Abstract
While progress has been made in treating cancer, cytotoxic chemotherapeutic agents are still the most widely used drugs and are associated with severe side-effects. Drugs that target unique molecular signalling pathways are needed for treating cancer with low or no intrinsic toxicity to normal cells. Our goal is to target hypoxic tumours and specifically the hypoxia inducible factor (HIF) pathway for the development of new cancer therapies. To this end, we have previously developed benzopyran-based HIF-1 inhibitors such as arylsulfonamide KCN1. However, KCN1 and its earlier analogs have poor water solubility, which hamper their applications. Herein, we describe a series of KCN1 analogs that incorporate a morpholine moiety at various positions. We found that replacing the benzopyran group of KCN1 with a phenyl group with a morpholinomethyl moiety at the para positions had minimal effect on potency and improved the water solubility of two new compounds by more than 10-fold compared to KCN1, the lead compound.
Keywords: Hypoxia, solubility, HIF-1
Introduction
Cancer is one of the leading causes of death, second only to heart disease1. One of the hallmarks of cancer is the formation of hypoxic areas inside of solid tumours2. This hypoxic tumour microenvironment leads to many changes such as the upregulation of pro-angiogenic and pro-glycolytic pathways, as well as increases in cell proliferation, genetic instability, and metastatic potential3. A major mediator of the hypoxic response is the hypoxia inducible factor (HIF) pathway4. HIF is a heterodimeric transcription factor consisting of two subunits, HIF-α, the stability of which is regulated by oxygen, and HIF-1β, which is constitutively expressed5. There are three known isoforms of HIF-α, HIF-1α, HIF-2α, and HIF-3α, with HIF-1α being the most commonly expressed and most extensively studied. Under normoxic conditions, HIF-α subunits are hydroxylated by a prolyl hydroxylase (PHD2) using molecular oxygen and then degraded via a VHL-dependent ubiquitination pathway6. Under hypoxic conditions, however, HIF-α subunits are stabilised, heterodimerise with HIF-1β and recruit co-activators such as p300 and CBP, to form active transcription complexes that bind to 5′-HREs (hypoxia response elements) in promoter regions of hypoxia-inducible genes7. Increased levels of HIF-1α are linked to cancer progression and poor patient outcome. Therefore, HIF is an attractive target for developing anti-cancer therapeutics8.
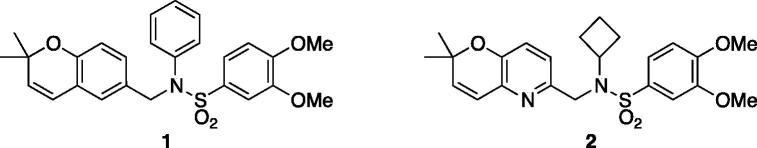
A library of 10,000 products containing the 2,2-dimethyl-2H-chromene moiety9 was screened for compounds with HIF inhibitory activity. This led to the identification of a compound designated KCN1 (Figure 1, 1, N-((2,2-dimethyl-2H-chromen-6-yl)methyl)-3,4-dimethoxy-N-phenylbenzenesulfonamide) showing potent inhibition activity (IC50 of ∼0.6 µM) in a HIF-dependent bioassay10.
Figure 1.
Lead compounds 1 (KCN1) and 2 (64b).
Further in vivo studies demonstrated 1’s very pronounced inhibitory activity against brain, and pancreatic cancers11. In addition, 1 was well tolerated in mice; daily treatments with 60 mg/kg for up to 12 weeks had minimal side effects11. Neither did 1 nor its analogs demonstrate cytotoxicity, indicating the selective inhibitory effects being based on pathways unique to cancer11. Such results strongly suggest that this is a very promising class of compounds and warrant further studies. In fact, a previously synthesised and analysed class of analogs has been developed, which led to the discovery of 64b (Figure 1, 2, N-cyclobutyl-N-((2,2-dimethyl-2H-pyrano[3,2-b]pyridin-6-yl)methyl)-3,4-dimethoxybenzenesulfonamide) with an IC50 value of ∼0.3 µM.12 However, 1 and its analogs possess poor solubility in water (0.009 µg/mL)11. Therefore, dissolution in DMSO is necessary for in vitro assays and cremophor:ethanol-based formulations are needed for in vivo models. Such a formulation introduces undesirable properties12. It is well known that the successful development of potential therapeutics relies on more parameters than potency alone. Other properties, including solubility, can play a critical role. Therefore, we are interested in designing water-soluble analogs of 1 and 2 to address this critical aspect of drug development.
Materials and methods
Synthesis
General methods and materials
All commercial chemicals were of reagent grade from VWR (Radnor, PA), Aldrich (St. Louis, MO), or Oakwood Chemicals (Estill, SC), and were used without further purification unless otherwise indicated. 1H and 13C spectra were obtained on a Bruker 400 NMR spectrometer at 400 and 100 MHz, respectively, in deuterated solvent with TMS (δ = 0.00 ppm) or deuterated solvent as internal reference. For all reactions, analytical grade solvent was used. Anhydrous solvents were used for all moisture-sensitive reactions. The Mass Spectrometry Facilities at Georgia State University obtained high-resolution mass spectra on a Waters Micromass Q-TOF (ESI) instrument.
Typical procedure for morpholine substitution (8a–c)
Benzyl bromide (1 equivalent) was dissolved in acetonitrile. Morpholine (1.1 equivalents) and K2CO3 (2 equivalents) were added and the reaction was stirred overnight at room temperature. The reaction was filtered through Celite and concentrated to give the product in quantitative yield.
4-(4-Bromobenzyl)morpholine (8a)
1H NMR (CDCl3): δ 7.41 (d, J = 8 Hz, 2H), 7.19 (d, J = 8 Hz, 2H), 3.67 (t, J = 4 Hz, 4H), 3.41 (s, 2H), 2.40 (s, 4H) ppm. 13C NMR (CDCl3): δ 137.0, 131.4, 130.8, 120.9, 66.9, 62.6, 53.6 ppm. HRMS (ESI) m/z calculated for C11H15NOBr [(M + H)+] 256.0337, found 256.0333.
4-(3-Bromobenzyl)morpholine (8b)
1H NMR (CDCl3): δ 7.46 (s, 1H), 7.32 (d, J = 8 Hz, 1H), 7.20 (d, J = 7 Hz, 1H), 7.12 (t, J = 8 Hz, 1H), 3.64 (d, J = 4 Hz, 4H), 3.39 (s, 2H), 2.37 (s, 4H) ppm. 13C NMR (CDCl3): δ 140.4, 131.9, 130.2, 129.8, 127.6, 122.5, 66.9, 62.7, 53.6 ppm. HRMS (ESI) m/z calculated for C11H15NOBr [(M + H)+] 256.0337, found 256.0348.
4-(2-Bromobenzyl)morpholine (8c)
1H NMR (CDCl3): δ 7.52 (d, J = 8 Hz, 1H), 7.46 (d, J = 7 Hz, 1H), 7.26 (t, J = 7 Hz, 1H), 7.08 (t, J = 7 Hz, 1H), 3.71–3.68 (m, 4H), 3.57 (s, 2H), 2.49–2.48 (m, 4H) ppm. 13C NMR (CDCl3): δ 137.2, 132.8, 130.8, 128.5, 127.2, 124.7, 67.0, 62.2, 53.6 ppm. HRMS (ESI) m/z calculated for C11H15NOBr [(M + H)+] 256.0337, found 256.0348.
Typical procedure for lithium halogen exchange to form aldehydes (9a–c)
Arylbromide (1 equivalent) was dissolved in anhydrous THF under N2 and cooled in a dry ice and acetone bath for 30 min before treatment with n-buLi (1.4 equivalents). After 30 additional minutes, anhydrous DMF (1.4 equivalents) was added and stirring continued 1 h. The reaction was quenched with saturated NH4Cl, taken up in ethyl acetate, washed with brine, dried over Mg2SO4, and concentrated in vacuo. Purification by column chromatography was performed in 4:1 hexanes/ethyl acetate.
4-(Morpholinomethyl)benzaldehyde (9a)
Yield: 74%. 1H NMR (CDCl3): δ 9.96 (s, 1H), 7.81 (d, J = 8 Hz, 2H), 7.49 (d, J = 8 Hz, 2H), 3.68–3.68 (m, 4H), 3.54 (s, 2H), 2.43 (m, 4H) ppm. 13C NMR (CDCl3): δ 191.9, 145.3, 135.6, 129.8, 129.5, 66.9, 63.0, 53.6 ppm. HRMS m/z calculated for C12H16NO2 [(M + H)+] 206.1181, found 206.1182.
3-(Morpholinomethyl)benzaldehyde (9b)
Yield: 88%. 1H NMR (CDCl3): δ 9.92 (s, 1H), 7.77 (s, 1H), 7.69 (d, J = 8 Hz, 1H), 7.54 (d, J = 8 Hz, 2H), 7.41 (t, J = 8 Hz, 1H), 3.63 (m, 4H), 3.50 (s, 2H), 2.39 (m, 4H) ppm. 13C NMR (CDCl3): δ 192.2, 138.8, 136.5, 135.2, 130.2, 129.0, 128.7, 66.7, 62.6, 53.4 ppm. HRMS m/z calculated for C12H16NO2 [(M + H)+] 206.1181, found 206.1183.
2-(Morpholinomethyl)benzaldehyde (9c)
Yield: 85%. 1H NMR (CDCl3): δ 10.37 (s, 1H), 7.81 (d, J = 8 Hz, 1H), 7.44 (d, J = 8 Hz, 1H), 7.37–7.33 (m, 2H), 3.76 (s, 2H), 3.58–3.57 (m, 4H), 2.40–2.39 (m, 4H) ppm. 13C NMR (CDCl3): δ 192.0, 140.4, 135.0, 133.2, 130.6, 129.4, 127.9, 67.0, 66.9, 60.0, 53.5, 53.3 ppm. HRMS m/z calculated for C12H16NO2 [(M + H)+] 206.1181, found 206.1186.
Procedure for 2,2-dimethyl-2H-chromene-6-carbaldehyde (12)
Synthesised and purified as described in previous examples13. Yield: 37% over two steps. 1H NMR (CDCl3): δ 9.83 (s, 1H), 7.64 (d, J = 8 Hz, 1H), 7.52 (s, 1H), 6.87 (d, J = 8 Hz, 2H), 6.37 (d, J = 10 Hz, 1H), 5.70 (d, J = 10 Hz, 1H), 1.47 (s, 6H) ppm.
Typical procedure for reductive amination with aniline (10a–d, 14a)
Aldehyde (1 equivalent), NaBH4 (1.5 equivalents), and InCl3 (0.15 equivalents) were dissolved in anhydrous ACN under inert gas. Aniline (1.5 equivalents) was added and the reaction was stirred until completion as monitored by TLC (typically ∼20 min). The reaction was quenched with saturated NH4Cl, taken up in ethyl acetate, washed with brine, dried over MgSO4, and concentrated. Column chromatography (1:1 hexane/ethyl acetate) was used to yield the final pure product.
N-(4-(Morpholinomethyl)benzyl)aniline (10a)
Yield: 60%. 1H NMR (CDCl3): δ 7.23–7.17 (m, 4H), 6.79–6.66 (m, 5H), 4.34 (s, 2H), 3.74 (m, 4H), 3.52 (s, 2H), 2.74 (m, 4H) ppm. 13C NMR (CDCl3): δ 148.2, 138.4, 136.8, 129.5, 129.3, 127.5, 118.6, 117.6, 115.1, 112.9, 67.0, 63.2, 53.6, 48.1 ppm. HRMS m/z (ESI) calculated for C18H23N2O [(M + H)+] 283.1810, found 283.1805.
N-(3-(Morpholinomethyl)benzyl)aniline (10b)
Yield: 60%. 1H NMR (CDCl3): δ 7.36–7.17 (m, 6H), 6.76–6.65 (m, 3H), 4.35 (s, 2H), 3.73–3.72 (m, 4H), 3.52 (s, 2H), 2.45 (m, 4H) ppm. 13C NMR (CDCl3): 148.1, 139.5, 138.1, 129.3, 128.6, 128.3, 128.1, 126.4, 117.6, 112.9, 67.0, 63.4, 53.6, 48.3 ppm. HRMS (ESI) m/z calculated for C18H23N2O [(M + H)+] 283.1810, found 283.1809.
N-(2-(Morpholinomethyl)benzyl)aniline (10c)
Yield: 54%. 1H NMR (CDCl3): δ 7.44 (d, J = 7 Hz, 1H), 7.32–7.22 (m, 5H), 6.75 (d, J = 7 Hz, 3H), 5.37 (bs, 1H), 4.39 (s, 2H), 3.75 (m, 4H), 3.57 (s, 2H), 2.51 (m, 4H) ppm. 13C NMR (CDCl3): 148.6, 138.9, 135.8, 131.5, 130.0, 129.3, 128.2, 127.2, 117.4, 113.1, 67.1, 61.7, 53.5, 46.9 ppm. HRMS (ESI) m/z calculated for C18H23N2O [(M + H)+] 283.1810, found 283.1805.
N-(4-Morpholinobenzyl)aniline (10d)
Yield: 25%. 1H NMR (CDCl3): δ 7.33 (d, J = 8 Hz, 2H), 7.22 (t, J = 8 Hz, 2H), 6.94 (d, J = 8 Hz, 2H), 6.76 (t, J = 7 Hz, 1H), 6.68 (d, J = 8 Hz, 2H), 4.28 (s, 2H), 4.00 (bs, 1H), 3.91–3.90 (m, 4H), 3.19–3.18 (m, 4H) ppm. 13C NMR (CDCl3): δ 150.6, 148.3, 130.8, 129.3, 128.7, 117.5, 115.9, 112.9, 67.0, 49.5, 47.8 ppm. HRMS (ESI) m/z calculated for C17H21N2O [(M + H)+] 269.1654, found 269.1659.
N-((2,2-Dimethyl-2H-chromen-6-yl)methyl)aniline (14a)
Yield: 80%. 1H NMR (CDCl3): δ 7.26–7.21 (m, 3H), 6.97 (d, J = 7 Hz, 1H), 6.87 (d, J = 8 Hz, 1H), 6.79–6.72 (m, 3H), 6.39 (d, J = 10 Hz, 1H), 5.68 (d, J = 10 Hz, 1H), 4.37 (s, 2H), 4.06 (bs, 1H) 1.52 (s, 6H) ppm. 13C NMR (CDCl3): δ 150.8, 148.5, 130.6, 129.2, 128.8, 126.5, 125.5, 122.5, 121.1, 120.5, 117.4, 113.2, 76.4, 43.1, 28.1 ppm. HRMS (ESI) m/z calculated for C18H20NO [(M + H)+] 266.1545, found 266.1548.
Typical procedure for reductive amination with cyclobutyl and alkylmorpholino amines (11a–d, 13a–b, 14b)
Aldehyde (1 equivalent) and amine (1 equivalent) were dissolved in anhydrous MeOH under inert gas and the reaction was stirred overnight at room temperature. NaBH4 (1.6 equiv.) was added and the reaction stirred for an additional hour. The reaction was quenched with NaOH (1 M), stirred for an hour, then taken up in ethyl acetate, washed with brine, dried over MgSO4, concentrated, and taken directly to the next step without further purification.
N-(4-(Morpholinomethyl)benzyl)cyclobutanamine (11a)
Crude yield: 89%. 1H NMR (CDCl3): δ 7.32–7.26 (m, 4H), 3.69–3.68 (m, 4H), 3.47 (s, 2H), 3.29 (quintet, J = 7 Hz, 1H), 2.42 (m, 4H), 2.22–2.21 (m, 2H), 1.63–1.62 (m, 4H) ppm. 13C NMR (CDCl3): δ 139.3, 136.3, 129.4, 128.1, 67.0, 63.2, 53.6, 50.8, 31.2, 31.1, 15.0, 14.8 ppm. HRMS (ESI) m/z calculated for C16H25N2O [(M + H)+] 261.1967, found 261.1961.
N-(3-(Morpholinomethyl)benzyl)cyclobutanamine (11b)
Crude yield: 88% unpurified. 1H NMR (CDCl3): 7.32–7.19 (m, 4H), 3.69 (m, 6H), 3.49–3.48 (m, 2H), 3.30 (quintet, J = 7 Hz, 1H), 2.43 (m, 4H), 2.24–2.20 (m, 2H), 1.74–1.63 (m, 4H) ppm. 13C NMR (CDCl3): 140.3, 137.9, 129.0, 128.6, 127.8, 127.1, 66.9, 63.1, 53.7, 53.6, 51.0, 31.1, 14.8 ppm. HRMS (ESI) m/z calculated for C16H25N2O [(M + H)+] 261.1967, found 261.1963.
N-(2-(Morpholinomethyl)benzyl)cyclobutanamine (11c)
Crude yield: 94%. 1H NMR (CDCl3): δ 7.30–7.16 (m, 4H), 3.67 (s, 2H), 3.64 (s, 4H), 3.49 (s, 2H), 3.28–3.27 (m, 1H), 2.53 (s, 1H), 2.43 (s, 4H), 2.19–2.17 (m, 2H), 1.70–1.63 (m, 4H) ppm. 13C NMR (CDCl3): δ 140.3, 135.7, 131.3, 130.6, 127.9, 126.7, 67.0, 61.7, 53.9, 53.4, 49.7, 31.0, 15.1 ppm. HRMS (ESI) m/z calculated for C16H25N2O [(M + H)+] 261.1967, found 261.1962.
N-(4-Morpholinobenzyl)cyclobutanamine (11d)
Crude yield: 90%. 1H NMR (CDCl3): δ 7.22 (d, J = 8 Hz, 2H), 6.87 (d, J = 8 Hz, 2H), 3.86–3.84 (m, 4H), 3.62 (s, 2H), 3.28 (quintet, J = 6.8 Hz, 1H), 3.14–3.11 (m, 4H), 2.22–2.19 (m, 2H), 1.70–1.62 (m, 4H) ppm. 13C NMR (CDCl3): δ 150.3, 131.9, 129.1, 115.7, 66.9, 53.5, 50.4, 49.5, 31.1, 14.8 ppm. HRMS (ESI) m/z calculated for C15H23N2O [(M + H)+] 247.1810, found 247.1819.
N-((2,2-dimethyl-2H-chromen-6-yl)methyl)-2-morpholinoethanamine (13a)
Crude yield: 90%. 1H NMR (CDCl3): δ 7.16 (dd, J = 8, 22 Hz, 1H), 7.00 (d, J = 8 Hz, 1H), 6.69 (d, J = 8 Hz, 1H), 6.27 (d, J = 10 Hz, 1H), 5.57 (d, J = 10 Hz, 1H), 3.72–3.64 (m, 6H), 3.09 (s, 1H), 2.66–2.64 (m, 2H), 2.47–2.44 (m, 2H), 2.35 (m, 4H), 1.39 (s, 6H) ppm. 13C NMR (CDCl3): δ 152.0, 130.9, 128.9, 128.7, 126.2, 122.2, 121.5, 116.1, 76.1, 66.9, 57.9, 53.6, 53.2, 44.9, 27.9 ppm. HRMS (ESI) m/z calculated for C18H27N2O2 [(M + H)+] 303.2073, found 303.2063.
N-((2,2-Dimethyl-2H-chromen-6-yl)methyl)-3-morpholinopropan-1-amine (13b)
Crude yield: 89%. 1H NMR (CDCl3): δ 7.18–7.10 (m, 1H), 6.98 (d, J = 8 Hz, 1H), 6.67 (d, J = 8 Hz, 1H), 6.24 (d, J = 10 Hz, 1H), 5.55 (d, J = 10 Hz, 1H), 3.68–3.62 (m, 6H), 2.63 (m, 2H), 2.37–2.35 (m, 4H), 1.67–1.58 (m, 2H), 1.58 (m, 2H), 1.37 (s, 6H) ppm. 13C NMR (CDCl3): δ 151.9, 132.3, 130.9, 128.8, 126.1, 122.3, 121.5, 116.1, 73.9, 66.9, 57.3, 53.7, 47.9, 29.6, 27.9, 26.4 ppm. HRMS (ESI) m/z calculated for C19H29N2O2 [(M + H)+] 317.2229, found 317.2237.
N-((2,2-Dimethyl-2H-chromen-6-yl)methyl)aniline (14a)
Crude yield: 90%. 1H NMR (CDCl3): δ 7.26–7.21 (m, 3H), 6.96 (d, J = 7 Hz, 1H), 6.87 (d, J = 8 Hz, 1H), 6.79–6.72 (m, 3H), 6.39 (d, J = 10 Hz, 1H), 5.68 (d, J = 10 Hz, 1H), 4.73 (s, 2H), 4.06 (bs, 1H), 1.52 (s, 6H) ppm. 13C NMR (CDCl3): δ 150.8, 148.5, 130.6, 129.2, 128.8, 126.5, 125.5, 122.5, 121.1, 120.5, 117.4, 113.2, 76.4, 43.1, 28.2 ppm. HRMS (ESI) m/z calculated for C18H20NO [(M + H)+] 266.1545, found 266.1548.
N-((2,2-Dimethyl-2H-chromen-6-yl)methyl)cyclobutanamine (14b)
Crude yield: 98%. 1H NMR (CDCl3): δ 7.03 (d, J = 8 Hz, 1H), 6.95 (s, 1H), 6.72 (d, J = 8 Hz, 1H), 6.31 (d, J = 10 Hz, 1H), 5.60 (d, J = 10 Hz, 1H), 3.56 (s, 2H), 3.32–3.26 (m, 1H), 2.22 (m, 2H), 1.72–1.69 (m, 4H), 1.42 (s, 6H) ppm. 13C NMR (CDCl3): δ 151.9, 132.5, 130.8, 128.9, 126.2, 122.3, 121.2, 116.1, 76.1, 53.5, 50.5, 31.1, 27.9, 14.8 ppm. HRMS (ESI) m/z calculated for C16H22NO [(M + H)+] 244.1701, found 244.1697.
Typical procedure for sulfonylation with 3,4-dimethoxybenzenesulfonyl chloride (3a–d, 4a–d, 5a–b)
Amine (1 equivalent) was dissolved in DCM. K2CO3 (2 equivalents) and 3,4-dimethoxybenzenesulfonyl chloride (2 equivalents) were added. The reaction was stirred overnight at room temperature, then washed with brine, dried over MgSO4, and concentrated. The product was purified by column chromatography in 4:1 or 1:1 hexane/ethyl acetate.
3,4-Dimethoxy-N-(4-(morpholinomethyl)benzyl)-N-phenylbenzenesulfonamide (3a)
Yield: 11%. 1H NMR (CDCl3): δ 7.36 (d, J = 7 Hz, 1H), 7.35 (s, 1H), 7.22–7.20 (m, 6H), 7.04–7.02 (m, 2H), 6.97–6.94 (m, 2H), 4.72 (s, 1H), 3.98 (s, 3H), 3.77 (s, 3H), 3.70–3.69 (m, 4H), 3.44 (s, 2H), 2.40 (m, 4H) ppm. 13C NMR (CDCl3): δ 152.6, 148.7, 139.2, 135.0, 130.2, 129.2, 129.0, 128.8, 128.4, 127.8, 127.5, 121.4, 110.4, 110.4, 66.9, 63.0, 56.2, 56.01, 54.4, 53.5 ppm. HRMS (ESI) m/z calculated for C26H31N2O5S [(M + H)+] 483.1954, found 483.1956.
3,4-Dimethoxy-N-(3-(morpholinomethyl)benzyl)-N-phenylbenzenesulfonamide (3b)
Yield: 64%. 1H NMR (CDCl3): δ 7.36–7.31 (m, 2H), 7.17–7.09 (m, 7H), 6.98–6.91 (m, 4H), 4.69 (s, 2H), 3.95 (s, 3H), 3.73 (s, 3H), 3.63 (t, J = 4 Hz, 4H), 3.39–3.37 (m, 2H), 2.27 (m, 4H) ppm. 13C NMR (CDCl3): δ 152.7, 148.8, 139.1, 137.8, 135.9, 130.1, 129.6, 129.1, 128.8, 128.6, 128.4, 127.8, 127.6, 121.5, 110.5, 67.0, 63.2, 56.3, 56.2, 54.5, 53.5, 48.5 ppm. HRMS (ESI) m/z calculated for C26H31N2O5S [(M + H)+] 483.1948, found 483.1928.
3,4-Dimethoxy-N-(2-(morpholinomethyl)benzyl)-N-phenylbenzenesulfonamide (3c)
Yield: 47%. 1H NMR (CDCl3): δ 7.36 (d, J = 8 Hz, 1H), 7.21–7.18 (m, 4H), 7.10–7.09 (m, 3H), 7.03–7.01 (m, 2H), 6.95–6.93 (m, 2H), 4.97 (s, 2H), 3.97 (s, 3H), 3.75 (s, 3H), 3.62 (m, 4H), 3.48 (s, 2H), 2.35 (m, 4H) ppm. 13C NMR (CDCl3): δ 152.7, 148.8, 139.5, 136.1, 135.2, 130.8, 130.2, 129.7, 128.9, 128.8, 127.8, 127.4, 127.3, 121.7, 110.7, 110.5, 67.2, 61.1, 56.3, 56.2, 53.6, 51.3, 31.0, 30.8, 13.6 ppm. HRMS (ESI) m/z calculated for C26H31N2O5S [(M + H)+] 483.1948, found 483.1941.
3,4-Dimethoxy-N-(4-morpholinobenzyl)-N-phenylbenzenesulfonamide (3d)
Yield: 40%. 1H NMR (CDCl3): δ 7.35 (d, J = 8 Hz, 1H), 7.21 (m, 3H), 7.12 (d, J = 7 Hz, 2H), 7.01–6.93 (m, 4H), 6.76 (d, J = 7 Hz, 2H), 4.65 (s, 2H), 3.97 (s, 3H), 3.84–3.83 (m, 4H), 3.77 (s, 3H), 3.11–3.10 (m, 4H) ppm. 13C NMR (CDCl3): δ 152.5, 150.5, 148.7, 139.2, 130.3, 129.6, 129.1, 128.7, 127.7, 127.1, 121.4, 115.3, 110.4, 66.8, 56.2, 56.1, 54.1, 49.1 ppm. HRMS (ESI) m/z calculated for C25H29N2O5S [(M + H)+] 469.1797, found 469.1796.
N-Cyclobutyl-3,4-dimethoxy-N-(4-(morpholinomethyl)benzyl)benzenesulfonamide (4a)
Yield: 46%. 1H NMR (CDCl3): δ 7.43 (dd, J = 8, 2 Hz, 1H), 7.34–7.25 (m, 5H), 6.94 (d, J = 9 Hz, 1H), 4.39 (s, 2H), 4.27 (quintet, J = 9 Hz, 1H), 3.96 (s, 3H), 3.92 (s, 3H), 3.75–3.72 (m, 4H), 3.51 (s, 2H), 2.46 (s, 4H), 1.99–1.94 (m, 4H), 1.57–1.52 (m, 2H) ppm. 13C NMR (CDCl3): δ 152.4, 149.0, 137.8, 132.0, 129.4, 127.0, 120.9, 110.6, 109.8, 66.9, 63.0, 56.2, 56.2, 53.5, 52.9, 48.2, 29.2, 15.0 ppm. HRMS (ESI) m/z calculated for C24H33N2O5S [(M + H)+] 461.2110, found 461.2102.
N-Cyclobutyl-3,4-dimethoxy-N-(3-(morpholinomethyl)benzyl)benzenesulfonamide (4 b)
Yield: 81%. 1H NMR (CDCl3): δ 7.43 (dd, J = 8, 2 Hz, 1H), 7.30–7.21 (m, 5H), 6.95–6.93 (d, J = 8 Hz, 1H), 4.39 (s, 2H), 4.28 (quintet, J = 8 Hz, 1H), 3.95 (s, 3H), 3.91 (s, 3H), 3.71 (t, J = 4 Hz, 4H), 3.49 (s, 2H), 2.43 (s, 4H), 1.99–1.92 (m, 4H), 1.55–1.48 (m, 2H) ppm. 13C NMR (CDCl3): δ 152.4, 149.0, 138.7, 137.9, 131.9, 128.4, 128.1, 127.8, 126.0, 120.9, 110.5, 109.7, 67.0, 63.3, 56.2, 56.2, 53.6, 52.9, 48.3, 29.2, 15.0 ppm. HRMS (ESI) m/z calculated for C24H33N2O5S [(M + H)+] 461.2110, found 461.2112.
N-Cyclobutyl-3,4-dimethoxy-N-(2-(morpholinomethyl)benzyl)benzenesulfonamide (4c)
Yield: 63%. 1H NMR (CDCl3): δ 7.56 (d, J = 8 Hz, 1H), 7.47 (dd, J = 8, 2 Hz, 1H), 7.31–7.27 (m, 2H), 7.17 (d, J = 7 Hz, 1H), 6.95 (d, J = 8 Hz, 1H), 4.68 (s, 2H), 4.45 (quintet, J = 8 Hz, 1H), 3.96 (s, 3H), 3.92 (s, 3H), 3.65 (m, 4H), 3.50 (bs, 2H), 2.42 (bs, 4H), 1.93–1.90 (m, 4H), 1.56–1.50 (m, 2H) ppm. 13C NMR (CDCl3): δ 152.4, 149.0, 138.4, 133.4, 132.1, 130.6, 128.0, 127.4, 126.4, 121.0, 110.5, 109.7, 67.1, 61.6, 56.3, 56.2, 53.5, 52.7, 44.5, 29.0, 15.1 ppm. HRMS (ESI) m/z calculated for C24H33N2O5S [(M + H)+] 461.2110, found 461.2095.
N-Cyclobutyl-3,4-dimethoxy-N-(4-morpholinobenzyl)benzenesulfonamide (4d)
Yield: 70%. 1H NMR (CDCl3): δ 7.41 (dd, J = 8, 2 Hz, 1H), 7.25–7.21 (m, 3H), 6.92 (d, J = 9 Hz, 1H), 6.86 (d, J = 9, 2H), 4.32 (s, 2H), 4.20 (quintet, J = 8 Hz, 1H), 3.93 (s, 3H), 3.88 (s, 3H), 3.86–3.46 (m, 4H), 3.15–3.12 (m, 4H), 2.02–1.90 (m, 4H), 1.54–1.45 (m, 2H) ppm. 13C NMR (CDCl3): δ 152.4, 150.4, 149.0 132.2, 129.8, 128.2, 120.9, 115.6, 110.6, 109.7, 66.9, 56.2, 56.1, 52.9, 49.4, 48.0, 29.7, 29.3, 15.1 ppm. HRMS (ESI) m/z calculated for C23H31O5N2S [(M + H)+]: 446.1948, found 447.1949.
N-((2,2-Dimethyl-2H-chromen-6-yl)methyl)-3,4-dimethoxy-N-(2-morpholinoethyl)benzenesulfonamide (5a)
Yield: 49%. 1H NMR (CDCl3): δ 7.45 (d, J = 8 Hz, 1H), 6.93 (t, J = 9 Hz, 2H), 6.88–6.85 (m, 1H), 6.67 (d, J = 8 Hz, 1H), 6.22 (d, J = 10 Hz, 1H), 5.59 (d, J = 7 Hz, 1H), 4.23 (s, 2H), 3.93 (s, 3H), 3.89 (s, 3H), 3.59–3.56 (m, 4H), 3.19 (t, J = 7 Hz, 2H), 2.30 (t, J = 7 Hz, 2H), 2.25 (s, 4H), 1.23 (s, 6H) ppm. 13C NMR (CDCl3): δ 152.7, 152.5, 149.1, 131.8, 131.3, 129.1, 128.3, 126.5, 122.0, 121.4, 121.0, 116.3, 110.6, 109.8, 66.8, 57.3, 57.3, 56.3, 56.2, 53.6, 52.2, 44.4, 27.9 ppm. HRMS (ESI) m/z calculated for C26H35N2O6S [(M + H)+] 503.2210, found 503.2204.
N-((2,2-Dimethyl-2H-chromen-6-yl)methyl)-3,4-dimethoxy-N-(3-morpholinopropyl)benzenesulfonamide (5b)
Yield: 15%. 1H NMR (CDCl3): δ 7.43 (d, J = 8 Hz, 1H) 6.97 (d, J = 8 Hz, 1H), 6.94 (d, J = 8 Hz, 1H), 6.87 (s, 1H), 6.68 (d, J = 8 Hz, 1H), 6.23 (d, J = 10 Hz, 1H), 4.19 (s, 2H), 3.94 (s, 3H), 3.90 (s, 3H), 3.60 (s, 4H), 3.12 (t, J = 8, 2H), 2.22 (s, 4H), 2.18 (t, J = 7, 2H), 1.59–1.52 (m, 2H), 1.40 (s, 6H) ppm. 13C NMR (CDCl3): δ 152.7, 152.4, 149.1, 131.6, 131.3, 129.3, 128.4, 126.5, 121.9, 121.3, 121.0, 116.3, 110.6, 109.8, 66.9, 56.3, 56.2, 55.9, 53.4, 52.0, 46.2, 28.0, 25.4 ppm. HRMS (ESI) m/z calculated for C27H37N2O6S [(M + H)+] 517. 2367, found 517.2366.
Typical procedure for sulfonylation with 4-morpholinosulfonyl chloride (6a–b)
Amine (1 equivalent) was dissolved in dichloroethane. Pyridine (3 equivalents) and 4-morpholinosulfonyl chloride (1.3 equivalents) were added. The reaction was refluxed for 2 days, then concentrated, taken up in ethyl acetate, washed with saturated NH4Cl and brine, then dried over MgSO4, and concentrated. The residue was then purified by column chromatography in 4:1 hexane/ethyl acetate.
N-((2,2-Dimethyl-2H-chromen-6-yl)methyl)-N-phenylmorpholine-4-sulfonamide (6a)
Yield: 17%. 1H NMR (CDCl3): δ 7.32–7.25 (m, 5H), 6.90 (d, J = 8 Hz, 1H), 6.83 (s, 1H), 6.65 (d, J =8 Hz, 1H), 6.25 (d, J = 10 Hz, 1H), 5.60 (d, J = 10 Hz, 1H), 4.70 (s, 2H), 3.63–3.62 (m, 4H), 3.17 (m, 4H), 1.42 (s, 6H) ppm. 13C NMR (CDCl3): δ 131.0, 129.6, 129.2, 129.1, 127.9, 126.9, 122.1, 116.2, 66.3, 56.3, 46.5, 28.0 ppm. HRMS (ESI) m/z calculated for C22H27N2O4S [(M + H)+] 415.1692, found 415.1695.
N-Cyclobutyl-N-((2,2-dimethyl-2H-chromen-6-yl)methyl)morpholine-4-sulfonamide (6b)
Yield: 16%. 1H NMR (CDCl3): δ 7.04 (dd, J = 8, 2 Hz, 1H), 6.95 (s, 1H), 6.72 (d, J = 8 Hz, 1H), 6.30 (d, J = 10 Hz, 1H), 5.61 (d, J = 10 Hz, 1H), 4.35 (s, 2H), 4.19 (quintet, J = 8 Hz, 1H), 3.63 (t, J = 5 Hz, 4H), 3.09 (t, J = 5 Hz, 4H), 2.13 – 2.06 (m, 4H), 1.60 (m, 2H), 1.41 (s, 6H) ppm. 13C NMR (CDCl3): δ 152.4, 131.2, 130.6, 128.2, 125.5, 122.4, 121.4, 116.4, 76.4, 66.4, 53.0, 48.8, 46.2, 29.6, 28.1, 14.8 ppm. HRMS (ESI) m/z calculated for C20H29N2O4S [(M + H)+] 393.1843, found 393.1834.
Lipophilicity and solubility prediction
The in silico log D and log S values of all analogs were predicted using Calculator Plugins from MarvinSketch 4.3.0, 2017, ChemAxon (http://www.chemaxon.com), with results detailed in Table 1. Graphical representations of the log D and log S from pH 0 to 14 are provided in the Supplemental Information.
Table 1.
Structures, HRE-luciferase reporter inhibitory activity, cLog D, and cLog S of analogs.
| Compound | Structure | IC50 (μM) | cLog D | cLog S |
|---|---|---|---|---|
| 1 | 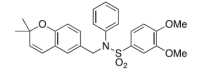 |
∼0.6 | 4.99 | −6.37 |
| 2 | 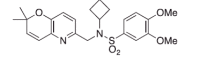 |
∼0.3 | 3.34 | −4.53 |
| 3a | 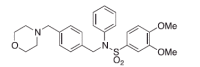 |
0.9 | 3.69 | −4.39 |
| 3b | 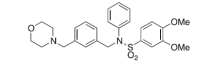 |
>5 | 3.71 | −4.41 |
| 3c | 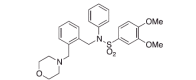 |
>5 | 3.80 | −4.50 |
| 3d | 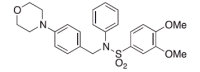 |
3.8 | 3.98 | −5.19 |
| 4a | 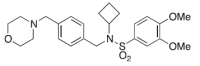 |
1.0 | 2.94 | −3.36 |
| 4b | 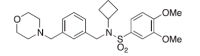 |
>5 | 2.96 | −3.38 |
| 4c | 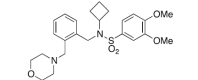 |
>5 | 3.05 | −3.47 |
| 4d | 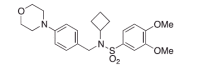 |
2.6 | 3.24 | −4.15 |
| 5a | 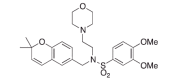 |
>5 | 3.13 | −4.23 |
| 5b | 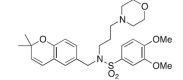 |
>5 | 3.17 | −4.37 |
| 6a | 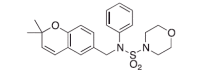 |
>5 | 2.97 | −5.17 |
| 6b | 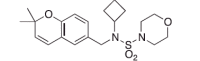 |
>5 | 2.22 | −4.12 |
Luciferase assay
These analogs were first evaluated for their ability to inhibit hypoxia-induced HIF transcriptional activity in LN229-HRE-luciferase glioma cells as described previously10–12. Their inhibitory activities are presented as IC50 in Table 1.
Solubility studies using dynamic light scattering
To further investigate the true enhancement of solubility, particle aggregation was examined using dynamic light scattering (DLS). Selected compounds were treated according to the following procedure:
All centrifuge tubes and cuvettes were rinsed with either DCM or water and then vacuum dried before use to remove dust and any particulates.
Stock solutions (10 mM) of each compound of interest were prepared in filtered DMSO.
Six dilutions (0, 10, 20, 30, 50, and 100 µM) were prepared in filtered de-ionised water with 1% DMSO and allowed to rest at room temperature for 24 h after vortex.
DLS analysis was performed for each concentration on the Brookhaven Instrument Corporation, NanoBrook 90Plus Particle Size Analyzer, Version 5.20 (Holtsville, NY).
Additional experiments were performed at specific concentrations for each compound as follows: 0, 1, 3, 10, and 20 µM concentrations of 1; 0, 10, 12, and 20 µM of 2; 0, 5, 7, and 10 µM of 3a; and 0, 10, 20, 30, and 50 µM concentrations of 4a.
Additional experiments were repeated in filtered PBS* with 1% DMSO as follows: 0, 0.5, 1, 2, 3, and 5 µM concentrations of 1; 0, 5, 7, 10, 12, and 15 µM of 2; 0, 10, 12, 15, and 20 µM of 3a; and 0, 10, 20, 30, and 50 µM concentrations of 4a.
*Experiments in PBS were carried out the same way as the experiments in water except DMSO stock solutions were made at 5 mM and the PBS diluted samples rested for 1 h before particle analysis.
Results and discussion
Design
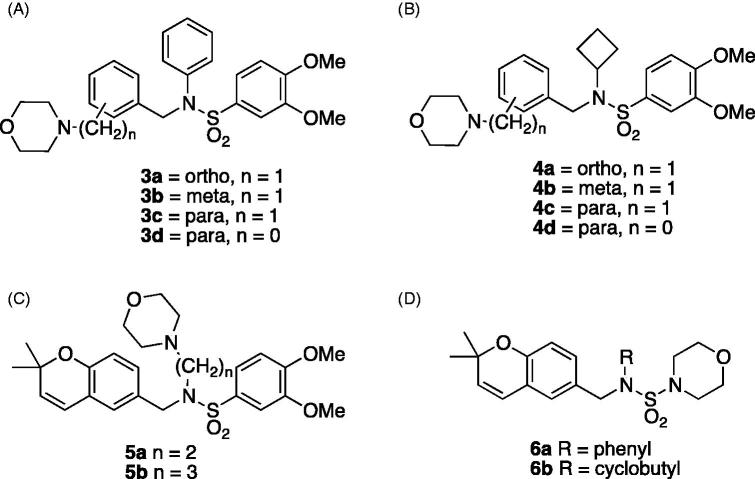
In considering ways to improve water solubility without compromising potency, we thought about introducing a commonly used morpholino moiety, which is known to help improve water solubility. In doing so, we were interested in searching for the optimal position, which would not negatively affect potency. Therefore, we devised four classes of compounds (Figure 2): Class A incorporates a morpholinomethylphenyl or morpholinophenyl moiety instead of the 2,2-dimethyl-2H-chromene moiety and maintains the N-phenyl group; Class B incorporates either a morpholinomethylphenyl or morpholinophenyl moiety instead of the 2,2-dimethyl-2H-chromene moiety and substitutes the N-phenyl group for an N-cyclobutyl group; Class C has either a 2,2-dimethyl-2H-chromene or N-(2,2-dimethyl-2H-pyrano[3,2-b]pyridin-6-yl) moiety and either an N-ethylmorpholino or N-propylmorpholino group instead of the N-phenyl; and Class D has the 2,2-dimethyl-2H-chromene moiety with a N-phenyl-morpholine-4-sulfonamide.
Figure 2.
Classes of analogs. (A) Class A, morpholinomethylphenyl in ortho, meta, or para positions, or morpholinophenyl in para position; (B) Class B, morpholinomethylphenyl in ortho, meta, or para positions, or morpholinophenyl in para position; (C) Class C, n = 2 or 3; (D) Class D.
Chemistry
Synthesis of Class A compounds (Scheme 1) was accomplished in four steps from 2-, 3-, or 4-bromomethylbenzylbromide 7a–c or in two steps from 4-morpholinobenzaldehyde 9d. Intermediates 7a–c were substituted with morpholine to yield morpholinomethylbenzylbromides 8a–c in quantitative yield. Next, the phenyl bromides 8a–c were converted to benzaldehydes 9a–cvia lithium-halogen exchange at −78 °C under inert gas. The aryllithium intermediate was treated with DMF as the electrophile in situ to generate the final benzaldehydes 9a–c. The aldehydes 9a–d underwent reductive amination with aniline to afford the secondary amines 10a–d. Finally, 10a–d were reacted with 3,4-dimethoxybenzenesulfonyl chloride to afford sulfonamides 2a–d. Class B compounds (Scheme 1(C)) were synthesised in almost the same fashion as Class A, except that reductive amination of 9a–d was with cyclobutylamine and was not catalysed by any Lewis acid.
Scheme 1.
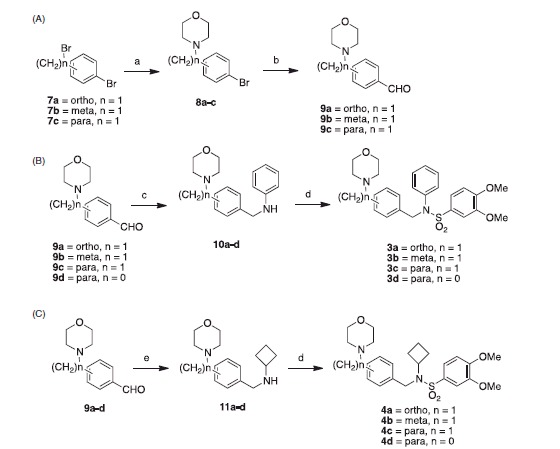
Synthesis of Class A & B compounds. (A) Synthesis of precursors. (B) Synthesis of Class A. (C) Synthesis of Class B. Reagents and conditions: (a) morpholine, K2CO3, ACN, room temperature, overnight; (b) BuLi, DMF, THF, −78 °C, 1 h; (c) aniline, InCl3, NaBH4, ACN, 20 min; (d) 3,4-dimethoxybenzenesulfonyl chloride, K2CO3, DCM, overnight; (e) cyclobutylamine, NaBH4, MeOH, overnight.
Class C compounds were synthesised (Scheme 2) from 2,2-dimethyl-2H-chromene-6-carbaldehyde 12, which was readily synthesised from published procedures13. The aldehyde 12 underwent reductive amination with either ethylaminomorpholine or propylaminomorpholine to give secondary amines 13a–b, which were then reacted with 3,4-dimethoxybenzenesulfonyl chloride to afford sulfonamides 5a–b.
Scheme 2.
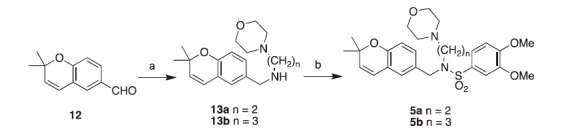
Synthesis of Class C compounds. Reagents and conditions: (a) amine, NaBH4, MeOH, overnight; (b) 3,4-dimethoxybenzenesulfonyl chloride, K2CO3, DCM, overnight.
Class D compounds were synthesised (Scheme 3) from 12 in two steps. First, 12 underwent reductive amination with either aniline or cyclobutylamine to give secondary amines 14a–b. Next, the amines 14a–b were reacted with 4-morpholinosulfonyl chloride to afford sulfonamides 6a–b.
Scheme 3.
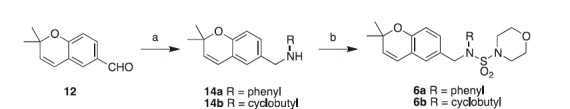
Synthesis of Class D compounds. Reagents and conditions: (a) aniline, InCl3, NaBH4, ACN, 20 min or cyclobutylamine, NaBH4, MeOH, overnight; (b) 4-morpholinosulfonyl chloride, pyridine, DCE, reflux 2 days.
Biology
All the analogs were assessed for their ability to inhibit the HIF-1 pathway using a luciferase reporter assay described previously10. This assay reports the ability for a compound to inhibit HIF transcriptional activity. However, it does not specifically reveal the mode of action at the biochemical level. As can be seen from Table 1, introduction of a morpholino unit on the sulfonamide nitrogen led to compounds (5) with substantially diminished activity. The same is true if the morpholino unit is directly attached to the sulfonyl group (6). In the two series of compounds (3, 4) with a substituted phenyl group replacing the benzopyran ring in 1, only introduction of the morpholino moiety at the para positions (3) allowed for the preservation of HIF inhibition activity. Indeed, compounds 3a and 3d, which have exchanged the benzopyran ring for a para-morpholinomethylphenyl and para-morpholinophenyl, respectively, exhibit IC50 values of 0.9 and 3.8 µM. Similarly, analogs 4a and 4d, which replace the N-phenyl with a N-cyclobutyl, but are otherwise structurally the same as 3a and 3d, have IC50 values of 1.0 and ∼2.6 µM, respectively. No other analogs synthesised in this work exhibited HIF inhibitory activity with IC50 lower than 5 µM, suggesting the importance of conserving electronic and/or steric effects para to the phenyl ring. In particular, compounds 3a and 4a are active within the same order of magnitude as 1, and are about threefold less active than the previously discovered 2 (IC50 = ∼0.3 µM)12. The improved potency of 3a and 4a over 3d and 4d suggests a possible role for flexibility of the ligand in the binding site.
To gain some initial understanding of lipophilicity and solubility, the predicted log D and log S values were calculated for 1, 2, and their analogs. Log P refers to a molecule’s partition coefficient, or the log of the ratio between its solubility in octanol versus water14. This is commonly used to indicate a candidate drug’s lipophilicity, and a log P or cLog P (calculated log P) less than 5 is generally considered “drug-like”15. For ionizable small molecules, log D is the distribution constant, which describes the partition coefficient at different pH levels16. A molecule’s water solubility is typically measured at room temperature (20–25 °C) in mol/L and represented as log S, or clog S when calculated computationally. Drugs on the market with a variety of structures typically possess a log S between −5 and −217.
Though several of the morpholine analogs have very drug-like properties, most are not active in the luciferase assay. Only 3a, 3d, 4a, and 4d are active toward the HIF pathway and only 3a and 4a show comparable IC50 values as 1. Therefore, we examined their solubility in water and phosphate buffered saline (PBS).
Solubility studies
To investigate the true enhancement of aqueous solubility, particle aggregation was examined using DLS. DLS can detect particle sizes in solution by measuring changes in scattered light in relation to the Brownian motion of particles18. It is commonly used to detect the particle sizes of various chemical and biological molecules, including small molecule inhibitors19. Though there are several methods for detecting solubility, we chose the DLS method due to its ease, reproducibility, minimal sample requirement, and relative sensitivity to small particles.
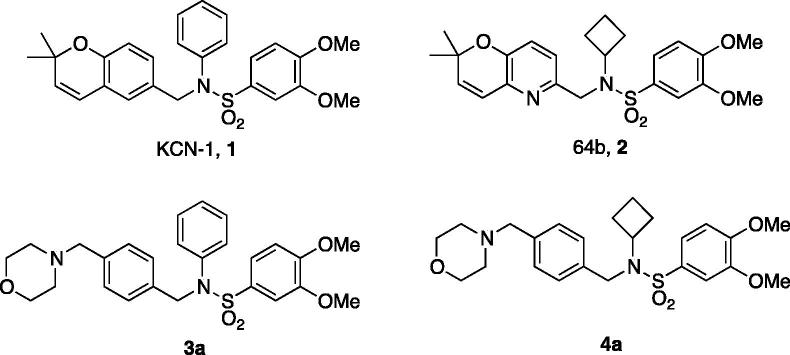
The active compounds 3a and 4a were compared to their non-morpholine containing counterparts, 1 and 2, respectively (Figure 3). Solutions of varying concentrations of each compound were made in either water or PBS with 1% DMSO. Each solution was measured in the particle size analyser to identify which samples showed formation of aggregates in solution. DLS measurements, summarised in Table 2, reveal that 3a forms aggregates at approximately 10 µM, an order of magnitude higher than 1, which is insoluble at just 1 µM in water. The N-cyclobutyl analog 4a forms aggregates in excess of 100 µM, significantly higher than its counterpart 2, which forms particles at a mere 10 µM. In PBS, the solubilities parallel those seen in the water solution, where 1 and 3a exhibit comparable particle formation at 1 µM and 15 µM, respectively. 2 shows particle formation at 10 µM, while 4a shows none at this concentration, as expected. Indeed, with a log D of 2.94 log S of −3.36 (Table 1), 4a is predicted to be quite soluble in aqueous solutions.
Figure 3.
Structures of compounds used for dynamic light scattering.
Table 2.
Measured solubility of selected compounds.
| Name | Concentration of particle appearance in water (μM) | Concentration of particle appearance in PBS (μM) |
|---|---|---|
| 1 | 1 | 1 |
| 2 | 10 | 10 |
| 3a | 10 | 15 |
| 4a | >100 | >50 |
The described results clearly indicate that (1) the benzopyran ring can be modified with minimal loss of activity and (2) the para position of the phenyl ring can tolerate substantial changes and can be used for improvement of water solubility. Such results will help future optimisation work.
Conclusion
Of the 12 new morpholine-containing analogs developed in this work, four demonstrate HIF inhibition in the low or sub-micromolar range. In particular, 3a and 4a both exhibit inhibition of HIF transcriptional activity with IC50 values of 0.9 and 1.0 µM, respectively. As expected, the in silico log P and log S values of these analogs are considered more favourable than lead compound 1 or its more potent analog 2, and are therefore likely to be more bioavailable. Following these indications, solubility as measured by particle detection with DLS reveal the exceptional solubility of analogs 3a and 4a over their non-morpholine containing predecessors 1 and 2. Particle formation of 4a is undetected in excess of 100 µM in water and 50 µM in PBS, while still displaying HIF inhibition in the same order of magnitude as lead 1. These results encourage exploration and use of more soluble moieties to further probe the SAR (structure–activity relationship) and SSR (structure–solubility relationship) of potential analogs.
Supplementary Material
Funding Statement
Financial support from the National Institutes of Health (1R01CA180805-01, 5R01CA176001-04), the CURE Childhood Cancer, Alan B. Slifka, Samuel Waxman Cancer Research, and V Foundations (to EGVM) and the Antinori Winship Invest pilot grant (to SK) are gratefully acknowledged. The laboratories of Didier Merlin and Hamed Laroui are especially acknowledged for the use of and guidance with their dynamic light scattering instruments. JHF and SKZ also acknowledge internal fellowships: Department of Education GAANN grant (P200A120122) with Barbara Baumstark as PI in support of JHF and GSU MBD and CDT programs to SKZ.
Acknowledgements
Financial support from the National Institutes of Health (1R01CA180805-01, 5R01CA176001-04), the CURE Childhood Cancer, Alan B. Slifka, Samuel Waxman Cancer Research, and V Foundations (to EGVM) and the Antinori Winship Invest pilot grant (to SK) are gratefully acknowledged. The laboratories of Didier Merlin and Hamed Laroui are especially acknowledged for the use of and guidance with their dynamic light scattering instruments. JHF and SKZ also acknowledge internal fellowships: Department of Education GAANN grant (P200A120122) with Barbara Baumstark as PI in support of JHF and GSU MBD and CDT programs to SKZ.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Siegel R, Jemal A.. Cancer Facts & Figures 2012. American Cancer Society: Atlanta; 2012. [Google Scholar]
- 2.Ruan K, Song G, Ouyang G.. Role of hypoxia in the hallmarks of human cancer. J Cell Biochem 2009;107:1053–62. [DOI] [PubMed] [Google Scholar]
- 3.(a) Vaupel P, Mayer A.. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 2007;26:225–39. (b) Melillo G.Inhibiting hypoxia-inducible factor 1 for cancer therapy. Mol Cancer Res 2006;4: 601,–5. (c) Wilson WR, Hay MP.. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11: 393–410. [Google Scholar]
- 4.Wang GL, Semenza GL.. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 1995;270:1230–7. [DOI] [PubMed] [Google Scholar]
- 5.Wang GL, Jiang BH, Rue EA, Semenza GL.. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995;92:5510–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maxwell PH, Wiesener MS, Chang G-W, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999;399:271–5. [DOI] [PubMed] [Google Scholar]
- 7.Wenger RH, Stiehl DP, Camenisch G.. Integration of oxygen signaling at the consensus HRE. Sci STKE 2005;2005:re12. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL.Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721–32. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaou KC, Pfefferkorn JA, Mitchell HJ, et al. Natural product-like combinatorial libraries based on privileged structures. 2. Construction of a 10‚000-membered benzopyran library by directed split-and-pool chemistry using NanoKans and optical encoding. J Am Chem Soc 2000;122:9954–67. [Google Scholar]
- 10.(a) Tan C, de Noronha RG, Devi NS, et al. Sulfonamides as a new scaffold for hypoxia inducible factor pathway inhibitors. Bioorg Med Chem Lett 2011;21:5528–32. (b) Narita T, Yin S, Gelin CF, et al. Identification of a novel small molecule HIF-1α translation inhibitor. Clin Cancer Res 2009;15: 6128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Wang W, Ao L, Rayburn ER, et al. KCN1, a novel synthetic sulfonamide anticancer agent: in vitro and in vivo anti-pancreatic cancer activities and preclinical pharmacology. PLoS One 2012;7:e44883 (b) Zhang Q, Kaluz S, Yang H, et al. Arylsulfonamide KCN1 inhibits in vivo glioma growth and interferes with HIF signaling by disrupting HIF-1α interaction with cofactors p300/CBP. Clin Cancer Res 2012;18: 6623,–33.(c) Yin S, Kaluz S, Devi NS, et al. Arylsulfonamide KCN1 inhibits in vivo glioma growth and interferes with HIF signaling by disrupting HIF-1a interaction with co-factors p300/CBP. Clin Cancer Res 2012;18: 6623–33. [Google Scholar]
- 12.Mooring SR, Jin H, Devi NS, et al. Design and synthesis of novel small-molecule inhibitors of the hypoxia inducible factor pathway. J Med Chem 2011;54:8471–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Ferguson J, De Los Santos Z, Devi N, et al. Examining the structure–activity relationship of benzopyran-based inhibitors of the hypoxia inducible factor-1 pathway. Bioorg Med Chem Lett 2017;27:1731–6. (b) Prado S, Janin YL, Saint-Joanis B, et al. Synthesis and antimycobacterial evaluation of benzofurobenzopyran analogues. Bioorg Med Chem 2007;15: 2177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Sangster J.Octanal–water partition coefficients of small organic compounds. J Phys Chem Ref 1989;18:1111–227. (b) Acree WE, Grubbs LM, Abraham MH. Prediction of partition coefficients and permeability of drug metabolites in biological systems with Abraham model solute descriptors derived from measured solubilities and water-to-organic solvent partition coefficients. In: Acree B, ed. Toxicity and drug testing. InTech. Available from: http://www.intechopen.com/books/toxicity-and-drug-testing/prediction-of-partition-coefficients-and-permeability-of-drug-molecules-in-biological-systems-with-a [Google Scholar]
- 15.Lipinksi CA, Lombardo F, Dominy BW, Feeney PJ.. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 1997;23:3–25. [DOI] [PubMed] [Google Scholar]
- 16.Bhal SK, Kassam K, Peirson IG, Pearl GM.. The rule of five revisited: applying log D in place of log P in drug-likeness filters. Mol Pharm 2007;4:556–60. [DOI] [PubMed] [Google Scholar]
- 17.Huuskonen J, Salo M, Taskinen J.. Neural network modeling for estimation of the aqueous solubility of structurally related drugs. J Pharm Sci 1997;86:450. [DOI] [PubMed] [Google Scholar]
- 18.Pecora R.Dynamic light scattering measurement of nanoparticles in liquids. J Nano Res 2000;2:123–31. [Google Scholar]
- 19.Berne BJ, Pecora R.. Dynamic light scattering: with applications to chemistry, biology, and physics. North Chelmsford: Courier Corporation; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.