Abstract
Background
This study compared the efficacy of catheter ablation of atrial fibrillation (AF) between impedance (IMP)‐guided and contact force (CF)‐guided annotation using the automated annotation system (VisiTag™).
Methods
Fifty patients undergoing pulmonary vein isolation (PVI) for AF were randomized to the IMP‐guided or CF‐guided groups. The annotation criteria for VisiTag™ were a 10 second minimum ablation time and 2 mm maximum catheter movement range. A minimum CF of 10 g was added to the criteria in the CF‐guided group. In the IMP‐guided group, a minimum IMP drop of over 5 Ω was added to the criteria.
Results
The rates of successful PVI after an initial ablation line were higher in the CF‐guided group (80% vs 48%, P = .018). Although average CF was similar between two groups, the average force‐time integral (FTI) was significantly higher in the CF‐guided group (298.3 ± 65. 2 g·s vs 255.1 ± 38.3 g·s, P = .007). The atrial arrhythmia‐free survival at 1 year demonstrated no difference between the two groups (84.0% in the IMP‐guided group vs 80.0% in the CF‐guided group, P = .737). If the use of any antiarrhythmic drug beyond the blanking period was considered as a failure, the clinical success rate at 1 year was 52.0% for the CF‐guided group vs 56.0% for the IMP‐guided group (P = .813).
Conclusions
Atrial fibrillation ablation using an automated annotation system guided by CF improved the success rate of PVI after the initial circumferential ablation. An IMP‐guided annotation combined with catheter stability criteria showed similar clinical outcomes as compared to the CF‐guided annotation.
Keywords: atrial fibrillation, catheter ablation, contact force, impedance, pulmonary vein
1. INTRODUCTION
Catheter ablation of atrial fibrillation (AF) has been performed as an effective treatment for patients with paroxysmal and persistent AF.1, 2 Pulmonary vein isolation (PVI) is an essential strategy for AF ablation, and pulmonary vein (PV) reconnections are considered as one of the main causes of recurrence after AF ablation.3, 4
During AF ablation, the location where the ablation is conducted is displayed through annotation tags using a three‐dimensional (3D) system to recognize where the energy delivery was performed in that area. However, manual annotations are usually performed subjectively and we are unable to identify how effectively the ablation has been performed at that location. To remedy this disadvantage, an automated annotation system (VisiTag™ module, Biosense Webster, Inc., Diamond Bar, CA) was recently developed to make an automatic annotation based on predefined criteria, including the catheter stability, contact force (CF), and impedance (IMP) drop. Recent studies using this automated annotation system have demonstrated that an automated annotation based on catheter stability combined with the IMP or CF information has reduced the conduction gaps or reconnections during PVI.5, 6, 7, 8 The objective of this study was to compare the efficacy of AF ablation between CF‐guided and IMP‐guided annotations using the automated annotation system.
2. METHODS
2.1. Study population
Fifty patients receiving ablation for paroxysmal or persistent AF were randomly assigned with the same number to two groups with CF‐guided ablation and IMP‐guided ablation. This study protocol was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center (November 11, 2014; DSMC 2014‐10‐052‐001). Informed consent was obtained from all patients.
2.2. Procedure
The administration of antiarrhythmic drug (AAD) was stopped for at least five times longer than the half‐life (except for amiodarone). Multislice computed tomography (CT) scans were performed one day before the procedure, and all patients received transesophageal echocardiography prior to the procedure. The ablation procedure was performed under conscious sedation with remifentanil and midazolam.
Three multipolar catheters were inserted through the left femoral region and placed in the right atrium, coronary sinus, and His bundle. Two 8.5 Fr nonsteerable long sheaths (Swartz™ Braided Transseptal Guiding Introducer SL1, St. Jude Medical, St. Paul, MN) were placed in the right atrium through the right femoral region and inserted into the left atrium (LA) using one or two transseptal punctures. After the transseptal puncture, an activated clotting time of over 350 seconds was maintained with intermittent boluses of intravenous heparin. The anatomical structure was identified through LA and PV angiography, and 3D mapping was conducted to map the anatomical structure of the LA and PVs. Then, the prescanned CT and 3D images were merged.
A point‐by‐point ablation was conducted at the junction between the LA and PVs by a circumferential PVI. The PVI was performed with the automated annotation program (VisiTag™ module) using a 3.5 mm irrigated ablation catheter with CF monitoring (THERMOCOOL® SMARTTOUCH™ catheter, Biosense Webster, Inc., Diamond Bar, CA).
The CF‐guided ablation group underwent a PVI guided by automated annotation criteria with a minimum time of 10 seconds, maximum range of 2 mm, CF over a time of 50%, and minimum CF of 10 g. The IMP‐guided ablation group underwent a PVI guided by annotation criteria with a minimum time of 10 seconds, maximum range of 2 mm, and minimum IMP decrease of over 5 Ω instead of the CF parameters. In the IMP‐guided group, the operators were blinded to the contact force data during the PVI. When an automated annotation was not taken even after a repeated energy application delivery, manual tagging was performed at that site and was included for the data analysis.
Ablation was performed in a power‐controlled mode using a power of 30 W for a duration of 25‐30 seconds. Ablation of the posterior wall was performed with a power of 20‐25 W for 15‐20 seconds. A successful PVI was defined by the absence of PV potentials recorded from a 20‐pole circular mapping catheter (LASSO® NAV or LASSO® NAV ECO, Biosense Webster, Inc., Diamond Bar, CA) positioned at each PV ostium. Linear ablation of the cavotricuspid isthmus (CTI) was performed in the patients who had a history of typical atrial flutter. For the patients with persistent AF, the operators routinely performed linear ablation of the CTI. Another additional linear ablation was left to the operator's discretion. PV reconnections and dormant conduction were confirmed after a waiting period of 30 minutes. Dormant conduction was assessed for each PV by an intravenous injection of 12 mg of adenosine. Further ablation was performed if there was a PV reconnection or dormant conduction.
2.3. Analysis of the ablation parameters
After the ablation procedure, a data analysis was conducted using the CARTO®3 System version 4.3 software. For the analysis, the PVs were divided into 18 segments along the anatomical ablation line (Figure 1). The data obtained for analysis included the ablation duration, power, average CF, and IMP drop at each point. The data were collected in accordance with the PV segmentation and used for the analysis.
Figure 1.
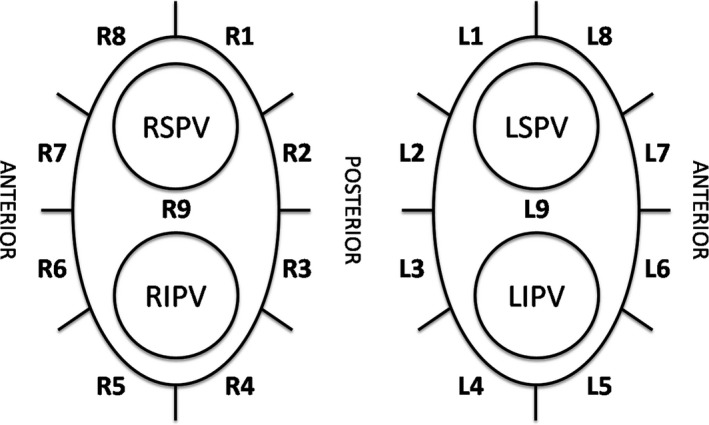
Segmentation of each pulmonary vein. R = right pulmonary vein, L = left pulmonary vein, RSPV = right superior pulmonary vein, RIPV = right inferior pulmonary vein, LSPV = left superior pulmonary vein, RIPV = right inferior pulmonary vein
2.4. Follow‐up
Antiarrhythmic agents were allowed during the first 3 months of the blanking period, and the maintenance of AADs was left to the physician's discretion. An ECG and 24 hour Holter monitoring were obtained right after the procedure and at 3, 6, and 12 months after the ablation. ECG and Holter monitoring were also performed whenever the patient had symptoms suggesting an arrhythmia recurrence. Recurrence of atrial arrhythmias was defined as the detection of AF or atrial tachycardia lasting more than 30 seconds on the ECG or 24 hour Holter monitoring.
2.5. Endpoints
The primary endpoint was to compare the success rates of the PVI obtained after creating the initial circumferential ablation line between the two groups. The secondary endpoints were the difference in the values of the ablation parameters and incidence of recurrence of atrial arrhythmias at 1 year between the two groups.
2.6. Statistical analysis
For the statistical analysis, a Student's t test or Mann‐Whitney U test was used for the comparison of the continuous variables between the two groups and a chi‐square or Fisher's exact test was used for the categorical data. Kaplan‐Meier survival estimates were used to compare the degree of recurrence of AF or the incidence of atrial arrhythmias. The data analyses were performed with IBM® SPSS® Statistics version 23 software (IBM Corp., Armonk, NY, USA).
3. RESULTS
3.1. Patient and procedural characteristics
The patient clinical characteristics are shown in Table 1. There were no significant differences in the baseline characteristics between the CF‐guided and IMP‐guided groups. The average procedure, ablation, and fluoroscopy times revealed no differences between the two groups. Furthermore, there were no differences in the additional ablation strategies between the two groups (Table 2).
Table 1.
Patient characteristics
| CF‐Guided (n = 25) | IMP‐Guided (n = 25) | P value | |
|---|---|---|---|
| Age | 58.5 ± 10.2 | 56.6 ± 7.7 | .467 |
| Male (%) | 20 (80.0) | 20 (80.0) | 1.000 |
| Paroxysmal AF (%) | 15 (60.0) | 12 (48.0) | .395 |
| Heart failure (%) | 3 (12.0) | 1 (4.0) | .609 |
| Hypertension (%) | 12 (48.0) | 9 (36.0) | .390 |
| Diabetes (%) | 3 (12.0) | 1 (4.0) | .609 |
| Stroke or TIA (%) | 2 (8.0) | 1 (4.0) | 1.000 |
| AF duration (mo) | 34.7 ± 47.0 | 31.9 ± 37.8 | .942 |
| CHA2DS2‐VASc score | 1.36 ± 1.32 | 0.84 ± 1.11 | .138 |
| Ejection fraction (%) | 62.7 ± 9.0 | 61.2 ± 10.6 | .682 |
| LA dimension (cm) | 4.23 ± 0.54 | 4.23 ± 0.52 | .973 |
| LA volume (mL) | 85.9 ± 28.4 | 80.5 ± 28.2 | .507 |
AF, atrial fibrillation; CF, contact force; IMP, impedance; LA, left atrium; TIA, transient ischemic attack.
Data are shown as the mean ± standard deviation or number (%).
Table 2.
Procedural characteristics
| CF‐Guided (n = 25) | IMP‐Guided (n = 25) | P value | |
|---|---|---|---|
| Procedure time (min) | 201.3 ± 40.4 | 196.7 ± 23.5 | .655 |
| Ablation time, total (min) | 95.3 ± 23.0 | 90.1 ± 19.4 | .446 |
| Ablation time, PVI (min) | 68.7 ± 13.7 | 73.3 ± 16.5 | .294 |
| Fluoroscopy time (min) | 25.5 ± 9.6 | 26.8 ± 6.5 | .603 |
| Roof line (%) | 2 (8.0) | 5 (20.0) | .417 |
| Mitral isthmus line (%) | 1 (4.0) | 5 (20.0) | .189 |
| CTI line (%) | 21 (84.0) | 21 (84.0) | 1.000 |
| SVC isolation (%) | 0 (0.0) | 1 (4.0) | 1.000 |
| Complication (%) | 0 (0.0) | 0 (0.0) |
CF, contact force; CTI, cavotricuspid isthmus; IMP, impedance; PVI, pulmonary vein isolation; SVC, superior vena cava.
Data are shown as the mean ± standard deviation or number (%).
3.2. Pulmonary vein isolation and reconnection data
After an initial circumferential ablation line was created, a PVI was achieved in 80% of the patients in the CF‐guided group (20 of 25 patients) and 48.0% of those in the IMP‐guided group (12 of 25 patients) (P = .018). In terms of each PV, a total of 200 PVs were included. After creating the initial ablation line, 93.0% of the PVs (93/100) in the CF‐guided group and 81% of the PVs (81/100) in the IMP‐guided group were isolated (P = .012). Among 100 total ipsilateral PV pairs, a successful PVI was obtained in 90% of the ipsilateral PVs (45/50) in the CF‐guided group and 66% (33/50) in the IMP‐guided group (P = .004) (Figure 2).
Figure 2.
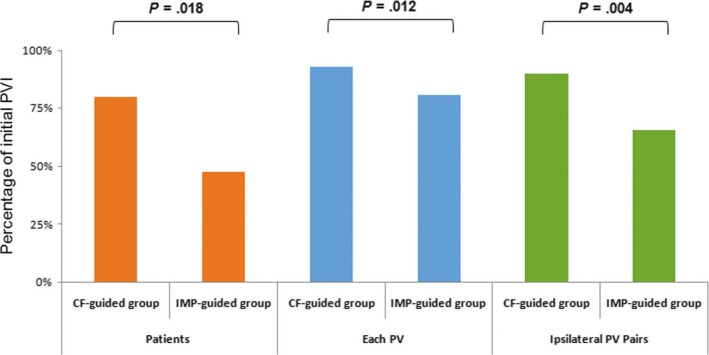
Success rate of the pulmonary vein isolation after creating the initial circumferential ablation line. PV, pulmonary vein; PVI, pulmonary vein isolation; CF, contact force; IMP, impedance
All PVs were successfully isolated by additional ablation. After a 30 minute waiting period, 16 patients (32%) had spontaneous pulmonary vein reconnections or dormant conduction without any difference in the incidence between the two groups. The location and number of conduction gaps also showed no differences between the two groups (1.3 ± 1.5 in the CF‐guided group vs 2.24 ± 2.1 in the IMP‐guided group, P = .096).
3.3. Ablation parameters
The comparison of the ablation parameters between the two groups is shown in Table 3. The average CF during the ablation was 12.7 ± 3.2 g in the CF‐guided group and 11.5 ± 2.2 g in the IMP‐guided group without any significant differences (P = .121). However, the average FTI was significantly higher in the CF‐guided group than IMP‐guided group (298.3 ± 65.2 g·s vs 255.1 ± 38.3 g·s, P = .007). The average CF was significantly higher at the left upper posterior wall (L1 & L2 segment) in the CF‐guided group. The CF‐guided group also had a significantly higher FTI level in the left posterior roof region (L1 segment). Figure 3 shows the distribution of the CF and FTI at each segment.
Table 3.
Ablation parameters
| CF‐Guided (n = 25) | IMP‐Guided (n = 25) | P value | |
|---|---|---|---|
| CF (g) | 12.7 ± 3.2 | 11.5 ± 2.2 | .121 |
| Time (s) | 23.6 ± 1.9 | 23.0 ± 2.1 | .332 |
| FTI (g·s) | 298.3 ± 65.2 | 255.1 ± 38.3 | .007 |
| IMP drop (Ohms) | 7.6 ± 2.9 | 7.4 ± 1.7 | .822 |
CF, contact force; FTI, force‐time integral; IMP, impedance.
Data are shown as the mean ± standard deviation or number (%).
Figure 3.
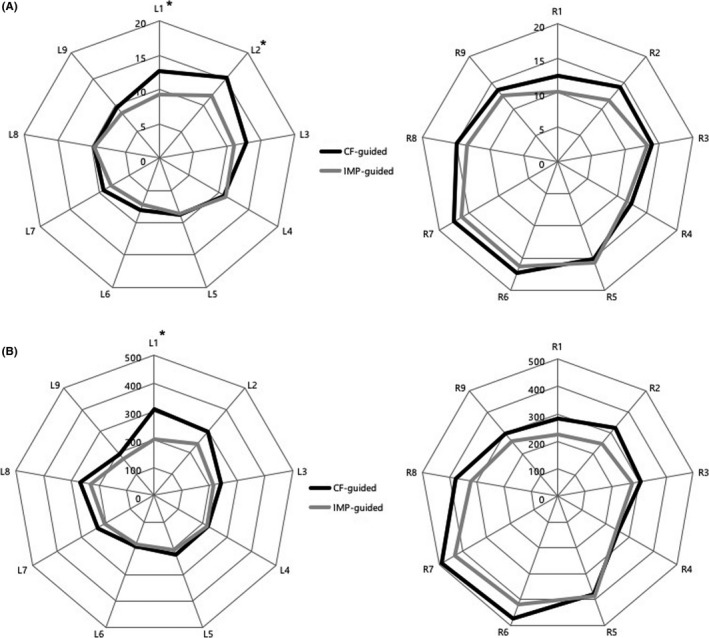
Radar chart for the distribution of the contact force (CF, g) (A) and force‐time integral (FTI, g·s) (B) at each segment of the pulmonary vein. L, left pulmonary vein; R = right pulmonary vein; IMP, impedance, *P < .05
3.4. Clinical follow‐up result
During a 1‐year follow‐up period, one patient in the CF‐guided group experienced a transient ischemic attack without any neurological sequelae. The atrial arrhythmia‐free survival after a single procedure at 1 year with or without AADs demonstrated no difference between the two groups (80.0% in the CF‐guided group vs 84.0% in the IMP‐guided group, P = .737) (Figure 4). In total, 34.1% of the patients without arrhythmia recurrences were taking antiarrhythmic medications (35.0% in the CF‐guided group vs 33.3% in the IMP‐guided group). If the use of any AAD beyond the blanking period was considered as a failure even if no AF was documented, the clinical success rate at 1 year was 52.0% for the CF‐guided group vs 56.0% for the IMP‐guided group (P = .813). The arrhythmia‐free survival rate at 12 months in the patients with paroxysmal AF was 86.7% in the CF‐guided group and 91.7% in the IMP‐guided group (P = .720). In the persistent AF patients, 70.0% in the CF‐guided group and 76.9% in the IMP‐guided group were free from atrial arrhythmias (P = .668). No patients received any redo procedures during 1 year of follow‐up.
Figure 4.
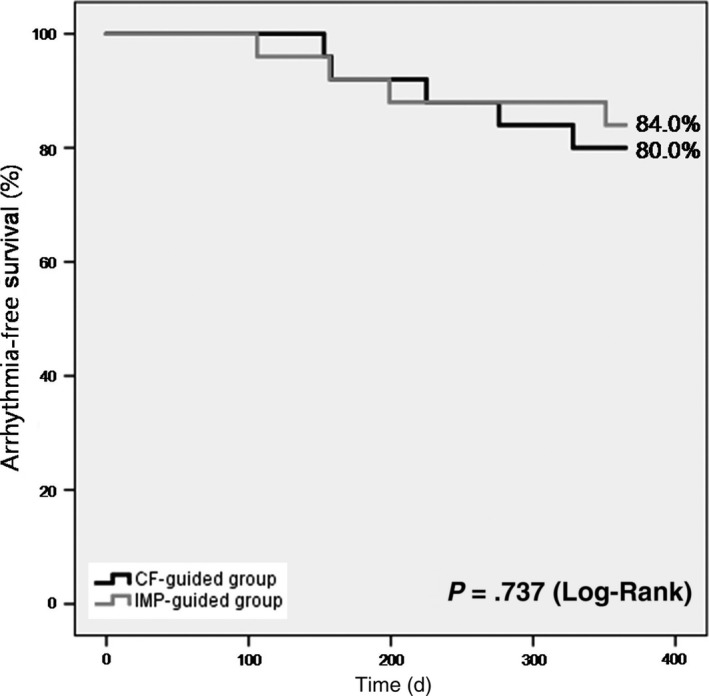
Arrhythmia‐free survival curves for atrial arrhythmias
4. DISCUSSION
4.1. Main findings
The present study demonstrated the following: (i) The PVI using an CF‐guided automated annotation system exhibited higher rates of a successful PVI after an initial circumferential ablation line, (ii) the CF‐guided group had a slightly higher CF value without any clinical significance, but had a significantly higher FTI level as compared to the IMP‐guided ablation, and (iii) the 1 year atrial arrhythmia recurrence rates were similar between the two groups.
4.2. Success rate of the PVI after creating an initial anatomical line
Contact force sensing catheters have allowed continuous monitoring of the CF during catheter ablation procedures. Previous studies demonstrated that the lesion size correlated well with the CF,9, 10 and PV isolation with a CF sensing catheter reduced any residual conduction gaps and the incidence of PV reconnections.11, 12 The present study showed that PV isolation with CF monitoring had a higher success rate of PV isolation after creating an initial circumferential ablation line. Although there was no statistical significance, the CF level was higher in the CF‐guided group than the IMP‐guided group. Because the FTI level is a summation of the CF and duration of the ablation, a nonsignificant higher level of the CF and longer duration of the RF energy delivery might have produced a significantly higher FTI level in the CF‐guided group. Higher FTI values are known to be associated with a durable PVI and better clinical outcomes.13, 14, 15 Therefore, the higher success rate of the PVI in the CF‐guided group might have been caused by the higher values of the FTI in this group.
4.3. Ablation with the automated annotation system
The automated annotation system (VisiTag™ module) displays tags automatically when predefined criteria are met. The parameters include the catheter position stability, CF over time, IMP drop, and target temperature. Furthermore, the color of the tag changes based on predefined parameters like the total time, CF, and FTI level.16
Stable and constant catheter contact is known to be correlated with the lesion size,17 but there were have been no tools to evaluate the catheter stability during the catheter ablation procedure. One of the most important pieces of information from the automated annotation system is the real‐time data about the catheter stability during the RF energy delivery. One recent study using the VisiTag™ module reported that the acute success rate of PV isolation was 74% with an automated annotation based on the stability parameters alone, and the addition of CF parameters increased the acute success rate to 92%.8 Another study5 showed that ablation using the automated annotation with a combination of stability and IMP criteria had a significantly higher initial success rate of the PV isolation as compared to the traditional manual annotation. Based on the results of these studies, automated annotation based on catheter stability criteria combined with the CF or IMP drop could be considered as a good tool to improve the efficacy of catheter ablation.
Recently, the ablation line contiguity index (ALCI) was introduced as a novel automated algorithm that combines the interlesion distance (contiguity) and measurements of the lesion depth (power, time, and CF).18 The ablation index (AI) incorporates the CF, power, and time in a weighted formula and overcomes the deficiencies of the FTI by adding information about the power.19 In addition to the AI, the ALCI provides additional information about the ablation contiguity and the ALCI is an accurate predictor of a durable lesion formation. However, the present study did not include an analysis of the AI and lesion contiguity because the parameters were not commercially available during the study period.
4.4. Impedance changes as a marker for a good contact force
Before there were CF sensing catheters, the contact between the tissue and ablation catheter could be identified by indirect methods such as a sense of tactile feeling, the size of the local electrogram, and the movement of the catheter tip shown on fluoroscopy. Another surrogate marker for good contact is the IMP changes during radiofrequency energy deliveries. Previous studies reported that a larger IMP decrease during the RF energy delivery was related to a good contact force and an ablation guided by an IMP drop improved the efficacy of the PVI.5, 20, 21, 22 Although the average FTI level and initial success rate of the PVI were lower, a PVI guided by an IMP drop had a similar CF as that for the CF‐guided ablation in the present study. This result also demonstrated the relationship between the IMP drop and CF and showed the importance of monitoring the IMP changes during the catheter ablation procedure.
The previous study showed that automated annotation guided by an IMP drop of over 5% (>7‐8 Ω) coupled with catheter stability reduced the conduction recovery and clinical recurrence.5 In the present study, the IMP‐guided ablation group underwent ablation guided by an annotation criterion with minimum IMP decreases of over 5 Ω (without CF information). In our experience, it took much longer for automated tagging using 10 Ω as the criterion and the annotation was occasionally not taken. Furthermore, the annotation using the 5 Ω criterion used in the present study resulted in a similar IMP drop as compared to the CF‐guided ablation. Therefore, a 5 Ω drop in the IMP could be a reasonable criterion for an automated annotation coupled with the catheter stability criteria.
4.5. Clinical atrial arrhythmia recurrence
Even though the acute success rate was higher in the CF‐guided group, the atrial arrhythmia recurrence rate at 1 year exhibited no differences between the two groups. AF ablation using CF sensing catheters with an optimal contact force had a reduced clinical recurrence of atrial arrhythmias during the follow‐up.23, 24, 25 However, some studies demonstrated no benefit of CF sensing catheters in terms of the clinical AF recurrences.12, 26 Ullah et al12 suggested that an acute improvement in the initial success rate in a CF‐guided ablation is canceled out by further ablation targeting pulmonary reconnections combined with an adenosine injection after a waiting period. In the present study, we also evaluated the PV reconnections and dormant conduction after a 30 minutes waiting period and eliminated any remnant PV conduction. In addition, even though the CF information was blinded, the other group underwent PV isolation guided by an IMP drop with stability monitoring. This could have had a positive impact on reducing the clinical recurrences in the IMP‐guided ablation group. These results imply that AF ablation with real‐time monitoring of a stable catheter position and the additional information of the CF or IMP drop could reduce the clinical recurrences of atrial arrhythmias and improve the clinical outcomes.
4.6. Clinical implications
Atrial fibrillation ablation with an automated annotation system based on CF and catheter stability criteria is an efficient way to improve the clinical outcomes and preferred technique in our institution. However, CF sensing catheters are not routinely available in clinical practice around the world and there could be a situation we had to use standard catheters. Catheter ablation using an automated annotation based on IMP drop and catheter stability can be used as an alternative method for CF‐guided ablation if CF sensing catheters are not available.
4.7. Limitations
Because the primary endpoint of this study was to compare the success rate of an acute PV isolation, the sample size was relatively small and not enough to demonstrate the long‐term clinical outcomes. Even though there were no statistical differences between the two groups, the type of AF included both paroxysmal and persistent AF.
The CF and FTI values were relatively low as compared to the previous reports. In our opinion, the reason for that was because the procedures were performed with nonsteerable sheaths and under conscious sedation without a jet ventilator. Nonetheless, the initial success rate of the PV isolation and 1 year clinical outcome were comparable to the previous studies. Those results suggest that the optimal CF and FTI values might be reevaluated when performing PVI with an automated annotation system that provides real‐time stability monitoring.
The recurrence of atrial arrhythmias was evaluated by ECGs or 24 hour Holter monitoring, and patients who were taking AADs were included in the evaluation. This might have caused an underestimation of the recurrence rate of atrial arrhythmia recurrences during the follow‐up.
5. CONCLUSION
Contact force ‐guided AF ablation with an automated annotation system improved the acute success rate of the PVI after creating an initial anatomical ablation line as compared to an IMP‐guided ablation. An IMP‐guided annotation combined with catheter stability criteria had a similar atrial arrhythmia‐free survival rate at 1 year as compared to the CF‐guided annotation.
CONFLICT OF INTEREST
HS Park received research funding from Biosense Webster Inc.
ACKNOWLEDGMENT
We appreciate Mr. John Martin's linguistic assistance.
Park H‐S, Kim I‐C, Cho Y‐K, et al. Comparison of the efficacy between impedance‐guided and contact force‐guided atrial fibrillation ablation using an automated annotation system. J Arrhythmia. 2018;34:239–246. https://doi.org/10.1002/joa3.12054
Funding information
The funding support for the trial was provided by an Investigator‐Initiated Study Grant from Biosense Webster.
ClinicalTrials.gov Identifier: NCT02364401
REFERENCES
- 1. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sauer WH, McKernan ML, Lin D, Gerstenfeld EP, Callans DJ, Marchlinski FE. Clinical predictors and outcomes associated with acute return of pulmonary vein conduction during pulmonary vein isolation for treatment of atrial fibrillation. Heart Rhythm. 2006;3:1024–8. [DOI] [PubMed] [Google Scholar]
- 4. Cappato R, Negroni S, Pecora D, et al. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003;108:1599–604. [DOI] [PubMed] [Google Scholar]
- 5. Anter E, Tschabrunn CM, Contreras‐Valdes FM, Buxton AE, Josephson ME. Radiofrequency ablation annotation algorithm reduces the incidence of linear gaps and reconnection after pulmonary vein isolation. Heart Rhythm. 2014;11:783–90. [DOI] [PubMed] [Google Scholar]
- 6. Fujiwara R, Imamura K, Kijima Y, et al. The importance of catheter stability evaluated by Visitag(TM) during pulmonary vein isolation. J Interv Card Electrophysiol. 2016;46:161–6. [DOI] [PubMed] [Google Scholar]
- 7. Okumura Y, Watanabe I, Iso K, et al. Clinical utility of automated ablation lesion tagging based on catheter stability information (VisiTag Module of the CARTO 3 System) with contact force‐time integral during pulmonary vein isolation for atrial fibrillation. J Interv Card Electrophysiol. 2016;47:245–52. [DOI] [PubMed] [Google Scholar]
- 8. Asbach S, Lang C, Trolese L, Bode C, Schluermann F. Automated lesion annotation during pulmonary vein isolation: influence on acute isolation rates and lesion characteristics. J Interv Card Electrophysiol. 2016;47:349–56. [DOI] [PubMed] [Google Scholar]
- 9. Ikeda A, Nakagawa H, Lambert H, et al. Relationship between catheter contact force and radiofrequency lesion size and incidence of steam pop in the beating canine heart: electrogram amplitude, impedance, and electrode temperature are poor predictors of electrode‐tissue contact force and lesion size. Circ Arrhythm Electrophysiol. 2014;7:1174–80. [DOI] [PubMed] [Google Scholar]
- 10. Yokoyama K, Nakagawa H, Shah DC, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008;1:354–62. [DOI] [PubMed] [Google Scholar]
- 11. Itoh T, Kimura M, Tomita H, et al. Reduced residual conduction gaps and favourable outcome in contact force‐guided circumferential pulmonary vein isolation. Europace. 2016;18:531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ullah W, McLean A, Tayebjee MH, et al. Randomized trial comparing pulmonary vein isolation using the SmartTouch catheter with or without real‐time contact force data. Heart Rhythm. 2016;13:1761–7. [DOI] [PubMed] [Google Scholar]
- 13. Neuzil P, Reddy VY, Kautzner J, et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol. 2013;6:327–33. [DOI] [PubMed] [Google Scholar]
- 14. Kautzner J, Neuzil P, Lambert H, et al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace. 2015;17:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reddy VY, Shah D, Kautzner J, et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm. 2012;9:1789–95. [DOI] [PubMed] [Google Scholar]
- 16. Lin T, Ouyang F, Kuck KH, Tilz R. THERMOCOOL(R) SMARTTOUCH(R) CATHETER ‐ The evidence so far for contact force technology and the role of VISITAG MODULE. Arrhythm Electrophysiol Rev. 2014;3:44–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah DC, Lambert H, Nakagawa H, Langenkamp A, Aeby N, Leo G. Area under the real‐time contact force curve (force‐time integral) predicts radiofrequency lesion size in an in vitro contractile model. J Cardiovasc Electrophysiol. 2010;21:1038–43. [DOI] [PubMed] [Google Scholar]
- 18. El Haddad M, Taghji P, Phlips T, et al. Determinants of acute and late pulmonary vein reconnection in contact force‐guided pulmonary vein isolation: identifying the weakest link in the ablation chain. Circ Arrhythm Electrophysiol. 2017;10:e004867. [DOI] [PubMed] [Google Scholar]
- 19. Das M, Loveday JJ, Wynn GJ, et al. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace. 2017;19:775–83. [DOI] [PubMed] [Google Scholar]
- 20. Reichlin T, Knecht S, Lane C, et al. Initial impedance decrease as an indicator of good catheter contact: insights from radiofrequency ablation with force sensing catheters. Heart Rhythm. 2014;11:194–201. [DOI] [PubMed] [Google Scholar]
- 21. Reichlin T, Lane C, Nagashima K, et al. Feasibility, efficacy, and safety of radiofrequency ablation of atrial fibrillation guided by monitoring of the initial impedance decrease as a surrogate of catheter contact. J Cardiovasc Electrophysiol. 2015;26:390–6. [DOI] [PubMed] [Google Scholar]
- 22. Strickberger SA, Vorperian VR, Man KC, et al. Relation between impedance and endocardial contact during radiofrequency catheter ablation. Am Heart J. 1994;128:226–9. [DOI] [PubMed] [Google Scholar]
- 23. Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART‐AF trial. J Am Coll Cardiol. 2014;64:647–56. [DOI] [PubMed] [Google Scholar]
- 24. Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force‐sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation. 2015;132:907–15. [DOI] [PubMed] [Google Scholar]
- 25. Afzal MR, Chatta J, Samanta A, et al. Use of contact force sensing technology during radiofrequency ablation reduces recurrence of atrial fibrillation: a systematic review and meta‐analysis. Heart Rhythm. 2015;12:1990–6. [DOI] [PubMed] [Google Scholar]
- 26. Stabile G, Solimene F, Calo L, et al. Catheter‐tissue contact force values do not impact mid‐term clinical outcome following pulmonary vein isolation in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2015;42:21–6. [DOI] [PubMed] [Google Scholar]


