Abstract
This study aimed to determine antiradical (DPPH• and •OH) and acetylcholinesterase (AChE) inhibitory activities along with chemical composition of autochtonous fungal species Trametes versicolor (Serbia). A total of 38 phenolic compounds with notable presence of phenolic acids were identified using HPLC/MS-MS. Its water extract exhibited the highest antiradical activity against •OH (3.21 μg/mL), among the rest due to the presence of gallic, p-coumaric and caffeic acids. At the concentration of 100 μg/mL, the same extract displayed a profound AChE inhibitory activity (60.53%) in liquid, compared to donepezil (89.05%), a drug in clinical practice used as positive control. The flavonoids baicalein and quercetin may be responsible compounds for the AChE inhibitory activity observed. These findings have demonstrated considerable potential of T. versicolor water extract as a natural source of antioxidant(s) and/or AChE inhibitor(s) to be eventually used as drug-like compounds or food supplements in the treatment of Alzheimer’s disease.
Keywords: AChE inhibitory activity, Alzheimer’s disease, anti •OH activity, Trametes versicolor, water extract
Introduction
It is well-known that fungal organisms possess a tremendous biochemical potential and produce a range of secondary metabolites thanks to their sessile style of life, mainly phenolic compounds1, such as phenolic acids2–6 and flavonoids3 as well as terpenoids4 , 7 , 8. In recent years, polysaccharides and polysaccharide–protein complexes found in medicinal mushrooms have attracted a great attention of the scientific audience worldwide, due to their numerous bioactivities, especially antioxidant and antiradical5 , 9 , 10. The presence and composition of these compounds in fungi mainly depend not only on genetic determinants, but also on ecological factors, including special environmental conditions in specific habitats and the life style (saprotrophic, parasitic and symbiotic/e.g. mycorrhizal)11.
Trametes versicolor (L.) Lloyd (1920), commonly called Turkey tail, is a widespread white-rot lignicolous fungal species that grows on many deciduous trees (oak, Prunus) and some conifers (fir and pine trees), with basidiums mostly appearing on stubs and trunks throughout a year. It is an inedible species; however, it is one of the most known medicinal mushrooms in the world12. Recent in vitro and in vivo studies have shown that T. versicolor possesses many medicinal properties, such as antitumour13, antimicrobial14, anti-diabetic15, immunostimulant16, AChE inhibitory17 and antioxidant activities5 , 9 , 10 , 12. Bioactive compounds detected in this species mostly belong to a group of proteins and polysaccharides, with the polysaccharide krestin and polysaccharopeptide PSP being the most studied ones18. The fact that large number of diseases and medical conditions are associated with oxidative stress has provoked numerous investigations of new sources of natural antioxidants, in particular microorganisms and mushrooms2 , 6. Their advantage lies in the fact that they could be easily used as functional foods. A higher consumption of mushrooms in a regular diet, which is considered useful in the prevention of many diseases caused by oxidative stress, represents an alternative treatment based on natural products.
Reactive oxygen species (ROS) are formed as by-products in the processes of cellular metabolism including a vast number of free radicals, such as superoxide anion (O2−•), hydroxyl radical (•OH) and hydrogen peroxide (H2O2). An excessive amount of ROS leads to a process known as oxidative stress, which can be provoked by tissue inflammation caused by extreme exercise or environmental factors such as radiation, xenobiotics, smoking and drugs4. Uncontrolled production of free radicals leads to damage of the cellular components such as lipids, proteins and DNA10, which may cause more than 100 diseases, including several forms of cancer19, diabetes20, cardiovascular21, neurodegenerative diseases22, with stress on Alzheimer’s disease (AD), as one of the most progressive (leading) health problems in the world16 , 17.
The etiology of AD is poorly understood. The oldest hypothesis links its cause with reduced synthesis of the neurotransmitters acetylcholine, noradrenaline and serotonin in the brain23. Deficiency in acetylcholine, which breaks down into inactive metabolites choline and acetate, is directly connected with the enzyme acetylcholinesterase (AChE) activity. AChE inhibitors (AChEi) are actually compounds that are used in the treatment of AD. Although these inhibitors do not stop the progression of disease, they can alleviate certain symptoms24. However, considering that common AChEi-based drugs (galanthamine, tacrine, donepezil and rivastigmine) have limited effectiveness and numerous side effects, there is a real need for novel AD therapeutics25. In the fungal kingdom, Flammulina velutipes, Lentinus edodes and many Pleurotus species have stood out for their ability to prevent or slow down the progression of neurodegenerative diseases24.
Taking into account relevant literature data and bioactive potential of wild-growing autochthonous medicinal mushrooms, the aim of the present study was to investigate antiradical (DPPH• and •OH) and AChE inhibitory activities of autochthonous fungal species T. versicolor originated from Serbia at the same time assessing its total contents of phenolics and polysaccharides. The overall aim was to estimate its potential as a possible source of bioactive compounds to be used as functional foods.
Materials and methods
Biological material and extract preparation
Fruiting bodies of T. versicolor were collected from Fruška Gora Mountain (Iriški Venac) in January 2014. Determination and identification of the collected material was carried out in the microbiological laboratory at the Department of Biology and Ecology, University of Novi Sad. Voucher specimens are deposited in the Herbarium of the University of Novi Sad (BUNS), under the number 12–00706.
Fresh material was lyophilized (Alpha 2–4 LDplus, Christ GmbH, Switzerland), grounded to a fine powder (IKA A11 basic, Germany) and kept in dark bottles at room temperature until further use. For the preparation of the H2O extract, 5 g of freeze-dried fruiting bodies was macerated with 100 mL of boiling water and stirred in a mechanical stirrer at 120 rpm (New Brunswick Scientific, Edison, NY), at room temperature for 24 h.
For the preparation of methanolic (MeOH) and ethanolic (EtOH) extracts, 5 g of fungal material was mixed with 100 mL of 100% methanol (Sigma Aldrich, Darmstadt, Germany) or 96% ethanol (Zorka Pharma, Serbia) and stirred at 120 rpm at room temperature for 72 h. After filtration (Filters Fioroni, France), H2O extracts were lyophilized, while MeOH and EtOH extracts were evaporated (Bűchi R-210, Switzerland) until the dry weight (dw) is reached. Finally, all extracts were redissolved in 100% DMSO (Baker Analyzed Reagent, JT Baker, Netherlands) to achieve the concentration of 20% (w/V). Polysaccharide (PSH) extracts were prepared according to the procedure of Ren et al27.
Determination of TPhC
TPhC was determined using the Folin-Ciolcateu (FC) reagent28, based on the detection of phenols that form a colored complex with the FC reagent, whose absorbance was read at 760 nm (MultiscanGO, ThermoScientific, Waltham, MA). Gallic acid (GA) was used for the preparation of calibration curve (0.1–1000 μmol/L). The content of TPhC was expressed as mg eq. gallic acid (GAE)/g dw of the extracts.
Determination of TFC
Determination of TFC in extracts was determined spectrophotometrically29. TFC was calculated on the basis of the calibration curve for quercetin (QUE) standard solution. The result was expressed as the mean of three measurements (mg eq. QUE/g dw).
Determination of TPSH
Determination of TPSH was performed by the phenol–sulfuric acid test (PSA), which was modified and adapted to experiment using microtiter plates30. The absorbance was measured at 490 nm. Standard of glucose (Merck Ltd., Stara Pazova, Serbia) was made at an initial concentration of 10 mg/mL, while a standard curve was made by diluting the stock solution in order to get the total sugar content, which was expressed as mg eq. glucose (GLUE)/g dw
HPLC-MS/MS determination of the phenolic compounds
Reference standards for the phenolic compounds were obtained from Sigma-Aldrich Chem (Steinheim, Germany), Fluka Chemie gmbh (Buchs, Switzerland) or from Chromadex (Santa Ana CA). HPLC grade methanol was purchased from J. T. Baker (Deventer, The Netherlands), while p.a. formic acid and DMSO from Merck (Darmstadt, Germany).
The phenolic profile of extracts was determined by HPLC-MS/MS31. The extracts were diluted with mobile phase solvents A (water) and B (methanol) (1:1) to obtain 2 mg/mL. Fifteen working standards (from 1.53 to 25.0 × 103 ng/mL) were prepared by serial dilutions (1:1) of the standard mixture with solvents A (water) and B (methanol) (1:1). The samples and standards were analyzed using the Agilent Technologies 1200 Series high-performance liquid chromatograph coupled with the Agilent Technologies 6410A Triple Quad tandem mass spectrometer with an electrospray ion source, controlled by the Agilent Technologies MassHunter Workstation software – Data Acquisition (ver. B.03.01). 5 μL of the samples were used, while the compounds were separated on the Zorbax Eclipse XDB-C18 (50 mm ×4.6 mm, 1.8 μm) rapid resolution column held at 50 °C. The mobile phase was delivered at a flow rate of 1 mL/min in gradient mode (0 min 30% B, 6 min 70% B, 9 min 100% B, 12 min 100% B, re-equilibration time 3 min). Eluted compounds were detected by ESI-MS, using the ion source parameters as follows: nebulization gas (N2) pressure 40 psi, drying gas (N2) flow 9 L/min and temperature 350 °C, capillary voltage 4 kV, negative polarity. Data were acquired in a dynamic MRM mode, using the optimized compound-specific parameters (retention time, precursor ion, product ion, fragmentor voltage, collision voltage). For all the compounds, peak areas were determined using the Agilent MassHunter Workstation software – Qualitative Analysis (ver. B.04.00). Calibration curves were plotted and the OriginLabs Origin Pro (ver. 8.0) software was used to calculate the concentration of the samples.
Characterization of polysaccharides
Planar Chromatography (PC) – Samples of the PSH extract were hydrolyzed with 2 M trifluoroacetic acid (TFA) at 120 °C for 16 h. The resultant hydrolysate was analyzed by descending paper chromatography, with ethyl acetate–pyridine–water as a mobile phase (2.5:1: 2.5, v/v/v). Monosaccharide components were identified by PC chromatography in a hydrolyzed PS extract of the test sample by comparison of chromatographic mobility with authentic monosaccharide standards.
Structured instrumental characterization of PSH – Organic microanalysis of elements was done for PSH. The content of C, H, N and S in the polysaccharide extract was analyzed using the automatic analyzer Vario EL III CHNS/O elemental analyzer (Elementar, Germany).
Fourier transform infrared spectroscopy (FTIR)
FTIR spectra of the PS extract were recorded in a range of 400–4000 cm−1 using the KBr disc technique on the camera Nicolet 6700 FT (Thermo Scientific, Waltham, MA) in the technique of shooting ATR-FTIR (Attenuated Total Reflectance – Fourier Transform Infrared Spectroscopy).
Determination of RSC
DPPH• assay – DPPH radicals scavenging capacity (RSC) was determined by the DPPH method32, which is based on transformation of purple colored stable DPPH• into reduced yellow colored DPPH-H. The color change was measured at 515 nm. The reaction mixture contained 60 μL of DPPH reagent, 10 μL of the extract and 180 μL of MeOH. Butylated hydroxyanisole (BHA, Sigma-Aldrich, Darmstadt, Germany) was used as the standard antioxidant. All tests were performed in triplicate. Free radical scavenging capacity was determined by the following formula:
where A test and A control are the absorbances of study and control reactions. The result obtained using the regression analysis was expressed as an IC50 value (the concentration of the test substance at which 50% of the radicals is neutralized).
•OH assay – RSC of the •OH was determined by a modified method of Halliwell and Gutteridge33. •OH react with 2-deoxy-D-ribose and oxygen creating malondialdehyde (MDA), which is determined by the TBA (thiobarbituric acid) assay based on the spectrophotometric measurement of pink coloration formed by MDA and TBA. After incubation for 60 min at 37 °C and addition of ethylenediaminetetraacetic acid (EDTA) (Thermo Scientific, Waltham, MA) and TBA reagent, the pink color was developed and measured at 532 nm (Multiscan, Thermo Scientific, Waltham, MA). The RSC value was obtained according to the same formula used for the DPPH• assay.
AChE inhibitory activity
In solid
Inhibition of AChE was carried out as described by Marston et al.34, and the H2O and MeOH extracts were dissolved in MeOH to a stock concentration of 1 mg/mL after lyophilization and evaporation. Dilution series were made from this stock solution as follows: 0.001, 0.01 and 0.1 mg/mL. 10 μL of each solution was applied to TLC plates in order to test 100, 10, 1 and 0.1 μg of samples to detect the minimum concentration which inhibits AChE. Galanthamine (GA, Sigma-Aldrich, Darmstadt, Germany) was used as a positive control at concentrations of 1–0.001 μg. AChE inhibitory activity was detected by a white spot on a purple background after 1–2 min exposure.
In liquid
In vitro determination of AChE inhibitory activity35 adapted for the use in 96-well microplates was done. Acetylcholine iodide (AChI) originating from electric eel (Sigma Aldrich) was used as an artificial substrate for the enzyme AChE, which degrades this compound to acetate and thiocholine. In the next reaction, dithiobenzoate (DTNB) produced the yellow color with thiocholine which was measured at 412 nm at 25 °C. The reaction test wells contained 20 μL of the fungal extract, 150 μL of Ellman’s reagent and AChI and 50 μL of AChE (518 U/mL) and phosphate buffer (PB). All tests were performed in triplicate. Donepezil at 1 mg/mL (Donecept, Zdravlje Leskovac, Serbia) was used as a positive control.
The result expressed as a percentage (%) of AChE inhibition by the extract was calculated according to the following formula:
Statistical analysis
The results were reported as mean values ± standard deviation (SD). IC50 values were determined by the linear regression analysis of RSC (Microsoft Excel program for Windows, v. 2007 and Origin 8). The statistical analysis was performed using the one-way ANOVA (STATISTICA, StatSoft, Inc. (2012), version 10.0, www.statsoft.com). Tukey’s test was used to determine significant differences (p < .01) between the extracts.
Results and discussion
TPhC, TFC and TPSH contents
It has been previously demonstrated that bioactive compounds derived from fungi, such as phenolics, including phenolic acids and flavonoids4 , 36, polysaccharides9 and proteins37 or polysaccharide–protein complexes9 contribute significantly to the antioxidant activity of fungal species.
The results obtained for the contents of TPh, TF and TSH are shown in Table 1. In relation to the values obtained for total contents of detected compounds, the extracts showed activity as follows: TPhC – H2O > EtOH > MeOH, TFC – MeOH > EtOH > H2O, and TPSH – H2O > EtOH > MeOH.
Table 1.
The chemical composition of T. versicolor extracts.
| TPhC(mg eq. GAE/g dw) | TFC(mg eq. QUE/g dw) | TPSH(mg eq. GLUE/g dw) | |
|---|---|---|---|
| H2O | 142.17 ± 3.08* | 1.56 ± 0.48† | 58.60 ± 2.16* |
| EtOH | 71.55 ± 3.00† | 4.13 ± 0.91* | 33.80 ± 4.45‡ |
| MeOH | 64.76 ± 2.08† | 5.13 ± 0.70* | 46.49 ± 4.96† |
Each value is expressed as mean ± SD.
*, †, ‡Significant differences between extracts were determined by the Tukey HSD test at p < .01.
Means with different letters within a column are significantly different.
H2O: water extract of T. versicolor; EtOH: ethanolic extract of T. versicolor; MeOH: methanolic extract of T. versicolor; TPhC: total phenol content; TFC: total flavonoid content; TPSH: total polysaccharides.
The H2O extract showed the highest TPhC (142.17 mg eq. GAE/g dw) which was actually twice bigger compared to the EtOH and MeOH extracts. Thus, TPhC may be considered as the possible main components for RSC activity observed (Table 3). Furthermore, the examined MeOH sample had twice higher TPhC (64.76 mg eq. GAE/g dw) than the same extract from China (23.28 mg eq. GAE/g dw)2. The TPhC of T. versicolor EtOH extract analyzed herein (71.55 mg eq. GAE/g dw) showed a much higher value than the same extract from Turkey (9.58 mg eq. GAE/g extract)17. The highest content of TFC was recorded in the MeOH extract (5.13 mg eq. QUE/g dw) (Table 1).
Table 3.
Antiradical activity (EC50) and acetylholinesterase inhibitory activity of PSH, H2O, EtOH and MeOH extracts of T. versicolor.
| DPPH· (μg/mL) | •OH (μg/mL) | AChE in solid (μg) | AChE in liquid (%) | |
|---|---|---|---|---|
| PSH | 950.16 ± 2.43¶ | 109.00 ± 1.16¶ | – | nd |
| H2O | 14.89 ± 1.36a | 3.21 ± 0.10a | 10 | 60.53 ± 2.12 |
| EtOH | 155.61 ± 2.62‡ | 46.52 ± 0.18‡ | 44.35 ± 2.06 | |
| MeOH | 51.57 ± 3.14† | 16.45 ± 0.24† | nd | nd |
| BHA | 8.62 ± 0.50 | 8.67 ± 0.58 | – | – |
| Galanthamine | – | – | 0.001 | – |
| Donepezil | – | – | – | 89.05 ± 1.35 |
Each value is expressed as mean ± SD.
a, †, ‡, ¶Means with different letters within a column are significantly different.
(Tukey’s HSD test, p < .01).
nd: not detected; PSH: polysaccharide extract of T. versicolor; H2O: water extract of T. versicolor; EtOH: ethanolic extract of T. versicolor; MeOH: methanolic extract of T. versicolor.
HPLC-MS/MS determination
Among 45 investigated phenols, a total of 38 compounds were identified and quantified by HPLC-MS/MS in all the extracts examined (Table 2). A total of 20 flavonoid aglycones and glycosides, 1 biflavonoid (amentoflavone), 2 isoflavonoids (daidzein and genisein), 3 coumarins, 6 hydroxybenzoic and 4 hydroxycinammic acids, quinic acid and 5-O-caffeoylquinic acid were found. Among the investigated samples, the EtOH and MeOH extracts were the richest in phenolics, expressing TPhC of 776.29 and 514.66 μg/g, respectively.
Table 2.
Quantification of particular phenolics by HPLC-MS/MS.
| Extract (μg/g dw) |
|||||
|---|---|---|---|---|---|
| Class | Compound | PSH | H2O | EtOH | MeOH |
| Flavones | Apigenin | 1.57 | 1.71 | 0.93 | 0.23 |
| Baicalein | 43.00 | 21.60 | 8.04 | 3.63 | |
| Luteolin | 2.01 | 1.47 | 1.05 | 1.19 | |
| Chrysoeriol | 1.68 | 1.79 | 1.21 | 0.74 | |
| Vitexin | 2.06 | 1.56 | 1.98 | 1.19 | |
| Apigenin-7-O-Glucoside | 2.37 | 1.41 | 1.34 | 0.54 | |
| Luteolin-7-O-glucoside | 0.78 | 0.91 | 0.78 | 0.26 | |
| Apiin | 2.86 | 1.82 | 2.07 | 0.86 | |
| Baicalin | 9.95 | 10.7 | 8.88 | 6.27 | |
| Flavonols | Kaempferol | 1.69 | 2.15 | 1.96 | 1.58 |
| Quercetin | 33.70 | 31.20 | 29.90 | 29.30 | |
| Isorhamnetin | 21.40 | 14.60 | 9.36 | 8.97 | |
| Quercitrin | 0.89 | 1.62 | 1.81 | 1.93 | |
| Kaempferol-3-O-Glucoside | 1.68 | 1.71 | 1.86 | 0.80 | |
| Hyperoside | 0.85 | 0.68 | 0.11 | 0.43 | |
| Quercetin-3-O-Glucoside | 0.96 | 0.31 | 1.72 | 0.59 | |
| Rutin | 1.01 | 1.11 | 1.34 | 0.53 | |
| Flavanone | Naringenin | 1.82 | 1.70 | 1.82 | 1.06 |
| Flavanols | Catechin | nd | 17.20 | 5.91 | 21.90 |
| Epicatechin | 3.92 | nd | nd | nd | |
| Biflavonoid | Amentoflavone | 32.40 | 17.20 | 7.79 | 6.60 |
| Isoflavonoids | Daidzein | 0.86 | 0.53 | nd | nd |
| Genistein | 0.51 | 0.37 | 0.30 | 0.21 | |
| Hydroxybenzoic acids | p-Hydroxybenzoic acid | 10.10 | 141.00 | 465.00 | 184.00 |
| Protocatechuic acid | 2.06 | 1.82 | 18.20 | 8.57 | |
| Gentisic acid | nd | nd | 32.10 | 24.90 | |
| Vanillic acid | nd | nd | 45.60 | 44.70 | |
| Gallic acid | 9.30 | 11.40 | 16.80 | 22.00 | |
| Syringic acid | nd | 6.02 | 59.10 | 30.10 | |
| Hydroxycinnamic acids | p-Coumaric acid | 1.56 | 1.21 | 1.49 | 1.28 |
| o-Coumaric acid | 0.81 | 1.16 | 0.79 | 0.74 | |
| Ferulic acid | 2.12 | nd | 2.66 | 2.12 | |
| Caffeic acid | 1.72 | 1.53 | 3.26 | 2.33 | |
| Coumarins | Esculetin | nd | 0.78 | 26.20 | 2.87 |
| Scopoletin | 4.22 | 1.66 | 0.59 | nd | |
| Umbeliferon | 1.40 | 1.13 | 1.37 | 1.13 | |
| Cyclohexanecarboxylic acid | Quinic acid | 2.72 | 80.40 | 8.84 | 98.70 |
| Chlorogenic acid | 5-O-caffeoylquinic acid | 2.29 | 2.93 | 4.13 | 2.41 |
| Total | 206.27 | 384.39 | 776.29 | 514.66 | |
nd – not detected, peak not observed; the concentration is lower than the LOD.
Bold numbers indicate the highest values of the respective compound.
PSH: polysaccharide extract of T. versicolor; H2O: water extract of T. versicolor; EtOH: ethanolic extract of T. versicolor; MeOH: methanolic extract of T. versicolor.
The EtOH extract contained most of analyzed phenolic acids, with the highest amount of p-hydroxybenzoic acid, syringic acid and vanillic acid. The highest TFC was detected in the H2O and PSH extracts. Among flavonoids, the most prevalent were baicalein, catechin, isorahmetin and epicatechin, while epicatechin was detected only in the PSH extract. While several phenolic substances such as gallic, caffeic and protocatechuic acids had already been detected in autochtonous T. versicolor 5, p-coumaric acid along with all the flavonoids were detected herein for the first time. More precisely, some flavonoid compounds had been previously found in fungi, such as catechin, naringenin, quercetin, rutin and kaempferol3, a more fruitful detection of phenolic compounds proceeded in this work. Indeed, flavonoids (eight flavones, six flavonols, flavanone, flavanols, biflavonoid and isoflavonoids), phenolic acids (hydroxybenzoic, hydroxicynnamic, cyclohexanecarboxylic and chlorogenic acids) and coumarins of this species have not been reported so far. Although some authors claim that mushrooms do not contain flavonoids, since they are not plants and cannot synthesize them38, we have to point out that we have detected a variety of these compounds in the fruiting bodies of T. versicolor. The possibility for the accumulation of these compounds from the substrate may be strictly strain-specific and influenced by specific environmental conditions in the fungal habitat (soil and wood)39. Furthermore, some polyphenols detected herein (e.g. quercetin and kaempferol) together with some others found in plants (huperzine A, tri- and tetra-hydroxyflavones) have shown to express acetylcholinesterase inhibitory activity40 , 41. Similarly, the AChE inhibition had also been previously observed in the case of flavonoids, such as catechin and amentoflavone26 , 42. In the present study, both these flavonoid compounds were identified in the extracts examined (Table 2). Hence, we can assume that they may be responsible molecules for AChE inhibitory activity potential of T. versicolor.
Characterization of PSH
The T. versicolor acid hydrolyzed PSH extract was identified using PC chromatography. The main polysaccharide component was D-glucose; D-galactose was less prevalent, while D-mannose, L-fucose and D-xylose were found only in traces. This is in agreement with the results obtained by other authors10. The presence of additional sugar compounds and uronic acid was not observed in the aforementioned sample.
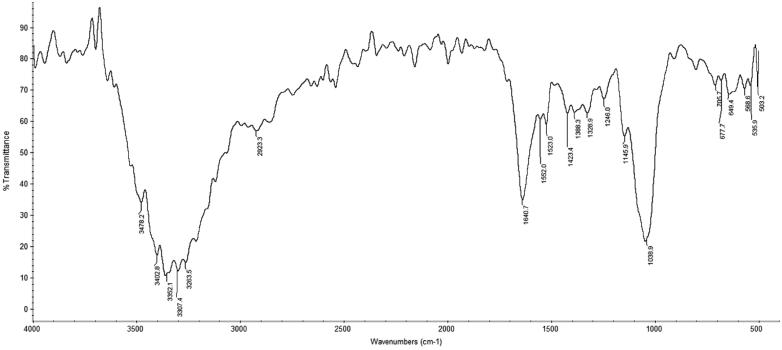
The FTIR spectrum of PSH showed absorption bands typical for a polysaccharide structure (Figure 1). In addition to polysaccharides, the extract contained a mixture of protein and polyphenolic compounds in varying degrees.
Figure 1.
FTIR spectrum of the polysaccharide extract of Trametes versicolor. FTIR assignments, wave number (cm−1): 3000–3500 cm−1 stretching vibration O–H and N–H; 2920–2950 cm−1 C–H stretching vibration; 1630–1650 cm−1 is characteristic of the absorbed water; 1200–1500 cm−1 C–H deformation vibrations; 1030–1070 cm−1 and 1150 cm−1 stretching vibrations of glicosidic linkage (C–O–C) and C–O–H, respectively; 850 cm−1 and 950 cm−1 indicate the presence of α-glycoside bond; 890 cm−1 indicates the presence of β-glycoside bond; 1630–1655 cm−1 corresponds to the amide I band while the peak at about 1550 cm−1 (1500–1600 cm−1) refers to the amide II band. The presence of pigments is characterized with frequencies in the range (1600–1650 cm−1) indicative for the aromatic C = C double bond conjugated with the C = O and/or –COO groups; the area of 1310–1410 cm−1 is specific to OH groups of the phenolic compounds.
In addition to the absorption peaks specific for polysaccharides, the FTIR spectrum included absorption peaks typical for proteins and polyphenolic compounds, taking into account some of the overlapped absorptions. The bands in the area of 1310–1410 cm−1 were specific for the OH groups belonging to phenolic compounds. The obtained results are in good accordance with published experimental data for the same fungal species9.
There is a growing body of evidence that antioxidant activity of polysaccharides depends on their structural features, like molecular weight, monosaccharide composition, configuration and type of glycosyl bonds6 , 9 , 27. Polysaccharides with lower molecular weight or those with a β-configuration in the pyranose form show a higher antioxidant activity than α-glycans27. For RSC on DPPH•, the conformation of T. versicolor PSH is of greater importance than the composition of monosaccharides9 which is consistent with the assumptions that relation of OH/CH groups is of less importance than structural characteristics based on α- and β-glycoside bonds27.
The total content of macroelements in the PSH extract was as follows: nitrogen (N = 3.36%), carbon (C = 38.85%) and oxygen (H = 6.38%), while microanalysis pointed out that PSH extract did not contain sulfur. The obtained value for N indicated the presence of proteins. The fungal protein content analysis was based on the conversion factor of 4.3843.
RSC on DPPH• and •OH
The results of antiradical activity of all three crude extracts along with PSH fraction of T. versicolor are presented in Table 3. All extracts were arranged in separate statistical groups based on the expressed activities of both the radical species, which is as follows: H2O > MeOH > EtOH > PSH.
The highest RSC on •OH and DPPH• obtained for the H2O extract (IC50 = 3.21 and 14.89 μg/mL, respectively) was similar to the activity of the commercial antioxidant BHA (8.62 μg/mL) (Table 3). The MeOH extract was also an efficient scavenger, while the EtOH extract exhibited three times lower RSC. The obtained RSC value for the MeOH extract was lower than previously observed one for 70% MeOH (IC50 = 239.30 μg/mL)5. The difference may have been caused by the choice of the solvent concentration used during extraction. As stated in literature till date5 , 9 we found that RSC of fungal extracts on DPPH• increases with increasing concentrations of extracts.
It has been well established that phenolic substances are strongly associated with antiradical activity3–5. Quercetin and rutin, derivatives of quinic acid, showed high scavenging DPPH• activity44. Antiradical activity actually depends on the basic structural organization of the phenolic compounds44 , 45. Both the substituents on the phenyl ring and conjugated carbon skeleton were previously found to be of importance for this particular activity45 , 46. Consequently, quercetin, kaempferol and myrcetin (flavonols with a free 3-hydroxy group) exhibited better antiradical activity compared to flavonoids with glycosylated OH group. Sroka (2014) emphasized potent anti DPPH• activity of gallic and caffeic acids, the compounds identified in T. versicolor H2O extract. On the other hand, gallic, p-coumaric and caffeic acids are known due to their anti •OH potential46.
High correlations between TFC and DPPH• (R 2 = 0.97 and 0.96, respectively) for the EtOH extract were observed. In case of the MeOH extract, the highest correlation was found for TPSH and DPPH• (R 2 = 0.88). On the other hand, for the H2O extract only TPSH displayed a moderate correlation with DPPH• (R 2 = 0.66) (Table 4). These correlations point out the roles of flavonoids and polysaccharides in the EtOH and H2O & MeOH extracts, respectively.
Table 4.
Correlation (R 2) between content of compounds (TF, TPh, TPR and PSH) and antiradical activities.
| TPhC | TFC | TPSH | ||
|---|---|---|---|---|
| H2O | R2 | |||
| DPPH• | 0.53 | 0.03 | 0.66 | |
| •OH | 0.50 | 0.42 | 0.30 | |
| EtOH | ||||
| DPPH• | 0.79 | 0.97* | 0.75 | |
| •OH | 0.58 | 0.89 | 0.75 | |
| MeOH | ||||
| DPPH• | 0.87 | 0.48 | 0.88 | |
| •OH | 0.27 | 0.03 | 0.07 |
Significant correlation coefficient (R 2) (p < .05).
Bold numbers indicate the highest values.
TPhC: total phenol content; TFC: total flavonoid content; TPSH: total polysaccharides; H2O: water extract of T. versicolor; EtOH: ethanolic extract of T. versicolor; MeOH: methanolic extract of T. versicolor.
Inhibition of acetylcholinesterase
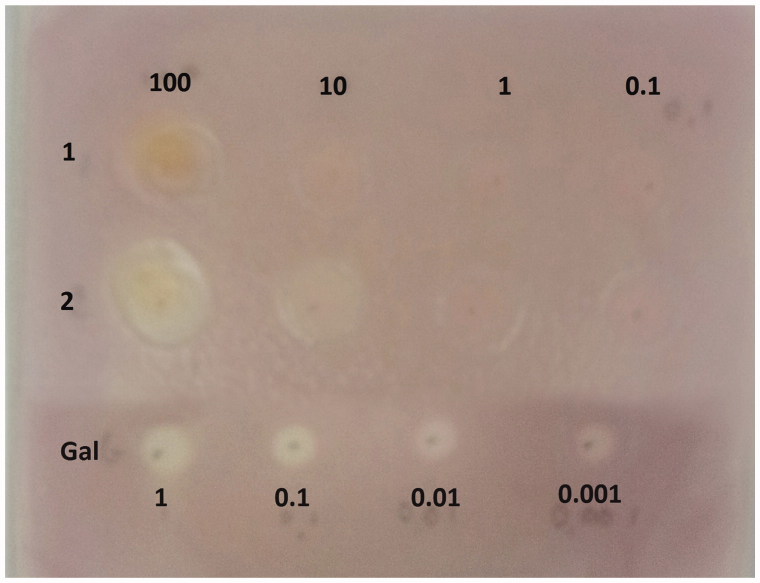
Qualitative and quantitative inhibition of AChE activity by the aforementioned extracts was examined using thin-layer chromatography (TLC) and confirmed by the appearance of white halos in places of the enzyme inhibition by the microdilution assay in vitro. Among two of the selected crude extracts (H2O and MeOH), only the H2O extract exhibited AChE inhibitory activity at 10 μg (Figure 2). The PSH, H2O and EtOH extracts were analyzed by the quantitative microdilution using Ellman’s assay in vitro as well. At 100 μg/mL, a strong degree of inhibition (60.53%) was expressed, compared to donepezil (89.05%), which is commonly used as an AChE inhibitor in the treatment of AD. The analyzed EtOH extract (44.35%, at 500 μg/mL) displayed a two times higher AChE inhibitory activity compared with T. versicolor EtOH extract previously studied (28.35%, at the same concentration)17. The values obtained for AChE inhibitory activity of the extracts (H2O and EtOH) were IC50 = 78.01 μg/mL and IC25 = 383.96 μg/mL, respectively. This inhibition was dependent on the concentration, as it was the case of quercetin reported by Zhang et al.44 which inhibited AChE 58.80% at the concentration of 40 μg/mL.
Figure 2.
In solid AChE inhibitory activity of selected T. versicolor extracts. 1 – methanolic extract (MeOH) of T. versicolor; 2 – water extract (H2O) of T. versicolor; Gal – galanthamine, used as a positive control.
Examining AChE inhibitory activity of different phenolic compounds, Balkis et al.47 found baicalein as the most potent inhibitor. Indeed, apigenin, baicalein, kaempferol and quercetin expressed inhibitory effect above 90% at 100 μM. In addition to this, daidzein also inhibited the respective enzyme, at the lower extent. Finally, p-hydroxybenzoic acid (presented in T. versicolor bioactive extract) is known to exhibit the aforementioed bioactivity48.
Taken all together, it was found that the flavonoids baicalein (21.60 μg/g dw) and quercetin (31.20 μg/g dw) may be the responsible compounds for the AChE inhibitory activity. While alkaloid compounds represent the main group of AChEi49, those not belonging to this structural class include terpenoids, flavonoids and other phenolic substances50. The inhibition of AChE activity reported herein may be caused by some complexes. It is well-known that polyphenolic and polysaccharide compounds are linked to each other resulting in, for example, triterpenoids that may be dissolved in H2O7. Indeed, the water soluble triterpenoid compounds in the H2O extract may also contribute both to the antioxidant activity and synergistic effect of these compounds with polysaccharides, phenols and flavonoids as suggested by other authors in the field7.
Conclusions
All the examined extracts exhibited a significant antiradical activity. Among them, the most potent was the H2O extract. Furthermore, the H2O extract showed the best AChE inhibitory activity. This is the first record of AChE inhibitory activity of the T. versicolor H2O extract: baicalein and quercetin were responsible for the inhibition of AChE activity. This extract actually contained 35 phenolic compounds. Among the rest, the identified compounds included daidzein (isoflavone molecule not presented in alcoholic extracts) and amentoflavone and catechin (presented in twice bigger concentrations, compared with the EtOH extract). Taken all together, the antiradical and AChE inhibitory activities displayed by phenols and flavonoids may be attributed also to polysaccharides. As a consequence, T. versicolor should be considered as an alternative source of bioactive substances to be used in the treatment of AD and other neurodegenerative diseases.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This study was carried out within the projects supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Nos. 172053 and 172058).
References
- 1. Glumac M, Pejin B, Karaman M, et al. Lignicolous fungi hydrodistilled extracts may represent a promising source of natural phenolics. Nat Prod Res 2016; DOI: 10.1080/14786419.2016.1212036. [DOI] [PubMed] [Google Scholar]
- 2. Mau JL, Lin HC, Song SF.. Antioxidant properties of several specialty mushrooms. Food Res Int 2002;35:519–26. [Google Scholar]
- 3. Kim MY, Seguin P, Ahn JK, et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea . J Agric Food Chem 2008;56:7265–70. [DOI] [PubMed] [Google Scholar]
- 4. Ferreira ICFR, Barros L, Abreu RMV.. Antioxidants in wild mushrooms. Curr Med Chem 2009;16:1543–60. [DOI] [PubMed] [Google Scholar]
- 5. Karaman M, Jovin E, Malbaša R, et al. Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytother Res 2010;24:1473–81. [DOI] [PubMed] [Google Scholar]
- 6. Khatua S, Paul S, Acharya K.. Mushroom as the potential source of new generation of antioxidant: a review. Res J Pharm Technol 2013;6:496–505. [Google Scholar]
- 7. Hsu WK, Hsu TH, Lin FY, et al. Separation, purification, and α-glucosidase inhibition of polisaccharides from Coriolus versicolor LH1 mycelia. Carbohyd Polym 2013;92:297–306. [DOI] [PubMed] [Google Scholar]
- 8. Pejin B, Jovanovic K, Mojovic M, et al. New and highly potent antitumor natural products from marine-derived fungi: covering the period from 2003 to 2012. Curr Top Med Chem 2013;13:2745–66. [DOI] [PubMed] [Google Scholar]
- 9. Kozarski M, Klaus A, Niksic M, et al. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor . J Food Compos Anal 2012;26:144–53. [Google Scholar]
- 10. Sun X, Sun Y, Zhang Q, et al. Screening and comparison of antioxidant activities of polysaccharides from Coriolus versicolor . Int J Biol Macromol 2014;69:12–9. [DOI] [PubMed] [Google Scholar]
- 11. Chaumenton JP, Chauveau C, Chavant L.. Water-soluble polysaccharides excreted by mycelium of higher fungi: relationship with taxonomy and physiology. Biochem Syst Ecol 1993;21:227–39. [Google Scholar]
- 12. Kamiyama M, Horiuchi M, Umano K, et al. Antioxidant/anti-inflammatory activities and chemical composition of extracts from the mushroom Trametes versicolor . Int J Nutr Food Sci 2013;2:85–91. [Google Scholar]
- 13. Luo KW, Yue GGL, Ko CH, et al. In vivo and in vitro anti-tumor and anti-metastasis effects of Coriolus versicolor aqueous extract on mouse mammary 4T1 carcinoma. Phytomedicine 2014;21:1078–87. [DOI] [PubMed] [Google Scholar]
- 14. Helba L, Vuković N, Petrová J, et al. Antimicrobial activity of crude methanolic extracts from Ganoderma lucidum and Trametes versicolor . Anim Sci Biotechnol 2014;47:89–93. [Google Scholar]
- 15. Liu YT, Sun J, Luo ZY, et al. Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem Toxicol 2012;50:1238–44. [DOI] [PubMed] [Google Scholar]
- 16. Trovato A, Siracusa R, Di Paola R, et al. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: relevance to Alzheimer’s disease pathogenesis. Neurotoxicology 2016;53:1–358. [DOI] [PubMed] [Google Scholar]
- 17. Orhan I, Üstün O.. Determination of total phenol content, antioxidant activity and AChE inhibition in selected mushrooms from Turkey. J Food Compos Anal 2011;24:386–90. [Google Scholar]
- 18. Wan JMF. Polysaccharide Krestin (PSK) and Polysaccharopeptide (PSP) In: Kastin AJ, ed. Handbook of biologically active peptides: fungal peptides. USA: Elsevier Inc; 2013:180–184. [Google Scholar]
- 19. Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer . Chem Biol Interact 2006;160:1–40. [DOI] [PubMed] [Google Scholar]
- 20. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiologicalfunctions and human disease. Int J Biochem Cell B 2007;39:44–84. [DOI] [PubMed] [Google Scholar]
- 21. Shah AM, Channon KM.. Free radicals and redox signalling in cardiovascular disease. Heart 2004;90:486–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moreira PI, Santos MS, Oliveira CR, et al. Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol Disord Drug Targets 2008;7:3–10. [DOI] [PubMed] [Google Scholar]
- 23. Bryne GJA. Treatment of cognitive impairment in Alzheimer's disease. Aust J Hosp Pharm 1998;28:261–6. [Google Scholar]
- 24. Filho JMB, Medeiros KCP, Diniz MF, et al. Natural products inhibitors of the enzyme AChE. Braz J Pharmacog 2006;16:258–85. [Google Scholar]
- 25. Chopra K, Misra S, Kuhad A. Current perspectives on pharmacotherapy of Alzheimer’s disease. Expert Opin Pharmacother 2011;12:335–50. [DOI] [PubMed] [Google Scholar]
- 26. Williams P, Sorribas A, Howes MJ.. Natural products as a source of Alzheimer’s drug leads. Nat Prod Rep 2011;28:48–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ren L, Hemar Y, Perera CO, et al. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact Carbohydr Dietary Fibre 2014;3:41–51. [Google Scholar]
- 28. Singleton VL, Orthofer RRM.. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol 1999;299:152–78. [Google Scholar]
- 29. Chang CC, Yang MH, Wen HM, et al. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 2002; 10:178–82. [Google Scholar]
- 30. Masuko T, Minami A, Iwasaki N, et al. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem 2005;339:69–72. [DOI] [PubMed] [Google Scholar]
- 31. Orčić D, Francišković M, Bekvalac K, et al. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem 2014;143:48–53. [DOI] [PubMed] [Google Scholar]
- 32. Espin CJ, Soler-Rivas G, Wichers JH.. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agr Food Chem 2000;48:648–56. [DOI] [PubMed] [Google Scholar]
- 33. Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview . Meth Enzymol 1990;186:1–85. [DOI] [PubMed] [Google Scholar]
- 34. Marston A, Kissling J, Hostettmann K.. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem Analysis 2002;13:51–4. [DOI] [PubMed] [Google Scholar]
- 35. Ellman GL, Courtney KD, Andres V, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95. [DOI] [PubMed] [Google Scholar]
- 36. Karaman M, Stahl M, Vulić J, et al. Wild-growing lignicolous mushroom species as sources of novel agents with antioxidative and antibacterial potentials. Int J Food Sci Nutr 2014;65:311–9. [DOI] [PubMed] [Google Scholar]
- 37. Erjavec J, Kos J, Ravnikar M, et al. Proteins of higher fungi-from forest to application. Trends Biotechnol 2012;30:259–73. [DOI] [PubMed] [Google Scholar]
- 38. Gil-Ramírez A, Pavo-Caballero C, Baeza E, et al. Mushrooms do not contain flavonoids. J Funct Foods 2016;25:1–13. [Google Scholar]
- 39. Karaman M, Matavulj M.. Macroelements and heavy metals in some lignicolous and tericolous fungi. Matica Srpska Proceedings for Natural Sciences 2005;108:255–67. [Google Scholar]
- 40. Ebrahim A, Schluesener H.. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res Rev 2012;11:329–45. [DOI] [PubMed] [Google Scholar]
- 41. Choi D, Lee Y, Hong J, et al. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res Bull 2012;87:144–53. [DOI] [PubMed] [Google Scholar]
- 42. Mandel S, Youdim M.. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases . Free Radic Biol Med 2004;37:304–17. [DOI] [PubMed] [Google Scholar]
- 43. Silva EG, Dias ES, Siqueira FG, et al. Chemical analysis of fructification bodies of Pleurotus sajor-caju cultivated in several nitrogen concentrations. Cienc Tecnol Aliment 2007;27:72–5. [Google Scholar]
- 44. Zhang XD, Liu XQ, Kim YH, et al. Chemical constituents and their acetyl cholinesterase inhibitory and antioxidant activities from leaves of Acanthopanax henryi: potential complementary source against Alzheimer’s disease. Arch Pharm Res 2014;37:606–16. [DOI] [PubMed] [Google Scholar]
- 45. Mathew S, Abraham TE, Zakaira ZA.. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J Food Sci Technol 2015;52:5790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sroka Z. Antioxidative and antiradical properties of plant phenolics . Z Naturforsch C J Biosci 2014;60:833–43. [DOI] [PubMed] [Google Scholar]
- 47. Balkis A, Tran K, Lee YZ, et al. Screening flavonoids for inhibition acetylcholinesterase identified baicalein as the most potent inhibitor. J Agr Sci 2015; 7:26–35. [Google Scholar]
- 48. Szwajgier D. Anticholinesterase activity of selected phenolic acids and flavonoids – interaction testing in model solutions. Ann Agr Env Med 2015;22:690–4. [DOI] [PubMed] [Google Scholar]
- 49. Teles APC, Takahashi JA. Paecilomide, a new acetylcholinesterase inhibitor from Paecilomyces lilacinus . Microbiol Res 2013;168:204–10. [DOI] [PubMed] [Google Scholar]
- 50. Murray AP, Faraoni MB, Castro MJ, et al. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr Neuropharmacol 2013;11:388–413. [DOI] [PMC free article] [PubMed] [Google Scholar]