Abstract
Retinitis pigmentosa (RP) is an inherited retinal dystrophy that courses with progressive degeneration of retinal tissue and loss of vision. Currently, RP is an unpreventable, incurable condition. We propose glycogen synthase kinase 3 (GSK-3) inhibitors as potential leads for retinal cell neuroprotection, since the retina is also a part of the central nervous system and GSK-3 inhibitors are potent neuroprotectant agents. Using a chemical genetic approach, diverse small molecules with different potency and binding mode to GSK-3 have been used to validate and confirm GSK-3 as a pharmacological target for RP. Moreover, this medicinal chemistry approach has provided new leads for the future disease-modifying treatment of RP.
Keywords: GSK-3 inhibitors, retinal diseases, retinitis pigmentosa, glaucoma
Introduction
Retinal neurodegenerative diseases like age-related macular degeneration, glaucoma, diabetic retinopathy and retinitis pigmentosa (RP) are disorders that have different etiology and pathogenesis and where the progressive impairment of diverse retinal cell types leads to vision loss. However, at the cellular and molecular level, the response to retinal injury is similar in all of them, and results in morphological and functional impairment of retinal cells. Interestingly, an inflammatory response, oxidative stress and activation of apoptotic pathways are common features in all these diseases 1 . Altogether they represent a major cause of blindness and a public health challenge. Up to date, there is not effective treatment for their neurodegenerative component, although many lines of basic research are devoted to identify their basic pathology and to develop effective treatments 2 .
Among other retinal degenerative disorders, RP and glaucoma course with neuronal death of photoreceptors and retinal ganglion cells, respectively. RP, together with other inherited retinal dystrophies, comprises a genetically heterogeneous, although clinically similar, group of neurodegenerative retinal disorders caused by mutations in more than 250 different genes. These genetic defects disrupt the function of photoreceptor cells which gradually causes night blindness, progressive constriction of the visual field and eventually blindness 3 . The progression of visual loss rate varies within individuals. Most people with RP are legally blind by the age of 40. RP is categorized as a rare disease, with a prevalence reported of one case out of the 3000–4000 individuals 4 .
The retina is a highly specialized sensory tissue that belongs to the central nervous system. It may be considered as an accessible, external part of the brain, as both derive from the most rostral neural tube 5 . In fact, the retina and the rest of the central nervous system share identical physiological and pathological molecular pathways. Consequently, diseases of the brain and the retina show similarities. For example, neuroinflammation appears as a relevant process for retina and brain neurodegenerative diseases 1 , 6 and may provide a promising target when looking for therapeutic treatments for the central nervous system in general, and for the retina in particular 7 .
Glycogen synthase kinase 3 (GSK-3) is a constitutively active Ser/Thr kinase ubiquitously expressed in the human body. Although it was first identified as an enzyme involved in glycogen synthesis 8 , its involvement in the regulation of multiple physiological functions has been well-characterized. GSK-3 has been categorized as a central kinase in a large number of prevalent diseases such as psychiatric and neurodegenerative diseases, diabetes and cancer 9 . Inflammation has been postulated as a link for all these diseases-mediated by GSK-3 malfunction 10 . The recent discovery of cellular location of GSK-3 in retina, both in Müller and photoreceptor cells 11 , led to recently propose GSK-3 as a key player in retinal neuronal death both in early diabetic retinopathy and retinal ischemic injury 12 , 13 . Moreover, lithium which among many different targets is a weak GSK-3 inhibitor (IC50 = 2 mM) has been used to reduce intraocular pressure in a rat model of glaucoma when it was intraperitoneally administered 14 . Additionally, valproic acid, that has been reported as an indirect inhibitor of GSK-3 through direct inhibition of histone deacetylase 15 , showed a short-term benefit for RP patients 16 , 17 , although the mechanism underlying this effect is not well-understood 18 .
Based in our previous experience with GSK-3 inhibitors as drug candidates for neurodegenerative disorders 19 , and in the search for RP therapies, our goal is to study whether GSK-3 inhibitors could be systemically administered as a potential therapy for RP. In this communication, we report ex vivo studies that show the potential of GSK-3 inhibitors to protect retinal cells and, thus, their potential to be translated into treatments to delay vision loss, particularly in RP.
Methods
Chemistry
Compounds 1, 2 and tideglusib have been synthesized in our laboratories following their previous described procedures 20 .
Biology
Animals
The rd10 mouse model of RP is a homozygous recessive mutant for phosphodiesterase 6b (Pde6brd10/rd10) on a C57BL/6J background. It was kindly provided by Bo Chang from The Jackson Laboratory (Bar Harbor, ME). Wild type (WT) control mice of the same background were obtained from The Jackson Laboratory. All animals were housed and handled in accordance with the ARVO statement for the use of animals in ophthalmic and vision research, European Union guidelines, and those of the local ethics committees of the CSIC and the Comunidad de Madrid. Mice were bred in the CIB core facilities on a 12/12-h light/dark cycle.
Retina explant cultures
P23 rd10 and P42–43 WT mice were euthanized, and their eyes were enucleated. Retinas were dissected and cultured on Teflon filters in R16 medium as described 21 (See also Figure 2). For the glaucoma model, WT retinas were treated with 50 µM NMDA during 48 h, with a medium change after 24 h. The rd10 mouse retinas were cultured for 24 h. Compound 1 was employed at 3.2 µM, compound 2 at 10 µM and tideglusib at 10 µM. Retinas were subsequently fixed in 4% (wt/vol) paraformaldehyde in phosphate buffer 0.1 M, pH 7.4 for 1 h at RT and processed for the detection of cell death.
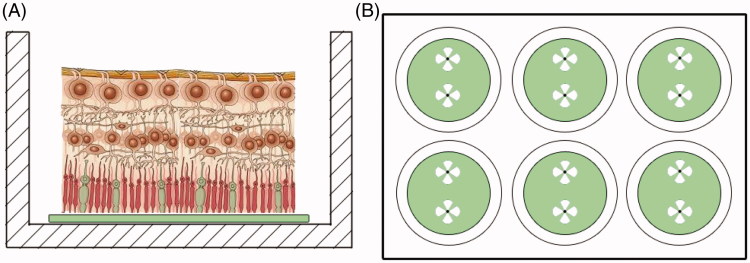
Figure 2.
Organotypic culture design. (A) The retinas were mounted with the photoreceptors in direct contact to the Teflon disc. (B) After extraction from the eyeball, four cuts were made in the retina to facilitate attachment. Two retinas were cultured in each well.
Cell death visualization and counting
Ganglion cell and photoreceptor cell death was visualized by DNA fragmentation assay terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (DeadEnd Fluorometric TUNEL system; Promega, Madison, WI) as described 22 . After labeling, the retinas were mounted in Fluoromount-G (Southern Biotechnology, Birmingham, AL), stained with DAPI, and analyzed on a laser confocal microscope (TCS SP5; Leica, Microsystems, Wetzlar, Germany). Image acquisition was performed in four areas of each retina. Serial optical sections were acquired in the depth of the ganglion cell layer or the outer nuclear layer, as determined in z-axis retinal sections, to ensure that TUNEL-positive nuclei belong to ganglion cells or to photoreceptors, respectively. Direct counting of TUNEL-positive cells was carried out on merged images using ImageJ v1.48.s software. Paired statistical analysis was performed using the Wilcoxon signed-rank test.
Results and discussion
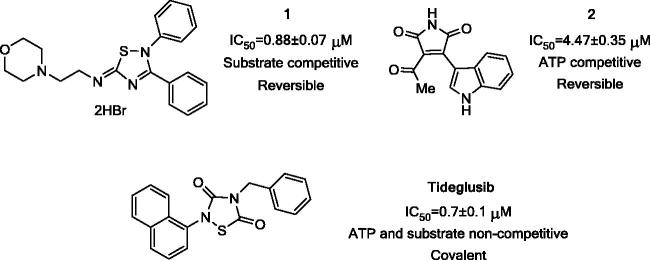
In order to confirm the therapeutic relevance of GSK-3 modulation on retinal pathologies, we employed a chemical genetic approach in ex vivo studies. The chemical genetic rationale postulates that different small chemical probes assayed in different in vitro, ex vivo and/or in vivo studies contribute to decipher the role of a potential therapeutic target 23 . Consequently, here we selected three chemically diverse small heterocyclic molecules designed and synthetized in our laboratory that target GSK-3 by different mechanism of inhibition (Figure 1): a substrate competitive inhibitor with an iminothiadiazole scaffold 1 , 24 an ATP competitive inhibitor belonging to the maleimide heterocyclic family 2 , 25 and tideglusib, a non-ATP, non-substrate competitive GSK-3 inhibitor currently in clinical trials for autism spectrum disorders 26 . Tideglusib is a thiadiazolidindione (TDZD) and currently the most advanced compound in clinical development among the selected GSK-3 inhibitors. Additionally, 1, 2 and Tideglusib have previously been tested in cell cultures and animal models showing no toxicity 27–30 .
Figure 1.
Chemical structures of the selected candidates and their GSK-3 inhibition features.
First we assayed the two more novel inhibitors (1 and 2) in retinal explants obtained from rd10 and cultured over Teflon discs (Millipore), as exemplified in Figure 2.
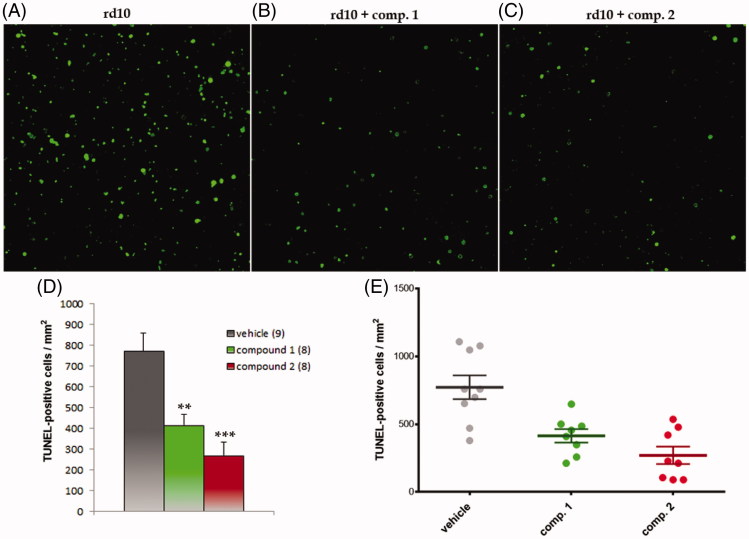
The rd10 mouse retinal explants are a RP disease model in which there is intrinsic photoreceptor cell death. The retinas were dissected at postnatal day P23, at the peak of cell death 31 , and cultured in the absence or presence of compounds 1 and 2. Cell death was visualized by TUNEL and quantified. Both GSK-3 inhibitors significantly reduced photoreceptor cell death (Figure 3) elicited a neuroprotective action in the RP model. Further, they suggest a novel potential role of GSK-3 inhibition on the treatment of this retinal pathology.
Figure 3.
GSK-3 inhibitors decreased photoreceptor cell death in rd10 mouse retinal explants. Representative images of groups (A) vehicle, (B) treatment with compound 1 and (C) treatment with compound 2. D–E. Graphic representation of data: (D) mean ± standard error is represented for each experimental group. The number in brackets corresponds to the number of retinas; (E) Individual retinal values are depicted. Significances were calculated with t student test **: p < 0.01, ***: p < 0.001.
Next, the retinal explants were obtained from wild type (WT) mice and cultured as above in the presence of N-methyl-D-aspartate (NMDA) 32 , a glutamate receptor agonist. This excitotoxic treatment mimics glaucoma-related damage of the retinal ganglion cells and has been used to validate the potential of GSK-3 inhibitors in retinal cells protection.
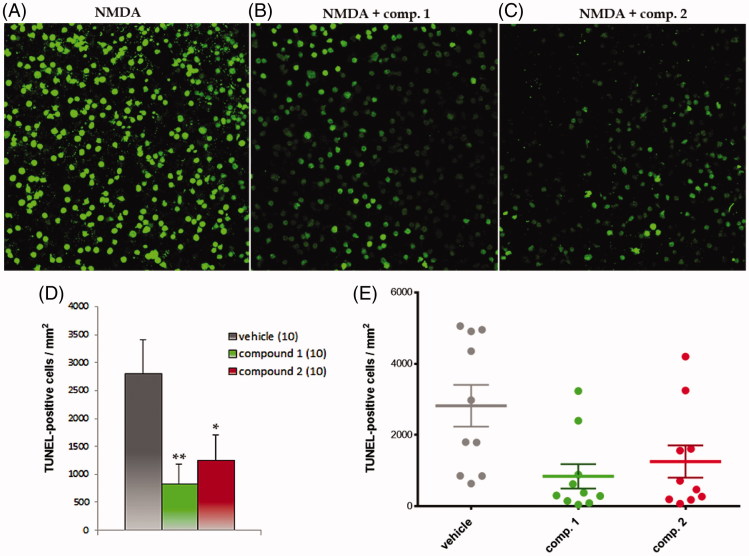
NMDA treatment induced massive cell death in the ganglion cell layer that was significantly reduced when the retinas were co-incubated with either one of the two GSK-3 inhibitors (Figure 4). These results confirmed the neuroprotective effect of GSK-3 inhibition in retinal tissue, since the two pharmacological probes, with different chemical scaffolds but with the common property of being selective GSK-3 inhibitors, reduced significantly retinal cell death 33 .
Figure 4.
GSK-3 inhibitors decreased NMDA-induced retinal ganglion cell death. Representative images of groups (A) NMDA, (B) NMDA + compound 1 and (C) NMDA + compound 2. D–E. Graphic representation of data: (D) mean ± standard error is represented for each experimental group. The number in brackets corresponds to the number of retinas; (E) Individual retinal values are depicted. Significances were calculated with t student test *: p < 0.05, **: p < 0.01.
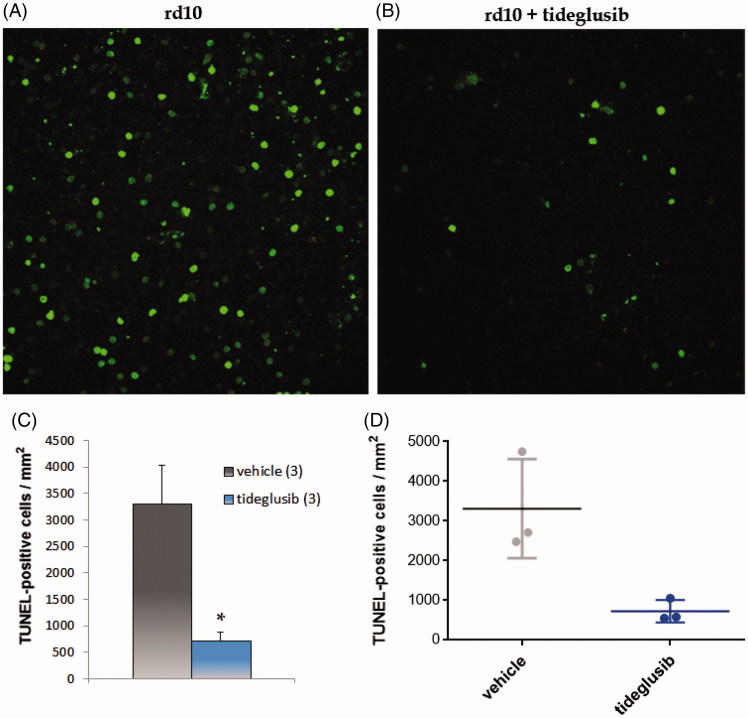
Finally, we confirmed the pro-survival effect of GSK-3 inhibition in the RP model with a third GSK-3 inhibitor, tideglusib. This compound has a chemical scaffold different to the other two inhibitors described above (compounds 1 and 2) and shows a non-ATP, non-substrate competitive mechanism of GSK-3 inhibition. The treatment of rd10 mouse retinal explants with tideglusib significantly reduced photoreceptor cell death (Figure 5), an observation that reinforces the role of GSK-3 as pharmacological target in retinal RP neuroprotection. Further, it opens an interesting translational opportunity. Tideglusib is an oral drug that has shown a wide safety window in human clinical trials both in Alzheimer’s disease and progressive supranuclear palsy 34 , and it is currently on clinical trials for autism spectrum disease 26 . In the light of the results described here, we propose the use of tideglusib for the treatment of retinal diseases, such as RP.
Figure 5.
Tideglusib decreased photoreceptor cell death in rd10 mouse retinal explants. Representative images of groups (A) vehicle, (B) tideglusib. C–D. Graphic representation of data: (C) mean ± standard error is represented for each experimental group. The number in brackets corresponds to the number of retinas; (D) Individual retinal values are depicted. Significances were calculated with t student test *: p < 0.05.
Conclusions
During the last years a large body of evidence shows that GSK-3 pathway is altered in neurodegenerative conditions of the central nervous system and small molecules targeting GSK-3 selectively have been developed as disease-modifying drugs for several unmet diseases. However, the role of GSK-3 inhibitors in retinal pathologies has not been extensively studied although they may represent potential therapeutic candidates for retinal degeneration therapy. Hereby, we have shown that GSK-3 inhibition results in a protection of retinal ganglion cells and photoreceptor cells, an observation made with three different inhibitors in two different ex vivo models of retinal pathologies, including RP. The use of mechanistically and structurally diverse GSK-3 inhibitors allows us to state that the neuroprotective effect was produced by the target inhibition, highly reducing the possibilities of cross-reactivity based on the structure or inhibition type. The results of these studies suggest that GSK-3 inhibitors are a potential pharmacotherapeutic strategy to rescue photoreceptor cells, as well as retinal ganglion cells, from a progressive degeneration such the one present in RP. Furthermore, results obtained upon tideglusib treatment in the rd10 model leads us to propose this compound as a clinical lead for RP.
Finally, the transient benefit observed in RP patients after oral intake of indirect GSK-3 inhibitor valproic acid, suggests and supports the clinical therapeutic potential of GSK-3 inhibitors for the treatment of RP. Together with the results here shown, selective GSK-3 modulation emerges as a new therapeutic target for the treatment of RP, opening new lines of research to discover an effective pharmacotherapy for RP and other retinal diseases.
Funding Statement
This work has been partially funded by the Spanish MINECO grants (SAF2012-37979-C03-01 to AM and SAF2013-41059-R to EJdlR).
Acknowledgements
JZD acknowledges a pre-doc grant in the FPU program to the Spanish MECD.
Disclosure statement
The authors report no conflict of interest.
References
- 1. Cuenca N, Fernandez-Sanchez L, Campello L, et al. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res 2014;43:17–75. [DOI] [PubMed] [Google Scholar]
- 2. Smith J, Ward D, Michaelides M, et al. New and emerging technologies for the treatment of inherited retinal diseases: a horizon scanning review. Eye (Lond) 2015;29:1131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Natarajan S. Retinitis pigmentosa: a brief overview. Indian J Ophthalmol 2011;59:343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chizzolini M, Galan A, Milan E, et al. Good epidemiologic practice in retinitis pigmentosa: from phenotyping to biobanking. Curr Genomics 2011;12:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. London A, Benhar I, Schwartz M.. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol 2013;9:44–53. [DOI] [PubMed] [Google Scholar]
- 6. Hippert C, Graca AB, Barber AC, et al. Müller glia activation in response to inherited retinal degeneration is highly varied and disease-specific. PLoS One 2015;10:e0120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jindal V. Interconnection between brain and retinal neurodegenerations. Mol Neurobiol 2015;51:885–92. [DOI] [PubMed] [Google Scholar]
- 8. Embi N, Rylatt DB, Cohen P.. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem 1980;107:519–27. [PubMed] [Google Scholar]
- 9. Martinez A, Castro A, Dorronsoro I, Alonso M.. Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Med Res Rev 2002;22:373–84. [DOI] [PubMed] [Google Scholar]
- 10. Jope RS, Yuskaitis CJ, Beurel E.. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 2007;32:577–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perezleon JA, Osorio-Paz I, Francois L, Salceda R. Immunohistochemical localization of glycogen synthase and GSK3β: control of glycogen content in retina. Neurochem Res 2013;38:1063–9. [DOI] [PubMed] [Google Scholar]
- 12. Li Z, Ma L, Chen X, et al. Glycogen synthase kinase-3: a key kinase in retinal neuron apoptosis in early diabetic retinopathy. Chin Med J (Engl Ed) 2014;127:3464–70. [PubMed] [Google Scholar]
- 13. Russo R, Adornetto A, Cavaliere F, et al. Intravitreal injection of forskolin, homotaurine, and L-carnosine affords neuroprotection to retinal ganglion cells following retinal ischemic injury. Mol Vis 2015;21:718–29. [PMC free article] [PubMed] [Google Scholar]
- 14. Sun XB, Lu HE, Chen Y, et al. Effect of lithium chloride on endoplasmic reticulum stress-related PERK/ROCK signaling in a rat model of glaucoma. Pharmazie 2014;69:889–93. [PubMed] [Google Scholar]
- 15. De Sarno P, Li X, Jope RS.. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology 2002;43:1158–64. [DOI] [PubMed] [Google Scholar]
- 16. Kumar A, Midha N, Gogia V, et al. Efficacy of oral valproic acid in patients with retinitis pigmentosa. J Ocul Pharmacol Ther 2014;30:580–6. [DOI] [PubMed] [Google Scholar]
- 17. Iraha S, Hirami Y, Ota S, et al. Efficacy of valproic acid for retinitis pigmentosa patients: a pilot study. Clin Ophthalmol 2016;10:1375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daly C, Yin J, Kennedy BN.. Histone deacetylase: therapeutic targets in retinal degeneration. Adv Exp Med Biol 2016;854:455–61. [DOI] [PubMed] [Google Scholar]
- 19. Martinez A, Perez DI, Gil C.. Lessons learnt from glycogen synthase kinase 3 inhibitors development for Alzheimer's disease. Curr Top Med Chem 2013;13:1808–19. [DOI] [PubMed] [Google Scholar]
- 20. Martínez A, Dorronsoro DI, Alonso CM, Panizo DPG, Fuertes HA, Perez PMJ, et al. Thiadiazolidinones as gsk-3 inhibitors, WO2005097117; 2005. [Google Scholar]
- 21. Arroba AI, Wallace D, Mackey A, et al. IGF-I maintains calpastatin expression and attenuates apoptosis in several models of photoreceptor cell death. Eur J Neurosci 2009;30:975–86. [DOI] [PubMed] [Google Scholar]
- 22. Corrochano S, Barhoum R, Boya P, et al. Attenuation of vision loss and delay in apoptosis of photoreceptors induced by proinsulin in a mouse model of retinitis pigmentosa. Investig Ophthalmol Vis Sci 2008;49:4188–94. [DOI] [PubMed] [Google Scholar]
- 23. Kawasumi M, Nghiem P.. Chemical genetics: elucidating biological systems with small-molecule compounds. J Investig Dermatol 2007;127:1577–84. [DOI] [PubMed] [Google Scholar]
- 24. Palomo V, Perez DI, Perez C, et al. 5-imino-1,2,4-thiadiazoles: first small molecules as substrate competitive inhibitors of glycogen synthase kinase 3. J Med Chem 2012;55:1645–61. [DOI] [PubMed] [Google Scholar]
- 25. Perez DI, Palomo V, Perez C, et al. Switching reversibility to irreversibility in glycogen synthase kinase 3 inhibitors: clues for specific design of new compounds. J Med Chem 2011;54:4042–56. [DOI] [PubMed] [Google Scholar]
- 26. https://clinicaltrials.gov/ct2/show/NCT02586935?term=tideglusib&rank=2 [accessed July 2016]. [Google Scholar]
- 27. Redondo M, Palomo V, Brea J, et al. Identification in silico and experimental validation of novel phosphodiesterase 7 inhibitors with efficacy in experimental autoimmune encephalomyelitis mice. ACS Chem Neurosci 2012;3:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morales-Garcia JA, Luna-Medina R, Alonso-Gil S, et al. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem Neurosci 2012;3:963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sereno L, Coma M, Rodriguez M, et al. A novel GSK-3beta inhibitor reduces Alzheimer's pathology and rescues neuronal loss in vivo. Neurobiol Dis 2009;35:359–67. [DOI] [PubMed] [Google Scholar]
- 30. Bolos M, Fernandez S, Torres-Aleman I.. Oral administration of a GSK3 inhibitor increases brain insulin-like growth factor I levels. J Biol Chem 2010;285:17693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barhoum R, Martinez-Navarrete G, Corrochano S, et al. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience 2008;155:698–713. [DOI] [PubMed] [Google Scholar]
- 32. Xin H, Yannazzo JA, Duncan RS, et al. A novel organotypic culture model of the postnatal mouse retina allows the study of glutamate-mediated excitotoxicity. J Neurosci Methods 2007;159:35–42. [DOI] [PubMed] [Google Scholar]
- 33. Ha S, Ryu HY, Chung KM, et al. Regulation of autophagic cell death by glycogen synthase kinase-3β in adult hippocampal neural stem cells following insulin withdrawal. Mol Brain 2015;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoglinger GU, Huppertz HJ, Wagenpfeil S, et al. Tideglusib reduces progression of brain atrophy in progressive supranuclear palsy in a randomized trial. Mov Disord 2014;29:479–87. [DOI] [PubMed] [Google Scholar]