Abstract
Identifying possible new biological activities of psychoactive substances belonging to various chemical classes may lead to a better understanding of their mode of action and side effects. We report here that amines structurally related to amphetamine, a widely used psychoactive substance, such as amphetamine, methamphetamine, phentermine, mephentermine, and chlorphenteramine, potently activate several carbonic anhydrase (CA, EC 4.2.1.1) isoforms involved in important physiological functions. Of the 11 investigated human (h) isoforms, the widespread hCA I and II, the secreted hCA VI, as well as the cytosolic hCA XIII, and membrane-bound hCA IX and XIV were poorly activated by these amines, whereas the extracellular hCA IV, the mitochondrial enzymes hCA VA/VB, the cytosolic hCA VII, and the transmembrane isoform hCA XII were potently activated. Some of these enzymes are abundant in the brain, raising the possibility that some of the cognitive effects of such psychoactive substances might be related to their activation of these enzymes.
Keywords: Carbonic anhydrase, activator, amphetamine, methamphetamine, phentermine
1. Introduction
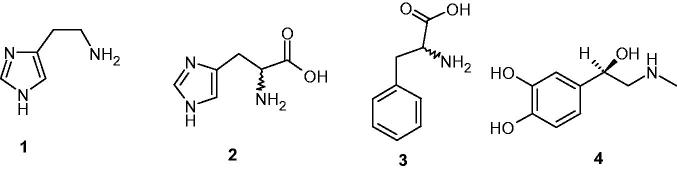
Drug abuse, especially by young people, constitutes a serious social problem worldwide, with a significant increase in the use/abuse of both “classical” psychoactive substances, such as cocaine, amphetamines, and cannabinoids, as well as new synthetic molecules belonging to a vast array of chemical families, some of which are poorly characterised from the pharmacological and toxicological viewpoints1–3. Our interest in this type of compounds is connected to the fact that many of the “classical” drug abuse compounds are primary, secondary, or tertiary amines incorporating the phenethylamine scaffold (such as amphetamine and methamphetamine)1,2. We have showed in earlier works4–6 that this type of amines, possessing the general formula Ar–CH2CH(R)NHR′, where Ar is an aromatic or heterocyclic ring; R is H, methyl, COOH, or another small compact group (see Figure 1 for some relevant examples), and R′ is H or methyl (with phenethylamine the simplest representative), effectively activate the zinc enzyme carbonic anhydrase (CA, EC 4.2.1.1)7–10 involved in a host of physiologic and pathologic processes in organisms all over the phylogenetic tree11,12.
Figure 1.
Examples of CAAs: histamine 1, histidine 2, phenylalanine 3, (both L- and D-enantiomers possess this activity), and L-adrenaline 4.
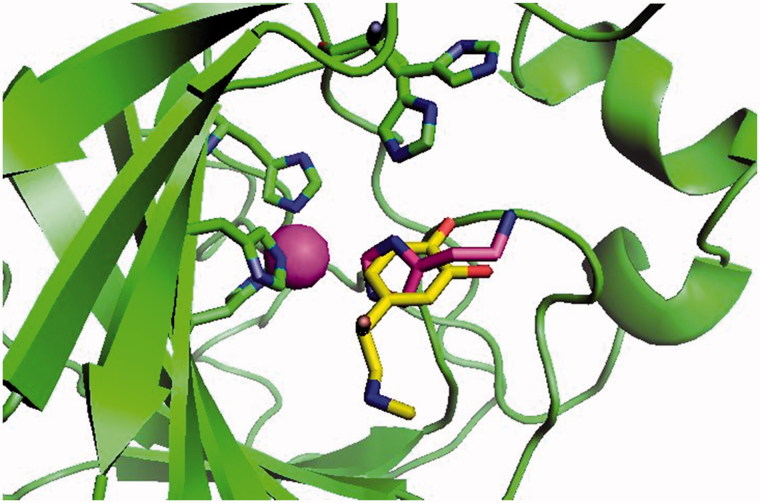
CA activators (CAAs) were a controversial issue for a long time: reported in 1941 by three different groups13–16, this phenomenon was considered an artefact due to improper experimental measurements for decades5,6, more exactly till 1997, when the first X-ray crystal structure of a CA – activator adduct was reported by some of us5. Indeed, histamine 1, one of the first CAAs investigated15 was shown to bind at the entrance of the CA active site cavity (Figure 2) where it participates to the catalytic cycle, more precisely to the rate-determining step of it, which is a proton transfer reaction between a water molecule coordinated to the zinc ion from the enzyme active site to the reaction medium, which generates the zinc hydroxide, nucleophilic species of the enzyme4–7. In the catalytic cycle (in the absence of activators), this function is played by the imidazole moiety of a His residue, with a pKa around 7, placed in the middle of the active site, which for most human (h) CA isoforms is His64 (hCA I numbering system)7. In the presence of CAAs, this proton transfer process is facilitated by the parallel, activator pathway which leads to a facilitated formation of the nucleophilic species of the enzyme4–7. Many other X-ray crystal structures of CA – activator adducts have been reported since 1997, such as for example the ones with L-and D-histidine 217,18, L- and D-phenylalanine 319, L-adrenaline 420, D-Trp21, etc. Drug design studies of amine/amino acid based CAAs were also reported22,23. In all these adducts for which the X-ray crystal structure was reported, it has been observed that the activator binds at the entrance of the active site, in a region of the cavity where inhibitors were never observed until 2009, from which they actively participate to the proton shuttling between the active site and the reaction medium5,17–21. In 2009, we have reported the X-ray crystal structure of a coumarin derivative acting as CA inhibitor (CAI) with a totally new mechanism of action24. Interestingly, the hydrolysed coumarin, which is the real CAI, binds in the same active site region as the activators, but not having a proton shuttling moiety in its molecule, does not activate but inhibit the enzyme24,25.
Figure 2.
Binding of histamine 1 (magenta, PDB ID: 1AVN)5 and L-adrenaline (yellow, PDB ID: 2HKK)204 to hCA II, as determined by X-ray crystallography. The Zn(II) ion from the enzyme active site is the magenta sphere, with its three His ligands in green (His94, 96, and 119). His64, the natural proton shuttling residues, is shown (in green) in both its “in” and “out” conformations, above the activator molecules, which are bound at the entrance of the cavity.
As mentioned above, many psychoactive compounds possess the phenethylamine scaffold and the general formula Ar–CH2CH(R)NHR′, and they were never investigated as potential CAAs. Here, we report the first such study, showing that amphetamine, methamphetamine, phentermine, mephentermine, and chlorphenteramine, potently activate several CA isoforms, some of which are highly abundant in the brain, where they play important functions connected to cognition and memory, among others26,27.
2. Experimental
2.1. Chemistry
Compounds 1–4 were commercially, highest purity available derivatives from Sigma-Aldrich (Milan, Italy) and were used without further purification. Amines 5–9 were certified standards from the standards collection of University of Florence, Struttura di Tossicologia Forense, furnished under authorisation from the Italian Ministry of Health by LGC standards SRL (Milan, Italy).
2.2. Carbonic anhydrase assay
A stopped-flow method28 has been used for assaying the CA catalysed CO2 hydration activity with Phenol red as indicator, working at the absorbance maximum of 557 nm, following the initial rates of the CA-catalysed CO2 hydration reaction for 10–100 s. For each activator at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of activator (0.1 mM) were prepared in distilled–deionised water and dilutions up to 0.1 nM were done thereafter with the assay buffer. The activation constant (KA), defined similarly with the inhibition constant KI, was obtained by considering the classical Michaelis–Menten equation (Equation (1)), which has been fitted by non-linear least squares by using PRISM 3:
| (1) |
where [A]f is the free concentration of activator.
Working at substrate concentrations considerably lower than KM ([S] ≪ KM), and considering that [A]f can be represented in the form of the total concentration of the enzyme ([E]t) and activator ([A]t), the obtained competitive steady-state equation for determining the activation constant is given by Equation (2)29–34:
| (2) |
where v0 represents the initial velocity of the enzyme-catalysed reaction in the absence of activator. All CA isozymes used in the experiments were purified recombinant proteins obtained as reported earlier by our group29–34.
3. Results and discussion
3.1. Chemistry
Amines 5–9 are widely used psychoactive substances (Figure 3). They include primary amines such as amphetamine 5 and phentermine 7, secondary ones such as methamphetamine 6 and mephentermine 8, as well as a tertiary amine, chlorphenteramine 9, which unlike compounds 5–8, which possess the general formula mentioned in the introduction, typical to most CAAs investigated so far4–6, incorporates a dimethylamino moiety linked by a three carbon atoms chain to a bulky aromatic moiety of the 4-chlorophenyl type. Furthermore, 9 also incorporates a second bulky moiety, the 2-pyridyl one, making it an interesting candidate to be tested as a CAA, with structural features not encountered in other such derivatives investigated earlier.
Figure 3.
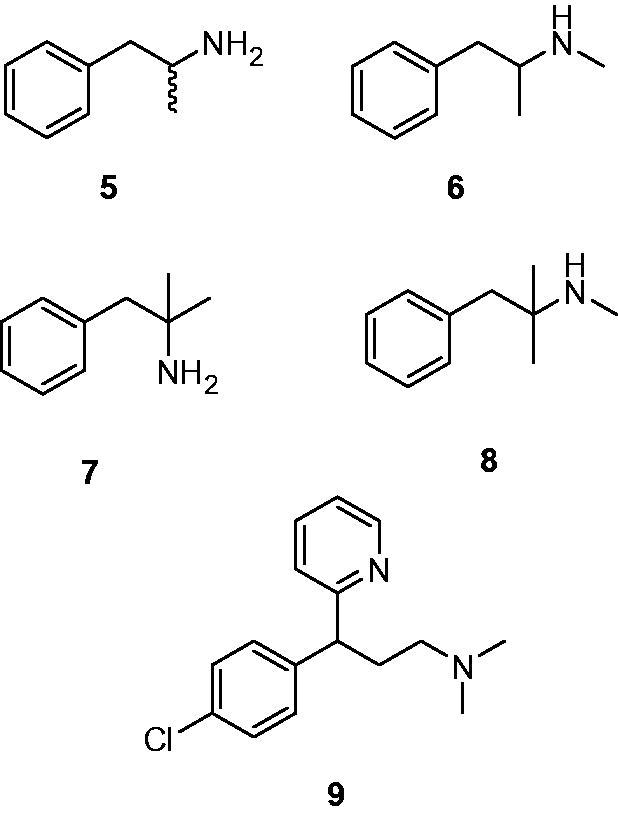
Structure of psychoactive substances investigated as CAAs in the present article: amphetamine 5, methamphetamine 6, phentermine 7, mephentermine 8, and chlorphenteramine 9.
3.2. CA activation
Amines 5–9 are strong central nervous system (CNS) stimulants and were originally used as drugs, for the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, obesity, nasal congestion, and depression35–37. They interfere with the catecholamine neurotransmitters norepinephrine and dopamine metabolism, by the activation of a trace amine receptor31, which leads to an increase of monoamine and excitatory neurotransmitter activity in the brain, leading to emotional and cognitive effects such as euphoria, change in desire for sex, increased wakefulness, accompanied by improved cognitive control38–40. However, drug addiction is a serious risk with the recreational use of these substances, with high doses leading to psychosis, such as delusions and paranoia, as well as many other serious side effects41.
We investigated the CA activating properties of amines 5–9 against 11 catalytically active and physiologically relevant hCA isoforms, that is, the cytosolic hCA I, II, VII, and XIII, the membrane-associated hCA IV, the mitochondrial hCA VA and VB, the secreted hCA VI, as well as the transmembrane isoforms hCA IX, XII, and XIV7–12,42,43. It should be noted that the tissue distribution and physiological roles of these isoforms are very heterogeneous/complex, but many of them are abundant in the brain43–45. Indeed, the human brain as well as the choroid plexus contain a multitude of CA isoforms, although their precise functions are poorly understood44. For example, hCA II, the physiological dominant isoform is abundant in the choroid plexus, oligodendrocytes, myelinated tracts, astrocytes, and myelin sheaths in the vertebrates brain44. The membrane-associated CA IV is located on the luminal surface of cerebral capillaries, and associated with the blood–brain barrier, being also concentrated in layers III and VI within the cortex, hippocampus, and thalamus of all investigated mammals44,45. Other studies demonstrated the presence of the mitochondrial CA VA in the nervous tissues, where the enzyme is expressed in astrocytes and in neurons, being probably involved in biosynthetic processes such as neoglucogenesis, lipogenesis, etc36,37. The expression of CA VII is of particular interest, as relatively high levels have been observed throughout the cortex, hippocampus and thalamus (CA VII also shows a very good catalytic activity for the physiological reaction)44 and is probably the target for CA inhibitors acting as anti-neuropathic pain and anti-epileptic agents44,45. CA IX was shown to be overexpressed in many neurologic cancers such as glioma, ependymoma, hemangioblastoma, meningioma as well as choroid plexus tumors42. CA XII is also connected to cancers and has the same expression pattern as CA IX in brain tumors42–44. However, CA XII is also present in normal tissues and a high level of this isoform was reported in the choroid plexus42. CA XIV is expressed in nuclei and nerve tracts associated with pontine, medullary, and hippocampal functions43. CA XIV was also shown to be located on the plasma membrane of some neurons and on axons of both mouse and human brain43,44. With so many CA isoforms in the CNS it is rather difficult to ascertain their precise physiological role, but some indirect information may be drawn from the fact that positively-charged, membrane-impermeant CAAs (or CAIs) do not show central nervous system effects when administered intra-peritoneally, which may be explained by the fact that intracellular (cytosolic or mitochondrial) CA isoforms are involved in the activation/inhibition phenomena. As mentioned briefly above, CA activation in the brain leads to enhanced memory, through the activated extracellular signal-regulated kinase (ERK) pathway, which is involved in a critical step for memory formation in the cortex and the hippocampus, two brain areas known to play a crucial role in memory processing, as recently demonstrated by one of our groups27.
CA activation data against these 11 isoforms with psychotropic amines 5–9 and standard CAAs 1–4 are shown in Tables 1 and 2.
Table 1.
CA activation of isoforms hCA I, II, VII, and XIII (cytosolic) and IV (membrane-associated) with compounds 1–9, by a stopped-flow CO2 hydrase assay28.
|
KAa (µM) |
|||||
|---|---|---|---|---|---|
| Compound | hCA I | hCA II | hCA IV | hCA VII | hCA XIII |
| 1 | 2.1 | 125 | 25.3 | 37.5 | 4.7 |
| 2 (L-His) | 0.03 | 0.90 | 7.3 | 0.92 | 0.13 |
| 3 (L-Phe) | 0.07 | 0.013 | 36.3 | 10.9 | 1.02 |
| 4 | 0.09 | 96 | 45 | 60 | nt |
| 5 | >150 | >150 | 0.094 | 0.91 | 24.1 |
| 6 | >150 | >150 | 0.051 | 0.93 | 25.6 |
| 7 | >150 | >150 | 0.074 | 0.89 | 54.2 |
| 8 | >150 | >150 | 1.03 | 0.64 | 48.3 |
| 9 | >150 | >150 | 0.055 | 0.098 | 79.5 |
Table 2.
CA activation of isoforms hCA VA, VB (mitochondrial), VI (secreted), and IX, XII, and XIV (trans-membrane) with compounds 1–9, by a stopped-flow CO2 hydrase assay28.
|
KAa (µM) |
||||||
|---|---|---|---|---|---|---|
| Compound | hCA VA | hCA VB | hCA VI | hCA IX | hCA XII | hCA XIV |
| 1 | 0.010 | 3.52 | 6.50 | 35.1 | 27.9 | 0.010 |
| 2 (L-His) | 1.34 | 0.97 | 32.0 | 9.71 | 37.5 | 0.90 |
| 3 (L-Phe) | 9.81 | 10.45 | 1.23 | 16.3 | 1.38 | 0.24 |
| 4 | 63 | 67 | nt | 0.87 | 0.41 | 36.1 |
| 5 | 0.81 | 2.56 | >150 | >150 | 0.64 | 9.15 |
| 6 | 0.92 | 0.78 | >150 | >150 | 0.80 | 7.38 |
| 7 | 0.53 | 0.62 | >150 | 34.6 | 3.24 | 12.7 |
| 8 | 0.37 | 0.24 | >150 | 25.8 | 6.12 | 18.1 |
| 9 | 0.31 | 0.75 | >150 | 34.1 | 0.97 | 6.81 |
The following structure–activity relationship (SAR) for the activation of these enzymes with psychotropic amines 5–9 can be drawn from data of Tables 1 and 2:
The cytosolic, widespread isoforms hCA I and II were not significantly activated by these amines up to concentrations as high as 150 µM, whereas hCA XIII, another cytosolic isoform with a more particular expression pattern (as it is widespread in many organs but with quite low expression level)42 is moderately activated by amines 5–9, with activation constants in the range of 24.1–79.5 µM. The best activators of hCA XIII were amphetamine 5 and methamphetamine 6, whereas phentermine 7, mephentermine 8, and chlorphenteramine 9 showed a decreased potency. It is interesting to note the differences between the activating effects of amines 5–9 and the standard CAAs 1–4: hCA I for example is effectively activated by compounds 1–4, whereas hCA II is poorly activated by histamine 1 and L-adrenaline 4, but highly effectively activated by amino acids such as L-His and D-Phe (Table 1). hCA XIII is also rather well activated by 1–3 (L-adrenaline’s effects on this isoform were not investigated).
hCA VII, the brain-associated cytosolic CA isoform46, known to be involved in the antiepileptic and antineuropathic pain effects of the CAIs44,45, was effectively activated by the psychotropic amines investigated here, with KAs ranging between 98 nM and 0.93 µM. The most effective hCA VII activator was chlorphenteramine 9 whereas the remaining derivatives, possessing the phenethylamine scaffold showed rather similar, submicromolar activation constants (KAs of 0.64–0.93 µM). No major differences in the activating capacity were observed between the primary and secondary amines, or between the derivatives possessing the relatively not sterically hindered α-methyl group near the amino functionality, such as 5 and 6, compared to the sterically hindered, structurally related amines 7 and 8 (Table 1). It should also be noted that except L-His, which has the same level of activity as the psychotropic amines investigated here, the other standard CAAs, such as 1, 3, and 4, were quite ineffective as hCA VII activators (Table 1).
The membrane-associated, rather widespread (in endothelial cells of blood capillaries in many organs such as lungs, gastro-intestinal tract, brain, epithelial cells of gallbladder, renal tubules, reproductive organs, etc.)43 isoform hCA IV was the most sensitive one to activation by amines 5–9, which showed KAs ranging between 51 nM and 1.03 µM. The best hCA IV activator was methamphetamine 6 and chlorphenteramine 9 (KAs of 51–55 nM), followed by phentermine 7 and amphetamine 5 (KAs of 74–94 nM). The sterically hindered (at the amino moiety) mephentermine 8 was the least effective CAA with a KA of 1.03 µM. There is a huge difference of activity between the psychotropic amines investigated here 5–9, which act as efficient or highly efficient hCA IV activators, and compounds 1–4, which were quite ineffective such agents with KAs in the range of 7.3–45 µM (Table 1).
The two mitochondrial isoforms hCA VA and VB, involved in many metabolic reactions (ureagenesis, lipogenesis, neoglucogenesis, etc.)47 and also present in the brain43, were also effectively activated by amines 5–9, with KAs ranging between 0.24 and 2.56 µM (Table 2). The best activators against these isoforms were mephentermine 8 against hCA VB (KA of 240 nM) and chlorphenteramine 9 against hCA VA (KA of 310 nM), with the remaining compounds (except amphetamine 5 against hCA VB which showed a KA of 2.56 µM) showing a rather effective, submicromolar activation profile against both isoforms and with a flat SAR (KAs ranging in a narrow interval of 0.37–0.92 µM, Table 2). It should be noted that apart histamine 1, which is an effective hCA VA activator (KA of 10 nM), the standard CAAs 2–4 were generally less effective mitochondrial CA activators compared to the psychotropic amines 5–9 (Table 2).
Similar to hCA I and II, the secreted isoform hCA VI (present in the saliva and milk)30, was not significantly activated by amines 5–9 up until 150 µM concentration of activator, whereas compounds 1–3 showed a much better activating efficacy (Table 2).
The tumor-associated, transmembrane isoform hCA IX was not significantly activated by amphetamine 5 and methamphetamine 6 (KAs >150 µM), whereas the remaining psychotropic amines 7–9 showed weak activating effects, with KAs of 25.8–34.6 µM. However, the second tumour-associated, transmembrane isoform hCA XII showed a very different activation profile with these compounds compared to hCA IX. Indeed, amphetamine 5, methamphetamine 6, and chlorphenteramine 9 were submicromolar activators (KAs of 0.64–0.97 µM) whereas the sterically hindered amines 7 and 8 were one order of magnitude less efficient as hCA XII activators (KAs of 3.24–6.12 µM). The primary amines 5 and 7 were in this case more effective activators compared to the corresponding secondary amines 6 and 8. Among the standard CAAs, only L-adrenaline 4 showed the same level of activity as amines 5 and 6, the remaining derivatives 1–3 being generally less effective as hCA XII activators (Table 2).
hCA XIV, another transmembrane isoform not connected with tumours, and present in neurons, hepatocytes, renal tubules42,43, was moderately activated by amines 5–9, which showed KAs of 6.81–18.1 µM. The best hCA XIV activator was chlorphenteramine 9 whereas the least effective one was mephentermine 8. Some of the standard CAAs, such as histamine 1 showed much more effective, low nanomolar activating effects against this isoform.
4. Conclusions
We investigated psychotropic amines based on the phenethylamine scaffold, such as amphetamine 5, methamphetamine 6, phentermine 7, mephentermine 8, and the structurally diverse chlorphenteramine 9, for their activating effects on 11 CA isoforms of human origin, hCA I, II, VII, and XIII (cytosolic isoforms), the membrane-associated hCA IV, the mitochondrial hCA VA, and VB, the secreted hCA VI, as well as the transmembrane isoforms hCA IX, XII, and XIV. The widespread hCA I and II, the secreted hCA VI, as well as the cytosolic hCA XIII and membrane-bound hCA IX and XIV were poorly activated by these amines, whereas the extracellular hCA IV, the mitochondrial enzymes hCA VA/VB, the cytosolic hCA VII, and the transmembrane isoform hCA XII were potently activated. Some of these enzymes (hCA VII, VA, VB, XII) are abundant in the brain, raising the possibility that some of the cognitive effects of such psychoactive substances might be related to the activation of these enzymes. Furthermore, unlike the CAIs which are in clinical use for decades for a multitude of applications47a,48–61, the CAAs started to be considered only recently for possible pharmacologic applications in memory/cognition therapy27. This work may bring new lights on the intricate relationship between CA activation by this type of compounds and the multitude of pharmacologic actions that they can elicit.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.(a) European Drug report 2016: Trends and Developments. European Monitoring Centre for Drugs and drug Addicion (EMCDDA). Available from: http://www.emcdda.europa.eu/system/files/publications/2637/TDAT16001ENN.pdf.(b) European Drug report 2015: Trends and Developments. European Monitoring Centre for Drugs and drug Addicion (EMCDDA). Available from: http://www.emcdda.europa.eu/system/files/publications/974/TDAT15001ENN.pdf. [Google Scholar]
- 2.Bertol E, Pascali J, Palumbo D, et al. 3-MeO-PCP intoxication in two young men: first in vivo detection in Italy. Forensic Sci Int 2017;274:7–12. [DOI] [PubMed] [Google Scholar]
- 3.Bertol E, Vaiano F, Mari F, et al. Advances in new psychoactive substances identification: the U.R.I.To.N. Consortium. J Enzyme Inhib Med Chem 2017;32:841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clare BW, Supuran CT.. Carbonic anhydrase activators. 3: structure–activity correlations for a series of isozyme II activators. J Pharm Sci 1994;83:768–73. [DOI] [PubMed] [Google Scholar]
- 5.Briganti F, Mangani S, Orioli P, et al. Carbonic anhydrase activators: X-ray crystallographic and spectroscopic investigations for the interaction of isozymes I and II with histamine. Biochemistry 1997;36:10384–92. [DOI] [PubMed] [Google Scholar]
- 6.Temperini C, Scozzafava A, Supuran CT.. Carbonic anhydrase activation and the drug design. Curr Pharm Des 2008;14:708–15. [DOI] [PubMed] [Google Scholar]
- 7.Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68. [DOI] [PubMed] [Google Scholar]
- 8.Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO(2) capture. J Enzyme Inhib Med Chem 2013;28:229–30. [DOI] [PubMed] [Google Scholar]
- 9.Supuran CT.Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88. [DOI] [PubMed] [Google Scholar]
- 10.Supuran CT.Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. [DOI] [PubMed] [Google Scholar]
- 11.Supuran CT.Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. [DOI] [PubMed] [Google Scholar]
- 12.Supuran CT.How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60. [DOI] [PubMed] [Google Scholar]
- 13.Leiner M, Leiner G.. Die Aktivatoren der Kohlensäureanhydrase. Naturwiss 1941;29:195–7. [Google Scholar]
- 14.Leiner M, Leiner G.. Die Messmethoden zur Untersuchung der katalytischen Wirksamkeit der Kohlensäureanhydrase. Biochem Z 1941;311:119–45. [Google Scholar]
- 15.Main RE, Locke A.. Activation of carbonic anhydrase by histamine. J Biol Chem 1941;140:LXXXI. [Google Scholar]
- 16.Kiese M.Die Aktivierung der Kohlensäureanhydrase. Naturwiss 1941;29:116–17. [Google Scholar]
- 17.(a) Temperini C, Scozzafava A, Supuran CT.. Carbonic anhydrase activators: the first X-ray crystallographic study of an adduct of isoform I. Bioorg Med Chem Lett 2006;16:5152–6. (b) Temperini C, Scozzafava A, Puccetti L, Supuran CT.. Carbonic anhydrase activators: X-ray crystal structure of the adduct of human isozyme II with L-histidine as a platform for the design of stronger activators. Bioorg Med Chem Lett 2005;15: 5136–41. [DOI] [PubMed] [Google Scholar]
- 18.Temperini C, Scozzafava A, Vullo D, Supuran CT.. Carbonic anhydrase activators. Activation of isozymes I, II, IV, VA, VII, and XIV with l- and d-histidine and crystallographic analysis of their adducts with isoform II: engineering proton-transfer processes within the active site of an enzyme. Chemistry 2006;12:7057–66. [DOI] [PubMed] [Google Scholar]
- 19.Temperini C, Scozzafava A, Vullo D, Supuran CT.. Carbonic anhydrase activators. Activation of isoforms I, II, IV, VA, VII, and XIV with L- and D-phenylalanine and crystallographic analysis of their adducts with isozyme II: stereospecific recognition within the active site of an enzyme and its consequences for the drug design. J Med Chem 2006;49:3019–27. [DOI] [PubMed] [Google Scholar]
- 20.Temperini C, Innocenti A, Scozzafava A, et al. Carbonic anhydrase activators: L-Adrenaline plugs the active site entrance of isozyme II, activating better isoforms I, IV, VA, VII, and XIV. Bioorg Med Chem Lett 2007;17:628–35. [DOI] [PubMed] [Google Scholar]
- 21.Temperini C, Innocenti A, Scozzafava A, Supuran CT.. Carbonic anhydrase activators: kinetic and X-ray crystallographic study for the interaction of D- and L-tryptophan with the mammalian isoforms I–XIV. Bioorg Med Chem 2008;16:8373–8. [DOI] [PubMed] [Google Scholar]
- 22.Scozzafava A, Supuran CT.. Carbonic anhydrase activators: high affinity isozymes I, II, and IV activators, incorporating a beta-alanyl-histidine scaffold. J Med Chem 2002;45:284–91. [DOI] [PubMed] [Google Scholar]
- 23.(a) Ilies M, Banciu MD, Ilies MA, et al. Carbonic anhydrase activators: design of high affinity isozymes I, II, and IV activators, incorporating tri-/tetrasubstituted-pyridinium-azole moieties. J Med Chem 2002;45:504–10.(b) Supuran CT, Scozzafava A.. Carbonic anhydrase activators: amino acyl/dipeptidyl histamine derivatives bind with high affinity to isozymes I, II and IV and act as efficient activators. Bioorg Med Chem 1999;7: 2915–23. [DOI] [PubMed] [Google Scholar]
- 24.Maresca A, Temperini C, Vu H, et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009;131:3057–62. [DOI] [PubMed] [Google Scholar]
- 25.(a) Maresca A, Temperini C, Pochet L, et al. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–44.(b) Temperini C, Innocenti A, Scozzafava A, et al. The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J Med Chem 2010;53: 850–4. [Google Scholar]
- 26.Sun MK, Alkon DL.. Carbonic anhydrase gating of attention: memory therapy and enhancement. Trends Pharmacol Sci 2002;23:83–9. [DOI] [PubMed] [Google Scholar]
- 27.Canto de Souza L, Provensi G, Vullo D, et al. Carbonic anhydrase activation enhances object recognition memory in mice through phosphorylation of the extracellular signal-regulated kinase in the cortex and the hippocampus. Neuropharmacology 2017;118:148–56. [DOI] [PubMed] [Google Scholar]
- 28.Khalifah RG.The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73. [PubMed] [Google Scholar]
- 29.(a) Vullo D, Nishimori I, Scozzafava A, Supuran CT.. Carbonic anhydrase activators: Activation of the human cytosolic isozyme III and membrane-associated isoform IV with amino acids and amines. Bioorg Med Chem Lett 2008;18:4303–1. (b) Vullo D, Innocenti A, Nishimori I, et al. Carbonic anhydrase activators: activation of the human isoforms VII (cytosolic) and XIV (transmembrane) with amino acids and amines. Bioorg Med Chem Lett 2007;17: 4107,–12.(c) Parkkila S, Vullo D, Puccetti L, et al. Carbonic anhydrase activators: activation of isozyme XIII with amino acids and amines. Bioorg Med Chem Lett 2006;16: 3955–9. [Google Scholar]
- 30.(a) Nishimori I, Onishi S, Vullo D, et al. Carbonic anhydrase activators. The first activation study of the human secretory isoform VI. Bioorg Med Chem 2007;15:5351–7.(b) Pastorekova S, Vullo D, Nishimori I, et al. Carbonic anhydrase activators: activation of the human tumor-associated isozymes IX and XII with amino acids and amines. Bioorg Med Chem 2008;16: 3530,–6.(c) Vullo D, Nishimori I, Innocenti A, et al. Carbonic anhydrase activators: an activation study of the human mitochondrial isoforms VA and VB with amino acids and amines. Bioorg Med Chem Lett 2007;17: 1336–40. [Google Scholar]
- 31.Le Duc Y, Licsandru E, Vullo D, et al. Carbonic anhydrases activation with 3-amino-1H-1,2,4-triazole-1-carboxamides: discovery of subnanomolar isoform II activators. Bioorg Med Chem 2017;25:1681–6. [DOI] [PubMed] [Google Scholar]
- 32.Licsandru E, Tanc M, Kocsis I, et al. A class of carbonic anhydrase I – selective activators. J Enzyme Inhib Med Chem 2017;32:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Legrand YM, Petit E, et al. Dynamic encapsulation and activation of carbonic anhydrase in multivalent dynameric host matrices. Chem Commun (Camb) 2016;52:4053–5. [DOI] [PubMed] [Google Scholar]
- 34.Abdelrahim MY, Tanc M, Winum JY, et al. Dominant behaviours in the expression of human carbonic anhydrase hCA I activity. Chem Commun (Camb) 2014;50:8043–6. [DOI] [PubMed] [Google Scholar]
- 35.(a) Heal DJ, Smith SL, Gosden J, Nutt DJ.. Amphetamine, past and present – a pharmacological and clinical perspective. J Psychopharmacol 2013;27:479–96.(b) Bidwell LC, McClernon FJ, Kollins SH.. Cognitive enhancers for the treatment of ADHD. Pharmacol Biochem Behav 2011;99: 262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.(a) Brams M, Mao AR, Doyle RL.. Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder. Postgrad Med 2008;120:69–88.(b) Forray A, Sofuoglu M.. Future pharmacological treatments for substance use disorders. Br J Clin Pharmacol 2014;77: 382,–400.(c) Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry 2008;47: 21–31. [Google Scholar]
- 37.Rogge G, Jones D, Hubert GW, et al. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci 2008;9:747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.(a) Jing L, Li JX.. Trace amine-associated receptor 1: a promising target for the treatment of psychostimulant addiction. Eur J Pharmacol 2015;761:345–52.(b) Miller GM.The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem 2011;116: 164,–76.(c) Borowsky B, Adham N, Jones KA, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA 2001;98: 8966–71. [Google Scholar]
- 39.Malenka RC, Nestler EJ, Hyman SE.. Higher cognitive function and behavioral control In: Sydor A, Brown RY, eds. Molecular neuropharmacology: a foundation for clinical neuroscience. 2nd ed.New York (NY): McGraw-Hill Medical; 2009:318–21. [Google Scholar]
- 40.Spencer RC, Devilbiss DM, Berridge CW.. The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biol Psychiatry 2015;77:940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carvalho M, Carmo H, Costa VM, et al. Toxicity of amphetamines: an update. Arch Toxicol 2012;86:1167–231. [DOI] [PubMed] [Google Scholar]
- 42.(a) Pan PW, Parkkila AK, Autio S, et al. Brain phenotype of carbonic anhydrase IX-deficient mice. Transgenic Res 2012;21:163–76.(b) Hilvo M, Supuran CT, Parkkila S.. Characterization and inhibition of the recently discovered carbonic anhydrase isoforms CA XIII, XIV and XV. Curr Top Med Chem 2007;7: 893–9. [DOI] [PubMed] [Google Scholar]
- 43.(a) Halmi P, Parkkila S, Honkaniemi J.. Expression of carbonic anhydrases II, IV, VII, VIII and XII in rat brain after kainic acid induced status epilepticus. Neurochem Int 2006;48:24–30.(b) Parkkila S, Parkkila AK, Rajaniemi H, et al. Expression of membrane-associated carbonic anhydrase XIV on neurons and axons in mouse and human brain. Proc Natl Acad Sci USA 2001;98: 1918,–23.(c) Ghandour MS, Parkkila AK, Parkkila S, et al. Mitochondrial carbonic anhydrase in the nervous system: expression in neuronal and glial cells. J Neurochem 2000;75: 2212–20. [Google Scholar]
- 44.(a) Thiry A, Dognè JM, Masereel B, Supuran CT.. Carbonic anhydrase inhibitors as anticonvulsant agents. Curr Top Med Chem 2007;7:855–64.(b) Supuran CT.Acetazolamide for the treatment of idiopathic intracranial hypertension. Expert Rev Neurother 2015;15: 851–6. [DOI] [PubMed] [Google Scholar]
- 45.(a) Carta F, Di Cesare ML, Pinard M, et al. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg Med Chem 2015;23:1828–40.(b) Supuran CT.Carbonic anhydrase inhibition and the management of neuropathic pain. Expert Rev Neurother 2016;16: 961–8. [DOI] [PubMed] [Google Scholar]
- 46.(a) Ruusuvuori E, Li H, Huttu K, et al. Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J Neurosci 2004;24:2699–707.(b) Ruusuvuori E, Huebner AK, Kirilkin I, et al. Neuronal carbonic anhydrase VII provides GABAergic excitatory drive to exacerbate febrile seizures. EMBO J 2013;32: 2275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.(a) Scozzafava A, Supuran CT, Carta F.. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat 2013;23:725–35.(b) Supuran CT.Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Expert Opin Emerg Drugs 2012;17: 11–15. [DOI] [PubMed] [Google Scholar]
- 48.Briganti F, Pierattelli R, Scozzafava A, Supuran CT.. Carbonic anhydrase inhibitors. Part 37. Novel classes of isozyme I and II inhibitors and their mechanism of action. Kinetic and spectroscopic investigations on native and cobalt-substituted enzymes. Eur J Med Chem 1996;31:1001–10. [Google Scholar]
- 49.Supuran CT, Clare BW.. Carbonic anhydrase inhibitors–Part 57: quantum chemical QSAR of a group of 1,3,4-thiadiazole-and 1,3,4-thiadiazoline disulfonamides with carbonic anhydrase inhibitory properties. Eur J Med Chem 1999;34:41–50. [Google Scholar]
- 50.Capasso C, Supuran CT.. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32. [DOI] [PubMed] [Google Scholar]
- 51.Masini E, Carta F, Scozzafava A, Supuran CT.. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Pat 2013;23:705–16. [DOI] [PubMed] [Google Scholar]
- 52.Puccetti L, Fasolis G, Vullo D, et al. Carbonic anhydrase inhibitors. Inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with Schiff’s bases incorporating chromone and aromatic sulfonamide moieties, and their zinc complexes. Bioorg Med Chem Lett 2005;15:3096–101. [DOI] [PubMed] [Google Scholar]
- 53.Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Pat 2013;23:737–49. [DOI] [PubMed] [Google Scholar]
- 54.Scozzafava A, Menabuoni L, Mincione F, Supuran CT.. Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyamino–polycarboxylate tails and of their metal complexes possessing long-lasting, topical intraocular pressure-lowering properties. J Med Chem 2002;45:1466–76. [DOI] [PubMed] [Google Scholar]
- 55.Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005;15:3102–8. [DOI] [PubMed] [Google Scholar]
- 56.Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013)). Expert Opin Ther Pat 2013;23:681–91. [DOI] [PubMed] [Google Scholar]
- 57.Supuran CT, Nicolae A, Popescu A.. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;31:431–8. [Google Scholar]
- 58.Fabrizi F, Mincione F, Somma T, et al. A new approach to antiglaucoma drugs: carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J Enzyme Inhib Med Chem 2015;27:138–47. [DOI] [PubMed] [Google Scholar]
- 59.Pacchiano F, Aggarwal M, Avvaru BS, et al. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb) 2010;46:8371–3. [DOI] [PubMed] [Google Scholar]
- 60.Dubois L, Peeters S, Lieuwes NG, et al. Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol 2011;99:424–31. [DOI] [PubMed] [Google Scholar]
- 61.Carta F, Scozzafava A, Supuran CT. Sulfonamides: a patent review (2008–2012)). Expert Opin Ther Pat 2012;22:747–58. [DOI] [PubMed] [Google Scholar]