Abstract
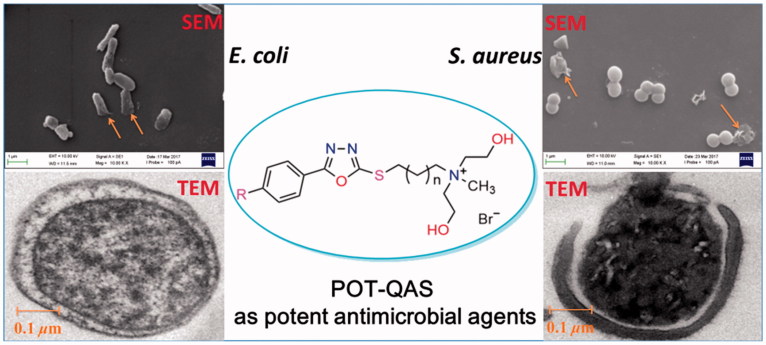
Twenty-four novel 5-phenyl-1,3,4-oxadiazole-2-thiol (POT) analogues, benzo[d]oxazole-2-thiol, benzo[d]thiazole-2-thiol and 5-methyl-1,3,4-thiadiazole-2-thiol-substituted N,N-bis(2-hydroxyethyl) quaternary ammonium salts (QAS) (5a-d, 6a-d, 7a-d, 10a-d, 13a-d, 16a-d) were prepared and characterised by FTIR, NMR and elemental analysis. Part of target compounds (5d, 6d, 7d, 10d, 13d, 16d) displayed potent antimicrobial effect against ten common pathogens (S. aureus, α-H-tococcus, β-H-tococcus, E. coli, P. aeruginosa, Proteus vulgaris, Canidia Albicans, Cytospora mandshurica, Physalospora piricola, Aspergillus niger) and had relatively low cytotoxity against two human cell lines (HaCat and LO2). TEM and SEM images of E. coli and S. aureus morphologies treated with 7d showed that the antibacterial mechanism might be the QAS fixing on cell wall surfaces and puncturing to result in the release of bacterial cytoplasm. This study provides new information of QAS, which could be used to design novel antimicrobial agents applied in clinic or agriculture.
Keywords: 5-Phenyl-1,3,4-oxadiazole-2-thiol; quaternary ammonium salts; antibacterial activity; antifungal activity; cytotoxicity
Introduction
As one of the leading causes of death worldwide, outbreaks of infectious diseases triggered by bacteria, viruses and fungi lead to over one-fourth of global deaths annually 1 , 2 . Thus, there is an urgent demand for exploring more efficient, broad-spectrum and long-lasting antimicrobial agents. Because of excellent antibacterial activities, quaternary ammonium salts (QAS) are widely used by the pharmaceutical industry for their recognised activity against fungi, bacteria, viruses and parasites 3 . For example, QASs are used as antiseptics and disinfectants of medical apparatus and instruments, and a variety of clinical application, such as preoperative disinfection of unbroken skin and trauma 4–9 . With more and more resistant organisms continue to emerge on clinic, the ideal antimicrobial agents should have to possess strong antibacterial activity, be safe in relation to humans and should not persist in the environment for a long time 10 . Some of the quaternary ammonium compounds fulfil these conditions have been invented and received increasing attentions as antibacterial agents 11–13 . Especially, long-chain alkyl-QAS compounds show unsurpassed antibacterial effects. It is well-known that the antibacterial activity and drug toxicity are influenced by the alkyl chain length of these agents 14 , 15 . As the growth of the alkyl chains, the antibacterial activities gradually increase, until reaching a reasonable limit. Unfortunately, drug toxicity of QAS bearing longer-chain alkyl groups also strengthen as well as benzyl-QAS. Tremendous research efforts have been devoted to development of alternative antibacterial therapeutics that are not easily forming resistance, such as quaternary ammonium salts (QAS), which destruct cell membrane of bacteria and induce the leakage of intracellular components from bacterial cells 16 . Thus, it becomes urgent to design and prepare one kind of novel antibacterial with higher antibacterial activities and lower drug toxicity. Many discrete QAS 17 and polymerised quaternised materials (such as quaternised chitosan 18 , 19 ) were reported with good antibacterial activities because of quick disinfection and sterilisation.
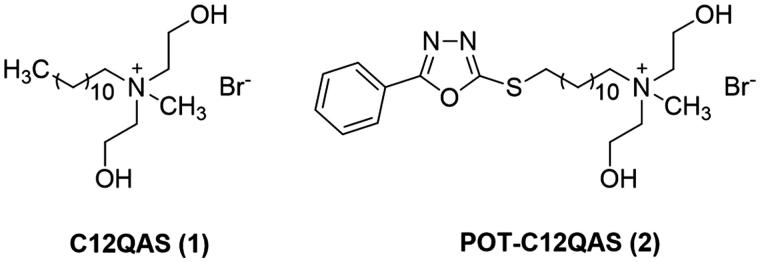
In the previous work of our group, N-methyl-N-R-N,N-bis(2-hydroxyethyl) ammonium bromides (BNQAS, C12QAS, C14QAS, C16QAS, C18QAS) were prepared from N-methyldiethanolamine and halohydrocarbon with long-chain alkyl bromides 20 . These QASs showed good antibacterial abilities against E. coli, S. aureus, B. subtilis and antifungal activities against Cytospora mandshurica, Botryosphaeria ribis, Physalospora piricola and Glomerella cingulata. The strategy provided a facile way to design and develop new types of antimicrobial agents for application in preventing the fruit rot, especially apple. Meanwhile, these QASs also were used to prepare nontoxic O-quaternised antimicrobial chitosan materials 21 . Simultaneously, C12QAS (1, Figure 1) with the best antibacterial abilities was also used to prepare novel block antibacterial polyurethane (BAPU) and terminated antibacterial polyurethane (TAPU) through prepolymerisation reaction 19 . However, QAS monomers display higher cytotoxicity towards normal cell lines 20 , which may limit their clinical utility as antibacterial material. So it is a big challenge that how to improve their antimicrobial activities on the basis of lower cytotoxicity.
Figure 1.
Representative target compounds in previous work.
Motivated by our interest in the antimicrobial agents, chemical modifications of QAS monomers were carried out immediately. Due to the quaternary ammonium group was a key active group, N,N-bis(2-hydroxyethyl) ammonium bromide of C12QAS (1, Figure 1) was retained. Studied through literature researches, we found many 1,3,4-oxadiazole-2-thiol derivatives were reported as potent inhibitors of Gram-negative and Gram-positive bacteria 22–25 . Very recently, we tried to change long-chain alkyl group of C12QAS (1, Figure 1) into 5-phenyl-1,3,4-oxadiazole-2-thiol (POT) 26 substituted alkyl groups and synthesised dozens of POT-QASs 27 . Among these, bearing N,N-bis(2-hydroxyethyl) and POT groups, POT-C12QAS (2, Figure 1) displayed unsurpassed antimicrobial activities and low cytotoxicity. The structure–activity relationships indicated that POT and flexible dihydroxyethyl group in QAS were necessary for antibacterial activities.
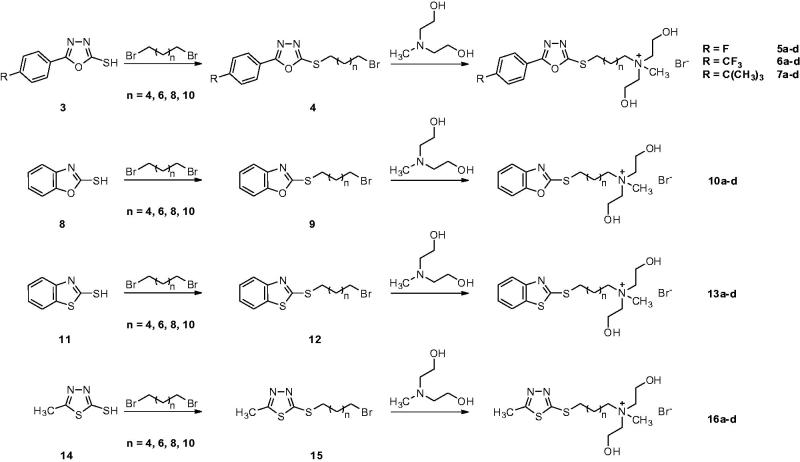
Furthermore, in order to improve antimicrobial activities and decrease cytotoxicity, we tried to modify or replace the POT fragment in POT-QAS with 5-(4-fluorophenyl)-1,3,4-oxadiazole-2-thiol (POTF), 5-(4-trifluoromethyl phenyl)-1,3,4-oxadiazole-2-thiol (POTT), 5-(4-tert-butylphenyl)-1,3,4-oxadiazole-2-thiol (POTB), benzo[d]oxazole-2-thiol, benzo[d]thiazole-2-thiol and 5-methyl-1,3,4-thiadiazole-2-thiol fragments. Based on the consideration, six series of novel QASs (5a-d, 6a-d, 7a-d, 10a-d, 13a-d, 16a-d, Scheme 1) were prepared. These novel QASs were characterised with proton nuclear magnetic resonance (1H NMR and 13C NMR) pectroscopy, stransmission Fourier transform infrared (FTIR) spectra, elemental analyses and melting point. Their antibacterial, antifungal properties and cytotoxicity were tested and the antibacterial mechanism of quaternary ammonium salts to E. coli and S. aureus was explored by SEM and TEM.
Scheme 1.
The synthesis and structures of six series of noval QASs.
Results and discussion
Structural analysis
In this study, 24 5-phenyl-1,3,4-oxadiazole-2-thiol (POT) analogues, benzo[d]oxazole-2-thiol, benzo[d]thiazole-2-thiol and 5-methyl-1,3,4-thiadiazole-2-thiol substituted N,N-bis(2-hydroxyethyl) quaternary ammonium salts (5a-d, 6a-d, 7a-d, 10a-d, 13a-d, 16a-d) were prepared from Aromatic thiols, α,ω-dibromoalkanes and N-methyldiethanolamine in good yields (Scheme 1). Their structures were characterised by FTIR, NMR and elemental analysis. From the FTIR spectra, strong characteristic absorption band of hydroxyl and stretching vibration band of C-O bonds can be found. For example, the two bands of 5a lie in 3307 cm−1 and 1054 cm−1, respectively, which demonstrates the existence of –OH of QAS. In addition, the distinct band in 788 cm−1 belongs to the stretching vibration band of linear paraffin in FTIR spectra of 5a. In other QAS, the stretching vibration bands of linear paraffin are 731 cm−1 (6a), 739 cm−1 (7a), 756 cm−1 (10a), 741 cm−1 (13a) and 729 cm−1 (16a), respectively. In 1H NMR spectra, the single peak of hydroxy of 5a can be found at δ 5.31 ppm, the multiple peaks of benzene ring disappeared in 1H NMR spectra at δ 8.16–7.22 ppm, and the single peaks of methyl of 5a at δ 3.06 ppm. The similar structure characteristic also can prove their structure for other compounds and they are shown in experimental part. Additionally, 13C NMR, element analysis further confirms the correctness of their structures.
Antimicrobial properties
In this paper, antimicrobial activities of 24 QASs were detected by quantitative tests. The data of minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for 24 QASs against Gram-positive bacteria (S. aureus, α-H-tococcus, β-H-tococcus), Gram-negative bacteria (E. coli, P. aeruginosa, Proteus vulgaris) and fungi (Canidia albicans, Cytospora mandshurica, Physalospora piricola, Aspergillus niger) were obtained as shown in Tables 1 and 2, respectively. Benzalkonium chloride (BZK) and chlorhexidine acetate (CA) was selected as positive controls. Representative compound POT-C12QAS (2) which synthesised and evaluated in previous work was also taken as a reference compound in MIC, MBC and cytotoxicity tests.
Table 1.
Minimal inhibitory concentration (MIC) of QASs (μg/mL).
| Compd | S. aureus | α-H-tococcus | β-H-tococcus | E. coli | P. aeruginosa | Proteus vulgaris | Canidia albicans | Cytospora mandshurica | Physalospora piricola | Aspergillus niger |
|---|---|---|---|---|---|---|---|---|---|---|
| BZK | 3.125 | 3.125 | 1.56 | 25 | 25 | 25 | 12.5 | 6.25 | 3.125 | 3.125 |
| CA | 3.125 | 6.25 | 1.56 | 6.25 | 25 | 12.5 | 12.5 | 6.25 | 1.56 | 3.125 |
| 2 | 6.25 | 6.25 | 6.25 | 25 | 12.5 | 25 | 6.25 | 3.125 | 3.125 | 3.125 |
| 5a | 100 | 50 | 25 | 100 | 100 | 100 | 50 | 50 | 12.5 | 50 |
| 5b | 25 | 12.5 | 12.5 | 25 | 50 | 50 | 25 | 25 | 6.25 | 50 |
| 5c | 25 | 12.5 | 6.25 | 25 | 50 | 50 | 25 | 12.5 | 3.125 | 25 |
| 5d | 12.5 | 12.5 | 6.25 | 25 | 50 | 25 | 12.5 | 12.5 | 3.125 | 25 |
| 6a | 50 | 50 | 25 | 50 | 100 | 100 | 50 | 12.5 | 6.25 | 25 |
| 6b | 25 | 12.5 | 12.5 | 25 | 50 | 50 | 25 | 12.5 | 3.125 | 12.5 |
| 6c | 25 | 12.5 | 6.25 | 25 | 50 | 25 | 25 | 12.5 | 3.125 | 12.5 |
| 6d | 6.25 | 6.25 | 3.125 | 25 | 50 | 25 | 12.5 | 6.25 | 1.56 | 6.25 |
| 7a | 50 | 25 | 12.5 | 50 | 50 | 50 | 25 | 12.5 | 12.5 | 25 |
| 7b | 25 | 12.5 | 6.25 | 25 | 50 | 25 | 12.5 | 6.25 | 3.125 | 12.5 |
| 7c | 12.5 | 12.5 | 3.125 | 25 | 50 | 25 | 6.25 | 6.25 | 1.56 | 6.25 |
| 7d | 6.25 | 6.25 | 1.56 | 12.5 | 25 | 12.5 | 6.25 | 3.125 | 1.56 | 6.25 |
| 10a | 100 | 50 | 25 | 100 | 100 | 100 | 50 | 50 | 25 | 50 |
| 10b | 50 | 25 | 12.5 | 50 | 50 | 50 | 50 | 25 | 12.5 | 25 |
| 10c | 25 | 25 | 6.25 | 50 | 50 | 50 | 25 | 6.25 | 6.25 | 12.5 |
| 10d | 12.5 | 12.5 | 6.25 | 25 | 25 | 25 | 12.5 | 6.25 | 3.125 | 6.25 |
| 13a | 50 | 50 | 12.5 | 50 | 100 | 100 | 25 | 25 | 25 | 25 |
| 13b | 25 | 25 | 12.5 | 50 | 50 | 50 | 25 | 6.25 | 6.25 | 12.5 |
| 13c | 12.5 | 25 | 6.25 | 25 | 25 | 25 | 12.5 | 3.125 | 3.125 | 6.25 |
| 13d | 6.25 | 12.5 | 1.56 | 25 | 25 | 25 | 6.25 | 1.56 | 3.125 | 3.125 |
| 16a | 100 | 50 | 50 | 100 | 200 | 100 | 50 | 50 | 25 | 50 |
| 16b | 50 | 50 | 25 | 50 | 100 | 50 | 25 | 25 | 12.5 | 25 |
| 16c | 25 | 25 | 6.25 | 50 | 50 | 50 | 25 | 12.5 | 12.5 | 12.5 |
| 16d | 12.5 | 12.5 | 6.25 | 25 | 50 | 25 | 12.5 | 12.5 | 6.25 | 12.5 |
Table 2.
Minimal bactericidal concentration (MBC) of QASs (μg/mL).
| Compd | S. aureus | α-H-tococcus | β-H-tococcus | E. coli | P. aeruginosa | Proteus vulgaris | Canidia albicans | Cytospora mandshurica | Physalospora piricola | Aspergillus niger |
|---|---|---|---|---|---|---|---|---|---|---|
| BZK | 3.125 | 3.125 | 1.56 | 25 | 25 | 25 | 25 | 6.25 | 3.125 | 6.25 |
| CA | 6.25 | 6.25 | 3.125 | 12.5 | 25 | 12.5 | 12.5 | 6.25 | 3.125 | 6.25 |
| 2 | 12.5 | 6.25 | 6.25 | 50 | 25 | 50 | 12.5 | 6.25 | 3.125 | 3.125 |
| 5a | 100 | 50 | 25 | 100 | 100 | 100 | 50 | 50 | 12.5 | 50 |
| 5b | 50 | 25 | 12.5 | 50 | 50 | 50 | 25 | 25 | 6.25 | 50 |
| 5c | 25 | 12.5 | 12.5 | 50 | 50 | 50 | 25 | 12.5 | 6.25 | 25 |
| 5d | 12.5 | 12.5 | 6.25 | 25 | 50 | 25 | 12.5 | 12.5 | 3.125 | 25 |
| 6a | 50 | 50 | 25 | 50 | 100 | 100 | 50 | 25 | 12.5 | 25 |
| 6b | 25 | 25 | 12.5 | 50 | 50 | 50 | 25 | 12.5 | 6.25 | 25 |
| 6c | 25 | 12.5 | 6.25 | 25 | 50 | 25 | 25 | 12.5 | 3.125 | 12.5 |
| 6d | 12.5 | 6.25 | 3.125 | 25 | 50 | 25 | 12.5 | 6.25 | 1.56 | 6.25 |
| 7a | 50 | 25 | 12.5 | 50 | 50 | 50 | 25 | 12.5 | 12.5 | 25 |
| 7b | 25 | 12.5 | 6.25 | 25 | 50 | 25 | 12.5 | 12.5 | 3.125 | 12.5 |
| 7c | 12.5 | 12.5 | 6.25 | 25 | 50 | 25 | 12.5 | 6.25 | 3.125 | 12.5 |
| 7d | 6.25 | 6.25 | 1.56 | 12.5 | 25 | 12.5 | 6.25 | 3.125 | 1.56 | 6.25 |
| 10a | 100 | 50 | 25 | 100 | 200 | 100 | 100 | 50 | 25 | 50 |
| 10b | 50 | 50 | 25 | 100 | 100 | 50 | 50 | 25 | 25 | 25 |
| 10c | 25 | 25 | 12.5 | 50 | 50 | 50 | 25 | 12.5 | 6.25 | 12.5 |
| 10d | 12.5 | 12.5 | 6.25 | 25 | 25 | 25 | 12.5 | 6.25 | 6.25 | 12.5 |
| 13a | 50 | 50 | 25 | 100 | 100 | 100 | 50 | 25 | 25 | 25 |
| 13b | 50 | 25 | 12.5 | 50 | 100 | 50 | 25 | 12.5 | 12.5 | 12.5 |
| 13c | 12.5 | 25 | 6.25 | 25 | 50 | 25 | 25 | 6.25 | 3.125 | 6.25 |
| 13d | 6.25 | 12.5 | 3.125 | 25 | 25 | 25 | 6.25 | 6.25 | 3.125 | 6.25 |
| 16a | 200 | 100 | 50 | 200 | 200 | 200 | 50 | 50 | 25 | 50 |
| 16b | 100 | 50 | 25 | 100 | 100 | 50 | 50 | 25 | 25 | 25 |
| 16c | 25 | 25 | 12.5 | 50 | 50 | 50 | 25 | 25 | 12.5 | 25 |
| 16d | 25 | 12.5 | 6.25 | 25 | 50 | 25 | 12.5 | 12.5 | 6.25 | 12.5 |
For the six series of QASs (5a-d, 6a-d, 7a-d, 10a-d, 13a-d, 16a-d), all of their structures contained nitrogen-containing heterocyclics and N,N-bis(2-hydroxyethyl) quaternary ammonium salt pharmacophores linked by medium- or long-chain alkyl groups (hexyl, octyl, decyl and dodecyl). Compared with our previous research, similar result was found that their MIC and MBC values decreased in turn following the increase of chain length. In other words, compounds 5d, 6d, 7d, 10d, 13d and 16d could regard as the most potent compounds in each series.
Although compounds 5a-d, 6a-d and 7a-d belonged to POT-QAS derivatives, they exhibited diverse antibacterial activities. For Gram-positive bacteria (S. aureus, α-H-tococcus, β-H-tococcus) and Gram-negative bacteria (E. coli, P. aeruginosa, Proteus vulgaris), compound 7d bearing with 5-(4-tert-butylphenyl)-1,3,4-oxadiazole-2-thiol (POTB) substituent group exhibited the best antibacterial activities among all six series (corresponding MIC values, Table 2). It is just similar to unmodified POT-C12QAS: ca. 6.25 μg/mL against S. aureus, α-H-tococcus ca. 1.56 μg/mL against β-H-tococcus; ca. 12.5 μg/mL against E. coli, Proteus vulgaris, ca. 25 μg/mL against P. aeruginosa, Table 1; MBC values were equal to corresponding MIC values, Table 2. It should be pointed out that the antibacterial activities of 7d were comparable to positive controls (BZK and CA), and more potent than unmodified POT-C12QAS (2). Furthermore, Introduced halogen substitution, especially fluoro substitution into the structure was hopeful to enhance the antibacterial effect 28 , 29 . But to our disappointment, fluoro (5d) or trifluoromethyl (6d) substituted derivatives did not exhibit desired antibacterial activities, and their MIC and MBC values only reached to medium level (e.g. MIC values for 5d: ca. 12.5 μg/mL against S. aureus, α-H-tococcus, ca. 6.25 μg/mL against β-H-tococcus; ca. 25 μg/mL against E. coli, Proteus vulgaris, ca. 50 μg/mL against P. aeruginosa, Table 1). These foregoing results suggest that electron-donating substituents (tertiary butyl) on POT might enhance antibacterial activities of POT-QAS; on the contrary, electron-withdrawing substituents (fluoro or trifluoromethyl) might give little contribution to the activities.
In order to investigate the influence of other nitrogen heterocyclics substituted in QAS, POT fragment was changed into benzo[d]oxazole-2-thiol (10a-d), benzo[d]thiazole-2-thiol (13a-d) or 5-methyl-1,3,4-thiadiazole-2-thiol (16a-d), respectively. Among these three non-POT series, compound 13d exhibited relatively potent antibacterial activities (MIC: ca. 6.25 μg/mL against S. aureus, ca. 12.5 μg/mL against α-H-tococcus, ca. 1.56 μg/mL against β-H-tococcus; ca. 25 μg/mL against E. coli, Proteus vulgaris, P. aeruginosa, Table 1; MBC: ca. 3.125 μg/mL against β-H-tococcus, other MBC values were equal to corresponding MIC values, Table 2), which was close to positive controls (BZK and CA). However, besides compound 10d possessed fairly activities against Gram-negative bacteria (MIC: ca. 25 μg/mL against E. coli, Proteus vulgaris, P. aeruginosa, Table 1; MBC values were equal to corresponding MIC values, Table 2), other non-POT derivatives did not exhibit noticeable activities. Based on the above results, POT-QAS derivatives were partially superior to non-POT-QAS derivatives, and this might be because POT-QAS derivatives with flexible alkyl chain length and POT fragment could more easily pass through cell membrane into the cell to damage bacteria 20 , 27 , 30–32 .
In the antifungal activity assay of the target compounds, four strains of common fungi in clinical practice and agriculture (Canidia albicans, Cytospora mandshurica, Physalospora piricola, Aspergillus niger) were tanken as the target fungi. As shown in Tables 1 and 2, to our delight, most compounds bearing with the long-chain alkyl group (dodecyl, e.g. 5d, 6d, 7d, 10d, 13d and 16d) displayed fairly good antifungal activities. The most noticeable compound was 13d (MIC: ca. 6.25 μg/mL against Canidia albicans, ca. 1.56 μg/mL against Cytospora mandshurica, ca. 3.125 μg/mL against Physalospora piricola, Aspergillus niger, Table 1; MBC: ca. 6.25 μg/mL against Cytospora mandshurica, other MBC values were equal to corresponding MIC values, Table 2), with a 2-mercaptobenzothiazole substituent in C12QAS, exhibited more potent activities than positive controls (BZK and CA). In addition, POT-C12QAS (2) derivative 7d (MIC: ca. 6.25 μg/mL against Canidia albicans, ca. 3.125 μg/mL against Cytospora mandshurica, ca. 1.56 μg/mL against Physalospora piricola, ca. 6.25 μg/mL against Aspergillus niger, Table 1; MBC values were equal to corresponding MIC values, Table 2) also displayed almost the same antifungal activities as positive controls (BZK and CA). These encouraging results indicated that either POTT (7d) or 2-mercaptobenzothiazole (13d) substituent in C12QAS could enhance the antifungal activities. More than that, both of which might develop into promising antifungal agents in clinic or agriculture.
Cytotoxicity
These six series of antimicrobial compounds may be applied to daily or clinical disinfectants for unbroken skin and trauma, or agricultural fungicides alternatively. Thus, their cytotoxicity for skin or body tissues are crucial. In this study, the cytotoxicity of target compounds was evaluated for human immortalised epidermal (HaCat) and human normal liver (LO2) cell line using MTT assay. The results were showed in Table 3 summarising the corresponding IC50 values of QASs. The IC50 values of 5a, 6a, 10a, 16a, 16 b were about more than 50 μg/mL for LO2 and HaCat. In addition, compounds 5 b, 6 b, 10 b, 13a, 16c, 16d also showed lower IC50 values (more than 25 μg/mL). These data demonstrated that compounds 5a, 6a, 16a, 16 b were non-toxic for LO2 and HaCat. Especially, lower-toxic compounds 5 b, 6 b, 16c, 16d with better activities might become superior antimicrobial agents. However, the IC50 values of each series for LO2 and HaCat decreased gradually and finally reached to 4.16 μg/mL. For example, the IC50 values of 7a-d were orderly 44.26 ± 3.31 μg/mL, 16.78 ± 1.35 μg/mL, 12.90 ± 1.37 μg/mL, 5.08 ± 0.51 μg/mL for LO2 as well as 23.84 ± 2.13 μg/mL, 9.05 ± 0.92 μg/mL, 6.94 ± 0.53 μg/mL,4.16 ± 0.43 μg/mL for HaCat, respectively. These results indicated that their cytotoxicity and antimicrobial activities synchronously increased following the increased of chain length from hexyl group to dodecyl group 27 , and all of them exhibited weaker cytotoxicity compared with positive controls (BZK and CA). In addition, it should be pointed out that compounds 7d and POT-C12QAS (2) with POT and dodecyl group displayed weaker cytotoxicity than N-methyl-N-dodecyl-N,N-bis(2-hydroxyethyl) ammonium bromides (C12QAS), which IC50 value was ca. 4.00 μg/mL 20 . This result proved that introduction of POT fragment in C12QAS could effectively reduce cytotoxicity and improve antimicrobial activities.
Table 3.
Cytotoxicity of QASs for HaCat and LO2 cells.
| IC50 (μg/mL) |
IC50 (μg/mL) |
IC50 (μg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|
| Compd | LO2 | HaCat | Compd | LO2 | HaCat | Compd | LO2 | HaCat |
| BZK | 2.12 ± 0.43 | 3.19 ± 0.26 | CA | 2.06 ± 0.06 | 2.54 ± 0.12 | 2 | 14.23 ± 2.15 | 18.27 ± 2.21 |
| 5a | 83.51 ± 5.67 | 76.52 ± 4.58 | 7a | 44.26 ± 3.31 | 23.84 ± 2.13 | 13a | 64.28 ± 4.12 | 45.06 ± 3.23 |
| 5b | 46.75 ± 3.32 | 50.83 ± 3.65 | 7b | 16.78 ± 1.35 | 9.05 ± 0.92 | 13b | 29.58 ± 2.86 | 17.84 ± 1.56 |
| 5c | 23.86 ± 1.68 | 19.39 ± 1.23 | 7c | 12.90 ± 1.37 | 6.94 ± 0.53 | 13c | 16.84 ± 1.61 | 10.51 ± 1.02 |
| 5d | 6.95 ± 0.62 | 6.14 ± 0.53 | 7d | 5.08 ± 0.51 | 4.16 ± 0.43 | 13d | 8.96 ± 0.46 | 6.02 ± 0.35 |
| 6a | 74.35 ± 4.12 | 62.57 ± 3.82 | 10a | 88.27 ± 5.89 | 54.62 ± 3.16 | 16a | >100 | >100 |
| 6b | 38.72 ± 2.92 | 32.57 ± 2.69 | 10b | 42.92 ± 2.37 | 30.38 ± 2.89 | 16b | 86.52 ± 5.33 | 65.38 ± 3.12 |
| 6c | 19.56 ± 1.62 | 12.06 ± 1.57 | 10c | 25.32 ± 1.78 | 15.86 ± 1.23 | 16c | 43.18 ± 2.67 | 36.87 ± 2.81 |
| 6d | 5.86 ± 0.61 | 5.25 ± 0.37 | 10d | 9.96 ± 0.76 | 7.14 ± 0.51 | 16d | 30.12 ± 2.32 | 27.03 ± 2.53 |
Haemolysis
The surfactant may be absorbed and penetrate to the cell membrane, where it makes osmotic phenomena by altering the permeability of membrane, which in turn causes the cellular lysis 33 . Haemolysis assay gives quantitative estimation about haemoglobin release when red blood cells are treated with QASs. Haemolytic assay of QASs at different concentration was carried out in Table 4. It was observed that the haemolysis rate of these samples increased successively following the increase of concentration, which showed concentration-dependent haemolysis. More importantly, all QASs did not cause haemolysis under the concentration of 50 μg/mL except 10d and 13d. For 5a-b, 6a-b, 10a-c, 13a-b, and 16a-d, their safe concentration can more than 100 μg/mL. Compared with BZK and CA, all QASs were safe to be used when they circulated into the blood in low concentration.
Table 4.
Haemolysis rate (%) of QASs with different concentration (μg/mL).
| Haemolysis rate (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Compd | 6.25 | 12.50 | 25.00 | 50.00 | 100.00 | 200.00 | 400.00 |
| BZK | 1.51 ± 0.31 | 1.39 ± 0.38 | 12.69 ± 1.87 | 63.85 ± 4.56 | 92.36 ± 3.78 | 96.28 ± 2.97 | 98.53 ± 1.23 |
| CA | 0.86 ± 0.28 | 2.51 ± 0.87 | 5.29 ± 1.22 | 37.05 ± 3.78 | 62.18 ± 3.53 | 86.02 ± 3.82 | 95.67 ± 3.54 |
| 2 | 0.18 ± 0.05 | 0.42 ± 0.13 | 0.93 ± 0.41 | 4.11 ± 0.89 | 17.07 ± 2.34 | 82.75 ± 3.18 | 90.61 ± 4.23 |
| 5a | 0.21 ± 0.03 | 0.53 ± 0.15 | 1.37 ± 0.87 | 1.54 ± 0.96 | 3.25 ± 1.23 | 6.21 ± 3.21 | 35.64 ± 4.87 |
| 5b | 0.29 ± 0.08 | 0.68 ± 0.23 | 1.87 ± 1.35 | 2.19 ± 1.29 | 3.92 ± 1.02 | 21.08 ± 4.55 | 65.49 ± 4.13 |
| 5c | 0.43 ± 0.16 | 0.75 ± 0.19 | 2.31 ± 0.67 | 4.89 ± 0.53 | 33.13 ± 4.87 | 52.28 ± 6.34 | 67.15 ± 5.89 |
| 5d | 0.49 ± 0.12 | 0.87 ± 0.27 | 2.43 ± 0.82 | 4.81 ± 0.74 | 32.41 ± 6.78 | 66.71 ± 5.21 | 66.92 ± 4.12 |
| 6a | 0.15 ± 0.08 | 0.35 ± 0.17 | 0.51 ± 0.19 | 0.99 ± 0.12 | 3.38 ± 1.23 | 7.09 ± 2.89 | 40.96 ± 5.32 |
| 6b | 0.18 ± 0.06 | 0.51 ± 0.08 | 0.43 ± 0.10 | 0.70 ± 0.18 | 4.61 ± 0.31 | 26.16 ± 3.68 | 80.37 ± 4.98 |
| 6c | 0.57 ± 0.15 | 0.42 ± 0.13 | 0.67 ± 0.13 | 3.56 ± 1.32 | 35.76 ± 5.10 | 82.52 ± 6.12 | 89.39 ± 4.35 |
| 6d | 0.64 ± 0.19 | 1.05 ± 0.28 | 2.02 ± 3.45 | 4.85 ± 4.65 | 36.43 ± 6.35 | 85.62 ± 4.86 | 92.46 ± 3.87 |
| 7a | 1.03 ± 0.56 | 2.50 ± 1.08 | 1.01 ± 0.58 | 1.98 ± 0.68 | 5.66 ± 1.23 | 20.76 ± 3.28 | 34.73 ± 4.78 |
| 7b | 1.41 ± 0.43 | 1.48 ± 0.68 | 2.20 ± 0.68 | 2.59 ± 0.89 | 13.84 ± 2.73 | 30.63 ± 3.76 | 85.75 ± 5.67 |
| 7c | 0.18 ± 0.06 | 0.75 ± 0.19 | 1.19 ± 0.76 | 1.92 ± 0.87 | 14.08 ± 0.69 | 38.82 ± 3.12 | 79.03 ± 4.08 |
| 7d | 1.51 ± 0.91 | 1.71 ± 0.12 | 2.32 ± 1.86 | 4.77 ± 1.57 | 12.54 ± 4.79 | 39.62 ± 3.93 | 87.75 ± 3.26 |
| 10a | 0.13 ± 0.06 | 0.40 ± 0.08 | 0.43 ± 0.10 | 0.83 ± 0.43 | 0.31 ± 0.11 | 1.27 ± 0.85 | 1.26 ± 0.82 |
| 10b | 0.17 ± 0.09 | 0.36 ± 0.09 | 0.12 ± 0.05 | 0.09 ± 0.02 | 0.85 ± 0.32 | 1.89 ± 0.72 | 1.52 ± 0.87 |
| 10c | 0.36 ± 0.08 | 1.37 ± 0.18 | 1.75 ± 0.53 | 3.87 ± 1.41 | 1.89 ± 0.67 | 34.67 ± 4.91 | 53.50 ± 6.04 |
| 10d | 1.28 ± 0.87 | 2.36 ± 1.23 | 3.31 ± 1.13 | 6.02 ± 1.11 | 39.95 ± 3.25 | 60.77 ± 4.37 | 74.32 ± 4.98 |
| 13a | 0.24 ± 0.05 | 0.27 ± 0.12 | 0.50 ± 0.12 | 0.81 ± 0.23 | 1.12 ± 0.87 | 1.23 ± 0.92 | 6.27 ± 2.32 |
| 13b | 0.37 ± 0.13 | 0.68 ± 0.37 | 1.03 ± 0.45 | 1.17 ± 0.74 | 4.05 ± 0.68 | 8.66 ± 1.85 | 44.95 ± 3.91 |
| 13c | 0.26 ± 0.05 | 0.70 ± 0.12 | 0.30 ± 0.09 | 1.40 ± 0.73 | 27.70 ± 3.96 | 51.09 ± 3.27 | 73.46 ± 4.76 |
| 13d | 0.56 ± 0.13 | 1.50 ± 0.72 | 3.98 ± 1.03 | 11.18 ± 2.86 | 48.65 ± 3.54 | 65.17 ± 3.91 | 83.68 ± 4.12 |
| 16a | 0.17 ± 0.04 | 0.21 ± 0.10 | 0.35 ± 0.12 | 0.47 ± 0.11 | 0.78 ± 0.17 | 1.23 ± 0.92 | 3.55 ± 1.03 |
| 16b | 0.19 ± 0.03 | 0.32 ± 0.26 | 0.45 ± 0.27 | 0.51 ± 0.76 | 0.80 ± 0.65 | 1.54 ± 0.92 | 4.37 ± 2.19 |
| 16c | 0.23 ± 0.09 | 0.44 ± 0.17 | 0.49 ± 0.21 | 0.58 ± 0.21 | 0.82 ± 0.27 | 1.63 ± 0.98 | 5.45 ± 1.72 |
| 16d | 0.28 ± 0.06 | 0.57 ± 0.22 | 0.73 ± 0.27 | 0.86 ± 0.51 | 1.53 ± 0.87 | 5.94 ± 2.13 | 21.39 ± 3.12 |
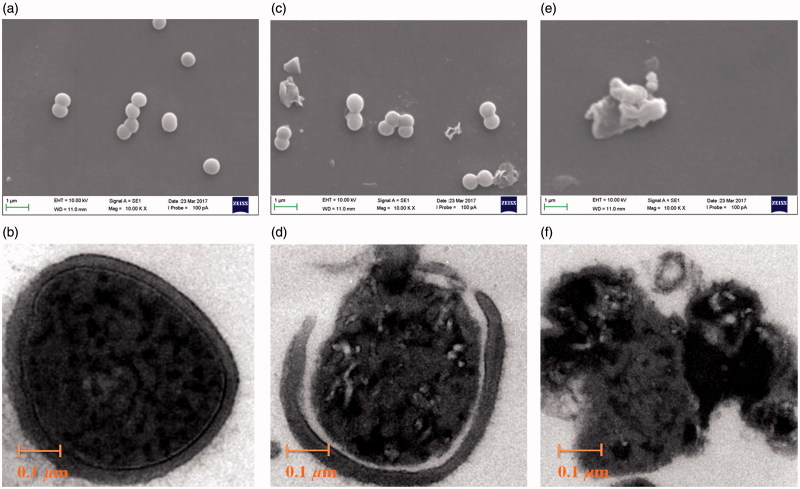
SEM images and TEM images of E. coli and S. aureus morphologies
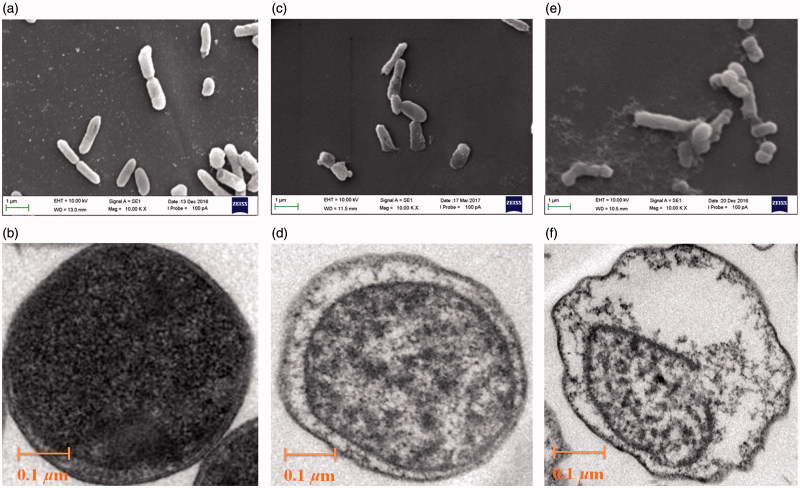
Treatment of bacteria with 7d caused physical and morphological alterations as well as cell wall surface deterioration, and very few intact cells were detected in samples of E. coli and S. aureus. Scanning electron microscope (SEM) was carried out to follow the morphological alterations of E. coli and S. aureus and the results are shown in Figures 2 and 3. The E. coli were uniformly rod-shaped in their morphology, they had typical dimensions and a smooth cell surface and were present in large numbers (Figure 2(a)). After contacting the addition of compound 7d (Figures 2(c) and 3(c)) for 4 h, bacteria reveal detrimental effect on the morphology of the cell membrane, showing a large surface collapse and wrinkled abnormalities on the morphology of E. coli and S. aureus. More obviously, bacterial cytoplasm release from cell membranes and bacteria have been on the verge of death. This may be caused by the antibacterial agents fixing on membrane surfaces 34 . As shown in Figure 2(e) and 3(e), all bacteria died after contacting compound 7d for 12 h. The alteration of E. coli and S. aureus morphology may be attributed to the puncturing of cell membranes to result in the release of bacterial cytoplasm 35 . Very few intact cells were detected in samples of E. coli and S. aureus treated with 7d.
Figure 2.
Images of E. coli obtained by SEM (a, c & e; scale bar = 1.0 μm) and TEM (b, d & f; scale bar = 0.1 μm) following treatment with culture medium MHB (control; a & b), compound 7d under the concentration 100 μg/mL for 4 h (c & d), 12 h (e & f).
Figure 3.
Images of S. aureus obtained by SEM (a, c & e; scale bar = 1.0 μm) and TEM (b, d & f; scale bar = 0.1 μm) following treatment with culture medium MHB (control; a & b), compound 7d under the concentration 100 μg/mL for 4 h (c & d), 12 h (e & f).
TEM analysis showed normal E. coli and S. aureus cell wall and cytoplasmic membrane were intact, and their cytoplasm was homogenously electron dense (Figure 2(b) and 3(b)). A lot of incomplete cells were detected in samples of E. coli and S. aureus treated with 7d (Figures 2(d,f) and 3(d,f)). Leakage of intracellular substance was observed in those cells that could be seen. Cells examined by TEM showed regional to complete loss of electron density of both their cell envelope and cytoplasm. In addition, large quantities of electron-dense extracellular material were observed 36 .
Conclusions
In this study, 24 N,N-bis(2-hydroxyethyl)-substituted quaternary ammonium salts bromides (5a-d, 6a-d, 7a-d, 10a-d, 13a-d, 16a-d) were prepared based on 5-phenyl-1,3,4-oxadiazole-2-thiol (POT) analogues, benzo[d]oxazole-2-thiol, benzo[d]thiazole-2-thiol, 5-methyl-1,3,4-thiadiazole-2-thiol and N,N-bis(2-hydroxyethyl) as well as several α,ω-dibromoalkanes. The results displayed that the antibacterial activities and antifungal activities of QASs were basically related to the introduction of POT, chain length and N,N-bis(2-hydroxyethyl) quaternary ammonium salts. The long-chain and two flexible hydroxyethyl groups in QAS could help QAS effectively pass through cell membrane into the cell to passivate enzyme and damage bacteria. Especially, compounds 5d, 6d, 7d, 10d, 13d, 16d showed unsurpassed antimicrobial activities against all the experimental bacteria and fungi. Meanwhile, introduction of POT fragment in QAS could effectively reduce cytotoxicity and improve antimicrobial activity. Haemolysis assay showed all QASs did not cause haemolysis under the concentration of 50 μg/mL, in which concentration all QASs were safe to be used as antibacterial and antifungal agents. SEM images and TEM images of E. coli and S. aureus morphologies of 7d showed antibacterial mechanism might be the antibacterial agents fixing on membrane surfaces and puncturing of cell membranes to result in the release of bacterial cytoplasm. This study provided a facile strategy to design and develop new types of antibacterial and antifungal agents for application as daily or clinical disinfectants, as well as agricultural fungicides.
Supplementary Material
Funding Statement
We are grateful for financial support from the National Natural Science Foundation of China (No. 21702018), the Foundation of Shandong province (Nos. ZR2013BM022, 2014CGZH1316, 2015GGX102013, BS2014YY049), the Foundation of Yantai city (Nos. 2016ZH078, 2013ZH095, 2014ZH092), and a project of Shandong province Higher Educational Science and Technology program (No. J17KA225).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1. Fan Z, Senapati D, Khan SA, et al. Popcorn-shaped magnetic core-plasmonic shell multifunctional nanoparticles for the targeted magnetic separation and enrichment, label-free SERS imaging, and photothermal destruction of multidrug-resistant bacteria. Chem Eur J 2013;19:2839–47. [DOI] [PubMed] [Google Scholar]
- 2. Xue Y, Xiao H, Zhang Y.. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int J Mol Sci 2015;16:3626–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duquebenítez SM, Ríosvásquez LA, Ocampocardona R, et al. Synthesis of novel quaternary ammonium salts and their in vitro antileishmanial activity and U-937 cell cytotoxicity. Molecules 2016;21:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shelton RS, Campen MG, Tilford CH, et al. Quaternary ammonium salts as germicides; non-acylated quaternary ammonium salts derived from aliphatic amines. J Am Chem Soc 1946;68:753–5. [PubMed] [Google Scholar]
- 5. McDonnell G, Russell AD.. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 1999;12:147–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Badawy HT, Pasetto P, Mouget JL, et al. Bacterial adhesion and growth reduction by novel rubber-derived oligomers. Biochem Biophy Res Commun 2013;438:691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu G, Wu D, Fu R.. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React Funct Polym 2007;67:355–66. [Google Scholar]
- 8. Gong SQ, Niu LN, Kemp LK, et al. Quaternary ammonium silane-functionalized, methacrylate resin composition with antimicrobial activities and self-repair potential. Acta Biomaterialia 2012;8:3270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Obłąk E, Piecuch A, Guz-Regner K, et al. Antibacterial activity of gemini quaternary ammonium salts. FEMS Microbiol Lett 2014;350:190–8. [DOI] [PubMed] [Google Scholar]
- 10. Lukáč M, Lacko I, Bukovský M, et al. Synthesis and antimicrobial activity of a series of optically active quaternary ammonium salts derived from phenylalanine. CEJC 2010;8:194–201. [Google Scholar]
- 11. Tischer M, Pradel G, Ohlsen K, et al. Quaternary ammonium salts and their antibacterial potential: Targets or nonspecific interactions. ChemMedChem 2012;7:22–31. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Song XJ, Yang RJ.. Synthesis and characterization of N,N-dimethyl dodecyl-(4-vinylbenzyl) ammonium chloride. Key Eng Mater 2014;575:71–5. [Google Scholar]
- 13. Babbs M, Collier HOJ, Austin WC, et al. Salts of decamethylene-bis-4-aminoquinaldinium (dequadin); a new antimicrobial agent. J Pharm Pharmacol 1956;8:110–19. [DOI] [PubMed] [Google Scholar]
- 14. Franklin TJ, Snow GA, Biochemistry of antibacterial action. 3rd ed London: Chapman and Hall; 1981;10. [Google Scholar]
- 15. Docherty KM, Kulpa CF. Jr, Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem 2005;7:185–9. [Google Scholar]
- 16. Zhang Y, Li YY, Li JH, et al. Synthesis and antibacterial characterization of waterborne polyurethanes with gemini quaternary ammonium salt. Sci Bull 2015;60:1114–21. [Google Scholar]
- 17. Ma S, Izutani N, Imazato S, et al. Assessment of bactericidal effects of quaternary ammonium-based antibacterial monomers in combination with colloidal platinum nanoparticles. Dent Mater J 2012;31:150–6. [DOI] [PubMed] [Google Scholar]
- 18. Sajomsang W. Synthetic methods and applications of chitosan containing pyridylmethyl moiety and its quaternized derivatives: a review. Carbohyd Polym 2010;80:631–47. [Google Scholar]
- 19. Wang CH, Hou GG, Du ZZ, et al. Synthesis, characterization and antibacterial properties of polyurethane material functionalized with quaternary ammonium salt. Polym J 2016;48:259–65. [Google Scholar]
- 20. Liu WS, Wang CH, Sun JF, et al. Synthesis, characterization and antibacterial properties of dihydroxy quaternary ammonium salts with long chain alkyl bromides. Chem Biol Drug Des 2015;85:91–7. [DOI] [PubMed] [Google Scholar]
- 21. Wang CH, Liu WS, Sun JF, et al. Non-toxic O-quaternized chitosan materials with better water solubility and antimicrobial function. Int J Biolog Macromol 2016;84:418–27. [DOI] [PubMed] [Google Scholar]
- 22. Siddiqa A, Rehman AU, Abbasi MA, et al. Synthesis and antibacterial screening of S-substituted derivatives of 5-(3,4-methylenedioxyphenyl)-1,3,4-oxadiazol-2-thiol. Pak J Pharm Sci 2016;29:27–34. [PubMed] [Google Scholar]
- 23. Salahuddin Mazumder A, Shaharyar M.. Synthesis, antibacterial and anticancer evaluation of 5-substituted (1,3,4-oxadiazol-2-yl)quinoline. Med Chem Res 2015;24:2514–28. [Google Scholar]
- 24. Khalid H, Aziz-ur-Rehman Abbasi MA, et al. Synthesis, spectral analysis and anti-bacterial study of N-substituted derivatives of 2-(5-(1-(phenylsulfonyl)piperidin-4-yl)-1,3,4-oxadiazol-2-ylthio)acetamide. J Saudi Chem Soc 2016;20:S615–23. [Google Scholar]
- 25. Aggarwal N, Kumar R, Dureja P, et al. Synthesis of novel nalidixic acid-based 1,3,4-thiadiazole and 1,3,4-oxadiazole derivatives as potent antibacterial agents. Chem Biol Drug Des 2012;79:384–97. [DOI] [PubMed] [Google Scholar]
- 26. Tripathi P, Pal A, Jancik V, et al. Metal-assisted transformation of N-benzoyldithiocarbazate to 5-phenyl-1,3,4-oxadiazole-2-thiol in the presence of ethylenediamine, and its first row transition metal complexes. Polyhedron 2007;26:2597–602. [Google Scholar]
- 27. Wang CH, Xie XR, Liu WS, et al. Quaternary ammonium salts substituted by 5-phenyl-1,3,4-oxadiazole-2-thiol as novel antibacterial agents with low cytotoxicity. Chem Biol Drug Des 2017;90:943–52. [DOI] [PubMed] [Google Scholar]
- 28. Kobal E. Synthesis and antibacterial activity of fluorobenzoylcephalosporins. Int J Pharm 1991;75:131–9. [Google Scholar]
- 29. Bi FC, Guo LW, Wang YH, et al. Design, synthesis and biological activity evaluation of novel 2,6-difluorobenzamide derivatives through FtsZ inhibition. Bioorg Med Chem Lett 2017;27:958–62. [DOI] [PubMed] [Google Scholar]
- 30. Aries V, Hill MJ.. Degradation of steroids by intestinal bacteria. II. Enzymes catalysing the oxidoreduction of the 3α-, 7α-and 12α-hydroxyl groups in cholic acid, and the dehydroxylation of the 7-hydroxyl group. BBA-Lipids Lipid Metab 1970;202:535–43. [DOI] [PubMed] [Google Scholar]
- 31. Ultee A, Bennik MHJ, Moezelaar R.. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 2002;68:1561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Makino M, Ohta S, Zenda H.. Study on new anti-rust disinfectants. III. Effect of alkyl chain length of N-alkyl-N-(2-hydroxy-3-phenoxy)propyl-N,N-dimethylammonium butyl phosphate on the antibacterial activity. Yakugaku Zasshi 1994;114:73–9. [DOI] [PubMed] [Google Scholar]
- 33. Li J, Guo YY.. Basic evaluation of typical nanoporous silica nanoparticles in being drug carrier: Structure, wettability and hemolysis. Mater Sci Eng C 2017;73:670–3. [DOI] [PubMed] [Google Scholar]
- 34. Sharma A, Bajpai VK, Baek K.. Determination of antibacterial mode of action of Allium sativum essential oil against foodborne pathogens using membrane permeability and surface characteristic parameters. J Food Safety 2013;33:197–208. [Google Scholar]
- 35. Wang J, Sun H, Gao X, et al. Enhancing antibiofouling performance of polysulfone (PSF) membrane by photo-grafting of capsaicin derivative and acrylic acid. Appl Surf Sci 2014;317:210–19. [Google Scholar]
- 36. Dholvitayakhun A, Trachoo N, Narkkong NA, et al. Using scanning and transmission electron microscopy to investigate the antibacterial mechanism of action of the medicinal plant Annona squamosa, Linn. J Herb Med 2017;7:31–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.