Summary
Objective
This study aimed to assess the relationship between weight gain from early adulthood and visceral fat accumulation.
Methods
The participants were 549 men aged 42 to 64 years who were randomly selected from the local resident registry for the National Institute for Longevity Sciences' neighbourhood. They were asked to recall their weight at 18 years of age, and then, post‐18 weight‐change values were calculated for each participant (their current weight minus their weight at 18). The participants were divided according to their median body mass index (BMI) at 18 years of age (initial BMI) (<20.14 and ≥20.14 kg m−2). Visceral fat area (VFA) and subcutaneous fat area (SFA) were measured on computed tomography scans.
Results
The participants with initial BMI of <20.14 kg m−2 exhibited greater post‐18 weight changes than those with initial BMI of ≥20.14 kg m−2. The participants' post‐18 weight‐change values were negatively correlated with their initial BMI and positively correlated with both VFA and SFA. The slope of the regression line for the relationship between post‐18 weight change and VFA was steeper in the participants with initial BMI of <20.14 kg m−2 (β = 4.36) than in those with initial BMI of ≥20.14 kg m−2 (β = 3.23).
Conclusions
Visceral fat accumulation is affected not only by an individual's post‐18 weight gain but also by their initial BMI. Men who were thin in early adulthood experienced greater weight gain‐associated VFA increases, but the same was not true for SFA.
Keywords: Visceral fat, weight at 18 years of age, weight gain
Introduction
Weight gain is commonly observed between early adulthood and middle age 1, 2, 3, 4. According to the National Health and Nutrition Examination Survey in 2014 (a cross‐sectional survey) 1, the mean body mass index (BMI) for Japanese men was 22.4 kg m−2 in the 20 to 29 years age group, 24.0 kg m−2 in the 40 to 49 years age group and 23.9 kg m−2 in the 50 to 59 years age group. According to the National Health Examination and the National Health and Nutrition Examination Surveys conducted in the USA from 1999 to 2002, the mean BMIs for men aged 20 to 29 years, 40 to 49 years and 50 to 59 years were 26.6, 28.4 and 28.7 kg m−2, respectively 2. In Norway, men aged 20–29 years exhibited a mean weight increase of 7.9 kg over 11 years 3. In a 14‐year follow‐up study conducted in South Wales, the mean BMI was 22.3 kg m−2 at 18 years, and the mean weight gain during the study period was 11.2 kg (standard deviation [SD]: 11.2) 4.
There are several causes of weight changes, such as variations in food intake or physical activity 5, smoking habits 6, 7, 8, and genetic factors 9. In a study of middle‐aged and elderly Japanese men, a combination of cholecystokinin 1 receptor and beta‐3 adrenergic receptor polymorphisms was found to be associated with a weight gain of ≥10 kg after age 18 9.
Marked weight gain is as harmful to health as being overweight or obese. In previous studies, weight gain from early adulthood to middle age was demonstrated to be closely associated with cardiovascular disease 10, 11, coronary heart disease 4, 12, 13, and diabetes in men 5. Chei et al. found that weight gain was associated with a two‐fold greater risk of coronary heart disease in Japanese men 11. Furthermore, the latter relationship was observed in men who had BMI of <21.7 kg m−2 at 20 years of age but not in those with BMI ≥21.7 kg m−2 at this age. They assumed that weight gain in initially lean men tends to favour upper abdominal fat accumulation over lower abdominal fat accumulation. Moreover, this tendency was weaker in men with higher BMI at 20 years of age. However, the latter study did not examine fat distribution, including visceral fat (VF) volume or area. Koh‐Banerjee et al. found that weight gain was associated with the risk of diabetes 5, but they only measured waist circumference; i.e., they did not assess VF accumulation. However, waist circumference is not necessarily an accurate measure of VF accumulation, as it is influenced by both VF and subcutaneous fat (SF). A previous study reported that VF accumulation was more strongly associated with numerous diseases than SF accumulation 14, 15.
Thus, the weight gain seen from early adulthood to middle age might be strongly associated with VF accumulation, especially in lean young adults. However, little is known about the association between such weight changes and VF accumulation. Therefore, the purpose of this study was to investigate the association between the weight change from 18 years of age to the present day (post‐18 weight gain) and VF area (VFA).
Methods
Participants
The participants were selected from among the 944 Japanese men (age range, 41 to 81 years) who participated in the first and second waves of the National Institute for Longevity Sciences‐Longitudinal Study of Aging (NILS‐LSA) 16. The NILS‐LSA involved gender‐stratified and age‐stratified samples from the neighbourhood of the NILS, who were randomly selected from the local resident registry in cooperation with the local government. The study participants were 549 men (age range, 42 to 64 years) for whom no measurements were missing (Figure 1). The participants who had quit smoking more than 1 year ago after smoking for more than 1 year were also excluded because smoking habits also affect weight changes. Many studies have indicated that smokers tend to have lower body weights than non‐smokers 7, and smoking cessation is known to be associated with substantial weight gain 6, 8. Weight gain was associated with increases in total cholesterol, triglyceride and β‐lipoprotein levels in men and women who ranged in age from 18 to 64 years but not in those who ranged in age from 65 to 88 years of age 17. Thus, men ranging in age from 65 to 82 years were excluded from this study.
Figure 1.
Sampling procedure.
Measurements
The measurements were obtained in 1 d in the second wave of the NILS‐LSA (April 2000 to May 2002). Body weight (kg) was measured in the morning after overnight fasting using digital scales while the participants were only wearing underwear. Waist circumference was measured at the level of the umbilicus with a fibreglass tape measure. VFA (cm2) and SF area (SFA; cm2) were measured on computed tomography scans of the abdomen taken at the umbilical level (L4 to L5). All of the computed tomography scans (SCT‐6800TX; Shimadzu, Japan) were performed with the participants in the supine position. VFA and SFA were calculated using computer software (Fat Scan; N2 Systems, Japan) 18 in accordance with a previously described procedure 19. Percentage body fat was measured using dual energy X‐ray absorptiometry (Hologic QDR‐4500). The participants' weights at 18 years of age were self‐reported via the first‐wave questionnaires (November 1997 to May 2000).
Data analysis
Body mass index at 18 years (the initial BMI) was calculated as the participant's weight (kg) at 18 years divided by their current height (m) squared. Post‐18 weight‐change values were calculated for each participant (the participant's current weight minus their weight at 18 years of age). Spearman's rank correlation coefficients were calculated for the relationships between post‐18 weight gain and VFA or SFA. The participants were grouped into two categories based on the median initial BMI (<20.14 and ≥20.14 kg m−2). The unpaired Student's t‐test was used to compare the post‐18 weight changes seen in the two groups. In each group, two‐way analysis of covariance (ancova) was used to evaluate the effects of the interactions between (i) the initial BMI and post‐18 weight gain and (ii) the initial BMI and current BMI on the participants' VFA and SFA after controlling for age. Regression lines were produced using multiple linear regression analysis, and the associated regression coefficients were compared. Comparisons between frequencies were performed using the chi‐squared test. The data were analysed using the SAS statistical software package (release 9.3; SAS Institute, Cary, NC, USA). Probability values of <0.05 were regarded as significant.
Ethics
This study was performed in accordance with the World Medical Association Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. The purpose of this study and the procedures for each examination were fully explained to the participants. All of the participants provided written informed consent. All data were analysed collectively, and the participants' privacy was protected. All of the procedures conducted in the NILS‐LSA were approved by the committee of ethics for human research at the National Center for Geriatrics and Gerontology.
Results
The participants' characteristics are shown in Table 1. Their mean initial BMI was 20.4 (SD: 2.3) skg m−2, and their mean current BMI was 23.3 (SD: 2.8) kg m−2. The mean post‐18 weight change was 8.0 (SD: 7.9) kg. The participants with initial BMI of <20.14 kg m−2 exhibited a greater mean post‐18 weight change than the participants with initial BMI of ≥20.14 kg m−2 (p < 0.001). The participants with initial BMI of <20.14 kg m−2 exhibited lower mean current BMI, VFA, SFA, and waist circumference values than the men with initial BMI of ≥20.14 kg m−2, but this was not true for percentage body fat. Table 2 shows data regarding post‐18 weight changes. Post‐18 weight increases of >10 kg were seen in 39% of men, and such increases were significantly more common among the men with initial BMI of <20.14 kg m−2 (49.3%) than among those with initial BMI of ≥20.14 kg m−2 (28.7%) (p < 0.001).
Table 1.
Participants' characteristics according to their BMI at 18 years of age
| BMI at 18 years of age | ||||
|---|---|---|---|---|
| All | <20.14 kg m−2 | ≥20.14 kg m−2 | ||
| n = 549 | n = 274 | n = 275 | p‐value | |
| Age (years) | 53.7 (6.5) | 53.4 (6.6) | 54.0 (6.3) | 0.331 |
| BMI (kg m−2) | 23.3 (2.8) | 22.4 (2.6) | 24.2 (2.6) | <0.001 |
| BMI at 18 years (kg m−2) | 20.4 (2.3) | 18.7 (1.0) | 22.1 (1.8) | <0.001 |
| Post‐18 weight change (kg) | 8.1 (7.9) | 10.3 (7.6) | 5.8 (7.6) | <0.001 |
| Visceral fat area (cm2) | 92.0 (45.3) | 86.8 (45.4) | 96.9 (44.6) | 0.009 |
| Subcutaneous fat area (cm2) | 114.6 (49.7) | 106.4 (46.2) | 122.4 (51.7) | 0.001 |
| Percentage body fat (%) | 20.8 (4.3) | 20.5 (4.3) | 21.1 (4.2) | 0.080 |
| Waist circumference (cm) | 85.2 (7.8) | 83.8 (7.6) | 86.6 (7.7) | <0.001 |
Data are shown as mean (standard deviation) values.
BMI, body mass index.
Table 2.
Frequency of weight change from 18 years of age to the present
| BMI at 18 years of age | |||||||
|---|---|---|---|---|---|---|---|
| Post‐18 weight change (kg) | All | <20.14 kg m−2 | ≥20.14 kg m−2 | ||||
| n | % | n | % | n | % | p‐value* | |
| <0.0 | 80 | 14.6 | 20 | 7.3 | 60 | 21.8 | <0.001 |
| 0–9.9 | 255 | 46.4 | 119 | 43.4 | 136 | 49.5 | |
| ≥10.0 | 214 | 39.0 | 135 | 49.3 | 79 | 28.7 | |
According to the chi‐squared test.
BMI, body mass index.
The post‐18 weight change was positively correlated with VFA, SFA, and waist circumference (after controlling for age and initial BMI), and it was negatively correlated with the initial BMI (after controlling for age) (Table 3). Two‐way ancova demonstrated that the interaction between the initial BMI (<20.14 and ≥20.14 kg m−2) and post‐18 weight change was significantly associated with VFA (p = 0.026), but not SFA (p = 0.554), after controlling for age (Table 4). The regression coefficient for the relationship between post‐18 weight change and VFA was significantly larger among the participants with initial BMI of <20.14 kg m−2 (β = 4.29, standard error [SE] = 0.24) than among those with initial BMI of ≥20.14 kg m−2 (β = 3.60, SE = 0.31) (Figure 2). In the same way, two‐way ancova demonstrated that the interaction between initial BMI (<20.14 and ≥20.14 kg m−2) and current BMI was not significantly associated with VFA (p = 0.584) or SFA (p = 0.436), after controlling for age (Table 4). However, the men with initial BMI of <20.14 kg m−2 demonstrated significantly larger (98.3 and 120.0 cm2, respectively) least means square values for VFA and SFA than the men with initial BMI of ≥20.14 kg m−2 (85.9 and 108.2 cm2, respectively).
Table 3.
Spearman's correlation coefficients for the relationships among the post‐18 weight change, BMI at 18 years old, visceral fat area, subcutaneous fat area and waist circumference after controlling for age and BMI at 18 years of age
| Post‐18 weight change (kg) | ||
|---|---|---|
| BMI at 18 years of age† | −0.344 | p < 0.001 |
| Visceral fat area (cm2)‡ | 0.691 | p < 0.001 |
| Subcutaneous fat area (cm2)‡ | 0.781 | p < 0.001 |
| Waist circumference (cm)‡ | 0.851 | p < 0.001 |
After controlling for age.
After controlling for age and BMI at 18 years of age.
BMI, body mass index.
Table 4.
Two‐way factorial anova results
| F‐value | p‐value | ||
|---|---|---|---|
| Visceral fat area | BMI at 18 years | 61.67 | <0.001 |
| Post‐18 weight change | 418.73 | <0.001 | |
| Age | 28.99 | <0.001 | |
| BMI at 18 years * post‐18 weight change | 5.01 | 0.026 | |
| Subcutaneous fat area | BMI at 18 years | 80.86 | <0.001 |
| Post‐18 weight change | 598.39 | <0.001 | |
| Age | 0.15 | 0.697 | |
| BMI at 18 years * post‐18 weight change | 0.35 | 0.554 | |
| Visceral fat area | BMI at 18 years | 0.00 | 0.967 |
| BMI | 526.89 | <0.001 | |
| Age | 19.12 | <0.001 | |
| BMI at 18 years * BMI | 0.30 | 0.584 | |
| Subcutaneous fat area | BMI at 18 years | 1.74 | 0.188 |
| BMI | 1,103.38 | <0.001 | |
| Age | 5.68 | 0.018 | |
| BMI at 18 years * BMI | 0.61 | 0.435 |
ANOVA, analysis of variance; BMI, body mass index.
Figure 2.
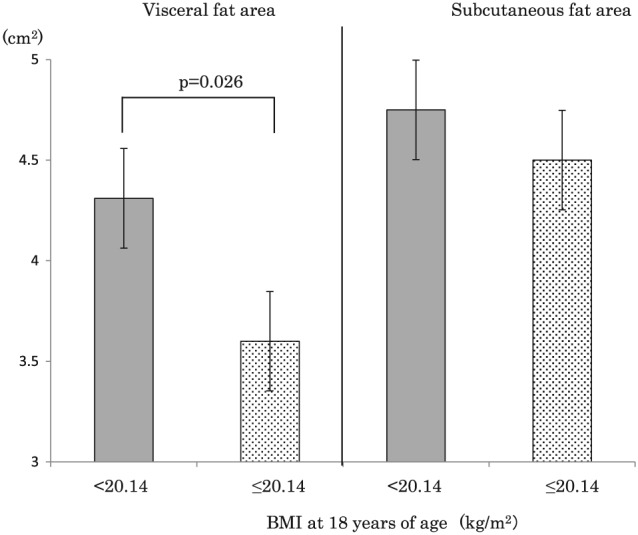
Estimated changes in visceral fat area and subcutaneous fat area according to each 1‐kg weight change as determined by regression analysis. BMI, body mass index.
Discussion
This study showed that the weight change that occurred in men during the period from 18 years of age to middle age (post‐18 weight change) was positively correlated with the increases in both VFA and SFA during the same period. However, VFA differed depending on the participants' weight at 18 years of age. The slope of the regression line for the relationship between post‐18 weight change (x) and VFA (y) was steeper in men with initial BMI of <20.14 kg m−2 (β = 4.29, SE: 0.24) than in those with initial BMI of ≥20.14 kg m−2 (β = 3.60, SE: 0.31). In other words, the VFA increase that accompanied the weight gain that occurred between the age of 18 and middle age was greater in men who were thin during early adulthood. Chei et al. found that weight gain of ≥10 kg was associated with a two‐fold greater risk of coronary heart disease in men with BMI of <21.7 kg m−2 at 20 years of age but not in men with BMI of ≥21.7 kg m−2 at 20 years of age 11. This result can be explained by the findings of the present study. Individuals with BMI of <21.7 kg m−2 at 20 years of age might possess more VF than those with BMI of ≥21.7 kg m−2. Furthermore, the post‐18 weight change was found to be negatively correlated with the initial BMI (r = −0.338) after controlling for age. This suggests that thin men are more likely to gain weight and accumulate VF during early adulthood than other men.
From the viewpoint of molecular biology, the total number of adipocytes increases in childhood and adolescence and then levels off and remains constant in adulthood 20. Subcutaneous adipose tissue storage acts as a buffer for excessive energy intake 21. In men who are thin when they are young, the number of fat cells might be relatively low. Therefore, fat cannot accumulate in SF cells alone and so might also accumulate in the abdominal cavity.
In order to prevent VF accumulation, it is important that men are not too thin during early adulthood and that their weight does not increase markedly between age 18 and middle age. Smoking habits also affect weight changes. In the present study, 39.0% of the participants were smokers, which was similar to the prevalence figure obtained in a national survey in 2016 (40 to 49 years of age: 41.1%; 50 to 59: 39.0%; 60 to 69: 28.9%) 1. Smoking might help to prevent weight gain or aid the maintenance of a low body weight 6, 7, 8. As a consequence, VFA might not increase in smokers. However, even if they exhibit little VF, smokers have higher blood haemoglobin A1c levels than those who do not smoke 22. Therefore, smoking in order to prevent weight gain is not advisable.
In this study, the association between VFA and current BMI was significantly stronger among the men with initial BMI of <20.14 kg m−2 than among the men with initial BMI of ≥21.14 kg m−2. In recent years, BMI and waist circumference have been used to diagnose obesity and abdominal adipose tissue accumulation. Post‐18 weight change might be another useful parameter for diagnosing obesity and abdominal adipose tissue accumulation. This highlights the fact that BMI does not provide any information about body composition or body fat distribution.
This study had some limitations. First, the participants' weights at 18 years of age were not measured; rather, they were self‐reported using a questionnaire. However, Tamakoshi et al. reported that individuals were able to recall their past weights with good accuracy over long periods 23. Casey et al. also detected strong correlations between measured weight at 18 years of age and self‐reported recalled weight at 50 years of age 24. Second, the timing of the participants' post‐18 weight changes was not examined. Previous studies have reported that weight fluctuations were associated with an increased risk of all‐cause mortality. Zhang et al. found that among Japanese men aged 40–49 years, the development of metabolic syndrome might explain the increased risk of cardiovascular disease, not only in people with overweight or obesity but also in normal‐weight people that exhibit large weight fluctuations 25.
In conclusion, in middle‐aged men, the weight change that occurs between 18 years of age and middle age was found to be positively correlated with the increases in VFA and SFA observed during the same period. SFA might increase with weight in a uniform manner. However, VF accumulation was affected not only by an individual's post‐18 weight gain but also by their initial BMI. Men who were thin in early adulthood experienced greater weight gain‐associated VFA increases, but the same was not true for SFA. Moreover, although the men with initial BMI of <20.14 kg m−2 exhibited lower current BMI, VFA, SFA and waist circumference values than the men with initial BMI of ≥21.14 kg m−2, the association between VFA and current BMI was stronger among the men with initial BMI of <20.14 kg m−2.
Conflicts of Interest Statement
None declared.
Acknowledgements
We would like to thank the participants and our colleagues at the NILS‐LSA. This study was supported by a Grant‐in‐Aid for Comprehensive Research on Aging and Health from the Ministry of Health, Labour and Welfare.
Koda, M. , Kitamura, I. , Okura, T. , Otsuka, R. , Ando, F. , and Shimokata, H. (2018) Men who were thin during early adulthood exhibited greater weight gain‐associated visceral fat accumulation in a study of middle‐aged Japanese men. Obesity Science & Practice, 4: 289–295. doi: 10.1002/osp4.270.
References
- 1. Ministry of Health and Welfare . The National Nutrition Survey, Japan in 2014. http://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h28-houkoku-06.pdf (access February 13, 2018)
- 2. Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960–2002. Adv Data 2004: 1–17. [PubMed] [Google Scholar]
- 3. Drøyvold WB, Nilsen TI, Krüger O, et al. Change in height, weight and body mass index: longitudinal data from the HUNT Study in Norway. Int J Obes (Lond) 2006; 30: 935–939. [DOI] [PubMed] [Google Scholar]
- 4. Yarnell JW, Patterson CC, Thomas HF, Sweetnam PM. Comparison of weight in middle age, weight at 18 years, and weight change between, in predicting subsequent 14 year mortality and coronary events: Caerphilly Prospective Study. J Epidemiol Community Health 2000; 54: 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koh‐Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol 2004; 159: 1150–1159. [DOI] [PubMed] [Google Scholar]
- 6. Brozek J, Keys A. Changes of body weight in normal men who stop smoking cigarettes. Science 1957; 125: 1203. [DOI] [PubMed] [Google Scholar]
- 7. Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health 1987; 77: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med 1991; 324: 739–745. [DOI] [PubMed] [Google Scholar]
- 9. Koda M, Ando F, Niino N, Shimokata H, Miyasaka K, Funakoshi A. Association of cholecystokinin 1 receptor and beta3‐adrenergic receptor polymorphisms with midlife weight gain. Obes Res 2004; 12: 1212–1216. [DOI] [PubMed] [Google Scholar]
- 10. Rosengren A, Wedel H, Wilhelmsen L. Body weight and weight gain during adult life in men in relation to coronary heart disease and mortality. A prospective population study. Eur Heart J 1999; 20: 269–277. [PubMed] [Google Scholar]
- 11. Chei CL, Iso H, Yamagishi K, Inoue M, Tsugane S. Body mass index and weight change since 20 years of age and risk of coronary heart disease among Japanese: the Japan Public Health Center‐based Study. Int J Obes (Lond) 2008; 32: 144–151. [DOI] [PubMed] [Google Scholar]
- 12. Walker M, Wannamethee G, Whincup PH, Shaper AG. Weight change and risk of heart attack in middle‐aged British men. Int J Epidemiol 1995; 24: 694–703. [DOI] [PubMed] [Google Scholar]
- 13. Galanis DJ, Harris T, Sharp DS, Petrovitch H. Relative weight, weight change, and risk of coronary heart disease in the Honolulu Heart Program. Am J Epidemiol 1998; 147: 379–386. [DOI] [PubMed] [Google Scholar]
- 14. Klein S. The case of visceral fat: argument for the defense. J Clin Invest 2004; 113: 1530–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 2005; 165: 777–783. [DOI] [PubMed] [Google Scholar]
- 16. Shimokata H, Ando F, Niino N. A new comprehensive study on aging – the National Institute for Longevity Sciences, Longitudinal Study of Aging (NILS‐LSA). J Epidemiol 2000; 10: S1–S9. [DOI] [PubMed] [Google Scholar]
- 17. Koda M, Ando F, Shimokata H, Kuzuya F. The effects of aging on the relationship between changes in body weight, serum lipid levels, and blood pressure. Nihon Ronen Igakkai Zasshi 1998; 35: 631–636. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 18. Yosizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology 1999; 211: 283–286. [DOI] [PubMed] [Google Scholar]
- 19. Okura T, Koda M, Ando F, Niino N, Shimokata H. Relationships of resting energy expenditure with body fat distribution and abdominal fatness in Japanese population. J Physiol Anthropol 2003; 22: 47–52. [DOI] [PubMed] [Google Scholar]
- 20. Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008; 453: 783–787. [DOI] [PubMed] [Google Scholar]
- 21. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000; 21: 697–738. [DOI] [PubMed] [Google Scholar]
- 22. Koda M, Kitamura I, Okura T, Otsuka R, Ando F, Shimokata H. The associations between smoking habits and serum triglyceride or hemoglobin A1c levels differ according to visceral fat accumulation. J Epidemiol 2016; 26: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tamakoshi K, Yatsuya H, Kondo T, et al. The accuracy of long‐term recall of past body weight in Japanese adult men. Int J Obes Relat Metab Disord 2003; 27: 247–252. [DOI] [PubMed] [Google Scholar]
- 24. Casey VA, Dwyer JT, Berkey CS, Coleman KA, Gardner J, Valadian I. Long‐term memory of body weight and past weight satisfaction: a longitudinal follow‐up study. Am J Clin Nutr 1991; 53: 1493–1498. [DOI] [PubMed] [Google Scholar]
- 25. Zhang H, Tamakoshi K, Yatsuya H, et al. Long‐term body weight fluctuation is associated with metabolic syndrome independent of current body mass index among Japanese men. Circ J 2005; 69: 13–18. [DOI] [PubMed] [Google Scholar]


