Abstract
The microenvironment of solid tumours is extremely acidic and this condition arises since the precancerous stage. This acidic milieu could therefore provide a useful target for both prophylactic and therapeutic approaches. In TRAMP transgenic mice, an in vivo model of prostate adenocarcinoma (AC), oral administration of alkaline water was devoid of unwanted side effects, and when started from an early age was as effective as NaHCO3 in significantly delaying tumour progression, while when started when prostate tumours were already present, a nonstatistically significant trend in the same direction was detected. These findings indicate that the use of alkalinizing drugs should be considered for chemoprevention and, in association with standard chemotherapy, for treatment of human prostate AC.
Keywords: Alkalinisation, chemoprevention, prostate tumour, TRAMP mice, tumour microenvironment
Introduction
During tumour development, the microenvironment becomes progressively acidic due to different and often concomitant mechanisms: local hypoxia resulting from poor blood perfusion, increased flux of carbons through fermentative glycolysis, and the release by cancer cells of lysosome content into the extracellular matrix (ECM)1–3. Indeed, extracellular acidosis in human solid tumours can reach pH values as low as 6.5. Likely, adaptation of cancer cells to an acid microenvironment occurs early during cancer progression, as pre-cancer cells undergo a metabolic switch in ATP generation, from oxidative phosphorylation to glycolysis. Since the latter pathway provides a much lower energy gain, transformed cells greatly increase their glucose uptake to meet their amplified metabolic requirements, resulting in intracellular lactate accumulation and the excretion of H+ by proton transporters4, causing progressive acidification of the extracellular milieu. This acidified habitat supports cancer cells with a stabilized glycolytic phenotype, which in turn leads to sustained generation of metabolic acids, even in well-oxygenated conditions, and to selection of cancer cells resistant to acid-mediated apoptosis5. It has been hypothesised that these mechanisms lead to a competitive advantage of cancer cells toward normal bystander cells that cannot survive in an increasingly acidic microenvironment1 , 2. Besides being toxic to normal cells, acidosis can stimulate invasion and metastatization by degrading and remodelling the ECM, increasing angiogenesis through the release of vascular endothelial growth factor, and inhibiting the immune response1 , 5–10.
It is likely that targeting the driver functions that confer selective advantages to tumour cells can be a suitable alternative approach for cancer therapy. The reversal of pH gradient in cancer cells is increasingly considered as a hallmark of virtually all cancers, and a potential target for new anti-tumours therapies11. In particular, alkalinizing treatment with existing molecules such as proton pump inhibitors (PPIs) and buffers, such as NaHCO3, citrate or TRIS has been a proposed for human therapy2. This approach has been supported by a clinical study on companion animals with spontaneous tumours in which the PPI lansoprazole, administered at high dose and combined with a water alkalizer, has proven effective in enhancing tumour response to metronomic chemotherapy12, and by two clinical trials, in either osteosarcoma13 or metastatic breast cancer patients14, where the administration of the PPI esomeprazole either improved the local effect of neoadjuvant chemotherapy or prolonged the time to progression and the overall survival rate in treated patients. Coming to the effect of alkalizer agents as monotherapy, it has been demonstrated that treatments with NaHCO3 or lysine inhibit human mammary and prostate metastases, respectively, in mouse xenograft models15 , 16, while the oral administration of a commercially available water alkalizer significantly reduced tumour growth in a syngeneic melanoma mouse model17. However these results should be confirmed in a more physiological model, i.e. in transgenic animals spontaneously developing tumours.
TRAMP mice are the best model available so far for pharmaceutical studies on prostate carcinoma, since 100% of these animals display spontaneous multistage prostate carcinogenesis, with histological and molecular features similar to those present in human prostate cancer18 , 19. Not surprisingly, the TRAMP model has been used to successfully test the chemopreventive efficacy of several natural anticancer agents such as green tea, grape, garlic, cabbage, tomato, hop20–26. The efficacy of microenvironment alkalization in such a model was assessed by Ibrahim-Hashim and co-authors. They found that NaHCO3 in drinking water prevented the onset of prostate cancer in transgenic TRAMP mice, albeit it was ineffective in treating established tumours27. Despite this excellent proof-of-principle demonstration, the authors themselves correctly stated that the administration of this regimen to humans would be unadvisable, due to the unwanted side effects resulting from sustained intake of high doses of NaHCO3. Moreover, sodium bicarbonate cannot be considered the ideal buffer for tumour treatment and prevention for many reasons. First, NaHCO3 is not a potent buffer molecule inasmuch as it can reach no more than pH 8.5 in a water solution. Moreover, it is unbalanced in term of electrolyte equilibrium, containing exclusively Na+, and therefore exposing to potential side effects in prolonged treatment regimens, including cardiovascular and renal dysfunctions. Lastly, at the concentration proposed in the Ibrahim-Hashim’s paper it would result disgusting when used for oral administration, independently from the disease condition. We have therefore tested in the same TRAMP model, the anti-tumour effect of a water alkalinizer (AlkaWater®) through which the best pH condition can be reached in water solution (from pH 9.0 to 10.0) depending on the tumour and systemic pH; moreover, the taste of alkalinized water is comparable to that of either tap and mineral water and the solution is balanced in terms of electrolytes, containing Na+, K+, Ca+ and Mg+. The results have shown that the administration of alkaline water was devoid of any unwanted side effects, and when started from an early age was as effective as NaHCO3, significantly delaying tumour progression, while when started when prostate tumours were already present showed a trend in the same direction.
Materials and methods
Cell culture and reagent
Tramp C1 prostate carcinoma cell lines (ATCC, Rockville, MD) were cultured in DMEM containing 10% FCS, glutamine and penicillin/streptavidin.
The alkaline stock solution contained NaCl and KOH (AlkaWater®) and was diluted 1:1000 in tap water to obtain a drinking solution at pH 9.5, and 1:300 for a solution at pH 10.5.
NaHCO3 was dissolved in tap water at the concentration of 200 mM, as previously described27.
Animals and anti-acid treatment
Animal studies and research protocols were reviewed and approved by the Ethics Committee of the IRCCS San Martino-IST and were conducted in accordance with the current Italian regulations and guidelines for the care and use of laboratory animals (D.L. 26/2014).
For in vivo studies, we used two different models, the spontaneous prostate tumour model developing in the transgenic TRAMP mice and xenograft model with TRAMP C1 cells.
TRAMP mice were maintained in heterozygosity by crossing C57Bl/6 TRAMP females with C57Bl/6 wild type males (Charles River Laboratories, Calco, Italy), and transgene verification was carried out when newborn mice reached 3 weeks of age using DNA obtained from tail clipping as previously described19. Transgene-positive mice were then randomly divided into four groups, each supplied with different types of water: the “control” group of 30 mice was administered with tap water; the “prevention” group of 59 mice with alkaline water at pH 9.5, starting at 4 weeks of age; the “therapy” group of 37 mice with alkaline water at pH 10.5, starting at 12 weeks of age; the “bicarbonate” group of 34 mice with NaHCO3, starting at 4 weeks of age. These time points were chosen accordingly with the progression of cancer in TRAMP mice, where high grade prostatic intraepithelial neoplasia (PIN) or well-differentiated prostate cancer is present in 10–12 weeks of age18. All animals were monitored daily for signs of suffering and, starting from 20 weeks of age, were checked by palpation for the development of precocious neuroendocrine tumours. Twenty-six percent of TRAMP mice of all groups displayed rapidly growing and very aggressive poorly differentiated neuroendocrine tumours that developed very early (24–29 weeks of age) respect to our chosen end point (32 weeks of age). These animals were not considered in our analysis, since these tumours are not comparable to the human pathology, their growth arise independently from atypical hyperplasia or other epithelial lesions28 and represent an extremely rare and advanced stage of carcinomas29. We instead took into the account neuroendocrine tumours that develop later, as consequence of stochastic events related to malignant progression30. Throughout the experiment, mice were fed with food and water ad libitum and water consumption was recorded. Mice were sacrificed by CO2 inhalation at 32 weeks of age and subjected to accurate necropsy. Body weight (bw) was registered before sacrifice. The entire urogenital (UG) apparatus, consisting of emptied bladder, urethra, seminal vesicles, testes and prostate was excised and weighed. The ratio between UG and bw (UG/bw) was then calculated. Seminal vesicles and testes were then removed and the prostate was fixed in 4% neutral buffered formalin and processed for histology and immunohistochemistry. Bladder was maintained to orient samples during embedding, so that sectioning was done starting from the dorsal part.
For the xenograft model, six 6-week-old C57/Bl6 male mice (Charles River Laboratories, Calco, Italy) were injected subcutaneously (s.c.) in the right flank with 6 × 106 TRAMP C1 cells suspended in 0.1 ml of PBS, a dose that is tumorigenic in 100% of syngeneic animals31. The animals were then randomly divided into two groups and either fed with pH 9.5 alkaline water, or with regular tap water. The mice were then regularly palpated to assess tumour latency. Tumour growth was recorded measuring nodule size with a calliper three times a week; when a nodule reached the size of 250 mm3, all animals were sacrificed and tumours were excised, measured, weighed and formalin fixed for further analyses.
Histology and immunohistochemical analysis
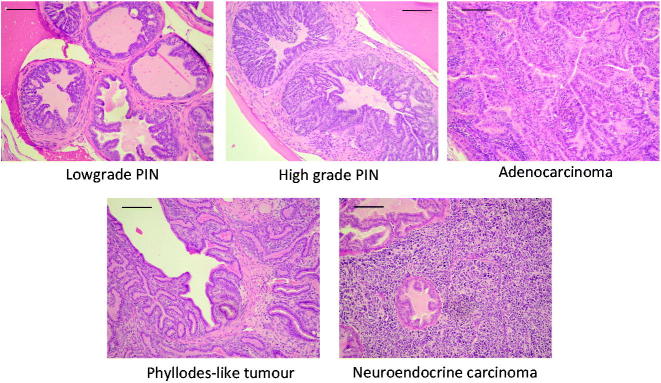
For histology and immunohistochemistry analysis, samples were fixed in 4% neutral buffered formalin, embedded in paraffin and cut to obtain 3–4 μm thick sections. Slides were then either stained with haematoxylin and eosin (H&E) for pathological analysis or processed for immunohistochemistry. All the evaluations were done in blind and scored independently by two investigators. Samples were examined for the presence of low or high grade PIN, well-differentiated adenocarcinoma (AC), phyllodes-like tumour (PHY) and neuroendocrine tumour (NE)19 , 30.
Immunohistochemistry was performed using rabbit monoclonal anti-Ki67 or rabbit polyclonal anti-CD3 (Abcam, Cambridge, UK). Primary antibodies were diluted 1:150 and 1:100 respectively in PBS containing 0.1%Tween20 and 1%BSA, and incubated for 1 h at RT. After washing, the slides were incubated with a biotinylated anti-rabbit secondary antibody (Pierce, Thermo Fisher Scientific, Waltham, MA) followed by peroxidase-conjugated streptavidin (BioSPA Biochemical, Milano, Italy). Samples were then stained using the Vectastain DAB Kit (Vector Laboratories, Burlingame, CA). To quantify proliferation, five randomly selected fields on each H&E-stained sections, from four different animals, were blindly photographed, with an oil-immersion 100× objective. Positive nuclei were counted and expressed as percentage of the total nuclei present in each field.
Statistical analysis
All statistical analyses were done with the IBM SPSS Statistic Software version 20 or 2 × 2 contingency table calculator (http://vassarstats.net/tab2x2.html).
Results
Alkaline water administration delays prostate AC progression in TRAMP mice
To evaluate toxicity of the treatment, we initially administered alkaline water at pH 10.5 to 5 C57/Bl6 male mice for two months, and looked for signs of toxicity in terms of pain, suffering or distress. In particular, we monitored behavioural signs and found no changes in posture, gait, activity and interactions with the environment. Moreover, we found no alteration in clinical signs, such as coat condition, nasal or ocular discharge, swollen eyes, increased respiratory rate, dyspnoea, tremors, excessive urination and consistency of faeces. Similarly, we did not register any significant difference in bw when treated mice were compared with control mice receiving tap water. At the time of necropsy, we macroscopically checked internal organs, with particular attention to liver and kidneys. No sign of toxicity was found (data not shown).
Afterward, we started to assess the therapeutic effect of alkaline water administration. Water consumption varied among the groups, with the NaHCO3-treated animals that doubled their intake (13.2 ± 1.47 ml/day/animal), compared to control group (7.25 ± 0.43 ml/day/animal). Conversely, the water intake for both the “prevention” and “therapy” groups was slightly lower (5.8 ± 1.12 ml/day/animal and 5.2 ± 0.66, respectively).
At the established experimental end point (32 weeks of age), we sacrificed and thoroughly necropsied all animals. We found no animals with evidence of oedema or abnormal organ size, in target and non-target organs, however we observed hydronephroses in 11 out of the 29 (38%) animals fed with NaHCO3.
The animals in the “prevention” group displayed a significant (p = .017) decrease in UG/bw, with respect to the animals in the “control” group, while no difference with the “control” group was found in the animals belonging to the “therapy” group (p = .26); the mice in the “bicarbonate” group showed a sharp decrease in the UG/bw ratio with respect to controls (p = .001) (Figure 1).
Figure 1.
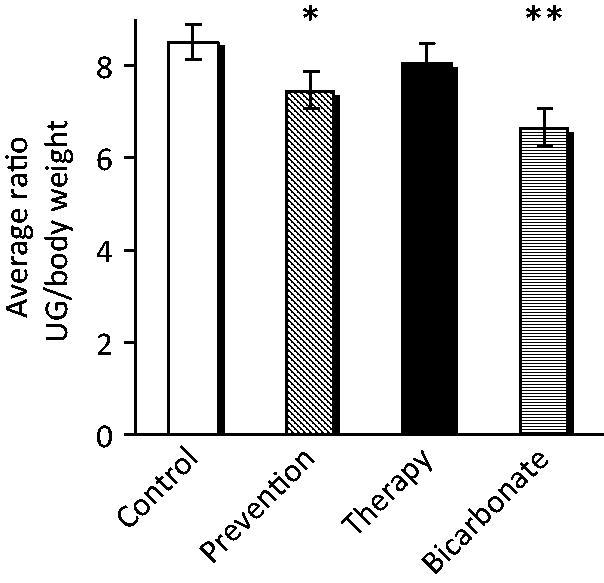
Administration of alkaline water to TRAMP mice reduces UG tract weight. The ratio between UG and bw in the different experimental groups is shown. Mean ± S.E. *p = .017; **p = .001.
All collected tumours were then examined for both histological and immunohistochemical analyses. Tissue sections, stained with H&E, were evaluated in a blind assay by two different researchers. In order to evaluate the progression of the disease, we recorded the different type of lesions found on each slide (Figure 2).
Figure 2.
Histological classification of tumour lesions. Examples of the different histological types are provided. Bar =20 μm.
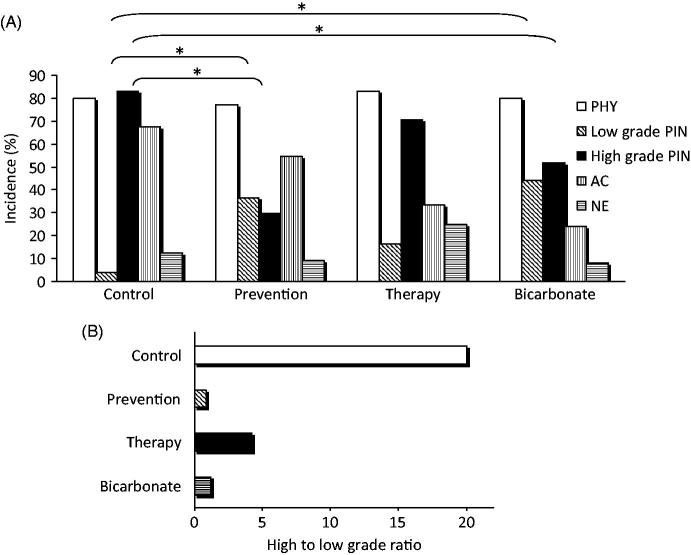
Overall tumour incidence was 100% in all groups but comparison between “control” and “prevention” groups demonstrated that our treatment produced a decrease in the incidence of high grade PIN from 83 to 29.5% (p = .001), and a corresponding increase in the incidence of low grade PIN from 4.17 to 36.4% (p = .001) (Figure 3(a)). Also the administration of alkaline water with the “therapy” scheme affected PIN progression, and albeit statistical significance was not achieved, there was a clear reduction in the high to low PIN ratio, as compared to the “control” group (Figure 3(b)). The “bicarbonate” group, showed a significant decrease, with respect to “control” group, in the incidence of high grade PIN (from 83 to 52% p = .020), and a corresponding increase in the incidence of low grade PIN (from 4.17 to 44% p = .01).
Figure 3.
Administration of alkaline water in TRAMP mice reduces tumour progression. (A) Distribution of the different tumour lesions in the experimental groups, calculated on the total number of mice in each group. The cumulative percentage surpasses 100% because in the same mouse different tumour types may co-exist. The asterisks indicate p < .05. (B) The ratio between high and low grade PIN in the different experimental groups is shown.
As it regards AC incidence, we found with respect to “control” group, a reduction of 12.8% in the “prevention” group, of 46.7% in the “therapy” group and of 61.6% in the “bicarbonate” group.
We detected no statistically significant difference in PHY or NE incidence among the groups.
Immunohistochemical analyses evidenced no changes in tumour cell proliferation, measured by Ki67 staining, as well as in the distribution and amount of tumour infiltrating T cells between treated group and controls (data not shown).
Alkaline water administration delays tumour growth in xenotransplants of an androgen independent prostate cancer cell line
C57/Bl6 wild type male mice injected s.c. with TRAMP C1 syngeneic cells, were randomly divided into two groups that received either tap water or alkaline water at pH 9.5, and tumour growth was monitored (Figure 4). The growth of tumour nodules in treated mice was delayed respect to control mice. The delay was statistically significant till day 14 from cell injection (p = .018, p = .001 and p = .017 for day 10, 12 and 14 respectively). From day 17, the difference became not statistically significant, due to increase in standard error. Histology on tumours collected from the mice at the end of experiment, showed no differences in the histological types between the two groups (data not shown). Also, immunohistochemistry did not show any difference in tumour cell proliferation and tumour infiltration by T cells between the two groups (data not shown).
Figure 4.
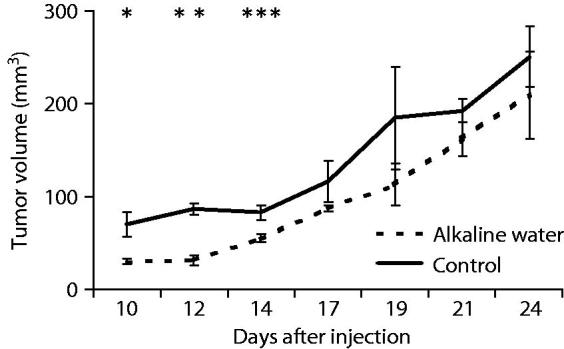
Alkaline water delays tumour growth in xenotransplants. The kinetics of tumour growth of TRAMP C1 cells injected subcutaneously into alkaline water-treated mice and into untreated mice is shown. Mean ± S.E. *p = .018; **p = .001; ***p = .017.
Discussion
The effect of alkaline water treatment confirms that the alkalinisation of the microenvironment has a prophylactic effect on prostate cancer progression, as already observed by Ibrahim-Hashim et al.27 with NaHCO3 using a small number of animals. We have followed this therapeutic strategy in a much larger cohort of mice and also using a different alkalinizing agent. We have found that oral administration of alkaline water or NaHCO3 to TRAMP mice, beginning at four weeks of age, are equal in inducing a delay in the progression of prostate AC, with reduction in the incidence over time of both high grade PIN and AC. Confirming previous results with NaHCO3, both the alkalinizing agents we tested the induced growing tumours to retain a more differentiated low grade PIN for longer.
However, the preventive treatment with alkaline water has the advantage to be devoid of the long-term unwanted side effects of a high dose NaHCO3. As a matter of fact, we have found hydronephrosis in a significant percentage of NaHCO3-treated mice. In these animals, an excess of water intake was also detected, suggesting possible long-term impact on blood pressure, and on cardiac and renal function. Instead, no tissue or organ impairment was detected in the two groups of mice treated with alkaline water. Indeed, the administration of high dose of NaHCO3 regimens to humans would be unadvisable27, making alkaline water a safer alternative.
The effect of the “therapy” scheme was less significant, however, there was a clear trend toward a delay in PIN progression and a reduction in AC incidence. This conclusion is supported by the results obtained with xenografts, a model of early tumour, which showed that alkaline water treatment delays tumour growth. These results are comparable to those obtained with NaHCO3 treatments in a mouse model of mammary tumour3. These findings are encouraging, since no therapeutic effect was detected when NaHCO3 was administered to TRAMP mice with the same scheme27.
It is of note that PHY tumours and NE tumours did not respond to the alkalinizing treatments. It remains to be elucidated whether these tumours do not rely on an acidic microenvironment for their growth, or if they have escape mechanisms that allow them to counter the effect of alkalinizing agents. However, the prostate tumours in humans are almost all ACs, the NE tumour accounting for about 4% of the total29 and the PHY tumours of the prostate being very rare32, therefore alkaline water may be considered a promising low-cost therapeutic approach that should be taken into account for prophylaxis and, combined with standard chemotherapy, for treatment of human prostate cancer. The few clinical trials available to date suggest that the anti-acidic/alkalinizing approach may well represent an efficient way to implement the existing anticancer therapies33 , 34.
Acknowledgements
We are grateful to Dr Michele Cilli for the help with the TRAMP colonies and Dr Rocco Palmisano for providing the AlkaWater® solution used in our experiments. We also thank our students (Eugenio Alberti and Angela Andrisani) for help with histology.
Disclosure statement
The authors report no conflicts of interest.
Funding
This work was supported in part by Ricerca Corrente Ministero della Salute 5xmille 2011 to OB.
References
- 1. Gatenby RA, Gillies RJ.. A microenvironmental model of carcinogenesis. Nat Rev Cancer 2008;8:56–61. [DOI] [PubMed] [Google Scholar]
- 2. Fais S, Venturi G, Gatenby B.. Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy. Cancer Metastasis Rev 2014;33:1095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Damaghi M, Tafreshi NK, Lloyd MC, et al. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat Commun 2015;6:8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spugnini EP, Sonveaux P, Stock C, et al. Proton channels and exchangers in cancer. Biochim Biophys Acta 2015;1848:2715–26. [DOI] [PubMed] [Google Scholar]
- 5. Gatenby RA, Gillies RJ.. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891–9. [DOI] [PubMed] [Google Scholar]
- 6. Lardner A. The effects of extracellular pH on immune function. J Leuk Biol 2001;69:522–30. [PubMed] [Google Scholar]
- 7. Xu L, Fukumura D, Jain RK.. Acidic extracellular pH induces vascular endothelial growth factor (VEGF) in human glioblastoma cells via ERK1/2 MAPK signaling pathway: mechanism of low pH-induced VEGF. J Biol Chem 2002;277:11368–74. [DOI] [PubMed] [Google Scholar]
- 8. Moellering RE, Black KC, Krishnamurty C, et al. Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis 2008;25:411–25. [DOI] [PubMed] [Google Scholar]
- 9. Estrella V, Chen T, Lloyd M, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 2013;73:1524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damaghi M, Wojtkowiak JW, Gillies RJ.. pH sensing and regulation in cancer. Front Physiol 2013;4:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 12. Spugnini EP, Buglioni S, Carocci F, et al. High dose lansoprazole combined with metronomic chemotherapy: a phase I/II study in companion animals with spontaneously occurring tumors. J Transl Med 2014;12:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrari S, Perut F, Fagioli F, et al. Proton pump inhibitor chemosensitization in human osteosarcoma: from the bench to the patients' bed. J Transl Med 2013;11:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang BY, Zhang J, Wang JL, et al. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J Exp Clin Cancer Res: CR 2015;34:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robey IF, Baggett BK, Kirkpatrick ND, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 2009;69:2260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ibrahim-Hashim A, Wojtkowiak JW, de Lourdes Coelho Ribeiro M, et al. Free base lysine increases survival and reduces metastasis in prostate cancer model. J Cancer Sci Ther 2011:Suppl 1(4). [PMC free article] [PubMed] [Google Scholar]
- 17. Azzarito T, Lugini L, Spugnini EP, et al. Effect of modified alkaline supplementation on syngenic melanoma growth in CB57/BL mice. PLoS One 2016;11:e0159763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gingrich JR, Barrios RJ, Kattan MW, et al. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res 1997;57:4687–91. [PubMed] [Google Scholar]
- 19. Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 1995;92:3439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pannellini T, Iezzi M, Liberatore M, et al. A dietary tomato supplement prevents prostate cancer in TRAMP mice. Cancer Prev Res (Philadelphia, PA) 2010;3:1284–91. [DOI] [PubMed] [Google Scholar]
- 21. Singh SV, Powolny AA, Stan SD, et al. Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res 2008;68:9503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta S, Hastak K, Ahmad N, et al. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA 2001;98:10350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pannellini T, Iezzi M, Liberatore M, et al. A dietary tomato supplement prevents prostate cancer in TRAMP mice. Cancer Prev Res (Phila) 2010;3:1284–91. [DOI] [PubMed] [Google Scholar]
- 24. Raina K, Singh RP, Agarwal R, Agarwal C.. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res 2007;67:5976–82. [DOI] [PubMed] [Google Scholar]
- 25. Singh SV, Warin R, Xiao D, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res 2009;69:2117. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vene R, Benelli R, Minghelli S, et al. Xanthohumol impairs human prostate cancer cell growth and invasion and diminishes the incidence and progression of advanced tumors in TRAMP mice. Mol Med 2012;18:1292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ibrahim-Hashim A, Cornnell HH, Abrahams D, et al. Systemic buffers inhibit carcinogenesis in TRAMP mice. J Urol 2012;188:624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiaverotti T, Couto SS, Donjacour A, et al. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol 2008;172:236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parimi V, Goyal R, Poropatich K, Yang XJ.. Neuroendocrine differentiation of prostate cancer: a review. Am J Clin Exp Urol 2014;2:273–85. [PMC free article] [PubMed] [Google Scholar]
- 30. Kaplan-Lefko PJ, Chen TM, Ittmann MM, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. The Prostate 2003;55:219–37. [DOI] [PubMed] [Google Scholar]
- 31. Foster BA, Gingrich JR, Kwon ED, et al. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res 1997;57:3325–30. [PubMed] [Google Scholar]
- 32. Bostwick DG, Hossain D, Qian J, et al. Phyllodes tumor of the prostate: long-term follow up study of 23 cases. J Urol 2004;172:894–9. [DOI] [PubMed] [Google Scholar]
- 33. Fais S, O'Driscoll L, Borras FE, et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano 2016;10:3886–99. [DOI] [PubMed] [Google Scholar]
- 34. Taylor S, Spugnini EP, Assaraf YG, et al. Microenvironment acidity as a major determinant of tumor chemoresistance: proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist Updat 2015;23:69–78. [DOI] [PubMed] [Google Scholar]