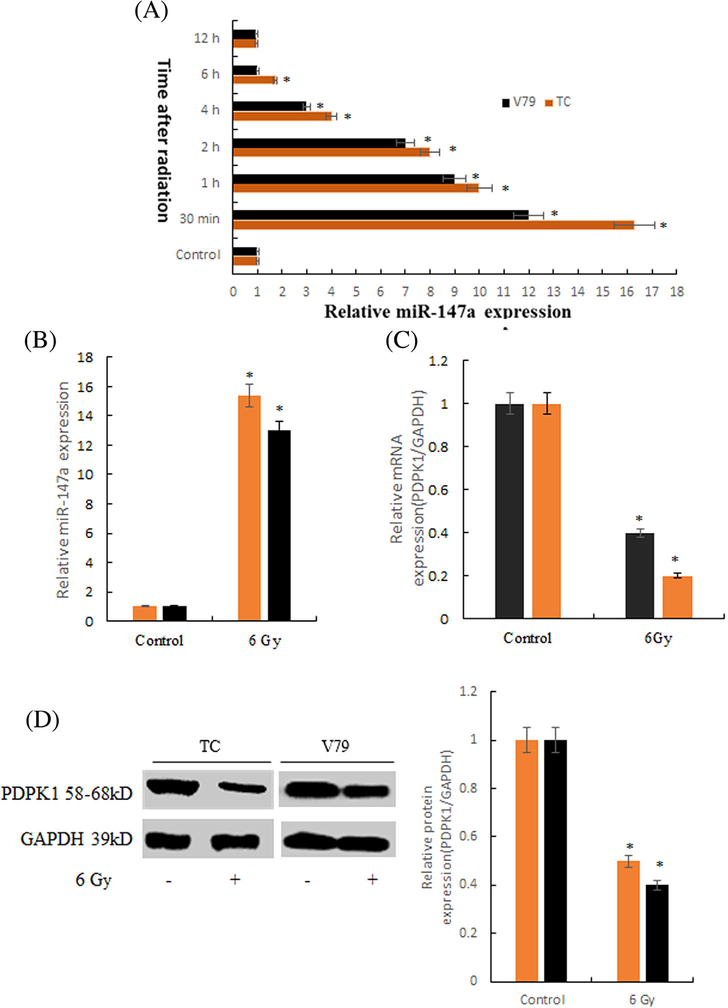
As shown in Figure 1A, the optimal detection time of miR-147a was 30min after radiation. As shown in Figure 1B-D, the expression level of miR-147a was significantly increased and the expression level of PDPK1 mRNA and protein was significantly decreased after radiation, compared with the normal group|.
FIGURE 1.
The expressions of miR-147a and PDPK1 after irradiation in thymus cells. A, The optimal detection time of miR-147a was 30min after radiation (*P < 0.01 compared with Control). B, The expression level of miR-147a after radiation (*P < 0.01 compared with Control). C, The expression level of PDPK1 mRNA after radiation (*P < 0.01 compared with Control). D, The expression level of PDPK1 protein after radiation (*P < 0.01 compared with Control)
3.2 | Bioinformatic prediction of binding sites between miR-147a and PDPK1
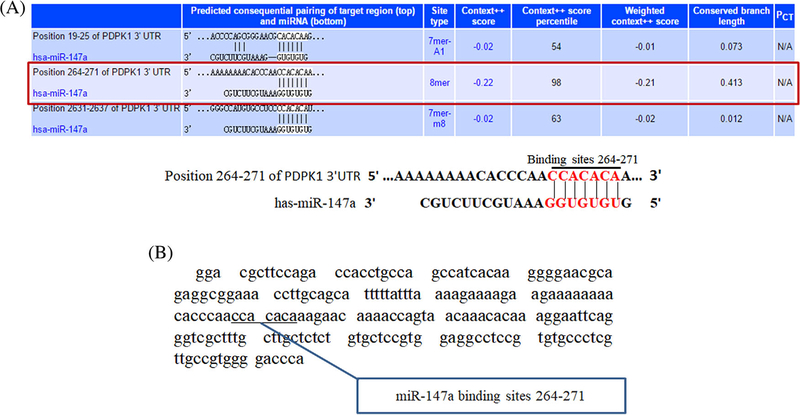
We use the miRNA database Targetscan to analyze and forecast the target genes of miR-147a. It is found that there were three miR-147a binding sites in the non-coding region (3′UTR) of PDPK1 (Figures 2A and 2B), And we chose the binding site with high score.
FIGURE 2.
Binding sites between miR-147a and PDPK1. A, Binding sites of miR-147a and PDPK1 3'UTR region. B, PDPK1 3′ UTR fragment marked with binding sites
3.3 | Construction of over expression plasmid
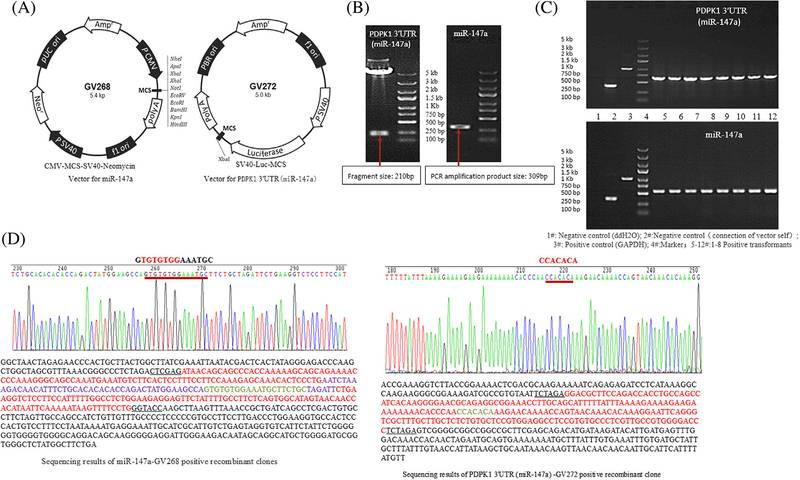
Overexpression plasmid vector information was shown in Figure 3A.
FIGURE 3.
Over expression plasmid construction. A, Vector information. B, Electrophoresis of the enzyme product containing PDPK1 3'UTR (miR-147a) gene sequence and electrophoresis of PCR amplified products of miR-147a. C, Identification of recombinant clones by PCR. D, Results of positive clone sequencing
3.3.1 | Acquisition of target gene fragments
The plasmid containing the gene sequence of PDPK1 3′UTR, was digested with XbaI/XbaI enzyme, and was treated with 1% agarose gel electrophoresis. As shown in Figure 3B, a fragment of the enzyme was shown near marker of 250 bp, consistent with the synthetic target gene PDPK1 of PDPK1 3′UTR, (miR-147a) size of 210 bp, the same as expected.
The primers were designed containing paired exchange bases, restriction sites, and containing 5′gene sequence. The miR-147a gene was amplified by PCR, and 1% agarose gel electrophoresis was performed. As shown in Figure 3B, a fragment of the enzyme was expressed near 300 bp. The size was consistent with target gene of 309 bp amplified by PCR, in line with expectation.
3.3.2 | Identification of recombinant clones by PCR
The target gene fragment was inserted into the linearized expression vector after recovery. That is to say, PDPK1 3′UTR was inserted into GV272 to construct PDPK1 3′UTR target sequence-reporter gene fusion vector of PDPK1 3′UTR —GV272. The recombinant clones were identified by PCR. As shown in Figure 3C, there was a positive transformation band between 500 and 750 bp, consistent with the design of the amplified product 667 bp.
PCR amplification products were exchanged with the vector, MiR-147a was inserted into GV268 to construct the fusion vector of miR-147a-GV268, and PCR was used to identify the recombinant clone.
As shown in Figure 3C, there was a positive conversion band between 500 and 750 bp, consistent with the design of the amplification product of 558 bp, in line with expectation.
3.3.3 | Positive clone sequencing
The results showed that the sequence of the inserted fragment was the same as that of pre-miR-147a and PDPK1 3′UTR (miR-147a) reported on Genbank, as shown in Figure 3D.
3.4 | PDPK1 was the target gene of miR-147a verified by dual luciferase reporter gene system experiments
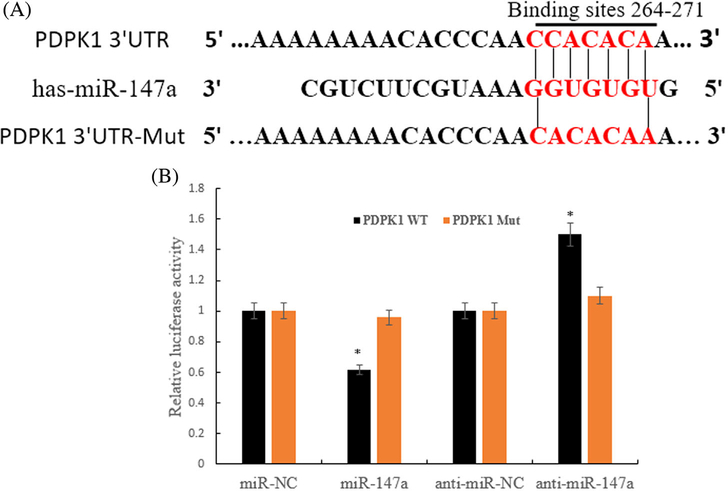
Luciferase activity showed that in the experimental groups transfected with wild type PDPK1 gene of 3′UTR plasmid, the luciferase activity decreased (or increased) significantly in miR-147a (or inhibitor) group compared with miR-NC (or anti-miR-NC); There was no significant difference between the miR-147a group (or inhibitor) and the miR-NC group (or anti-miR-NC) in the transfection of PDPK1–3′UTR-Mut gene vector (Figures 4A and 4B).
FIGURE 4.
Targeted binding of miR-147a and PDPK1. A, Wild type and mutant sequences of PDPK1–3′UTR and miR-147a binding sites; B, Overexpression or silencing of miR-147a (anti-miR-147a) effect on the activity of PDPK1–3′UTR verified by Dual-Luciferase Reporter Gene Assay (*P < 0.01 compared with NC)
These results indicate that miR-147a acted on the 3′UTR region of PDPK1 gene and inhibit the activity of it, which leads to the conclusion that PDPK1 was a target gene for direct regulation of miR-147a downstream.
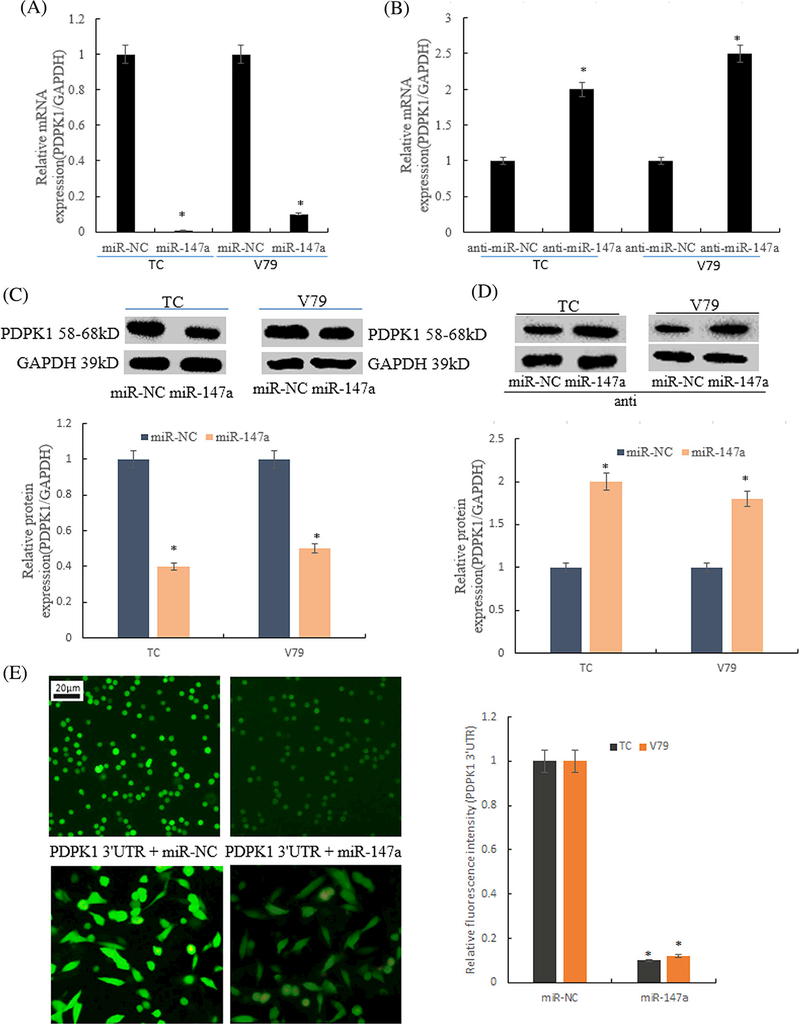
3.5 | MiR-147a negatively regulated the expression of PDPK1
QRT-PCR and Western blot were used to detect the expression of PDPK1 mRNA and protein after overexpression of miR-147a or silencing miR-147a in TC (thymus cells) and V79 cells. QRT-PCR results showed that in TC and V79 cells, the expression level of PDPK1 mRNA was down regulated in the group of miR-147a overexpression plasmid transfected by liposome 2000, (24 hole plate, each hole 0.5 μg), compared with miR-NC. However, the expression of PDPK1 in the TC and V79 cells was significantly higher than that of the control group after transfection with miR-147a inhibitor, and the difference was statistically significant, as shown in Figures 5A and 5B.
FIGURE 5.
Expression of PDPK1 negativly regulated by miR-147a. A, mRNA expression of PDPK1 was detected by qRT-PCR method after transfection of miR-147a overexpressing plasmid (*P < 0.01 compared with miR-NC); B, mRNA expression of PDPK1 was detected by qRT- PCR method after transfection of miR-147a inhibitor; C, Western blot method was used to detect the expression of PDPK1 in the cells transfected with miR-147a overexpression plasmid (*P < 0.01 compared with miR-NC); D, Western blot was used to detect the expression of PDPK1 protein in cells transfected with miR-147a inhibitor (*P < 0.01 compared with miR-NC); E, Fluorescence expression of PDPK1 3′UTR marked by GFP (*P < 0.01 compared with miR-NC)
The results of Western blot showed that the expression level of PDPK1 protein was down regulated after in miR-147a was overexpressed in TC and V79 cells. Conversely, after silencing miR-147a, the expression level of PDPK1 protein in the cells was up-regulated, as shown in Figures 5C and 5D. The activity of PDPK1 was reduced by miR-147a compared with miR-NC (Figure 5E).
These results further confirmed that PDPK1 was a direct target gene of miR-147a.
4.| DISCUSSION
Activation of PI3K/AKT signaling pathway can promote cell proliferation, inhibit apoptosis, promote cell cycle progression, and promote cell invasion and metastasis, which play an important role in radiation induced cancer cell resistance and normal cell injury. After irradiation, some cancer cells activated PI3K/AKT22-24 pathway, inducing radiation resistance to reduce the radiation sensitivity. But PI3K/AKT signaling pathway was inhibited in normal cells after radiation.18,19 PI3K/AKT inhibitor was given to thymus cells in advance, and the apoptosis rate of cells increased significantly after irradiation.25 With the AKT1 gene knockout by AKT1 shRNA, retinal pigment epithelial cells is very sensitive to ultraviolet radiation.26 The downstream effect of AKT activation is closely related to the inhibition of apoptosis pathway.27 Therefore, AKT is closely related to radiation damage.
Recent studies have found that many miRNA have high or low expression in many models of ionizing radiation injury, which indicates that miRNA is closely related to the occurrence, and development of radiation damage.10,11 The expression levels of let-7 family were reported by several studies to be significantly changed by IR.28–30 Moreover, the let-7 family is a tumor suppressor in regulating cell proliferation and differentiation. After IR exposure, miR-34a was up-regulated in diverse human cell lines and miR-21 was similarly up-regulated in various normal and cancer cell lines11,28,31 MiR-147 has been found to be involved in the regulation of differential gene expression in many pathological models. In the radiation injury model, the expression of MiR- 147 and PI3K/AKT was the opposite. MiR-147 significantly increased after radiation,17 but the PI3K/AKT signaling pathway was inhibited. MiR-147 inhibits the PI3K/AKT/ mTOR signaling pathway to prevented proliferation and migration of breast cancer cells, but its direct target was not elucidated.20,21 Gene silencing AKT1/2 produced a similar biological effect with that ofmiR-147, and the cells phenotype of AKT knockout was consistent with that of miR-147.32
So, we put forward the question, what is the relationship between PI3K/PDPK1/AKT and miR-147?
In order to find the answer to the question, we used bioinformatics techniques to analyze the relationship between miR-147 (a or b) and PI3K/PDPK1/AKT signaling pathway. Through the TargetScan target gene docking analysis, results were obtained as follows: (i) The mature sequence of miR- 147a was 5'-GUGUGUGGAAAUGCUUCUGC- 3′; (ii) PDPK1 mRNA 3′UTR region contains 3 potential miR- 147a binding sites (no miR-147b binding sites). These results suggest that miR-147a could be involved in the regulation of PDPK1 transcription by binding to the target site in PDPK1 mRNA 3′UTR, and then regulated AKT.
In this study, we focuses on the establishment and validation of mir147a and PDPK1 target relationships. It was found that the expression level of miR-147a was significantly increased and the expression level of PDPK1 mRNA and protein was significantly decreased after radiation in thymus cells. Bioinformatics analysis showed that there was a miR- 147a binding site in the non-coding region (3′ UTR) of PDPK1. In the experimental groups transfected with wild type PDPK1 gene of 3′ UTR plasmid, the luciferase activity decreased(or increased) significantly in miR-147a(or inhibitor) group compared with miR-NC (or anti-miR-NC); There was no significant difference between the miR-147a group(or inhibitor) and the miR-NC group(or anti-miR-NC) in the transfection of PDPK1–3′ UTR-Mut gene vector. So, it was concluded that PDPK1 was a target gene for direct regulation of miR-147a downstream. Verifying test results showed that the expression of PDPK1 mRNA and protein was reduced after overexpression of miR-147a, which was up-regulated after silencing miR-147a in TC and V79 cells.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation of China (81773358), the Young Teachers Plan of Higher Schools in Henan Province (2014GGJS-098), and Department of Science and Technology Research Project of Henan Province in China (142102310302).
Funding information
National Science Foundation of China, Grant number: 81773358; Young Teachers Plan of Higher Schools in Henan Province, Grant number: 2014GGJS-098; Department of Science and Technology Research Project of Henan Province in China, Grant number: 142102310302
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
- 1.Seyed JH. Trends in the development of radioprotective agents. Drug Discov. Today. 2007;12:794–805. [DOI] [PubMed] [Google Scholar]
- 2.Koturbash I, Zemp F, Kolb B, Kovalchuk O. Sex-specific radiationinduced microRNAome responses in the hippocampus, cerebellum and frontal cortex in a mouse model. Mutat Res Genet Toxicol Environ Mutagen. 2011;722:114–118. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 4.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. [DOI] [PubMed] [Google Scholar]
- 5.Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA. 2003;100:9779–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yekta S, Shih IH, Bartel DP. MicroRNAdirected cleavage of HOXB8 mRNA. Science. 2004;304:594–596. [DOI] [PubMed] [Google Scholar]
- 7.Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhry MA, Omaruddin RA, Brumbaugh CD, Tariq MA, Pourmand N. Identification of radiation-induced microRNA tran- scriptome by next-generation massively parallel sequencing. J Radiat Res. 2013;54:808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Wang W, Gu Q, et al. Protein and miRNA profiling of radiation induced skin injury in rats: the protective role of peroxiredoxin-6 against ionizing radiation. Free Radic Biol Med. 2014;69:96–107. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Zhang X, Tang X, Wang P, Wang H, Wang Y. MiR-21 is continually elevated long-term in the brain after exposure to ionizing radiation. Radiat Res. 2012;177:124–128. [DOI] [PubMed] [Google Scholar]
- 11.Girardi C, De Pitta C, Casara S, et al. Analysis of miRNA and mRNA expression profiles highlights alterations in ionizing radiation response of human lymphocytes under modeled microgravity. PLoS ONE. 2012;7:e31293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dangelmaier C, Manne BK. PDK1 selectively phosphorylates Thr(308) on Akt and contributes to human platelet functional responses. Thromb Haemost. 2014;111:508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Z, Cox-Hipkin MA, Windsor WT, Boyapati A. 3-phosphoi- nositide-dependent protein kinase-1 regulates proliferation and survival of cancer cells with an activated mitogen-activated protein kinase pathway. Mol Cancer Res. 2010;8:421–432. [DOI] [PubMed] [Google Scholar]
- 14.Qian XJ, Li XL, Xu X, Wang X, Feng QT, Yang CJ. α-SMA-Cre- mediated excision of PDK1 reveals an essential role of PDK1 in regulating morphology of cardiomyocyte and tumor progression in tissue microenvironment. Pathol Biol (Paris). 2015;63:91–100. [DOI] [PubMed] [Google Scholar]
- 15.Lawlor MA, Mora A, Ashby PR, et al. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21:3728–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mora A, Komander D, Van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Develop Biol. 2004;15:161–170. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Lu J, Hao L, Zheng Z, Zhang N, Wang Z. Discovery and characterization of miRNAs in mouse thymus responses to ionizing radiation by deep sequencing. Int J Radiat Biol 2016;11:1–10. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Wang R, Gao Y, et al. The protective role of interleukin-11 against neutron radiation injury in mouse intestines via MEK/ERK and PI3K/Akt dependent pathways. Dig Dis Sci. 2014;59:1406–1414. [DOI] [PubMed] [Google Scholar]
- 19.Li KR, Yang SQ, Gong YQ, et al. 3H-1 3H-1, 2-dithiole-3-thione protects retinal pigment epithelium cells against Ultra-violet radiation via activation of Akt-mTORC1-dependent Nrf2-HO-1 signaling. Sci Rep. 2016;6:25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uhlmann S, Mannsperger H, Zhang JD, et al. Global microRNA level regulation of EGFR-driven cell-cycleprotein network in breast cancer. Mol Syst Biol. 2012;8:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhang HE, Liu Z. MicroRNA-147 suppresses proliferation, invasion and migration through the AKT/mTOR signaling pathway in breast cancer. Oncol Lett. 2016;1:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YH, Wei MF, Wang CW, et al. Dual Phosphoinositide 3- kinase/mammalian target of rapamycin inhibitor is an effective radiosensitizer for colorectal cancer. Cancer Lett. 2015;357:582–590. [DOI] [PubMed] [Google Scholar]
- 23.Simpson DR, Mell LK, Cohen EE. Targeting the PI3K/AKT/ mTOR pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2015;51:291–298. [DOI] [PubMed] [Google Scholar]
- 24.Ettl T, Viale-Bouroncle S, Hautmann MG, et al. AKT and MET signalling mediates antiapoptotic radioresistance in head neck cancer cell lines. Oral Oncol. 2015;51:158–163. [DOI] [PubMed] [Google Scholar]
- 25.Xu P, Zhang WB, Cai XH, Qiu PY, Hao MH, Lu DD. Activating AKT to inhibitJNK by troxerutin antagonizes radiation-induced PTEN activation. Eur. J. Pharmacol. 2017;795:66–74. [DOI] [PubMed] [Google Scholar]
- 26.Gong YQ, Huang W, Li KR, et al. SC79 protects retinal pigment epithelium cells from UV radiation via activating Akt-Nrf2 signaling. Oncotarget. 2016;7:60123–60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Chen WR, Xing D. A pathway from JNK through decreased ERK and1168Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J Cell Physiol. 2012;227:1168–1178. [DOI] [PubMed] [Google Scholar]
- 28.Simone NL, Soule BP, Ly D, et al. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS ONE. 2009;4:e6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhry MA, Sachdeva H, Omaruddin RA. Radiation-induced micro-RNA modulation in glioblastoma cells differing in DNA- repair pathways. DNA Cell Biol. 2010;29:553–561. [DOI] [PubMed] [Google Scholar]
- 30.Templin T, Paul S, Amundson SA, et al. Radiation-induced micro- RNA expression changes in peripheral blood cells of radiotherapy patients. Int J Radiat Oncol Biol Phys. 2011;80:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikiforova MN, Gandi M, Kelly L, Nikiforov YE. MicroRNA dysregulation in human thyroid cells following exposure to ionizing radiation. Thyroid. 2011;21:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CG, McCarthy S, Gruidl M, Timme C, Yeatman TJ. MicroRNA-147 induces a mesenchymal-To-Epithelial transition (MET) and reverses EGFR inhibitor resistance. PLoS ONE. 2014;9: e84597. [DOI] [PMC free article] [PubMed] [Google Scholar]