Abstract
The Fontan operation creates a circulation characterized by elevated central venous pressure and low cardiac output. Over time, these characteristics result in a predictable and persistent decline in exercise performance that is associated with an increase in morbidity and mortality. A medical therapy that targets the abnormalities of the Fontan circulation might, therefore, be associated with improved outcomes. Udenafil, a phosphodiesterase type 5 inhibitor, has undergone phase I/II testing in adolescents who have had the Fontan operation and has been shown to be safe and well tolerated in the short-term. However, there are no data regarding the long-term efficacy of udenafil in this population. The Fontan Udenafil Exercise Longitudinal (FUEL) Trial is a randomized, double blind, placebo controlled phase III clinical trial being conducted by the Pediatric Heart Network in collaboration with Mezzion Pharma Co., Ltd. This trial is designed to test the hypothesis that treatment with udenafil will lead to an improvement in exercise capacity in adolescents who have undergone the Fontan operation. A safety extension trial, the FUEL Open-Label Extension Trial (FUEL OLE), offers the opportunity for all FUEL subjects to obtain open-label udenafil for an additional 12 months following completion of FUEL, and evaluates the long-term safety and tolerability of this medication. This manuscript describes the rationale and study design for FUEL and FUEL OLE. Together, these trials provide an opportunity to better understand the role of medical management in the care of those who have undergone the Fontan operation.
The Fontan circulation
When first described in 1971, the Fontan operation ushered in a new paradigm in the care of children born with the varied forms of complex congenital heart disease (CHD) characterized by a single effective pumping chamber(1,2). This procedure, which creates a total cavopulmonary connection, separates the systemic and pulmonary circuits and reduces both hypoxemia and ventricular volume overload. However, while life-saving or life-prolonging for many, the circulation created by the Fontan operation is not normal. After the Fontan, there is no ventricular pump to propel blood through the pulmonary arteries. Instead, blood returns to the lungs via passive flow from the systemic veins and is dependent on low pulmonary vascular resistance and optimal diastolic ventricular function. This creates a circulation characterized by elevated central venous pressure and chronically low cardiac output. Over time, these inherent characteristics of Fontan physiology result in a predictable, persistent deterioration of cardiovascular efficiency marked by a progressive decline in exercise performance as well as impaired ventricular and vascular function(3–8). Medical therapies that can favorably alter the deficiencies of the Fontan circulation might help to slow the decline associated with Fontan physiology and lead to improved outcomes over a lifetime(9).
Exercise performance in the Fontan circulation
The Fontan circulation significantly limits the ability to increase cardiac output in the setting of increased metabolic demand(10). In a two-ventricle circulation, the sub-pulmonary ventricle facilitates the increased blood flow and augmented preload required to increase cardiac output during exercise(11,12). In the single ventricle circulation, the central venous pressure provides the driving force that propels blood through the pulmonary vasculature. Central venous pressure can rise during periods of increased demand, but the increase in pressure is substantially less than that which can be generated by a sub-pulmonary ventricle(13). As a result of limited trans-pulmonary blood flow, ventricular preload and cardiac output are likewise limited(14).
These limitations of the Fontan physiology are demonstrated in evaluations of exercise performance. While many patients with CHD have lower maximal exercise capacity (maximal oxygen consumption; VO2 max) than age- and gender-matched peers, those with the Fontan physiology have among the lowest(15,16). In the Pediatric Heart Network’s (PHN) cross-sectional study of exercise performance in 411 children following Fontan, VO2 max was 65% of predicted for age and gender at 12 years of age and decreased progressively in the older cohorts(3,4). In multiple subsequent longitudinal studies, the progressive decline in exercise capacity has been confirmed, although the slope of the decline has varied by study, ranging from 0.8–2.6% of the predicted VO2 max per year(3,5–7).
The quantification of exercise capacity is not only a useful descriptor of functional capacity, it is also a reliable predictor of morbidity and mortality in those with congenital or acquired heart disease. In studies of young adults with CHD, a VO2 max of approximately 45–50% of predicted for age and gender (an absolute VO2 max of 18 to 20 ml/kg/min in young adults) appears to be the threshold value for an increased risk of symptomatic heart failure and death. This finding is consistent across many diagnostic categories of CHD(17–20). In one study of 321 subjects with Fontan physiology, the maximal VO2 was 52% of predicted at a mean age of 21 years. During a median follow up time of 21 months, 41% of patients required hospitalization for heart failure and 9% either died or underwent heart transplantation(19).
Pulmonary vasodilation and the Fontan circulation
In the absence of a sub-pulmonary ventricle, the importance of pulmonary vascular resistance (PVR) as a determinant of cardiac preload and output is magnified(14). Any small elevation in PVR necessitates an increase in central venous pressure to maintain cardiac output, and/or results in a decrease in cardiac output for any given central venous pressure. Likewise, any small decrease in PVR allows for a decrease in central venous pressure for any given cardiac output, and/or an increase in cardiac output for any given central venous pressure. Additional evidence suggests that even in the setting of a normal resting PVR, the typical decrease in PVR that occurs with exercise in two-ventricle circulations – facilitating substantial augmentation in cardiac output – does not occur during exercise in those with the Fontan circulation(21,22). Given the importance of PVR in the Fontan circulation, pulmonary vasodilators make intuitive sense as a class of drug that might be successful at improving the Fontan physiology.
Over the last decade, numerous studies have evaluated the acute, short-term, and medium-term impact of pulmonary vasodilators on Fontan physiology(23–31). While different classes of medications have been evaluated, phosphodiesterase-5 (PDE-5) inhibitors have the longest track record of use in pediatrics and have an excellent safety profile. PDE-5 inhibitors have been used for more than a decade as the first-line therapy for children with pulmonary arterial hypertension and have been associated with increased exercise performance as well as increased measures of quality of life in that group(32–35).
In children with Fontan physiology, there have been a number of acute and short-term efficacy studies of PDE-5 inhibitors, some that have suggested an improvement in exercise performance and others that have been equivocal(24,25,27,36). In the earliest publication, sildenafil was shown to improve exercise capacity and cardiac output after a single dose(36). In the largest and longest evaluation of sildenafil (6 weeks of therapy, 28 subjects), the authors found an improvement in submaximal exercise in those with heart failure (defined as a serum brain-type natriuretic peptide >100 pg/ml) but no improvement in VO2 max. Interestingly, the authors also found an improvement in ventricular performance as quantified by the myocardial performance index(26,27).
Study design and methods
Study overview
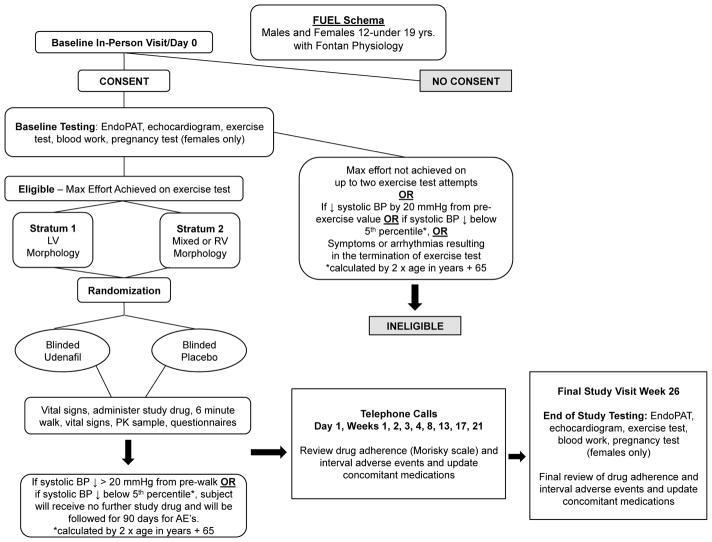
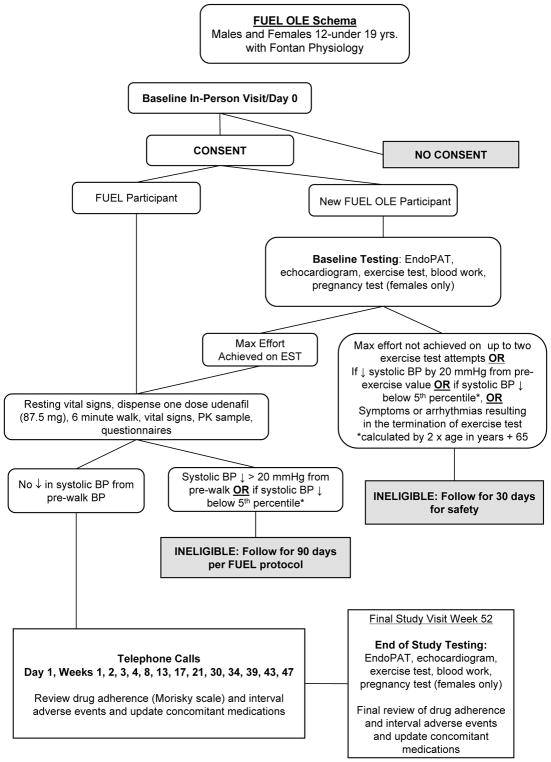
The Fontan Udenafil Exercise Longitudinal (FUEL) trial, a unique collaboration between the PHN and industry, is designed to test the hypothesis that treatment with udenafil will lead to an improvement in exercise capacity, as measured by VO2 max, in a cohort of adolescents who have undergone the Fontan operation. This trial has an enrollment goal of 400 adolescents who will be randomly assigned to receive udenafil or placebo for six months (Figure 1). This study is supported by the National Heart, Lung, and Blood Institute (NHLBI)-funded PHN in cooperation with the sponsor, Mezzion Pharma Co. Ltd under a Special Protocol Assessment through the Food and Drug Administration. We engaged with patient advocacy groups to help us understand potential barriers to recruitment as we prepared the protocol and recruitment materials. Thirty clinical centers throughout the United States, Canada, and the Republic of Korea are participating. A safety extension trial, the FUEL Open-Label Extension Trial (FUEL OLE) offers the opportunity for all FUEL subjects (regardless of their initial randomization assignment) to obtain open-label udenafil for an additional 12 months following completion of FUEL (Figure 2). The primary purpose of FUEL OLE is to collect additional safety data on the use of udenafil in at least 300 adolescents with Fontan physiology. Clinical efficacy parameters are also being re-evaluated at the conclusion of FUEL OLE to evaluate the durability of any changes noted in FUEL. Funding for this project was provided by the National Heart, Lung, and Blood Institute and by Mezzion Pharma Co. Ltd. (Seoul, Republic of Korea). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents. The clinicaltrials.gov reference numbers for FUEL and FUEL OLE are NCT0274115 and NCT03013751 respectively.
Figure 1.
Figure 2.
Rationale for FUEL and FUEL OLE
Despite the rationale and potential efficacy signals that PDE-5 inhibitors may improve Fontan physiology, there has not been a clinical trial adequately powered to definitively answer the question of medication efficacy. We proposed to test the long-acting PDE-5 inhibitor, udenafil, as a therapeutic agent to improve VO2 max in adolescent subjects with Fontan physiology. This multi-center, randomized, placebo-controlled clinical trial compares outcomes in those with Fontan physiology treated with six months of udenafil (87.5 mg twice daily) to those treated with placebo. Given the progressive decline of VO2 max in those with the Fontan circulation, the identification of a treatment that is safe and that can slow this decline could alter the fundamental trajectory of those who have undergone the Fontan operation. The primary aim of FUEL is to evaluate the effect of six months of treatment with udenafil (87.5 mg, twice daily) on exercise capacity in adolescents with Fontan physiology. Secondary aims include assessing the effect of udenafil on echocardiographic measures of ventricular performance, on vascular function, on biomarkers of heart failure, and on functional health status. Safety will be assessed in the FUEL trial and as the primary outcome of FUEL OLE.
Among PDE-5 inhibitors, udenafil is the only medication to have undergone phase I/II testing in patients with Fontan physiology(37). This trial, conducted in the PHN (NCT02201342), tested five different dosing strategies in cohorts of six subjects each. Once-daily and twice-daily dosing strategies were both studied at increasing dosing strengths. No drug-related serious adverse events were observed at any of the dosing regimens, and the 87.5 mg twice-daily regimen achieved the highest average serum concentrations of both udenafil and of its active metabolite. This dosing regimen was also associated with an improvement in the myocardial performance index, a marker of possible clinical efficacy. The results of the phase I/II trial led to the selection of the udenafil dosing regimen of 87.5 mg twice daily for this phase III clinical efficacy trial.
Patient selection
Inclusion and exclusion criteria are listed in Table 1. The decision to restrict the population to those older than 12 years of age and 132 cm or taller is based on the minimum size requirements needed to complete exercise testing, the primary outcome for this trial. In an effort to focus solely on the impact of udenafil on Fontan physiology, subjects with severe ventricular dysfunction, severe atrioventricular valve regurgitation, or those who are aerobically deconditioned beyond what would be expected solely on the basis of Fontan physiology are excluded from participation. This translates into excluding those with a recent history of a maximal VO2 below 50% predicted for age and gender. Below this threshold there is concern that co-morbidities outside of the inherent limitations of Fontan physiology could overwhelm the potential impact of udenafil thereby blunting the ability to discern a meaningful effect. To keep the mg/kg dose distribution within a relatively narrow range, inclusion is limited to those with a weight of 40 kg or greater, the minimal weight for an adult. All subjects who complete the FUEL protocol are eligible for enrollment in FUEL OLE. If fewer than 300 subjects choose to enroll in the open-label extension, the study will be opened to de novo Fontan patients who meet the eligibility criteria but did not participate in FUEL.
Table 1.
FUEL Inclusion and Exclusion Criteria
| Inclusion Criteria | |
| 1 | Males and females with Fontan physiology 12 – <19 years of age at enrollment |
| 2 | Participant consent or parental/guardian consent with participant assent |
| 3 | Participant fluent in English, Spanish, or Korean |
| Exclusion Criteria | |
| 1 | Weight <40 kg |
| 2 | Height <132 cm |
| 3 | Hospitalization for acute decompensated heart failure within the last 12 months |
| 4 | Current intravenous inotropic drugs |
| 5 | Undergoing evaluation for heart transplantation or listed for transplantation |
| 6 | Diagnosis of active protein losing enteropathy or plastic bronchitis within the last 3 years, or a history of liver cirrhosis |
| 7 | Known Fontan baffle obstruction, branch pulmonary artery stenosis, or pulmonary vein stenosis resulting in a mean gradient of >4 mmHg between the regions proximal and distal to the obstruction as measured by either catheterization or echocardiography, obtained prior to screening for the trial |
| 8 | Single lung physiology with greater than 80% flow to one lung |
| 9 | VO2 max less than 50% of predicted for age and gender at enrollment |
| 10 | Severe ventricular dysfunction assessed qualitatively by clinical echocardiography within six months prior to enrollment |
| 11 | Severe valve regurgitation, ventricular outflow obstruction, or aortic arch obstruction assessed by clinical echocardiography within six months prior to enrollment |
| 12 | Significant renal (serum creatinine >2.0), hepatic (serum AST and/or ALT >3 times upper limit of normal), gastrointestinal or biliary disorders that could impair absorption, metabolism or excretion of orally administered medications, based on laboratory assessment six weeks prior to screening for the trial |
| 13 | Inability to complete exercise testing at baseline screening |
| 14 | History of PDE-5 inhibitor use within 3 months before study onset |
| 15 | History of any other medication for treatment of pulmonary hypertension within 3 months before study onset |
| 16 | Known intolerance to oral udenafil |
| 17 | Frequent use of medications or other substances that inhibit or induce CYP3A4 |
| 18 | Current use of alpha-blockers or nitrates |
| 19 | Ongoing or planned participation in another research protocol that would either prevent successful completion of planned study testing or invalidate its results |
| 20 | Non-cardiac medical, psychiatric, and/or social disorder that would prevent successful completion of planned study testing or would invalidate its results |
| 21 | Cardiac care, ongoing or planned, at a non-study center that would impede study completion |
| 22 | For females: pregnancy at the time of screening, pregnancy planned before study completion, or refusal to use an acceptable method of contraception for study duration if sexually active |
| 23 | Inability to abstain from or limit intake of grapefruit juice during the duration of the trial |
| 24 | Refusal to provide written informed consent/assent |
| 25 | In the opinion of the primary care physician, the subject is likely to be non-compliant with the study protocol |
ALT – alanine aminotransferase; AST – aspartate aminotransferase; PDE-5 – phosphodiesterase type 5; CYP3A4 – cytochrome P450 3A4; VO2 max – maximal oxygen consumption
FUEL randomization and stratification
Eligible patients are randomly assigned to one of two treatment groups: udenafil or placebo. A stratified randomization scheme is being employed with randomly permuted blocks of varying sizes. Randomization assignments are generated by a web-based system at the data-coordinating center after confirmation of trial eligibility. Stratification is based on ventricular morphology - single left ventricle vs. single right or mixed. There is no pre-determined number in each morphological stratum, rather ventricular morphologies are divided evenly between those receiving placebo and those receiving udenafil. The rationale for this stratification is based on the results of the previous PHN Fontan cross-sectional studies (4,39). In these studies, there was a weak but significant difference in certain resting echocardiographic and exercise variables based on the morphology of the single ventricle. In addition, preliminary data on PDE-5 inhibitors suggest a difference in exercise performance based on morphology (23).
Drug initiation
Subjects undergo a blood draw to quantify hepatic and renal function, as well as a baseline exercise test prior to the first dose. All female study participants must have a negative urine or serum pregnancy test before participating in exercise testing or receiving study drug. Participants who meet inclusion criteria and achieve a maximal effort on the exercise test (respiratory exchange ratio >1.10) will receive their first dose of study drug. Participants who are unable to achieve a maximal effort on exercise testing, but who otherwise qualify for inclusion, are offered the opportunity to repeat the test one time within 2 weeks of the initial exercise test. If the participant achieves a maximal effort on this second test, they will be eligible for randomization. As a safety measure, if a participant demonstrates a fall in systolic blood pressure by 20 mmHg from the resting pre-exercise value, a fall in blood pressure below the 5th percentile for age (calculated by 2 x age in years + 65) at maximal exercise, or if the exercise test is terminated for arrhythmias or symptoms, the participant is not eligible for randomization.
After satisfactory completion of the exercise test, participants are eligible for randomization and study drug initiation at any time within seven days of the baseline testing. The first dose is administered in the presence of study personnel. At approximately two hours after the first dose, participants are asked to perform a self-limited 6-minute walk. As with the exercise test, if there is a significant drop in blood pressure during the 6-minute walk, participants are excluded from receiving any further study drug.
Subjects who consent to FUEL OLE upon completion of FUEL are able to use study measures collected at the 6-month FUEL visit as baseline measures for FUEL OLE, provided the study drug initiation visit is conducted within a reasonable time period (<2 months). Drug initiation procedures for FUEL OLE mirror those for FUEL. The baseline visit for de novo FUEL OLE subjects mirrors the baseline screening and drug initiation visit for FUEL.
Trial outcomes
Outcomes include measures obtained through exercise testing, echocardiography, and assessment of vascular function, as well as serum levels of brain type natriuretic peptide. Participants also complete assessments of functional health status. Safety data are collected during both FUEL and FUEL OLE when reported by participants and during prescribed phone calls between participants and study personnel. All testing performed at end-of-study will be compared to baseline testing. A complete list of outcome variables can be found in Table 2.
Table 2.
Outcome Measures
| Cardiopulmonary Exercise Testing | |
| Primary outcome | Change in VO2 max from baseline to six months |
| Secondary outcomes | Six-month change in:
|
| Echocardiography | |
| Primary outcome | Change in MPI from baseline to six months as determined by velocities obtained from blood pool Doppler assessment |
| Secondary outcomes | Six-month change in:
|
| Vascular Function | |
| Primary outcome | Change in EndoPAT® derived log-transformed reactive hyperemia index from baseline to six months |
| Serum Brain-Type Natriuretic Peptide | |
| Primary outcome | Change in the measured level of serum brain- type natriuretic peptide from baseline to six months |
| Functional Health Status | |
| Primary outcome | The change in functional health status from baseline to six months as measured by the full scale PedsQL assessment tool |
| Secondary outcomes | Six-month change in:
|
VO2 max – maximal oxygen consumption; VAT – ventilatory anaerobic threshold; MPI – myocardial performance index; AV – atrioventricular; PedsQL – Pediatric Quality of Life Inventory
Exercise
The primary outcome for the FUEL trial is the change in VO2 max from baseline to 26-week testing for those who receive study drug compared to those who receive placebo. VO2 max is the exercise measure that has been most closely correlated with morbidity and mortality in the Fontan population, and a positive change in VO2 max would suggest an improvement in overall physiology. Measurements of VO2 max occur locally at each study site using a standardized cycle ergometer ramp protocol as has been previously described in children and adolescents with Fontan physiology(38). Secondary exercise outcomes include additional measures of exercise performance at maximal effort and at the ventilatory anaerobic threshold.
Echocardiography
Echocardiographic images are obtained at each site according to a standardized protocol. These images are sent to a core lab with specific expertise in functional measurements. The primary echocardiographic endpoint is the myocardial performance index, a measure of global ventricular function that has been noted to improve with use of PDE-5 inhibitors in the Fontan population in previous studies(26,37). Secondary endpoints include tricuspid/mitral annular planar systolic excursion, a measure that has been linked to exercise performance(39), and the systolic to diastolic ratio, a measure that has been linked to ventricular end-diastolic pressure and to mortality in adults with Fontan physiology(40–42). Additional secondary endpoints will include estimates of cardiac output and measures of ventricular function obtained through Doppler tissue imaging techniques.
Vascular function
Peripheral vascular function is assessed using the EndoPAT® device. This device measures blood flow to the index finger before and after a period of induced distal limb ischemia, with control data measured from the contralateral (non-ischemic) limb. Ischemia is accomplished using a brachial blood pressure cuff inflated beyond the systolic blood pressure for a period of five minutes. Data from these tests are collected at each site and sent to a core lab for interpretation. The primary outcome for vascular function is the log-transformed reactive hyperemia index, which is obtained by comparing blood flow before and after limb ischemia, adjusted for the control arm, and has been shown to correlate with functional capacity and quality of life(8). An additional outcome measure of vascular function is the augmentation index, which is a measure of arterial stiffness.
Serum brain-type natriuretic peptide
Blood samples for the measurement of serum brain-type natriuretic peptide (BNP) are obtained at the baseline visit and at the 26-week visit for FUEL and the 52-week visit for FUEL OLE. These samples are sent to a core lab to determine serum BNP levels.
Functional health status
Functional health status is assessed using standardized instruments including the Pediatric Quality of Life Inventory™ (PedsQL) and the Pediatric Cardiac Quality of Life Inventory (PCQLI). The PedsQL and the PCQLI are administered at the baseline visit and at the 26-week visit for FUEL and the 52-week visit for FUEL OLE. Subsets of the PedsQL are also evaluated including the physical functioning score, the psychosocial functioning score, and the cardiac-specific quality of life score.
Masking
The study medication and the placebo are identical in appearance. Although the study is double-blinded, it is possible that some FUEL subjects may suspect they have been randomized to udenafil based on the side-effect profile. However, the randomization assignment is only seen by the statistician at the data coordinating center and is not available to the family, subject, study coordinator, investigators, or personnel administering the baseline or end-of-study testing. The exercise testing laboratories and the echocardiography and vascular core labs are likewise masked to group assignment. All participants and sites will remain masked to treatment group assignment until after all trial data are analyzed. Unmasking may occur when deemed necessary by the principal investigator, sponsor, or medical monitor in the event of a serious adverse event with a potential relationship to study drug.
Study measurements and subject follow-up
During the FUEL trial, drug accountability is assessed and measurements of blood pressure and heart rate are repeated at visits during week 2 and week 13. For female subjects, a urine pregnancy test is also repeated during these visits. Medication exposure is reviewed and adverse events are assessed weekly for four weeks and then monthly through the end of the 6-month trial. Adverse events are also assessed 24 hours following drug initiation. For subjects participating in FUEL OLE, the procedures for drug accountability are identical to those for FUEL, but the monthly assessment of adverse events continues through the end of the study. Adverse events are evaluated at one and three months after the last dose of study drug that an individual participant receives.
Statistical considerations
Primary outcome
A sample size of 200 subjects per arm was chosen to allow for 90% power to detect a mean treatment difference in change from baseline to 26-week testing in VO2 max of 10% while taking into account the assumptions listed below. These assumptions were based on historical data and were purposefully conservative to mitigate the risk of completing the study with inadequate power(3,4). Assumptions include:
Baseline standard deviation of VO2 max of 6.25 ml/kg/min
Correlation between baseline and 26-week testing within an individual of 0.33
Drop-out and incomplete testing rate of 10%
Failure to generate a maximal exercise test at the 26-week visit in 15% of subjects
With an anticipated population mean VO2 max of 28 mL/kg/min, this sample size would allow us to detect an improvement of 2.8 mL/kg/min in the udenafil group compared to zero change in the control group, assuming a Type 1 error of 0.05.
The primary analysis at study end will use an intention-to-treat model. The change in VO2 max will be calculated and analysis of covariance will be used to determine whether the mean change in maximal oxygen consumption from baseline to 26-weeks is significantly different between the udenafil and placebo groups after adjusting for baseline values of VO2 and ventricular morphology. Secondary analyses will be performed for pre-specified subgroups including ventricular morphology (left versus right or mixed). The subgroup analyses are exploratory since the trial isn’t powered to detect treatment differences within subgroups.
Secondary outcomes
Secondary exercise performance outcomes, most echocardiographic parameters, and vascular function measures are continuous data points and will be analyzed in the manner described for the primary outcome. If measures are highly skewed, then regression modeling will be conducted on a suitably transformed outcome, where possible, to achieve a distribution that is closer to normality (e.g., log-transformed BNP instead of raw values). Any echocardiographic indices that are not continuous (e.g. severity of atrioventricular valve regurgitation) will be assessed using a Cochran-Mantel-Haenszel test with ventricular morphology as the stratification factor. Treatment group differences for endpoints associated with quality of life instruments will be assessed with Friedman’s test with ventricular morphology as the stratification factor.
Interim analysis/safety monitoring
There are no efficacy interim analyses planned for this study. The PHN’s Data and Safety Monitoring Board (DSMB), established by NHLBI, routinely reviews study data at set intervals throughout the study period. The DSMB has the authority to recommend stopping the trial for safety concerns. Given the short duration of this clinical trial, there will not be an opportunity to review the data for futility. An independent medical monitor reviews all adverse events on a regular basis, including relationship to study drug.
Discussion
Choice of primary outcome
Studying the effect of any intervention in the Fontan population is difficult given the absence of readily measured, reliable and reproducible endpoints. This lack of clear endpoints for the study of Fontan physiology is primarily related to the small numbers of patients with this unique physiology and the slow rate of attrition (heart failure, death, and transplant), both in absolute terms and relative to other populations, such as adults with heart failure. The PHN Fontan Cross-Sectional and Longitudinal Studies were the largest attempts to characterize this group, but included several hundred rather than the several thousand subjects that might be included in studies of other forms of heart disease(3,4).
Given the difficulty with hard endpoints, exercise capacity is frequently used as a surrogate outcome in those with CHD. As noted, VO2 max has been shown to be a consistent and sensitive marker of deteriorating function, onset of symptoms of heart failure, and increasing risk for death in the population with the Fontan procedure(3,5–7,17–20). Although an alteration of the decline in exercise capacity over time was considered as the primary outcome, contemporary data from the PHN suggested that the rate of decline in the modern era might be more gradual than that reported for older cohorts, which would make it difficult to detect a change in the slope of decline within a reasonable study period(3). Given that limitation, a positive change in exercise capacity was determined to be a more practical endpoint.
Choice of trial duration
Although the acute benefit of PDE5 inhibitors and pulmonary vasodilators on Fontan physiology has been demonstrated by a number of prior studies(23–31), the important question is whether these benefits hold up over a longer period of time and whether they might be associated with a forestalling of the onset of the development of symptoms associated with “Fontan-failure.” Given the rate of the decline of exercise performance in those with Fontan physiology, an ideal study design would involve randomization and continuation of the study over many years. However, such a design would make recruitment and retention a potentially insurmountable challenge. The decision to choose a six-month study period was based on an attempt to balance the challenge of recruiting into a long-term study with the goal of observing the impact of udenafil over a meaningful period of time. The six-month time period should allow for a reasonable assessment of the impact of udenafil on exercise performance beyond the acute effect and for a “real-life” assessment of medication adherence and of the side effect profile.
Importance of Partnering with Patient Advocacy Groups
The FUEL trial is unique in scope and size in congenital cardiology and the success of the trial depends critically on the enthusiasm of patients and their families. By partnering with patient advocacy groups, we were better able to understand the barriers to participation created by the time consuming nature of the study procedures. This led to the creation of a travel fund to support families coming from a distance, and led to the distribution of portable devices to help provide reminders to assist with medication adherence. Using family feedback, we were also able to design subject compensation in a manner commensurate with the time and effort expended and to modify the protocol to try to minimize the disruption to the lives of the adolescent participants. In addition, the partnership with advocacy groups has facilitated a coordinated dissemination of information about the trial to potential participants who are followed by sites that are not participating in this trial. We were able to present a live webinar in which we reviewed the rationale and outline of the trial, and were then able to provide a link to the webinar to those potential participants who were not able to view the presentation as it occurred.
Importance of the clinical trial and timeline for completion
Since the initial description of the Fontan operation there have been numerous innovations that have allowed for improved perioperative and long-term survival for those born with single ventricle CHD(43–46). However, while early survival has improved, progressive morbidity is still pervasive(47,48). The elevated central venous pressure and low cardiac output that are hallmarks of the Fontan circulation create a unique form of chronic heart failure for which no specific treatment exists. If udenafil is confirmed to be a safe and well-tolerated chronic medication that improves exercise performance in Fontan physiology, it would have considerable potential to improve other outcomes for the growing population of children, adolescents, and adults with palliated single ventricle CHD. As of manuscript submission, 367 participants have been randomized at 30 sites and it is expected that recruitment will be completed by the end of June of 2018. This timeline would allow for the presentation of the primary results in the first quarter of 2019.
Limitations
The relatively small number of subjects with this rare condition will make it hard to detect improvements in the primary outcome that are smaller than anticipated, as well as subtle improvements in the secondary exercise and imaging outcomes. Although the trial is powered robustly for the primary outcome, it is limited in its enrollment goal by the reality that single ventricle heart disease is a rare pediatric disease. In addition, while the trial will have six months of safety data from FUEL and an additional 12 months of data from FUEL OLE, that amount of data is likely insufficient to adequately assess the long-term safety of udenafil. If udenafil is to be used extensively in those with Fontan physiology, on-going surveillance will be necessary to ensure long-term safety across the population.
Conclusions
While the Fontan operation has been life-saving for generations of children born with single ventricle CHD, there is a clear need to develop therapies aimed at improving this unique circulation. Medications that target PVR may be uniquely suited to improve Fontan physiology by lowering central venous pressure and increasing cardiac output. Among pulmonary vasodilators, PDE-5 inhibitors have a strong track record of safety and are currently the front-line therapy for children with pulmonary arterial hypertension. Udenafil is unique among PDE-5 inhibitors in that it has been studied in a phase I/II trial in adolescents who have undergone the Fontan operation. Given the theoretical benefit of udenafil in those with Fontan physiology, we have the opportunity to answer the question of clinical utility in the setting of relative equipoise among pediatric cardiologists, Fontan patients, and their families. The FUEL and FUEL OLE trials have the potential to make a substantial contribution to the care of those born with single ventricle heart disease who have survived beyond the Fontan operation.
Acknowledgments
Funding for this project was provided by the National Heart, Lung, and Blood Institute (NHLBI U01 HL068270, HL109741, HL109781, HL109816, HL109818, HL109777, HL109778, HL109673, HL109743, HL109737, HL068270) and by Mezzion Pharma Co. Ltd. (Seoul, Republic of Korea). The views expressed are those of the authors, and do not represent official positions of NHLBI or NIH.
We are grateful for the support of Mended Little Hearts, Sisters by Heart, and the Pediatric Congenital Heart Association.
Footnotes
Clinical Trial Numbers: NCT0274115 and NCT03013751
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–8. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreutzer G, Galindez E, Bono H, De Palma C, Laura JP. An operation for the correction of tricuspid atresia. The Journal of thoracic and cardiovascular surgery. 1973;66:613–21. [PubMed] [Google Scholar]
- 3.Atz AM, Zak V, Mahony L, et al. Longitudinal Outcomes of Patients With Single Ventricle After the Fontan Procedure. J Am Coll Cardiol. 2017;69:2735–2744. doi: 10.1016/j.jacc.2017.03.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes SM, McElhinney DB, Khairy P, Graham DA, Landzberg MJ, Rhodes J. Serial cardiopulmonary exercise testing in patients with previous Fontan surgery. Pediatric cardiology. 2010;31:175–80. doi: 10.1007/s00246-009-9580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giardini A, Hager A, Pace Napoleone C, Picchio FM. Natural history of exercise capacity after the Fontan operation: a longitudinal study. The Annals of thoracic surgery. 2008;85:818–21. doi: 10.1016/j.athoracsur.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins PC, Chinnock RE, Jenkins KJ, et al. Decreased exercise performance with age in children with hypoplastic left heart syndrome. The Journal of pediatrics. 2008;152:507–12. doi: 10.1016/j.jpeds.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein BH, Urbina EM, Khoury PR, et al. Endothelial Function and Arterial Stiffness Relate to Functional Outcomes in Adolescent and Young Adult Fontan Survivors. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg DJ, Paridon SM. Fontan circulation: the search for targeted therapy. Circulation. 2014;130:1999–2001. doi: 10.1161/CIRCULATIONAHA.114.013365. [DOI] [PubMed] [Google Scholar]
- 10.Gewillig M, Brown SC, Eyskens B, et al. The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg. 2010;10:428–33. doi: 10.1510/icvts.2009.218594. [DOI] [PubMed] [Google Scholar]
- 11.Stickland MK, Welsh RC, Petersen SR, et al. Does fitness level modulate the cardiovascular hemodynamic response to exercise? Journal of applied physiology. 2006;100:1895–901. doi: 10.1152/japplphysiol.01485.2005. [DOI] [PubMed] [Google Scholar]
- 12.Argiento P, Chesler N, Mule M, et al. Exercise stress echocardiography for the study of the pulmonary circulation. Eur Respir J. 2010;35:1273–8. doi: 10.1183/09031936.00076009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navaratnam D, Fitzsimmons S, Grocott M, et al. Exercise-Induced Systemic Venous Hypertension in the Fontan Circulation. Am J Cardiol. 2016;117:1667–1671. doi: 10.1016/j.amjcard.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Gewillig M, Goldberg DJ. Failure of the fontan circulation. Heart failure clinics. 2014;10:105–16. doi: 10.1016/j.hfc.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Kuebler JD, Chen MH, Alexander ME, Rhodes J. Exercise Performance in Patients with D-Loop Transposition of the Great Arteries After Arterial Switch Operation: Long-Term Outcomes and Longitudinal Assessment. Pediatric cardiology. 2016;37:283–9. doi: 10.1007/s00246-015-1275-5. [DOI] [PubMed] [Google Scholar]
- 16.Buys R, Cornelissen V, Van De Bruaene A, et al. Measures of exercise capacity in adults with congenital heart disease. International journal of cardiology. 2011;153:26–30. doi: 10.1016/j.ijcard.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham JW, Nathan AS, Rhodes J, Shafer K, Landzberg MJ, Opotowsky AR. Decline in peak oxygen consumption over time predicts death or transplantation in adults with a Fontan circulation. American heart journal. 2017;189:184–192. doi: 10.1016/j.ahj.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Diller GP, Dimopoulos K, Okonko D, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–35. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 19.Diller GP, Giardini A, Dimopoulos K, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. European heart journal. 2010;31:3073–83. doi: 10.1093/eurheartj/ehq356. [DOI] [PubMed] [Google Scholar]
- 20.Giardini A, Specchia S, Tacy TA, et al. Usefulness of cardiopulmonary exercise to predict long-term prognosis in adults with repaired tetralogy of Fallot. Am J Cardiol. 2007;99:1462–7. doi: 10.1016/j.amjcard.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein BH, Connor CE, Gooding L, Rocchini AP. Relation of systemic venous return, pulmonary vascular resistance, and diastolic dysfunction to exercise capacity in patients with single ventricle receiving fontan palliation. Am J Cardiol. 2010;105:1169–75. doi: 10.1016/j.amjcard.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Ridderbos FJ, Wolff D, Timmer A, et al. Adverse pulmonary vascular remodeling in the Fontan circulation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2015;34:404–13. doi: 10.1016/j.healun.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Mori H, Park IS, Yamagishi H, et al. Sildenafil reduces pulmonary vascular resistance in single ventricular physiology. International journal of cardiology. 2016;221:122–7. doi: 10.1016/j.ijcard.2016.06.322. [DOI] [PubMed] [Google Scholar]
- 24.Van De Bruaene A, La Gerche A, Claessen G, et al. Sildenafil improves exercise hemodynamics in Fontan patients. Circulation Cardiovascular imaging. 2014;7:265–73. doi: 10.1161/CIRCIMAGING.113.001243. [DOI] [PubMed] [Google Scholar]
- 25.Tunks RD, Barker PC, Benjamin DK, Jr, et al. Sildenafil exposure and hemodynamic effect after Fontan surgery. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2014;15:28–34. doi: 10.1097/PCC.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg DJ, French B, Szwast AL, et al. Impact of sildenafil on echocardiographic indices of myocardial performance after the Fontan operation. Pediatric cardiology. 2012;33:689–96. doi: 10.1007/s00246-012-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg DJ, French B, McBride MG, et al. Impact of oral sildenafil on exercise performance in children and young adults after the fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation. 2011;123:1185–93. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agnoletti G, Gala S, Ferroni F, et al. Endothelin inhibitors lower pulmonary vascular resistance and improve functional capacity in patients with Fontan circulation. The Journal of thoracic and cardiovascular surgery. 2017;153:1468–1475. doi: 10.1016/j.jtcvs.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 29.Hebert A, Mikkelsen UR, Thilen U, et al. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption) study. Circulation. 2014;130:2021–30. doi: 10.1161/CIRCULATIONAHA.113.008441. [DOI] [PubMed] [Google Scholar]
- 30.Schuuring MJ, Vis JC, van Dijk AP, et al. Impact of bosentan on exercise capacity in adults after the Fontan procedure: a randomized controlled trial. European journal of heart failure. 2013;15:690–8. doi: 10.1093/eurjhf/hft017. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes J, Ubeda-Tikkanen A, Clair M, et al. Effect of inhaled iloprost on the exercise function of Fontan patients: a demonstration of concept. International journal of cardiology. 2013;168:2435–40. doi: 10.1016/j.ijcard.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huddleston AJ, Knoderer CA, Morris JL, Ebenroth ES. Sildenafil for the treatment of pulmonary hypertension in pediatric patients. Pediatric cardiology. 2009;30:871–82. doi: 10.1007/s00246-009-9523-1. [DOI] [PubMed] [Google Scholar]
- 33.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. The New England journal of medicine. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 34.Travadi JN, Patole SK. Phosphodiesterase inhibitors for persistent pulmonary hypertension of the newborn: a review. Pediatric pulmonology. 2003;36:529–35. doi: 10.1002/ppul.10389. [DOI] [PubMed] [Google Scholar]
- 35.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117:1077–83. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 36.Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681–7. doi: 10.1093/eurheartj/ehn215. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg DJ, Zak V, Goldstein BH, et al. Results of a phase I/II multi-center investigation of udenafil in adolescents after fontan palliation. American heart journal. 2017;188:42–52. doi: 10.1016/j.ahj.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sleeper LA, Anderson P, Hsu DT, et al. Design of a large cross-sectional study to facilitate future clinical trials in children with the Fontan palliation. Am Heart J. 2006;152:427–33. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg DJ, French B, Szwast AL, et al. Tricuspid annular plane systolic excursion correlates with exercise capacity in a cohort of patients with hypoplastic left heart syndrome after Fontan operation. Echocardiography. 2016;33:1897–1902. doi: 10.1111/echo.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordina R, Ministeri M, Babu-Narayan SV, et al. Evaluation of the relationship between ventricular end-diastolic pressure and echocardiographic measures of diastolic function in adults with a Fontan circulation. International journal of cardiology. 2018 doi: 10.1016/j.ijcard.2018.02.045. [DOI] [PubMed] [Google Scholar]
- 41.Cordina R, von Klemperer K, Kempny A, et al. Echocardiographic Predictors of Mortality in Adults With a Fontan Circulation. JACC Cardiovascular imaging. 2017;10:212–213. doi: 10.1016/j.jcmg.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Friedberg MK, Silverman NH. The systolic to diastolic duration ratio in children with heart failure secondary to restrictive cardiomyopathy. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2006;19:1326–31. doi: 10.1016/j.echo.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 43.de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience The Journal of thoracic and cardiovascular surgery. 1988;96:682–95. [PubMed] [Google Scholar]
- 44.Bridges ND, Mayer JE, Jr, Lock JE, et al. Effect of baffle fenestration on outcome of the modified Fontan operation. Circulation. 1992;86:1762–9. doi: 10.1161/01.cir.86.6.1762. [DOI] [PubMed] [Google Scholar]
- 45.Norwood WI, Jacobs ML. Fontan’s procedure in two stages. American journal of surgery. 1993;166:548–51. doi: 10.1016/s0002-9610(05)81151-1. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien JE, Jr, Marshall JA, Young AR, Handley KM, Lofland GK. The nonfenestrated extracardiac Fontan procedure: a cohort of 145 patients. The Annals of thoracic surgery. 2010;89:1815–20. doi: 10.1016/j.athoracsur.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 47.Downing TE, Allen KY, Glatz AC, et al. Long-term survival after the Fontan operation: Twenty years of experience at a single center. The Journal of thoracic and cardiovascular surgery. 2017;154:243–253e2. doi: 10.1016/j.jtcvs.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 48.Rogers LS, Glatz AC, Ravishankar C, et al. 18 years of the Fontan operation at a single institution: results from 771 consecutive patients. J Am Coll Cardiol. 2012;60:1018–25. doi: 10.1016/j.jacc.2012.05.010. [DOI] [PubMed] [Google Scholar]