Abstract
Objective
The objective of this study was to describe the indications for postnatal cytomegalovirus (CMV) testing among very low-birth-weight (VLBW, birth weight [BW] < 1,500 g) infants, clinical characteristics of infected infants, and adverse outcomes associated with CMV infection.
Study Design
This is a single-center, retrospective study of 2,132 VLBW infants from 1999 to 2013.
Results
In this study, 145 (6.8%) infants out of 2,132 were evaluated for postnatal CMV infection and 27 (18.6%) infants out of 145 were infected. CMV-tested infants were of significantly lower gestational age and BW compared with untested VLBW infants (p < 0.001). Respiratory decompensation and thrombocytopenia were the findings most commonly associated with infection. CMV-infected infants had significantly more exposure to mechanical ventilation and longer duration of hospitalization. Adjusting for multiple predictors of respiratory morbidity, the incidence of bronchopulmonary dysplasia (BPD) was significantly elevated among infants diagnosed with postnatal CMV infection (odds ratio, 4.0 [95% confidence interval, 1.3–12.4); p, 0.02.)
Conclusion
Symptomatic postnatal CMV infection was diagnosed in 1.3% of VLBW infants, most commonly among infants with BW < 1,000 g with respiratory instability and thrombocytopenia. Similar to late-onset bacterial infection, symptomatic postnatal CMV infection may be an independent contributor to the development of BPD. This possibility should be addressed in a prospective study of extremely low BW infants.
Keywords: cytomegalovirus, breast milk, postnatal transmission, premature, bronchopulmonary dysplasia
Breast milk transmission of cytomegalovirus (CMV) is now recognized as the primary source of postnatal transmission of CMV to preterm infants.1,2 The clinical course of postnatally acquired CMV infection in very low-birth-weight (VLBW) premature infants ranges from asymptomatic infection to a severe sepsis-like illness characterized variably by neutropenia, thrombocytopenia, hepatitis, colitis, and pneumonitis, although rarely with mortality.2–4 The majority of prospective and case–control studies of postnatal CMV infection among VLBW infants have reported no difference in clinical outcomes,5–9 although recent studies have found negative impacts on long-term neuro-developmental outcomes.10–12 Prior studies have included few symptomatic CMV-positive infants, and many asymptomatic, infected infants identified only by prospective screening.7,9,13 These studies could reassure neonatal clinicians that most instances of postnatal CMV have little impact on VLBW infants. However, variable approaches to freezing, pasteurizing, or withholding breast milk for VLBW infants persist due to concerns about breast milk–acquired CMV infection.14–16 It is possible that even among similarly vulnerable infants, those with symptomatic postnatal CMV infection have a more severe infection, and/or altered immune response, that negatively impacts their subsequent health.15,17,18 In particular, postnatal CMV may cause an acute pneumonitis and respiratory deterioration that may contribute to long-term pulmonary morbidity.19
Universal prospective maternal and/or infant CMV screening among VLBW infants is not a standard practice in American neonatal intensive care units (NICUs). Yet, the recognition of symptomatic postnatal CMV infection might spare infants’ prolonged antibiotic treatment for undiagnosed sepsis-like symptoms and identify infants for whom antiviral therapy and/or more intensive auditory and neurodevelopmental follow-up care may be indicated. In the absence of universal screening, there is a need to define infants most likely to benefit from diagnostic evaluation of postnatal CMV. We reviewed 15 years of data at our institute to describe the indications prompting CMV testing among VLBW infants; the clinical characteristics associated with symptomatic CMV infection; and outcomes among the symptomatically infected infants.
Methods
Study Design
Retrospective study of VLBW infants born from January 1, 1999, to December 31, 2013, at the Brigham and Women’s Hospital (BWH), a tertiary care NICU receiving specialty consult services from Boston Children’s Hospital. All CMV testing was done at the discretion of the attending neonatol-ogist, prompted by the presence of clinical findings suggesting either congenital or postnatal infection. Both fresh and frozen breast milk were provided to preterm infants in the NICU without regard to maternal CMV immunity. All blood products administered were leukocyte-reduced and irradiated. The study was approved by the Institutional Review Boards at BWH and Boston Children’s Hospital, Boston, MA.
Data Sources
Hospital administrative and laboratory data were reviewed for the entire study cohort. CMV-tested VLBW infants were identified by review of the hospital virology database. Medical chart review was performed to identify the clinical find-ings that prompted CMV testing. Data for clinical outcomes for infants born between January 1, 2003, and December 31, 2013, were obtained by review of NICU databases.
Study Population and Definitions
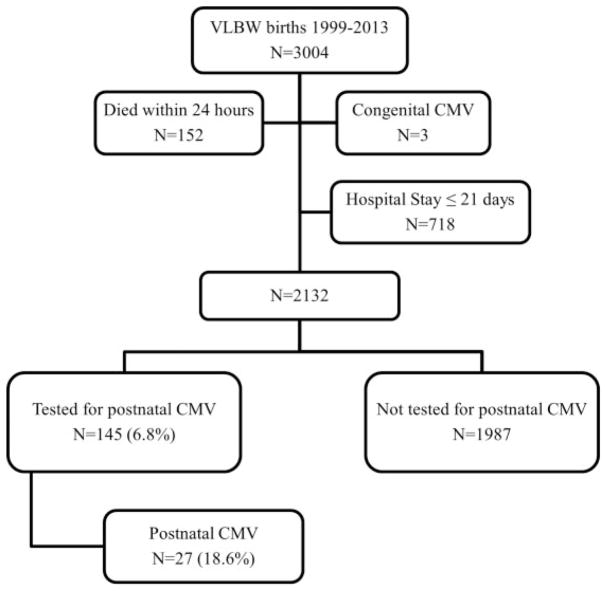
Study cohort consisted of all live-born VLBW infants who remained in the NICU at 21 days of age. CMV infection was diagnosed by shell vial assay or by viral culture of urine, blood, cerebrospinal fluid (CSF), or tracheal secretions. Infants diagnosed with congenital CMV were excluded (Fig. 1). The choice of 21 days as the cutoff for testing for postnatally acquired CMV is aligned with prior studies distinguishing congenital from breast milk–acquired CMV.1,14,15 To ensure this cutoff reflected a difference in clinical concern for congenital versus postnatal CMV infection, we collected the indications for the 155 VLBW infants tested for CMV before days of life (DOL) 21 and compared these to the indications for infants tested after 21 days. To avoid misclassification of congenitally infected infants, CMV-infected infants identified after 21 days of age were eliminated if cranial abnormalities suggestive of congenital infection were present, including microcephaly or intracranial calcifications on head ultrasound imaging done in the first week of life. Postnatal infection was defined as diagnosis at ≥ 21 days of age, prompted by clinical findings including: respiratory decline (increase in ventilatory support or supplemental oxygen ≥ 10% above baseline), abnormal liver function tests, colitis (bloody stool, abnormal abdominal exam or radiographs), and/or signs of bone marrow suppression (platelets < 150 × 103 cells/μL, white blood cell count < 5,000 cells/μL, absolute neutrophil count < 1,000 cells/μL). CMV testing was attributed to “late-onset sepsis evaluation” if it was sent with bacterial blood cultures due to nonspecific infant instability. For clinical outcome comparisons, the study cohort was restricted as follows: (1) infants born between January 1, 2003, and December 31, 2013, because complete information on the incidence of major morbidities before hospital discharge was only available during this period and (2) infants with birth weight (BW) < 1,250 g because all cases of CMV infection occurred in infants with BW < 1,250 g (N = 1,080 infants). To explore the association of CMV infection and bronchopulmonary dysplasia (BPD), the study cohort was further restricted to infants with a known respiratoryoutcome at 36 weeks corrected gestational age (CGA) (the age at which BPD is diagnosed) and to those discharged home before 36 weeks CGA without respiratory support (N = 923 infants).
Fig. 1.
Study population. Derivation of study population is shown. Infants were eliminated from the final cohort if they died; were discharged or transferred from the study NICU before 21 days of age; or were diagnosed congenital CMV. CMV, cytomegalovirus; VLBW, very low birth weight.
Definition of Clinical Outcomes
BPD was defined as requirement for supplemental oxygen and/or respiratory support at 36 weeks CGA; late-onset sepsis as blood culture with growth of pathogenic bacterial or fungal species after 72 hours of age. Other outcomes included: severe intraventricular hemorrhage (IVH > grade 2), periventricular leukomalacia (PVL), echocardiogram-confirmed patent ductus arteriosus (PDA), necrotizing enterocolitis (NEC) ≥ Bell stage 2, and retinopathy of prematurity (ROP) > stage 2.
Statistical Analysis
Differences between groups were assessed using chi-square tests, Fisher exact test, t-tests, or Wilcoxon test as appropriate; p-value < 0.05 was considered statistically significant. To determine the relation between postnatal CMV infection and BPD, we built a logistic regression model with BPD as an outcome and CMV status predictor, identifying variables significantly associated with BPD and adding them to the model in a stepwise manner. We retained variables with p-value < 0.05 in the model as predictors, and those which altered the estimate for CMV status by >10%, as confounders. All analysis was performed using SAS 9.3 (Cary, NC).
Results
Postnatal Cytomegalovirus Testing among Very Low-Birth-Weight Infants
During the 15 years of study period, a median 6.1% (inter-quartile range [IQR], 5.2–7.2) of VLBW infants hospitalized beyond 21 days were tested for postnatal CMV each year, with median 1.4% (IQR, 0.7–1.8) testing positive. No significant trend in the proportion tested or proportion positive was noted over the 15-year period. A total of 245 tests were sent from 145 VLBW infants, with mean 1.7 tests sent per infant (range, 1–5 tests). Table 1 demonstrates that VLBW infants tested for postnatal CMV were significantly smaller, with a lower gestational age (GA) at delivery and longer length of stay in the NICU, compared with those not tested.
Table 1.
Demographics of VLBW infants tested and not tested for postnatal CMV infection
| CMV tested N = 145 |
CMV not tested N = 1,987 |
p-Value | |
|---|---|---|---|
| Birth weight in g, mean (SD) | 822 (260) | 1,072 (266) | <0.001 |
| GA in wk, mean (SD) | 26.7 (2.1) | 28.3 (2.5) | <0.001 |
| Male gender, N (%) | 90 (62.1) | 981 (49.4) | 0.003 |
| Race | |||
| White | 55 (37.9) | 1,074 (54.1) | <0.001 |
| Black | 43 (29.7) | 372 (18.7) | |
| Others | 47 (32.4) | 541 (27.2) | |
| Multiple gestationa | 49 (33.8) | 834 (42.4) | 0.04 |
| Death | 4 (2.8) | 19 (1.0) | 0.13 |
| Median length of stay (IQR)b | 96 (80–109) | 64 (45–86) | <0.001 |
Abbreviations: CMV, cytomegalovirus; GA, gestational age per best obstetrical estimate; IQR, interquartile range; SD, standard deviation.
Nineteen infants with missing data.
Length of stay for infants being discharged home (excluding deaths).
Indications for Cytomegalovirus Postnatal Testing
Table 2 demonstrates that cholestasis (43.5%), late-onset sepsis evaluation (39.3%), and thrombocytopenia (37.9%) were the most commonly cited reasons for CMV testing. Laboratory findings prompting evaluation were either newly recognized by the medical care team within 7 days of testing or had persisted without apparent explanation for > 7 days. The median age at CMV testing was significantly older (by 11 days) among the 27 CMV-positive infants compared with the 118 infants that tested negative. Neutropenia and thrombo-cytopenia occurring ± 7 days of CMV testing were both significantly associated with infection (Table 2).
Table 2.
Indications and laboratory findings among infants tested for postnatal CMV
| CMV positive N = 27/tests = 48 |
CMV negative N = 118/tests = 197 |
p-Value | |
|---|---|---|---|
| Age at testing, median days (IQR) | 58 (51–74) | 47 (33–62) | < 0.001 |
| Times tested per patient, N (%) | |||
| 1 | 15 (55.5) | 77 (65.3) | 0.59 |
| 2 | 6 (22.2) | 15 (12.7) | |
| ≥ 3 | 6 (22.2) | 26 (22.0) | |
| Specimen tested, N (%) | |||
| Urine | 43 (89.6) | 184 (93.4) | 0.20 |
| Cerebrospinal fluid | 1 (2.1) | 2 (1.0) | |
| Tracheal aspirate | 2 (4.2) | 1 (0.5) | |
| Other | 2 (4.2) | 10 (5.1) | |
| Indication for testing, N (%) | |||
| Small for gestational age | 0 | 9 (7.6) | 0.14 |
| Thrombocytopenia | 18 (66.6) | 37 (31.4) | < 0.001 |
| Cholestasis | 8 (29.6) | 55 (46.6) | 0.11 |
| Part of late-onset sepsis evaluation | 13 (48.2) | 44 (37.3) | 0.23 |
| New cranial imaging abnormality | 1 (3.7) | 13 (11) | 0.25 |
| Other indicationsa | 3 (11.1) | 11 (9.3) | 0.78 |
| Incidence of abnormal laboratory values within ± 7 d of CMV test, N (%)b | |||
| Platelet < 100,000 | 14 (51.9) | 27 (28.1) | 0.02 |
| Leukocyte count < 5,000 | 2 (7.4) | 10 (10.5) | 1.0 |
| Absolute neutrophil count < 1,000 | 11 (40.7) | 15 (15.8) | 0.005 |
| Direct bilirubin > 1.0 | 7 (30.4) | 56 (67.5) | 0.001 |
| Aspartate transaminase > 150 | 8 (33.3) | 20 (23.5) | 0.33 |
| Alanine transaminase > 150 | 3 (12.5) | 5 (5.9) | 0.37 |
Abbreviations: CMV, cytomegalovirus; IQR, interquartile range.
Other indications among CMV-positive infants: rhinitis, hepatosplenomegaly, sibling tested for CMV. Among CMV-negative infants: retinal scarring, failed hearing screen, recurrent indirect bilirubinemia, rhinitis, diagnostic bronchial lavage sampling, liver calcifications, bloody stool, viral panel performed in response to breastmilk administration error, sibling tested for CMV.
Complete blood count was sent for 143 infants (27 CMV positive and 115 CMV negative), direct bilirubin in 106 infants (23 CMV positive and 83 CMV negative), liver transaminases in 109 infants (24 CMV positive and 85 CMV negative).
Among infants tested before 21 days, the median time was DOL 5 (IQR, 1–9), whereas the median time for testing beyond 21 days was DOL 51 (IQR, 35–64) (p < 0.001), demonstrating a wide gap aligned with testing for distinct indications. The most common clinical indication for early testing was small for GA status (124/175, 70.9%). Only 2 out of 155 infants tested before DOL 21 were found positive for congenital CMV infection (Fig. 1).
Clinical Characteristics and Management of Postnatal Cytomegalovirus Infection
The clinical condition at CMV diagnosis, findings of CMV-specific evaluation, and CMV treatment is provided for all 27 case infants in Table 3. All infants with postnatal CMV infection had BW < 1,250 g and were born < 32 weeks’ gestation; 21 out of 27 infants had BW < 1,000 g; 9 out of 27 infants had prior negative CMV testing before or after 21 DOL. All infants had been exposed to their mother’s fresh or frozen breast milk. CMV shell vial assay was positive in 24 of 27 infants; in three instances (cases 15, 24, and 26), urine shell vial results were negative but CMV culture results were reported as positive 10 to 21 days later. Four infants were tested after CMV was diagnosed in a twin sibling; 2 of 4 infants had the onset of respiratory or gastrointestinal abnormalities within several days of testing. Acute respiratory decompensation was present in 16 (59.3%) of 27 infants and symptoms consistent with colitis in 7 (25.9%) of 27 infants. Case infant 22 was initially evaluated for CMV due to acholic stools, but developed intestinal stricture and small bowel obstruction requiring surgical resection 1 month after CMV diagnosis. CMV inclusion bodies were reported on histopathology of the resected bowel. There were no deaths attributed to postnatal CMV infection before NICU discharge.
Table 3.
Clinical findings for CMV-positive infants
| Case | DOL | BW | GA | BMD | Clinical findings | Laboratory | VL | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1a | 49 | 765 | 28.2 | 44 | Respiratory decline Hepatosplenomegaly | ↓Plt ↑Dbili |
N/D | None |
| 2 | 51 | 955 | 27.3 | 20 | None | ↓Hct ↓ANC |
2.27b | None |
| 3 | 64 | 605 | 25.0 | 52 | Respiratory decline Bloody stool | ↓Plt | 51.88b | None |
| 4a | 68 | 780 | 25.0 | 49 | Twin sibling CMV+ | None | N/D | None |
| 5 | 52 | 1,230 | 29.0 | 17 | Respiratory decline Bloody stool | ↑LFT | 18.71b | GCV ×42 |
| 6 | 50 | 490 | 26.3 | 36 | Respiratory decline Bloody stool | ↓Plt ↑Dbili ↑LFT |
N/D | None |
| 7 | 50 | 670 | 26.0 | 44 | Respiratory decline Hepatosplenomegaly | ↓Plt ↑LFT |
< Assay | None |
| 8a | 54 | 1,240 | 28.3 | 48 | HSM | ↓Plt ↑LFT |
N/D | None |
| 9 | 49 | 770 | 26.5 | 40 | Respiratory decline Feeding intolerance | ↓Plt | 6.569b | GCV/VGC ×42 |
| 10a | 82 | 930 | 27.2 | 68 | Respiratory decline | None | N/D | None |
| 11 | 80 | 610 | 27.2 | 46 | Respiratory decline | ↓Plt ↑Dbili |
12.49b | GCV ×21 |
| 12a | 54 | 540 | 24.4 | 37 | Respiratory decline | ↓Plt | 24.68b | CMV Ig |
| 13 | 70 | 590 | 24.4 | 58 | New HUS finding | ↓Plt | 0.494b | GCV/VGCV ×42 |
| 14 | 40 | 650 | 26.0 | 34 | Respiratory decline Bloody stool | ↓Plt ↓Hct |
0.512b | None |
| 15a | 51 | 490 | 30.3 | 40 | Bloody stool | ↑Dbili ↑LFT |
N/D | None |
| 16 | 72 | 800 | 24.6 | 59 | None | ↑Dbili | 0.264b | None |
| 17 | 77 | 695 | 24.6 | 50 | Twin sibling CMV+ | None | N/D | None |
| 18a | 53 | 660 | 23.6 | 31 | Respiratory decline Bloody stool Hepatosplenomegaly | ↓Plt ↑LFT |
N/D | GCV/VGCV ×42 |
| 19 | 99 | 670 | 25.5 | 82 | Respiratory decline | ↓Plt ↓WBC ↓ANC |
N/D | None |
| 20a | 90 | 400 | 24.4 | 64 | Hepatosplenomegaly | ↓Plt ↑Dbili ↑LFT |
< 500c | VGCV ×60 |
| 21 | 57 | 680 | 26.3 | 51 | Respiratory decline | ↓Plt | 77,200c | CMV Ig VGCV ×38 |
| 22 | 55 | 1,070 | 27 | 34 | Respiratory decline Acholic stool | ↑Dbili | 200,000c | CMV Ig VGCV ×10 |
| 23 | 58 | 1,000 | 27 | 53 | Bloody stool | None | 1,800c | None |
| 24 | 65 | 830 | 25 | 56 | Respiratory decline | ↓Plt | < Assay | None |
| 25 | 50 | 1,050 | 26 | 39 | Respiratory decline Feeding intolerance | ↓Plt | < 2,000c | None |
| 26a | 74 | 560 | 27 | 69 | Respiratory decline | ↓Plt ↑Dbili |
< Assay | None |
| 27 | 42 | 1,120 | 28 | 39 | Respiratory decline | ↓Plt | 20,544c | CMV Ig VGCV ×18 |
Abbreviations: ***BMD, number of days fed breast milk before CMV testing (laboratory, abnormal laboratory findings within ± 7 days of CMV diagnosis); BW, birth weight; CMV, cytomegalovirus; CMV Ig, CMV-specific immunoglobulin; DOL, days of life; GA, gestational age; GCV, ganciclovir; HSM, hepatosplenomegaly; HUS, head ultrasound; N/D, not done; SD, standard deviation; VGCV, valganciclovir; VL, viral load.
Notes: Treatment expressed as total number of days of antiviral treatment is provided as “xN,” where N is the number of days of treatment with either antiviral medication. Abnormal laboratory tests were defined as follows: ↓Plt, platelet level < 150,000; ↓WBC, white blood count < 5,000; ↓ANC, absolute neutrophil count < 1,000; ↑LFT, alanine transaminase peak > 150 U/L and aspartate transaminase peak > 150 U/L; ↑Dbili, direct bilirubin level > 1.0 mg/dL. VL, viral load performed by hybrid capture in blood buffy coat. Viral load units changed during the period of study.
Infant with prior negative CMV testing.
Viral load expressed in pictograms/milliliter.
Viral load expressed in copies/milliliter.
Diagnostic evaluation varied over the study period. All infants had retinal exams and no instances of chorioretinitis were noted. Head imaging by computed tomography scan was done in four infants and by magnetic resonance imaging in nine infants, without specific findings attributable to CMV infection. Fifteen infants underwent lumbar punctures for CSF testing but only one was CMV-positive by CSF polymer-ase chain reaction (case 18). Antiviral treatment decisions were made on an individual basis in consultation with infectious disease physicians and 10 (37%) of 27 received some form of therapy. Criteria considered for treatment included evidence of CNS infection, blood viral load, overall severity of illness, and parental preference. Before 2005, infants received intravenous (IV) ganciclovir; after 2005, enteral valganciclovir was administered alone or in combination with IV ganciclovir.
Clinical Outcomes and Postnatal Cytomegalovirus Infection
To assess the impact of VLBW CMV infection, the clinical characteristics and major outcomes before hospital discharge were compared (Table 4). CMV-infected infants were of younger GA, lower BW, and were more likely to be male and/or black race compared with uninfected or untested infants (Table 4). Despite this, no differences were observed on univariate analysis in the incidence of multiple gestation, use of antenatal corticosteroids, delivery by cesarean section, low 5-minute Apgar score, or use of surfactant therapy or high-frequency ventilation (HFV). CMV-infected infants were not more likely to have severe IVH or PVL, early- or late-onset sepses, NEC, or severe ROP, and were no more likely to die before discharge. All infants passed hearing screen at the time of discharge except cases 18 and 25. However, CMV-infected infants had longer duration of mechanical ventilation (MV) and were significantly more likely to have BPD (odds ratio [OR] 5.6; 95% confidence interval [CI], 2.0–15.6) (Table 4).
Table 4.
Clinical characteristics and outcomes of postnatal CMV-positive infants
| CMV positive (N = 19) | CMV negative/not tested (N = 1,061) | p-Value | |
|---|---|---|---|
| BW in g, mean (SD) | 743 (208) | 926 (198) | < 0.001 |
| GA in wk, mean (SD) | 26.1 (1.7) | 27.4 (2.2) | 0.01 |
| Male gender, N (%) | 14 (73.7) | 527 (49.7) | 0.04 |
| Race, N (%) | |||
| White | 4 (21.1) | 565 (53.3) | 0.02 |
| Black | 8 (42.1) | 235 (22.2) | |
| Others | 7 (36.8) | 261 (24.6) | |
| Multiple gestation, N (%) | 9 (47.4) | 417 (39.3) | 0.48 |
| Antenatal steroid use, N (%) | 17 (89.5) | 939 (89.1) | 1.0 |
| Cesarean section, N (%) | 16 (84.2) | 849 (80.3) | 1.0 |
| 5-minute Apgar score < 5, N (%) | 3 (15.8) | 66 (6.2) | 0.11 |
| Surfactant use, N (%) | 17 (89.5) | 900 (85.1) | 1.0 |
| MV duration, N (%) | |||
| None | 0 | 137 (13.0) | 0.03 |
| ≤ 48 h | 3 (15.8) | 346 (32.7) | |
| > 48 h | 16 (84.2) | 575 (54.6) | |
| Any HFV, N (%) | 6 (31.6) | 244 (23.1) | 0.41 |
| Postnatal steroids, N (%) | 0 | 49 (4.6) | 1.0 |
| PDA ligation, N (%) | 6 (37.5) | 186 (31.7) | 0.62 |
| IVH ≥ Grade III or PVL, N (%) | 2 (10.5) | 116 (10.9) | 0.71 |
| BPD, N (%)a | 14 (73.7) | 303 (33.5) | <0.001 |
| Early-onset sepsis, N (%) | 0 | 30 (2.8) | 1.0 |
| Late-onset sepsis, N (%) | 4 (21.1) | 265 (25.3) | 0.80 |
| NEC, N (%) | 4 (21.1) | 101 (9.5) | 0.11 |
| ROP, N (%) | 5 (26.3) | 171 (17) | 0.35 |
| Median days of stay (IQR)b | 101 (92–119) | 78 (62–99) | 0.001 |
| Disposition, N (%) | |||
| Died | 0 | 15 (1.4) | 0.73 |
| Transfer | 4 (21.1) | 283 (26.7) | |
| Home | 15 (79) | 763 (71.9) | |
Abbreviations: BPD, bronchopulmonary dysplasia; BW, birth weight; CMV, cytomegalovirus; HFV, high-frequency ventilation; IVH, intraventricular hemorrhage; IQR, interquartile range; MV, mechanical ventilation; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity.
Note: Comparison includes infants with birth weight < 1,250 g, born from 2003 to 2013 (N = 1,080) (see “Methods” section).
A total of 157 infants were discharged/transferred (147) or died (10) before 36 weeks corrected and therefore could not be evaluated for the outcome of BPD. Other missing data are antenatal steroid administration (7), mode of delivery (3), surfactant (3), HFV (4), postnatal steroid treatment (7), MV (3), ROP (54), and PDA ligation (10).
Length of stay for infants discharged home.
To further explore the relationship of postnatal CMV with BPD, we assessed factors associated with increased risk of BPD, including BW and GA, MV, use of HFV, and postnatal steroids (Table 5). We then built a multivariate logistic regression model for BPD within our cohort as described in the “Methods” section. Race was retained in the model as a confounder for CMV status. Gender, multiple gestation, antenatal steroids, mode of delivery, 5-minute Apgar score, early-onset sepsis, IVH, PVL, PDA ligation or PDA medical treatment, and NEC were not significant predictors in the final model. After adjusting for each potential confounder, positive CMV status remained significant with adjusted OR 4.0 (95% CI, 1.3–12.4) (Table 5). In this retrospective study, we could not assess the precise relationship between time of CMV infection and development of BPD. Out of 19 infants, 6 were tested for CMV after 36 weeks CGA, which is the designated time for diagnosing BPD. On removing these infants, CMV remained significantly associated with BPD in the unadjusted model (OR, 4.5 (95% CI, 1.4–14.6); p, 0.01) and retained the same trend, although not statistically significant, in the adjusted model (adjusted OR, 3.0 (95% CI, 0.8–11.2); p, 0.096).
Table 5.
Multivariate regression model for BPD
| BPD (N = 317) | No BPD (N = 606) | OR (95% CI) | p-Value | Adjusted OR (95% CI) | Model p-value | |
|---|---|---|---|---|---|---|
| BW (g) | 795 (189) | 976 (181) | 0.3 (0.2–0.4) | < 0.001 | 0.7a (0.5–0.9) | 0.002 |
| GA (wk) | 25.9 (1.8) | 28.1 (2.1) | 0.6 (0.5–0.6) | < 0.001 | 0.8 (0.7–0.9) | < 0.001 |
| Race | ||||||
| White | 177 (55.8) | 290 (47.9) | Ref | – | Ref | – |
| Black | 75 (23.7) | 150 (24.8) | 0.8 (0.6–1.1) | 0.24 | 0.8 (0.5–1.2) | 0.20 |
| Others | 65 (20.5) | 166 (27.4) | 0.6 (0.5–0.9) | 0.01 | 0.8 (0.5–1.1) | 0.17 |
| MV ≥ 48 h | 270 (85.2) | 244 (40.3) | 8.5 (6.0–12.1) | < 0.001 | 2.1 (1.4–3.3) | < 0.001 |
| Any HFVa | 157 (49.3) | 71 (11.7) | 7.4 (5.3–10.3) | < 0.001 | 2.8 (1.9–4.1) | < 0.001 |
| Late-onset sepsis | 119 (37.5) | 93 (15.4) | 3.3 (2.4–4.6) | < 0.001 | 1.6 (1.1–2.4) | 0.01 |
| CMV positive | 14 (4.4) | 5 (0.8) | 6.4 (2.3–18.0) | < 0.001 | 4.0 (1.3–12.4) | 0.02 |
Abbreviations: BPD, bronchopulmonary dysplasia; BW, birth weight; CI, confidence interval; CMV, cytomegalovirus; GA, gestational age; HFV, high-frequency ventilation; MV, mechanical ventilation; OR, odds ratio; Ref, reference value.
Note: Model includes all infants with birth weight < 1,250 g, born from 2003 to 2013 with clinical status known at 36 weeks CGA (N = 923). Postnatal steroid treatment was given in 37 (11.8%) of BPD patients versus 10 (1.7%) of infants without BPD (p < 0.001). This variable was excluded from the final model due to collinearity with BPD as outcome.
Odds ratio for increase per 250 g birth weight.
Discussion
This study examines retrospective data over a 15-year period in a center where prospective maternal, infant, and breast milk CMV screening is not performed. CMV testing is done at caregiver discretion, prompted by specific infant signs or symptoms, a scenario that likely represents the reality in most centers in the United States that care for VLBW infants. To our knowledge, this is the largest single-center report of symptomatic postnatal CMV infection among VLBW infants. The incidence of symptomatic postnatal CMV in our center was 27 (1.3%) out of 2,132, consistent with recent population estimates of breast milk–acquired CMV sepsis-like syndrome.14 Our study provides neonatal clinicians with several important findings. First, postnatal CMV infection was not uncommon when clinically suspected, occurring in nearly 20% of tested infants. Second, consistent with prior reports, CMV infection occurred primarily among infants with BW < 1,000 g and exclusively among infants fed mother’s own milk.3,17,18,20 Third, we found no single clinical sign or symptom was specific for CMV infection, although significant thrombocytopenia (< 100,000) was present in approximately 50% of infected infants. Our work suggests that CMV testing should be included as part of antimicrobial stewardship when extremely low BW, breast milk–fed infants are evaluated for late-onset sepsis beyond DOL 21, as recognition of CMV infection may spare infants’ unnecessary prolonged antibiotic treatment. Finally, the incidence of BPD was significantly elevated among the postnatal CMV-infected infants, even after adjusting for GA and multiple other predictors of illness severity and respiratory morbidity.
Histologically, CMV infection in the lungs can result in diffuse alveolar damage and interstitial pneumonitis21 and clinically, a longer requirement for supplemental oxygen has been noted in CMV-infected infants,22 but prior studies have provided conflicting information regarding the association of postnatal CMV with BPD.7,9,19,21–23 Each of these studies prospectively screened VLBW infants and identified many infants with clinically silent CMV infection. In contrast, our study addressed infants with symptomatic CMV infection, compared them to a BW matched cohort, and controlled for multiple other factors known to be associated with BPD. Our work suggests that postnatal CMV infection, when symptomatic, may act in the same way that it is hypothesized that NEC and late-onset sepsis act to increase the risk of BPD—causing lung damage by a combination of direct pathogen effects, secondary inflammation, and/or increased exposure to MV and supplemental oxygen.
This study has several limitations due to the retrospective nature of the analysis. First, because universal prospective screening for CMV was not done in our center, only 1 out of 3 case infants had negative CMV testing before DOL 21. Given the approximately 0.4% incidence congenital CMV infection reported in prospective screening studies of both term and VLBW newborns, we estimate that 0 to 2 of our 27 cases of postnatal CMV could represent misclassified congenital CMV.24,25 Second, this study may also underestimate the incidence of symptomatic postnatal CMV infection. Our center does not routinely test for postnatal CMV when VLBW infants are evaluated for late-onset bacterial and fungal infection, and CMV may account for some cases of “culture-negative sepsis.” Misclassification of unrecognized, symptomatic postnatal CMV-infected infants as CMV negative could result in an underestimate of the impact of postnatal CMV on the development of BPD. Another significant limitation of this study is that the exact timing of onset of CMV infection cannot be determined with accuracy; we observed that testing for CMV was often delayed beyond the immediate symptomatic period. Therefore, we cannot firmly establish a direct chronology of CMV acquisition and development of BPD.
Breast milk feeding may be critical to the prevention of bacterial infection and NEC among preterm infants, and is important for neurodevelopment.26–28 There is current consensus that VLBW infants should be fed breast milk,28 but some centers routinely freeze breast milk due to concern for postnatal CMV.14–16 This practice does not reliably prevent CMV infection29 and potentially denies infants born to CMV-negative mothers the full immunologic benefit of fresh mother’s milk. Our work strongly supports the need for a multicenter, prospective study of infants with BW < 1,000 g to definitively establish the true risk/benefit balance of CMV exposure via fresh breast milk feeding to these infants.
Finally, in our center, infectious disease consultation was obtained to address management and antiviral therapy for case infants. The inconsistency in evaluation and treatment observed in this single-center study reflects the uncertainty surrounding the risks and benefits of antiviral treatment among VLBW infants. Recent data support the role of antiviral treatment for infants with symptomatic congenital CMV in improving hearing and neurodevelopmental outcomes.30–32 However, both ganciclovir and valganciclovir can have toxicities in infants31,32 and no studies have evaluated the safety, efficacy, or pharmacokinetics of antiviral therapy or CMV immune globulin in preterm infants with symptomatic postnatal CMV infection. Our study also highlights the need for research to provide rigorous evidence to guide care decisions for postnatal CMV among VLBW infants.
Acknowledgments
The authors have no financial relationships relevant to this article to disclose. This work was supported by the NIH grants K08AI087992 (S. R. P.); the Fred Lovejoy Research and Education Fund at Children’s Hospital Boston (S. A. M.); and a pilot grant from the Harvard Catalyst: The Harvard Clinical and Translational Science Center (NIH Grant #1 UL1 RR 025758–01 and financial contributions from participating institutions) (S. R. P. and K. M. P.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
References
- 1.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357(9255):513–518. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 2.Josephson CD, Caliendo AM, Easley KA, et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr. 2014;168(11):1054–1062. doi: 10.1001/jamapediatrics.2014.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miron D, Brosilow S, Felszer K, et al. Incidence and clinical manifestations of breast milk-acquired Cytomegalovirus infection in low birth weight infants. J Perinatol. 2005;25(5):299–303. doi: 10.1038/sj.jp.7211255. [DOI] [PubMed] [Google Scholar]
- 4.Hamprecht K, Maschmann J, Jahn G, Poets CF, Goelz R. Cytomega-lovirus transmission to preterm infants during lactation. J Clin Virol. 2008;41(3):198–205. doi: 10.1016/j.jcv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Jim WT, Shu CH, Chiu NC, et al. High cytomegalovirus load and prolonged virus excretion in breast milk increase risk for viral acquisition by very low birth weight infants. Pediatr Infect Dis J. 2009;28(10):891–894. doi: 10.1097/INF.0b013e3181a55c52. [DOI] [PubMed] [Google Scholar]
- 6.Vollmer B, Seibold-Weiger K, Schmitz-Salue C, et al. Postnatally acquired cytomegalovirus infection via breast milk: effects on hearing and development in preterm infants. Pediatr Infect Dis J. 2004;23(4):322–327. doi: 10.1097/00006454-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Neuberger P, Hamprecht K, Vochem M, et al. Case-control study of symptoms and neonatal outcome of human milk-transmitted cytomegalovirus infection in premature infants. J Pediatr. 2006;148(3):326–331. doi: 10.1016/j.jpeds.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi S, Kimura H, Oshiro M, et al. Transmission of cytomegalo-virus via breast milk in extremely premature infants. J Perinatol. 2011;31(6):440–445. doi: 10.1038/jp.2010.150. [DOI] [PubMed] [Google Scholar]
- 9.Capretti MG, Lanari M, Lazzarotto T, et al. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother’s milk: a prospective study. J Pediatr. 2009;154(6):842–848. doi: 10.1016/j.jpeds.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Goelz R, Meisner C, Bevot A, Hamprecht K, Kraegeloh-Mann I, Poets CF. Long-term cognitive and neurological outcome of preterm infants with postnatally acquired CMV infection through breast milk. Arch Dis Child Fetal Neonatal Ed. 2013;98(5):F430–F433. doi: 10.1136/archdischild-2012-303384. [DOI] [PubMed] [Google Scholar]
- 11.Dorn M, Lidzba K, Bevot A, Goelz R, Hauser TK, Wilke M. Long-term neurobiological consequences of early postnatal hCMV-infection in former preterms: a functional MRI study. Hum Brain Mapp. 2014;35(6):2594–2606. doi: 10.1002/hbm.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bevot A, Hamprecht K, Krägeloh-Mann I, Brosch S, Goelz R, Vollmer B. Long-term outcome in preterm children with human cytomeg-alovirus infection transmitted via breast milk. Acta Paediatr. 2012;101(4):e167–e172. doi: 10.1111/j.1651-2227.2011.02538.x. [DOI] [PubMed] [Google Scholar]
- 13.Nijman J, van Zanten BG, de Waard AK, Koopman-Esseboom C, de Vries LS, Verboon-Maciolek MA. Hearing in preterm infants with postnatally acquired cytomegalovirus infection. Pediatr Infect Dis J. 2012;31(10):1082–1084. doi: 10.1097/INF.0b013e31825eb3e5. [DOI] [PubMed] [Google Scholar]
- 14.Lanzieri TM, Dollard SC, Josephson CD, Schmid DS, Bialek SR. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics. 2013;131(6):e1937–e1945. doi: 10.1542/peds.2013-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luck S, Sharland M. Postnatal cytomegalovirus: innocent bystander or hidden problem? Arch Dis Child Fetal Neonatal Ed. 2009;94(1):F58–F64. doi: 10.1136/adc.2007.131623. [DOI] [PubMed] [Google Scholar]
- 16.Buxmann H, Falk M, Goelz R, Hamprecht K, Poets CF, Schloesser RL. Feeding of very low birth weight infants born to HCMV-seroposi-tive mothers in Germany, Austria and Switzerland. Acta Paediatr. 2010;99(12):1819–1823. doi: 10.1111/j.1651-2227.2010.01954.x. [DOI] [PubMed] [Google Scholar]
- 17.Gunkel J, Wolfs TF, de Vries LS, Nijman J. Predictors of severity for postnatal cytomegalovirus infection in preterm infants and implications for treatment. Expert Rev Anti Infect Ther. 2014;12(11):1345–1355. doi: 10.1586/14787210.2014.966080. [DOI] [PubMed] [Google Scholar]
- 18.Mehler K, Oberthuer A, Lang-Roth R, Kribs A. High rate of symptomatic cytomegalovirus infection in extremely low gestational age preterm infants of 22–24 weeks’ gestation after transmission via breast milk. Neonatology. 2014;105(1):27–32. doi: 10.1159/000355306. [DOI] [PubMed] [Google Scholar]
- 19.Sawyer MH, Edwards DK, Spector SA. Cytomegalovirus infection and bronchopulmonary dysplasia in premature infants. Am J Dis Child. 1987;141(3):303–305. doi: 10.1001/archpedi.1987.04460030081030. [DOI] [PubMed] [Google Scholar]
- 20.Okulu E, Akin IM, Atasay B, Ciftçi E, Arsan S, Türmen T. Severe postnatal cytomegalovirus infection with multisystem involvement in an extremely low birth weight infant. J Perinatol. 2012;32(1):72–74. doi: 10.1038/jp.2011.58. [DOI] [PubMed] [Google Scholar]
- 21.Papazian L, Fraisse A, Garbe L, et al. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology. 1996;84(2):280–287. doi: 10.1097/00000542-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Yeager AS, Palumbo PE, Malachowski N, Ariagno RL, Stevenson DK. Sequelae of maternally derived cytomegalovirus infections in premature infants. J Pediatr. 1983;102(6):918–922. doi: 10.1016/s0022-3476(83)80025-0. [DOI] [PubMed] [Google Scholar]
- 23.Prösch S, Lienicke U, Priemer C, et al. Human adenovirus and human cytomegalovirus infections in preterm newborns: no association with bronchopulmonary dysplasia. Pediatr Res. 2002;52(2):219–224. doi: 10.1203/00006450-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Pinninti SG, Ross SA, Shimamura M, et al. National Institute on Deafness and Other Communication Disorders CMV and Hearing Multicenter Screening (CHIMES) Study. Comparison of saliva PCR assay versus rapid culture for detection of congenital cytomegalovirus infection. Pediatr Infect Dis J. 2015;34(5):536–537. doi: 10.1097/INF.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner KM, Lee HC, Boppana SB, Carlo WA, Randolph DA. Incidence and impact of CMV infection in very low birth weight infants. Pediatrics. 2014;133(3):e609–e615. doi: 10.1542/peds.2013-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vohr BR, Poindexter BB, Dusick AM, et al. NICHD Neonatal Research Network. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118(1):e115–e123. doi: 10.1542/peds.2005-2382. [DOI] [PubMed] [Google Scholar]
- 27.Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O’Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol. 2007;27(7):428–433. doi: 10.1038/sj.jp.7211758. [DOI] [PubMed] [Google Scholar]
- 28.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 29.Maschmann J, Hamprecht K, Weissbrich B, Dietz K, Jahn G, Speer CP. Freeze-thawing of breast milk does not prevent cytomegalovi-rus transmission to a preterm infant. Arch Dis Child Fetal Neonatal Ed. 2006;91(4):F288–F290. doi: 10.1136/adc.2004.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver SE, Cloud GA, Sánchez PJ, et al. National Institute of Allergy, Infectious Diseases Collaborative Antiviral Study Group. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol. 2009;46(Suppl 4):S22–S26. doi: 10.1016/j.jcv.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimberlin DW, Lin CY, Sánchez PJ, et al. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomeg-alovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 32.Kimberlin DW, Jester PM, Sánchez PJ, et al. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Valganciclovir for symptomatic congenital cytomegalovi-rus disease. N Engl J Med. 2015;372(10):933–943. doi: 10.1056/NEJMoa1404599. [DOI] [PMC free article] [PubMed] [Google Scholar]