Abstract
This perspective represents a (highly personal) examination of the past, present and future of synthetic organic chemistry. The central thesis posits that the confluence of factors that led to the “Golden Age of Natural Product Synthesis” in the second half of the twentieth century can be traced back to the identification of the therapeutic potential of steroid hormones culminating in the introduction of oral contraceptives. The tremendous benefits of those activities to the development of organic synthesis as a vibrant discipline led to the exponential increase in strategies and methods and the ability to tackle, larger and larger molecules of greater and greater complexity. The existential challenge to the health of organic synthesis is whether a similarly dynamic future can be anticipated and if so, to what end and how. Musings on potential answers to those questions are presented.
Keywords: synthesis, steroids, function, history, natural products
Nowadays, the molecular program of chemistry has arrived at its successful termination
H. Primas (1982)
The reports of my death have been greatly exaggerated
Mark Twain (1897)
1. Introduction
The title of this perspective is an homage to Prof. Dr. Dieter Seebach who in 1990 published a tour de force analysis of the state of organic synthesis entitled “Organic Synthesis – Where Now?”.[1] Prof. Seebach was extremely well positioned both professionally and personally to undertake such a monumental challenge. Moreover, he had the courage and gravitas to prognosticate about its future. Anyone reading the accounts in this Rosarium Philosophorum on the same subject is well advised to understand the state of the art in 1990 so eloquently and comprehensively detailed in that review.
However, that article represents more than an authoritative disquisition on the immense field of organic synthesis, it also serves as a milestone in the evolution of the discipline. Indeed, on many occasions, intellectual leaders and practitioners have taken pause to evaluate the health of the field and provide perspective on its past, present and future. Such introspective analyses serve many functions; to provide a collective scorecard on the condition of the enterprise, to identify trends not easily discerned in the day-to-day progression of research agendas, and to allow future generations a historical reference point by which to measure progress in the field after an arbitrary period.
One of the first, and most often quoted expositions was published by R. B. Woodward in 1956 in “Perspectives in Organic Chemistry” which contained a collection of essays presented to Sir Robert Robinson on the occasion of his 70th birthday.[2] The range of topics and stature of the contributors are extraordinary from the theory of resonance (Pauling) to stereochemistry (Barton) to organometallic chemistry (Ziegler) to nucleic acids (Todd) to isotopes (Cornforth) to chemistry and genetics (Butenandt). Woodward’s essay on “Synthesis” served all three purposes with his characteristic insight and literary panache. He begins the essay with a highly positive evaluation:
It can scarcely be gainsaid that the successful outcome of a synthesis of more than thirty stages provides a test of unparalleled rigor……Since organic chemistry has produced syntheses of this magnitude, we can, by this yardstick, pronounce its condition good….”
After a thorough survey of the state of the field, he then ends the essay with a still more optimistic prognosis:
We shall leave it that the evidence is overwhelming that the creative function of organic chemistry will continue to augment Nature, with great rewards, for mankind and chemist in equal measure.
In the intervening years many other analyses and compilations of the successes and failures in organic synthesis have appeared, though for the most part, with a few notable exceptions, these have served only the first objective, namely a providing a summary of the accomplishments, with little analysis of the directions and remaining challenges.[3] Perhaps the most notable exception was a NATO ASI workshop that took place in 1994 in Ravello, Italy, organized by Chryssostomos Chatgilialoglu and Victor Snieckus, with the cryptic and controversial title “Chemical Synthesis: Gnosis to Prognosis”. The mandate of this workshop was,
To survey existing knowledge, assess current work, and discuss the future directions of chemical synthesis as it impinges on three exciting interdisciplinary themes of science in the 1990’s: bioactive molecules, man-made chemical materials, and molecular recognition.
As part of this workshop three panel discussions were organized, the first of which chaired by Prof. Clayton Heath-cock addressed the provocative question “As we head into the 21st Century, is there Still Value in Total Synthesis of Natural Products as a Research Endeavor?”. Full accounts of all 24 lectures as well as the transcripts from the three panel discussions are available.[4] Many of the key questions and arguments from the first panel discussion will serve as orienting guidelines for much of the content of this perspective and will be reproduced as needed. However, the reader is urged to make reference to the full text, now 20 years old, to appreciate both the amazing advances and the persistence of many challenges.
The same can be said of the Seebach essay in a more focused sense. The progress in organic synthesis in the intervening 28 years has been nothing short of spectacular. The power, efficiency, selectivity and understanding of synthetic organic chemistry have reached unimaginable heights. Furthermore, the trends in biology and materials science to become more and more molecular have necessarily required input from organic synthesis to advance those disciplines.
2. Structure of the Essay
If the points of debate from the panel discussion in Ravello serve as the conceptual guidelines for this essay, then the five questions posed by the editors of this collection of essays will serve as the structural guidelines. Those questions are:
How do you view the historical development of OS, and of your field in particular?
What have been the most significant contributions of OS to science/humankind?
How would you respond to comments that OS is merely a technology in service of other fields rather than an independent science?
In retrospect, were the key achievements of OS predictable?
Where may the discipline go in the next 20–30 years? What are the open questions?
These are weighty questions, but important ones to address and it will be very interesting to read the various opinions of the invited contributors which will no doubt represent a wide spectrum of thought.
Before entering into any discussion, it is imperative to establish clearly what “Organic Synthesis” means to this author. Whereas the term is often taken as a synonym for the total synthesis of natural products, that in my view is far too narrow. For the purposes of this essay, “Organic Synthesis” will mean the laboratory synthesis of any organic compound completely independent of its origin, composition or purpose. In fact, the bulk of the products of organic synthesis that will be discussed are not naturally occurring compounds. Nevertheless, it is completely understandable why the synthesis of natural products has figured so prominently in the evolution and development of the discipline which conveniently is the topic of the following section.
3. How Do You View the Historical Development of Organic Synthesis, and of Your Field in Particular?
And you may ask yourself – Well, how did I get here?
Talking Heads, Once in a Lifetime (1981)
3.1 In the Beginning
Although the conversion of ammonium cyanate to urea by Wöhler in 1828[5] serves as the consensus landmark for the beginning of organic synthesis, in reality it was much more important in providing the first evidence against the theory of vitalism than marking the beginning of a new field of endeavor.[6] Only in the later part of the 19th century, as a structural theory of organic chemistry took hold and the techniques for isolation and purification of compounds from natural sources appeared did the possibility of de novo synthesis become seriously considered, and that for the purposes of proof of composition and structure.
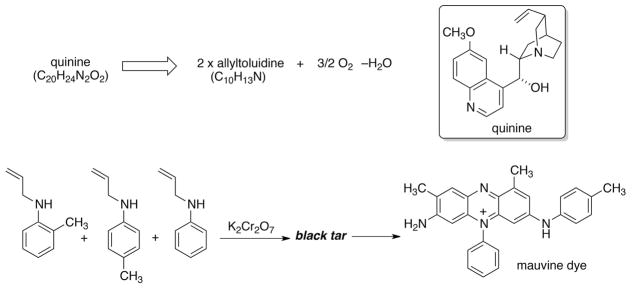
It is both instructive and amusing to recount the logic behind William Henry Perkin’s attempted synthesis of quinine in 1856. His supervisor, August Wilhelm von Hoffmann had published a hypothesis for the synthesis of the antimalarial on the basis of its molecular composition (C20H24N2O2) which entailed combining two molecules of allyltoluidine (C10H13N) with 3/2 O2 and loss of water (−H2O). The 18-year old Perkin used potassium dichromate as the oxygen source and unsurprisingly the experiments failed miserably (Scheme 1). However, upon cleaning up the tarry mess, the alcoholic extracts turned brilliant purple. In one of the most famous examples of serendipity, Perkin had synthesized mauveine dye which revolutionized the textile industry in England and launched the chemical industry.
Scheme 1.
Hoffmann/Perkin synthetic plan for quinine based solely on molecular formula.
Through the first half of the 20th century, the primary function of synthesis remained the proof of composition and structure. Although the methods for confirming these critical features were still primitive (melting point, refractive index, derivatization, elemental analysis, qualitative analysis), the predictability with which known reactions produced established outcomes was also becoming critical.
As is often the case, the mobilization of the resources in response to a major conflict can also stimulate technical advances. In the Second World War, the need to find a replacement for natural rubber drove the development of the polymer industry as did the need for quantities of penicillin and quinine to ameliorate the battlefield challenges of Allied Forces. Although not a single milligram of quinine was actually generated by the apocryphal claim[7] reported by the much celebrated and controversial[8] synthesis by Woodward and Doering in 1944, it does serve as an important landmark for the beginning of organic synthesis with a purpose beyond the establishment of structure.
For the next half century, often referred to as the “Golden Age of Natural Product Synthesis” (sometimes called the Woodwardian Era),[9] the challenges presented by the laboratory synthesis of larger and larger molecules of greater and greater complexity dominated and defined the field. Once again, with uncanny prescience and penetrating clarity, it was Woodward who anointed the new enterprise in much quoted footnotes in his synthesis of strychnine:[10]
It will not be lost upon the reader-nor was it on at least some of the observers of the chemical scene in the late nineteen forties –that the almost simultaneous outcomes of the decades-long chemical degradative assault, and the incomparably shorter X-ray crystallographic investigations, presaged a future in which so singular an edifice as the chemical structure determination of strychnine was unlikely to find parallel….
…This short history should give pause to those whose talent for despair is lavished upon an organic chemistry ornamented and supplemented, or as they fancy, burdened-by magnificent new tools which permit the establishment in days or weeks of enlightenments which once would have required months or years. While it is undeniable that organic chemistry will be deprived of one special and highly satisfying kind of opportunity for the exercise of intellectual élan and experimental skill when the tradition of purely chemical structure elucidation declines, it is true too that the not infrequent dross of such investigation will also be shed; nor is there any reason to suppose that the challenge for the hand and the intellect must be less, or the fruits less tantalizing, when chemistry begins at the advanced vantage point of an established structure.
Of course, men make much use of excuses for activities which lead to discovery, and the lure of unknown structures has in the past yielded a huge dividend of unsought fact, which has been of major importance in building organic chemistry as a science. Should a surrogate now be needed, we do not hesitate to advocate the case for synthesis.
3.2 Steroids Made it Possible[11a]
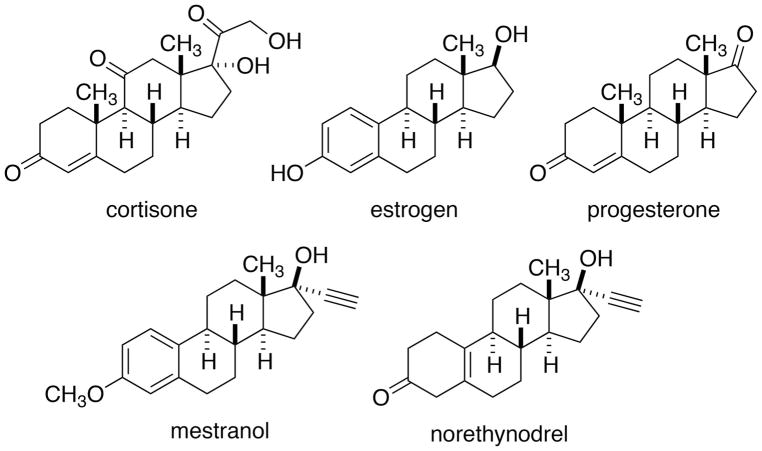
Organic synthesis is inexorably tied to the synthesis of natural products, from the repudiation of vitalism to the proof of unique chemical structure of all of the molecules of nature to their synthesis in service of therapeutic function. Arguably, the latter justification for synthesis is no more evident than in the enormous efforts beginning in the 1950’s toward the synthesis of steroids. By the end of the 1940’s various steroid compounds of the androgen, estrogen, progesterone and adrenocortical classes had been isolated from animal tissues, structurally characterized, and studied for medicinal properties. On the therapeutic front, the discovery by Mayo Clinic researchers that the corticosteroids such as cortisone were effective as anti-inflammatory agents for the treatment of debilitating diseases including rheumatoid arthritis constituted a major breakthrough and launched an overwhelming demand for cortisone (Figure 1).[12] However, cortisone was still being manufactured almost entirely as an extract from animal adrenal glands, making the supply very low and the cost very high.
Figure 1.
Structures of some therapeutically important steroids.
The decades beginning in the 1950’s represented an extraordinarily productive and successful epoch in the development of industrial syntheses of steroids. The myriad components of this amazing story have been chronicled in countless articles,[13] chapters,[14] books[11,15] and even popular accounts.[16] The central issue that drove much of this research was the subsequent discovery that steroids, in particular estrogen and progesterone, were implicated in female fertility and thus could be used to control human reproduction. The first commercial formulation (a combination of mestranol and norethynodrel) marketed as Enovid® from G. D. Searle, was approved for oral contraception in 1960. In the opinion of this author, the successful development of orally available, synthetic steroids to modulate the estrus cycle, is the single greatest gift of organic synthesis to mankind. The fact that an contribution of this significance has never been recognized by a Nobel Prize in either Chemistry or in Physiology or Medicine is clearly an egregious oversight or an accomplishment too fraught with complexities to award.
The spectacular success of steroid research provided the raison d’être for organic synthesis for much of the 1950’s and 1960’s, and justifiably so. The gleanings of research directed toward the synthesis of steroids constitute one of the richest contributions to the edifice of organic chemistry. Historians of science can draw a straight line between the discovery of the structure and properties of steroid molecules and the dramatic increase in research directed to the construction of fused six-membered rings that constitute three of the four rings of steroids. The famous Robinson annulation reaction was expressly devised to generate fused six ring portions of steroids.[17] In that context it cannot be overstated that the genesis of the entire domain of “organocatalysis” can trace its modern origins back to the proline-catalyzed Robinson annulation developed in the context of steroid synthesis independently by researchers at Hoffmann-La Roche[18a] and Schering AG.[18b]
The challenging D-ring of steroids, which is a five-membered ring, inspired scores of creative researchers to develop methods for the attachment of that unit in an efficient fashion.[19] The construction of five-membered rings is much more difficult because it does not arise from the natural reactivity pattern of common functional groups that are prone to construct six-membered rings. Accordingly, entirely new concepts of how to modulate chemical reactivity were introduced and refined to practical synthetic methods.[20]
The entire field of organic stereochemistry was built on the analysis of reactions and properties of steroids. The 1969 Nobel Prize in Chemistry was awarded jointly to Derek H. R. Barton and Odd Hassel “for their contributions to the development of the concept of conformation and its application in chemistry” This concept was developed from their studies of steroids which, because of their structural rigidity, availability and importance were the subjects of innumerable investigations that are now the foundation of conformational analysis.[21] Moreover, the concept of stereoelectronic effects was first articulated to explain the remarkable behavior of steroid ring rearrangements and substitution reactions.[22]
One of the earliest illustrations of the concept of “biomimetic synthesis” involved the demonstration of chemically initiated polyene cyclizations[23] from the brilliant studies of W. S. Johnson[23b] and E. E. van Tamelen.[23c] The laboratory synthesis of racemic progesterone by Johnson confirmed the “Stork-Eschenmoser” hypothesis for the stereochemical outcome of the polyene cyclization and demonstrated that enzymes are not essential to control the relative configuration of the products.[24]
One of the hottest topics in organic synthesis research today is the ability to carry out selective functionalization of inert C–H bonds.[25] This area as well finds its origin in steroid chemistry. Nature has evolved enzymatic pathways to introduce oxygen substituents at various locations of the steroid skeleton that lead to the diversity of the members of this family. These selective transformations inspired researchers such a Derek H. R. Barton[26a] and especially Ronald Breslow[26b] to invent methods to mimic the action of enzymes with new types of chemical transformations designed to introduce heteroatoms in the steroid skeleton.
Finally, the introduction of microbial oxidation of organic compounds[27] that provided the foundation for the emerging field of biocatalysis also can be traced back to landmark discoveries driven by the need to find readily available feedstocks for steroid synthesis and also provide hydroxylated derivatives, inaccessible by synthetic methods.[28] This challenge was of particular importance in the ability to convert readily available soy sterols to synthetic precursors of cortisone[29] and for the introduction of a C(11) hydroxyl group.[30]
This brief overview of the impact of organic chemical research inspired by the need to synthesize steroids is highly instructive for several reasons. Perhaps most striking is that no total synthesis of steroids ever became practical enough to be industrially viable. Nevertheless, a tremendous amount of synthesis was needed to modify readily available precursors to produce either the natural product (as in the case of cortisone) or the hundreds of analogs that have been marketed for various indications. One could argue that the “gleanings” of steroid research are far more significant than any of the total syntheses that served to demonstrate the limits of what could be done in the laboratory.
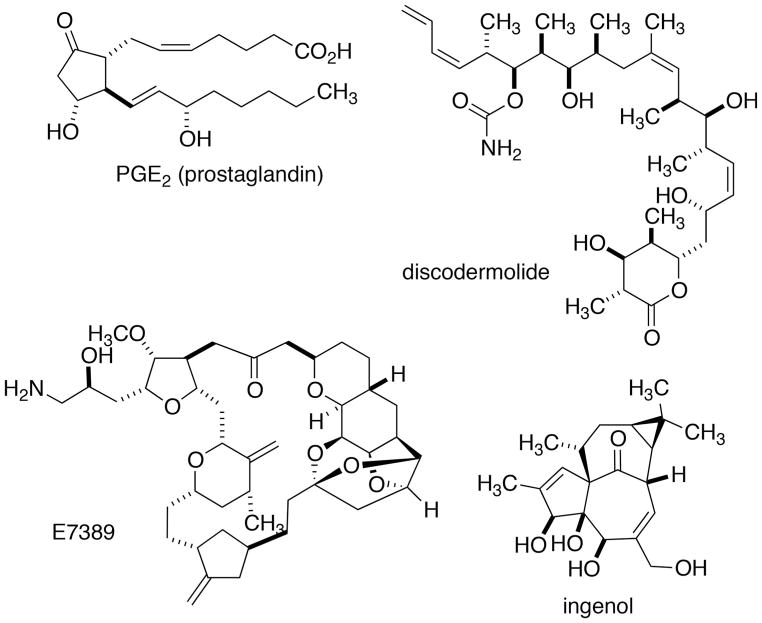
From this point forward, each generation of synthetic organic chemists was fully occupied with the next frontier as defined by the isolation and structure determination (now almost exclusively by spectroscopic means) of the next, more challenging class of molecules. And with each new structural family came new research questions that drove the field forward and contributed immensely to the edifice of knowledge even though, in almost every iteration, total synthesis rarely provided sufficient quantities for study, let alone production. Consider the decades of study on acyclic stereo-control, directed oxidation and reduction reactions, alkylation, aldol and allylation reactions in service of the synthesis of macrolide antibiotics and contrast that to the number of such therapeutic agents actually synthesized for medicinal use. Again and again, the answer comes back that the expansion of the field far outpaces the impact of the syntheses of the natural products. Of course, there are important exceptions when the natural material is extremely scarce, hard to source or unstable outside of its natural environment. Such notable exceptions include the prostaglandins,[31] discodermolide,[32] the Esai analog of the right half of halichondrin B (E7389)[33] and most recently, ingenol (Figure 2).[34]
Figure 2.
Natural and non-natural targets made available in quantity by synthesis.
All of these arguments make Woodward’s statement all the more prophetic:
Of course, men make much use of excuses for activities which lead to discovery, and the lure of unknown structures has in the past yielded a huge dividend of unsought fact, which has been of major importance in building organic chemistry as a science. Should a surrogate now be needed, we do not hesitate to advocate the case for synthesis.
Now, more that 50 years hence, what “excuses for activities which lead to discovery” would Woodward advocate in the coming decades? If still synthesis, then synthesis of what and what for? Perhaps the answers can be found in the Panel Discussion from Ravello in 1994.
3.3 As We Head into the 21st Century, is there Still Value in Total Synthesis of Natural Products as a Research Endeavor?
This provocative question stimulated a spirited discussion at Ravello among the participants. Clayton Heathcock opened the panel discussion with a summary of his own perspective on this important question and then solicited comments from the participants in response to five specific topics which are summarized below. The panelists for the discussion were Paul A. Bartlett (UC Berkeley), Derek H. R. Barton (Texas A&M University), Ronald Breslow (Columbia University), Albert Eschenmoser (ETH-Zürich), and Stephen Hanessian (University of Montreal). It is not possible to summarize the discussion so I will reproduce, verbatim, the summary composed by Professor Heathcock.[35]
There was general agreement that total synthesis still does have intrinsic value as a method of structure proof in many cases, particularly where stereochemistry is involved. It was also recognized that it is sometimes the only way to obtain sufficient amounts of rare natural products with which to carry out biological experiments.
Furthermore, there seemed to be agreement that this form of research is an excellent way to train students, particularly those headed for careers in pharmaceutical chemistry or the biological sciences. However, it was pointed out by several that we educators need to do more to teach our students just how to identify significant problems, and that we need to do more to encourage the kind of breadth that is increasingly necessary in the modem world of science.
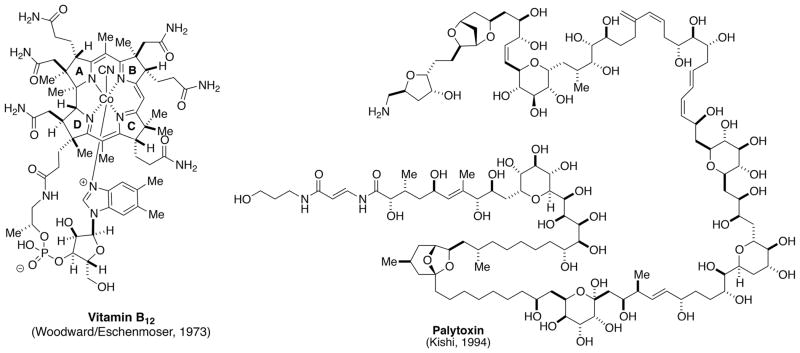
The case was strongly made that, in spite of the recent significant achievements in the arena of total synthesis, we still have far to go before we can accomplish practical syntheses of any desired structure, no matter how complicated. We have come a long way from Wohler’s synthesis of urea to the Woodward-Eschenmoser synthesis of vitamin B12 and Kishi’s synthesis of palytoxin. However, even these monumental synthetic feats are only big steps along the long road toward synthetic perfection. It will probably take another 150 years before chemists will be able to prepare non-biological compounds of comparable complexity in a truly practical manner. So there is continuing value in our trying to solve larger and larger problems by simpler and simpler means.
However, the point was also made that we must be aware of opinion in the community. Practitioners of multistep synthesis must continually question what they are doing; they must carefully evaluate their synthetic approaches to assure that each synthesis really does have the potential to teach us something new, be it a new method or a new strategy of synthesis. Professor Eschenmoser eloquently made the point that total synthesis has played an important role in the history of organic chemistry – the demystification of Nature. It would seem that the time has passed when we need to do fully planned synthesis solely for the purpose of convincing ourselves or the world that we can do it, that we can make anything if we are just willing to work hard enough.
The point was made that synthesis is a unique tool that chemists have, that this ability to make things to study, rather than just studying what Nature provides for us, sets chemists apart from other scientists. It was also pointed out that chemistry, unlike some of the other sciences, does not have an agenda of ‘big problems.’ Along these lines, Professor Eschenmoser challenged us to use synthesis to address a really big problem – understanding how life began! This bold suggestion, particularly his ambitious estimate that we might achieve a model for the kind of self-organization that may have led to the beginning of life in just a few decades, clearly caught the imagination of many participants in the discussion and no doubt provided an inspiration that many of us took with us as we returned home to our own laboratories.
Several of these conclusions deserve additional comment. First, the function of synthesis to confirm structure has surprisingly not disappeared despite the fantastic advances in spectroscopy as has been highlighted in recent structural revisions.[36] The perception that total synthesis of natural products provides an excellent educational experience has diminished in the last decade. Much of the change in emphasis results from a greater diversity of scientists in pharmaceutical industry and the realization that other skills and knowledge are also highly valuable for success in discovery and process chemistry environments.
The panel agreed that despite the landmark achievements of total synthesis (notable vitamin B12 and palytoxin, Figure 3) the field of synthetic organic chemistry is still a long way from the ideal of 100% yield, 100 % stereoselectivity with zero waste at every step (or better yet in a single step!).[37] Anyone who has worked in process development to produce ton quantities of a desired organic compound can readily attest to how far we are from achieving the ideal synthesis. The important question is what should we be doing to address all of those enormous shortcomings. That question will be addressed in the final section along with an expanded commentary on the future of organic synthesis as it was discussed at Ravello.
Figure 3.
Landmark achievements in the total synthesis of natural products.
Finally, the role of organic synthesis in the demystification of Nature cannot be overstated. It has evolved from the debunking of vitalism to the etiology of natural product structure to the understanding of the origin of life. As so eloquently stated by Professor Eschenmoser.[38]
The supreme property of chemical matter is its potency to have given rise to the emergence of life. Yet life’s origin continues to be one of the big unanswered questions of natural science. Life as we know it is a chemical life, thus chemistry is supposed to play a central role in the interdisciplinary effort to pursue the question of life’s origin as a scientific problem, implying that it may be amenable to a solution.
4. What Have Been the Most Significant Contributions of Organic Synthesis to Science/Humankind?
We change the way you live and die.
George M. Whitesides (2015)[39]
Modern life on this planet would be inconceivable without the contributions of organic synthesis and, again, this reality has been brilliantly articulated already 60 years ago by Wood-ward[2]
In the century that has passed since Berthelot’s words were uttered, organic chemistry has literally placed a new Nature beside the old….…the whole face and manner of society has been altered by its products. We are clothed, ornamented, and protected by forms of matter foreign to Nature; we travel and are propelled in, on, and by them, their conquest of our powerful insect enemies, their capacity to modify the soil and control its microscopic flora, their ability to purify and protect our water, have increased the habitable surface of the earth and multiplied our food supply; and the dramatic advances in synthetic medicinal chemistry comfort and maintain us, and create unparalleled social opportunities (and problems).
It is difficult to imagine another discipline that has had a greater impact on human life than chemistry and synthetic (organic) chemistry is the primary driver for the creation of all of those advances that depend on substances that have never before been created on Earth. As was alluded to in a previous section, the invention and introduction of oral contraception, squarely a product of synthetic organic chemistry, is in the opinion of this author the single greatest contribution to humankind.
5. How Would You Respond to Comments that Organic Synthesis is Merely a Technology in Service of Other Fields Rather Than an Independent Science?
Of all the scientific disciplines, chemistry seems to be the one particularly concerned about its public image.
The Public Image of Chemistry (2007)[40]
This loaded question does not ask if the assertion is true (which no chemist would ever concede), but rather how does one respond to this misconception. Chemistry is heralded as the “Central Science” for very good reason; it suffuses into so many other fields that have recently realized the molecular underpinnings of their discipline.[41] However, the impression is thus created that (synthetic) chemistry is an enabling technology to the peripheral sciences that rely on its ability to produce new forms of matter custom designed for their purposes. Thus, (synthetic organic) chemistry is lost at the center while the excitement happens at the leading edges occupied by other sciences. This perception will be true only if chemists do not actively engage in the kinds of interdisciplinary activities that are believed to be where the exciting things are happening. But as we all know that is not the case. Over the past three decades chemistry has become more and more interdisciplinary and chemists, particularly those with skills in synthesis have been able to make contributions undreamed of by biologists, or materials and polymer scientists. Even engineers have become more molecular thus demanding that chemistry, the quintessential molecular science be part of the frontier. But it is also incorrect to believe that at its core, synthetic chemistry is without enormous unanswered questions (more on that later). However, it is true that these more central activities are more difficult to communicate to the public.[42]
6. In Retrospect, Were the Key Achievements of Organic Synthesis Predictable?
The paths of research rarely lead in straightforward fashion from starting point to desired goal….Although intention predisposes the route, chance or occurrences along the way often enforce a change of course…..Along the way, we come upon various points of interest which invite us to linger a while.
Georg Wittig (1979)[43]
The Oxford English Dictionary defines “predict(able)” as:[44]
To state or estimate, especially on the basis of knowledge or reasoning, that (an action, event, etc.) will happen in the future or will be a consequence of something; to forecast, foretell, prophesy.
It is true that any vertical science that builds on itself might be seen as following predictable paths, but those activities are the logical progression of inquiry that take place after a new field is brought into view, often by accidental discovery. So many landmark developments in chemistry in general and synthetic organic chemistry in particular resulted from serendipitous discoveries by observant scientists that the topic has been brought to the dimensions of a book.[45] In a previous section, the serendipitous discovery of mauveine by William Henry Perkin was mentioned and the impact of that accident changed not only the textile and chemical industries, but also the social structure of mid-19th century Europe. Other accidents of great significance detailed in the book include: penicillin, Teflon™, synthetic rubber, artificial sweeteners, Rogain™ and Viagra™. The significance of these accidental discoveries was not always to open new fields of research, but rather had immediate commercial impact such as the last three examples. However, it is clear that the first three did launch entirely new fields in medicinal and polymer chemistry. Moreover, one of the greatest achievements of organo (metallic) synthesis was most certainly not predictable, namely the preparation and correct structural assignment of ferrocene, which launched the very rich field of metallocene chemistry.[46]
From a purely synthetic organic chemistry vantage, some of the most dramatic examples come from the life work of Georg Wittig, quoted above. Wittig clearly understood that it was impossible to predict the accidental discovery, but if one is very observant and chooses an interesting path (i.e. make excuses for activities which lead to discovery) then, as Pasteur famously wrote, chance favors the prepared mind.[47] And prepared Wittig was indeed! All of his most notable contributions including the eponymous carbonyl olefination and the [2,3]-sigmatropic rearrangement were discovered by accident as was the chemistry of ammonium ylides and benzynes.[48] Wittig was a modest man but also brutally honest. In today’s world we are discouraged from admitting that an important advance was not the result of planning and design, lest our creativity and scientific mettle be diminished.
Although it pains me to quote Donald Rumsfeld, the famous passage from his autobiography does capture the essence of the genesis of serendipity:[49]
[T]here are known knowns; there are things we know we know. We also know there are known unknowns; that is to say we know there are some things we do not know. But there are also unknown unknowns – the ones we don’t know we don’t know.
Most of the time in “normal science” (as defined by Thomas Kuhn)[50] we are investigating the “known unknowns”, i.e. unknown components of a known phenomenon that can be expected to be knowable. Such activities are essential and create the edifice of scientific knowledge and are an integral part of the Kuhn Cycle. However, “unknown unknowns” are discovered only by accident and we have no capacity for anticipating their existence or nature. These contributions are not part of Kuhn’s Cycle as they do not arise from model development, crisis and paradigm shift.
7. Where May the Discipline Go in the Next 20–30 Years? What are the Open Questions?
Making predictions is very difficult, especially about the future
Attributed to many from Yogi Berra to Neils Bohr
At the highest level, the future of synthetic organic chemistry can be seen to proceed in two directions, both vertically (empowering synthesis) and horizontally (enabling associated fields).
The vertical direction has as its “Holy Grail” the ability to construct any organic molecule according to the Hendrickson ideal, one step, 100% yield, 100% stereoselectivity, zero waste. If we accept that goal, then it is clear we are eons away from becoming a mature science. Ironically, that goal would constitute the realization of the Hoffmann/Perkin synthetic plan for quinine. At face value, it is a very reasonable proposition, but everything we currently know about structure and reactivity prevents us from formulating a plan that has anything above a zero probability of success. Nothing in our known knowns or known unknowns can imagine how to do this anymore than Wöhler could have imagined how to synthesize Vitamin B12. However, to define the problem, as Poincairé would say “is not to solve the difficulty, but only to baptize it”.[51] Thus, what “excuses for activities that lead to discovery” should we undertake to maximize the probability of discovering the unknown unknowns that will move us forward?
In the opinion of this author, we are already actively engaged in these activities. Across the worldwide enterprise, one sees a tremendous level of activity focused on improving synthetic efficiency (cascade reactions,[52] cooperative (synergistic) catalysis,[53] multicomponent reactions[54]) such that “streamlining synthesis” has become a buzzword. New mechanisms of reactivity are being explored as evidenced by the explosion of interest in photocatalysis,[55] mechanochemistry,[56] and frustrated Lewis pairs[57] to mention a few. Hybrid catalysis has shown remarkable potential by amalgamating existing enzymes with non-natural cofactors, which when combined with directed evolution create new, highly efficient transformations.[58] Finally, if one accepts the proposition that truly revolutionary changes come primarily from serendipitous discoveries, then, recent demonstrations of multidimensional screening,[59] accelerated serendipity[60] and high-throughput discovery[61] of new types of reactions hold extraordinary potential for identifying fundamentally new types of reactivity. These activities must not cease, nor be marginalized by other sciences or more importantly policy makers and funding agencies. Pushing the limits of synthetic efficiency and selectivity are the engines of discovery and ultimately serve both chemistry and the fields that now more than ever depend on its power.
In this context it is worth noting the role that synthetic strategy has evolved and influenced the development of new synthetic reactions. Whereas Woodward elevated strategy of synthesis to an art form, and reveled in the mystique associated with the inspired genius of his plans, it was E. J. Corey who formalized the process and from a very early time advocated for the use of computational analysis to enable and demystify the logic of synthesis.[62] Although the concept of retrosynthetic analysis can be traced back to the classic synthesis of tropinone by Sir Robert Robinson in 1917,[63] it was Corey’s systematic analysis that inspired widespread adoption of this approach. Unfortunately, for the most part, retrosynthetic analysis became nothing more than a reverse justification of an established forward synthetic route rather than what Corey initially intended. Corey’s objective was to use retrosynthetic algorithms to identify the most direct disconnections taking into account the introduction of stereo-centers and functionality without regard for the existence of known transformations. If the forward synthetic reactions did not exist to accomplish the best strategy, this then provided a justification and motivation for invention. Sadly, this strategy driven mechanism for reaction invention has been lost on the community and would serve an equally significant role as blind, high throughput discovery.
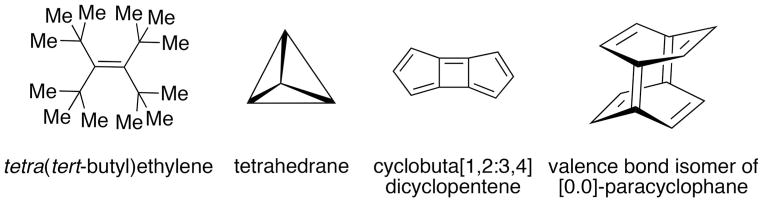
It should be noted that the activities described above all fall under the general heading of “methods of organic synthesis”. This field inarguably dominates all activity in the science of synthetic organic chemistry, especially when combined with catalysis. Clearly, the practitioners of synthetic organic chemistry have to a large extent eschewed the need to justify their development of new methods in the context of natural product structures. Although, this justification is often provided, to a lesser and lesser extent is the method actually used in such an exercise. This trend can be clearly seen as a normal progression of the field as the objective of synthesis evolves from reproducing the molecules of Nature to the synthesis of any molecular structure. Indeed, another role of organic synthesis in the vertical direction is to test the limits of structural theory which requires a completely different set of tools.[64] Unfortunately, this objective passed through its heyday in the 1970’s and 1980’s with such crowning achievements as the synthesis of fenestranes,[65] cubanes,[66] dodecahedrane[67] and [1.1.1]-propellane.[68] The demise of this activity is likely a consequence of the highly utilitarian focus on synthetic chemistry driven by federal funding agencies. Nevertheless, fascinating challenges still persist including tetra(tert-butyl)ethylene,[69] tetrahedrane,[70] cyclobuta[1,2:3,4]dicyclopentene[71] and the valence bond isomer of [0.0]-paracyclophane (Figure 4).[72]
Figure 4.
Molecules of theoretical interest, yet to be synthesized.
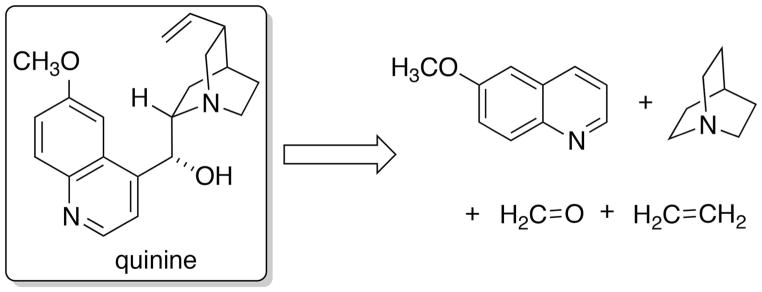
A word about catalysis and about the word catalysis. The entire domain of synthetic chemistry is dominated by catalysis, from the brutal generation of feedstocks from crude oil to the gentlest addition of hydrogen across double bonds. The impact of catalysis cannot be overstated and it will only increase in significance as new mechanisms of catalysis (including heterogeneous and biocatalysis) are developed. Although as coined by Berzelius to implicate “destruction”, catalysis as has been practiced for over a century has been central to the creation of new substances and processes.[73] Still, the ability to catalyze as yet unknown transformations falls under the known unknowns that just need the right circumstances to be discovered. An instructive example would be to modernize the Hoffmann/Perkin synthetic plan by applying some of the methods described above for reaction discovery. Of course, starting with allyltoluidine would be amazing, but let us consider a more modest construction involving the use of 6-methoxyquinoline, quinuclidine, ethylene and formaldehyde. The experimental design would be to combine these components in the presence of hundreds of combinations of transition metal catalysts, in different solvents at different temperatures with or without a dehydrogenating agent (Scheme 2). What is the probability that traces of quinine could be formed under some set of as yet unknown conditions? Most organic chemists would agree that it is nonzero, but this raises yet another challenge, one of analytical chemistry. How would one separate and structurally identify the myriad products formed in such an endeavor? Recent advances in high throughput experimentation and structure determination make this seemingly daunting scenario at least worthy of consideration.[74]
Scheme 2.
Synthesis of quinine for reaction discovery.
The horizontal direction (enabling other fields) will also provide incentives and inspiration for the advancement of synthetic chemistry. Just as the identification of a new natural product structure challenged the existing methods and strategies of synthesis, so should those molecules whose purpose is a specific function. It is tempting to speculate what Woodward himself would advocate for now as the new frontier for synthetic organic chemistry. Woodward’s interest, late in his life, on the design of organic superconductors[75] suggests that he would advocate for the “synthesis of function”.[76] The articulation of this important concept has been attributed to many but it is now firmly identified as the roadmap by which synthetic organic chemistry can impact (dare I say elevate) the allied sciences of biology, medicine, and materials science by providing custom-built molecules with exquisitely programmed function. But this venture will not reduce synthetic chemistry to a service role if the targets identified by practitioners of these disciplines are genuinely chemically agnostic. They should not consider whether such molecules are difficult to prepare (so long as they do not violate rules of structure and thermodynamics). The molecular design should be dictated solely by the desired function and not by ease of synthesis. As such, the challenge to the synthetic chemist is a target, not arising from the secondary metabolism of a living organism, but rather one invented by Man for which a purpose, a reason for synthesis is central to its existence. Again, it is clear that this kind of activity is already taking place in industries that rely on the macroscopic functional manifestation of molecular structure (pharmaceuticals, agrochemicals, high performance polymers, sensors, adhesives, structural materials, explosives, cosmetics, packaging, food additives, the list is endless). But rarely are these enterprises willing or able to invest the time and cost of inventing new chemistry to prepare the perfect molecule. However, such challenges present ideal opportunities for industrial/academic collaborations which must become more prevalent in an uncertain environment of government funding.
It will be in this union, that chemistry will ideally fulfill its sui generis character so clearly articulated by Berthelot, “chemistry creates its own object”.[77] Thus, one can safely prognosticate a brilliant future for synthetic organic chemistry now clearly guided by providing answers to the questions, “what shall we create” and “how shall we create it”. I look forward to being part of this future as long as possible.
Acknowledgments
The author is grateful to Dr. Jeffrey I. Seeman for helpful comments.
Biography
 Scott E. Denmark was born in Lynbrook, New York on 17 June 1953. He obtained an S.B. degree from MIT in 1975 (working with Richard H. Holm and Daniel S. Kemp) and his D. Sc. Tech. (under the direction of Albert Eschenmoser) from the ETH Zürich in 1980. That same year he began his career at the University of Illinois. He was promoted to associate professor in 1986 and to full professor in 1987, and since 1991 he has been the Reynold C. Fuson Professor of Chemistry. His research interests include the invention of new synthetic reactions, exploratory organoelement chemistry, and the origin of stereocontrol in fundamental carbon-carbon bond forming processes. Professor Denmark is currently the Editor in Chief of Organic Reactions and edited Volume 85 of Organic Syntheses. He served for six years as an Associate Editor of Organic Letters and for nine years as Editor of Topics in Stereochemistry. He is a Fellow of the Royal Society of Chemistry, was selected as an ACS Fellow in the inaugural year, 2009 and to the American Academy of Arts and Sciences in 2017.
Scott E. Denmark was born in Lynbrook, New York on 17 June 1953. He obtained an S.B. degree from MIT in 1975 (working with Richard H. Holm and Daniel S. Kemp) and his D. Sc. Tech. (under the direction of Albert Eschenmoser) from the ETH Zürich in 1980. That same year he began his career at the University of Illinois. He was promoted to associate professor in 1986 and to full professor in 1987, and since 1991 he has been the Reynold C. Fuson Professor of Chemistry. His research interests include the invention of new synthetic reactions, exploratory organoelement chemistry, and the origin of stereocontrol in fundamental carbon-carbon bond forming processes. Professor Denmark is currently the Editor in Chief of Organic Reactions and edited Volume 85 of Organic Syntheses. He served for six years as an Associate Editor of Organic Letters and for nine years as Editor of Topics in Stereochemistry. He is a Fellow of the Royal Society of Chemistry, was selected as an ACS Fellow in the inaugural year, 2009 and to the American Academy of Arts and Sciences in 2017.
Footnotes
Dedicated to Prof. Dr. Dieter Seebach on the festive occasion of his 80th birthday.
References
- 1.Seebach D. Angew Chem Int Ed. 1990;29:1320–1367. [Google Scholar]
- 2.Woodward RB. In: Perspectives in Organic Chemistry. Todd AR, editor. Interscience Publishers; 1956. pp. 155–184. [Google Scholar]
- 3.a) Hudlicky T, Reed JW. The Way of Synthesis. Wiley-VCH; Weinheim: 2007. [Google Scholar]; b) Nicolaou KC, Montagnon T. Molecules that Changed the World. Wiley-VCH; Weinheim: 2008. [Google Scholar]; c) Hanessian S, Giroux S, Berner BL. Design and Strategy in Organic Synthesis. Wiley-VCH; Weinheim: 2013. [Google Scholar]
- 4.Chatgilialoglu C, Snieckus V, editors. Chemical Synthesis, Gnosis to Prognosis. Vol. 320. Kluwer Academic Publishers; Dordrecht: 1996. Series E: Applied Sciences. [Google Scholar]
- 5.Wöhler F. Ann Phys Chem. 1828;12:253–256. [Google Scholar]
- 6.Normandin S, Wolfe CT. Vitalism and the Scientific Image in Post-Enlightenment Life Science, 1800–2010. Springer Verlag; 2013. [Google Scholar]
- 7.Woodward RB, Doering WE. J Am Chem Soc. 1944;66:849. [Google Scholar]
- 8.a) Seeman JI. Angew Chem Int Ed. 2007;46:1378–1413. doi: 10.1002/anie.200601551. [DOI] [PubMed] [Google Scholar]; b) Smith AC, Williams RM. Angew Chem Int Ed. 2008;47:1736–1740. doi: 10.1002/anie.200705421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodward RB. In: Architect and Artist in the World of Molecules. Benfey OT, Morris PJT, editors. Chemical Heritage Foundation; Philadelphia: 2001. [Google Scholar]
- 10.Woodward RB, Cava MP, Ollis WD, Hunger A, Daeniker HU, Schenker K. Tetrahedron. 1963:247–288. [Google Scholar]
- 11.a) Djerassi C. In: Steroids Made it Possible, In Profiles, Pathways and Dreams. Seeman JI, editor. American Chemical Society; Washington DC: 1990. [Google Scholar]; b) Djerassi C. This Man’s Pill. Oxford University Press; Oxford: 2001. [Google Scholar]; c) Djerassi C. From the Pill to the Pen. Imperial College Press; London: 2015. [Google Scholar]
- 12.Hetenyi G, Jr, Kars J. Perspect Biol Med. 1997;40:426–440. doi: 10.1353/pbm.1997.0006. [DOI] [PubMed] [Google Scholar]
- 13.a) Kametani T, Nemoto H. Tetrahedron. 1981;37:3–16. [Google Scholar]; b) Hirschmann R. 1992;57:579–592. doi: 10.1016/0039-128x(92)90012-x. [DOI] [PubMed] [Google Scholar]; c Hogg JA. Steroids. 1992;57:593–616. doi: 10.1016/0039-128x(92)90013-y. [DOI] [PubMed] [Google Scholar]
- 14.Nicolaou KC, Montagnon T. Steroids & the Pill, In Molecules that Changed the World. Chapt. 11 Wiley-VCH; Weinheim: 2008. [Google Scholar]
- 15.a) Djerassi C, editor. Steroid Reactions: An Outline for Organic Chemists. Holden-Day; New York: 1963. [Google Scholar]; b) Blickenstaff RT, Ghosh AC, Wolf GC. Total Synthesis of Steroids. Academic Press; New York: 1974. [Google Scholar]
- 16.a) Asbell B. The Pill: A Biography of the Drug that Changed the World. Random House; New York: 1995. [Google Scholar]; b) Eig J. The Birth of the Pill: How Four Crusaders Reinvented Sex and Launched a Revolution. W. W. Norton; New York: 2015. [Google Scholar]
- 17.a) Gawley RE. Synthesis. 1976:777–794. [Google Scholar]; b) Jung ME. Tetrahedron. 1976;32:3–31. [Google Scholar]
- 18.a) Hajos ZG, Parrish DR. J Org Chem. 1974;39:1612–1615. [Google Scholar]; b) Eder U, Sauer G, Wiechert R. Angew Chem, Int Ed Engl. 1971;10:496–497. [Google Scholar]
- 19.Taub D. In: Naturally Occurring Aromatic Steroids, In The Total Synthesis of Natural Products. ApSimon J, editor. Vol. 2. John Wiley & Sons; New York: 1973. pp. 641–725. [Google Scholar]
- 20.a) Wittig G, Davis P, Koenig G. Chem Ber. 1951;84:627–632. [Google Scholar]; b) Seebach D. Angew Chem, Int Ed Engl. 1979;18:239–258. [Google Scholar]
- 21.Barton DHR. Experientia. 1950;6:316–329. doi: 10.1007/BF02170915. [DOI] [PubMed] [Google Scholar]
- 22.a) Hirschmann R, Snoddy CS, Jr, Wendler NL. J Am Chem Soc. 1952;74:2693–2694. [Google Scholar]; b) Corey EJ. J Am Chem Soc. 1953;75:2301–2304. [Google Scholar]; c) Corey EJ. Experientia. 1953;9:329–331. doi: 10.1007/BF02155831. [DOI] [PubMed] [Google Scholar]
- 23.a) Yoder RA, Johnston JN. Chem Rev. 2005;105:4730–4756. doi: 10.1021/cr040623l. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Johnson WS. Acc Chem Res. 1968;1:1–8. [Google Scholar]; c) van Tamelen EE. Acc Chem Res. 1968;1:111–120. [Google Scholar]
- 24.Johnson WS. In: A Fifty-Year Love Affair with Organic Chemistry, In Profiles, Pathways and Dreams. Seeman JI, editor. American Chemical Society; Washington DC: 1998. [Google Scholar]
- 25.a) Newhouse T, Baran PS. Angew Chem Int Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Neufeldt SR, Sanford MS. Acc Chem Res. 2012;45:936–946. doi: 10.1021/ar300014f. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hartwig JF, Larsen MA. ACS Cent Sci. 2016;2:281–292. doi: 10.1021/acscentsci.6b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hartwig JF. Acc Chem Res. 2017;50:549–555. doi: 10.1021/acs.accounts.6b00546. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) He J, Wasa M, Chan KSL, Shao Q, Yu JQ. Chem Rev. 2017;117:8754–8786. doi: 10.1021/acs.chemrev.6b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.a) Barton DHR, Beaton JM. J Am Chem Soc. 1960;82:2461. [Google Scholar]; b) Breslow R, Corcoran R, Dale JA, Liu S, Kalicky P. J Am Chem Soc. 1974;96:1973–1974. [Google Scholar]
- 27.Gibson DT, editor. Microbial Degradation of Organic Compounds. Marcel Dekker; New York: 1984. [Google Scholar]
- 28.Charney W, Herzog HL. Microbial Transformations of Steroids – A Handbook. Academic Press; New York: 1967. [Google Scholar]
- 29.Wovcha MG, Antosz FJ, Knight JC, Kominek LA, Pyke TR. Biochim Biophys Acta. 1978;531:308–320. doi: 10.1016/0005-2760(78)90213-8. [DOI] [PubMed] [Google Scholar]
- 30.a) Peterson DH, Murray HC. J Am Chem Soc. 1952;74:1871–1872. [Google Scholar]; b) Peterson DH, Eppstein SH, Meister PD, Magerlein BJ, Murray HC, Leigh HM, Weintraub A, Reineke LM. J Am Chem Soc. 1953;75:412–415. [Google Scholar]
- 31.Bindra JS, Bindra R. Prostaglandin Synthesis. Academic Press; London: 1977. [Google Scholar]
- 32.Smith AB, III, Freeze BS. Tetrahedron. 2008;64:261–298. doi: 10.1016/j.tet.2007.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson KL, Henderson JA, Phillips AJ. Chem Rev. 2009;109:3044–3079. doi: 10.1021/cr900016w. [DOI] [PubMed] [Google Scholar]
- 34.Jørgensen L, McKerrall SJ, Kuttruff CA, Ungeheuer F, Felding J, Baran PS. Science. 2013;341:878–882. doi: 10.1126/science.1241606. [DOI] [PubMed] [Google Scholar]
- 35.Heathcock CH. As we head into the 21st Century, is there Still Value in Total Synthesis of Natural Products as a Research Endeavor? In: Chatgilialoglu C, Snieckus V, editors. Chemical Synthesis, Gnosis to Prognosis. Vol. 320. Kluwer Academic Publishers; Dordrecht: 1996. pp. 223–243. Series E: Applied Sciences. [Google Scholar]
- 36.Nicolaou KC, Snyder SA. Angew Chem Int Ed. 2005;44:1012–1044. doi: 10.1002/anie.200460864. [DOI] [PubMed] [Google Scholar]
- 37.a) Hendrickson JB. J Am Chem Soc. 1975;97:5784–5800. [Google Scholar]; b) Gaisch T, Baran PS. J Org Chem. 2010;75:4657–4673. doi: 10.1021/jo1006812. [DOI] [PubMed] [Google Scholar]
- 38.Eschenmoser A. Tetrahedron. 2007;63:12821–12844. [Google Scholar]
- 39.Whitesides GM. Angew Chem, Int Ed. 2015;54:3196–3209. doi: 10.1002/anie.201410884. [DOI] [PubMed] [Google Scholar]
- 40.Schummer J, Bensaude-Vincent B, Van Tiggelen B, editors. The Public Image of Chemistry. World Scientific Publishing; Singapore: 2007. [Google Scholar]
- 41.Brown TL, LeMay HE, Jr, Bursten BE, Murphy CJ, Woodward PM, Stoltzfus MW. Chemistry The Central Science. 14. Pearson: 2017. [Google Scholar]
- 42.For a recent discussion on this topic see: Overholser G. Bull Amer Acad Arts Sci. 2017;70:9–15.
- 43.Two Organic Chemists Share Nobel Prize. Chem Eng News. 1979;57:6–7. [Google Scholar]
- 44.Oxford English Dictionary. 2. XII. Oxford University Press; Oxford: 1989. p. 334. [Google Scholar]
- 45.Roberts RM. Serendipity: Accidental Discoveries in Science. John Wiley & Sons; New York: 1989. [Google Scholar]
- 46.Seeman JI, Cantrill S. Nat Chem. 2016;8:193–200. doi: 10.1038/nchem.2455. [DOI] [PubMed] [Google Scholar]
- 47.Pasteur L. Lecture. University of Lille; France: Dec 7, 1854. [Google Scholar]
- 48.From Diyls to Ylides to My IdyllWittig G. In: Nobel Lectures, Chemistry 1971–1980. Frängsmyr T, Forsén S, editors. World Scientific Publishing Co; Singapore: 1993.
- 49.Rumsfeld D. Known and Unknown: A Memoir. Penguin Books; 2011. [Google Scholar]
- 50.Kuhn T. The Structure of Scientific Revolutions. Univeristy of Chicago Press; Chicago: 1962. [Google Scholar]
- 51.Poincairé H. In: The Foundations of Science. Halsted GB, translator. The Science Press; New York: 1913. [Google Scholar]
- 52.a) Ho TL. Tandem Organic Reactions. John Wiley & Sons; New York: 1992. [Google Scholar]; b) Tietze LF, Brasche G, Gericke KM. Domino Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2006. [Google Scholar]; c) Nicolaou KC, Edmonds DJ, Bulger PG. Angew Chem, Int Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]; d) Pellissier H. Chem Rev. 2013;113:442–524. doi: 10.1021/cr300271k. [DOI] [PubMed] [Google Scholar]
- 53.a) Sammis GM, Jacobsen HDEN. J Am Chem Soc. 2004;126:9928–9929. doi: 10.1021/ja046653n. [DOI] [PubMed] [Google Scholar]; b) Allen AE, MacMillan DWC. Chem Sci. 2012;3:633–658. doi: 10.1039/C2SC00907B. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Pellissier H. Tetrahedron. 2013;69:7171–7210. [Google Scholar]; d) Inamdar SM, Shinde VS, Patil NT. Org Biomol Chem. 2015;13:8116–8162. doi: 10.1039/c5ob00986c. [DOI] [PubMed] [Google Scholar]
- 54.a) Herrera RP, Marquéz-López E, editors. Multicomponent Reactions. Wiley-VCH; Weinheim: 2015. [Google Scholar]; b) Zhu J, Wang Q, Wang M, editors. Multicomponent Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2015. [Google Scholar]
- 55.a) Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Photoredox Catalysis in Organic Chemistry (C Stephenson, T P Yoon) Acc Chem Res. 2016:49(6)–49(10). [Google Scholar]
- 56.a) Caruso MM, Davis DA, Shen Q, Odom SA, Sottos NR, White SR, Moore JS. Chem Rev. 2009;109:5755–5798. doi: 10.1021/cr9001353. [DOI] [PubMed] [Google Scholar]; b) Chauhan P, Chimni SS. Beilstein J Org Chem. 2012;8:2132–2141. doi: 10.3762/bjoc.8.240. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang GW. Chem Soc Rev. 2013;42:7668–7700. doi: 10.1039/c3cs35526h. [DOI] [PubMed] [Google Scholar]
- 57.a) Stephan DW, Erker G. Angew Chem Int Ed. 2010;49:46–76. doi: 10.1002/anie.200903708. [DOI] [PubMed] [Google Scholar]; c) Erker G, Stephan DW, editors. Top Curr Chem. 2013. Frustrated Lewis Pairs I; p. 332. [DOI] [PubMed] [Google Scholar]; c) Erker G, Stephan DW, editors. Top Curr Chem. 2013. Frustrated Lewis Pairs II; p. 334. [DOI] [PubMed] [Google Scholar]; d) Stephan DW. Acc Chem Res. 2015;48:306–316. doi: 10.1021/ar500375j. [DOI] [PubMed] [Google Scholar]; e) Stephan DW. Science. 2016;354:aaf7229. doi: 10.1126/science.aaf7229. [DOI] [PubMed] [Google Scholar]
- 58.a) Key HM, Dydio P, Clark DS, Hartwig JF. Nature. 2016;534:534–537. doi: 10.1038/nature17968. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang J, Liab G, Reetz MT. Chem Commun. 2017;53:3916–3928. doi: 10.1039/c7cc00368d. [DOI] [PubMed] [Google Scholar]; c) Prier CK, Zhang RK, Buller AR, Brinkmann-Chen S, Arnold FH. Nat Chem. 2017;9:629–634. doi: 10.1038/nchem.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.a) Beeler AB, Su S, Singleton CA, Porco JA. J Am Chem Soc. 2007;129:1413–1419. doi: 10.1021/ja0674744. [DOI] [PubMed] [Google Scholar]; b) Collins KD, Gensch T, Glorius F. Nat Chem. 2014;6:859–871. doi: 10.1038/nchem.2062. [DOI] [PubMed] [Google Scholar]
- 60.McNally A, Prier CK, MacMillan DWC. Science. 2011;334:1114–1117. doi: 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.a) Robbins DW, Hartwig JF. Science. 2011;333:1423–1427. doi: 10.1126/science.1207922. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Troshin K, Hartwig JF. Science. 2017;357:175–181. doi: 10.1126/science.aan1568. [DOI] [PubMed] [Google Scholar]
- 62.a) Corey EJ, Wipke WT. Science. 1969;166:178–192. doi: 10.1126/science.166.3902.178. [DOI] [PubMed] [Google Scholar]; b) Corey EJ, Long AK, Rubenstein SD. Science. 1985;228:408–418. doi: 10.1126/science.3838594. [DOI] [PubMed] [Google Scholar]; c) Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. John Wiley and Sons; New York: 1989. [Google Scholar]
- 63.Robinson R. J Chem Soc Trans. 1917;111:762–768. [Google Scholar]
- 64.a) Greenberg A, Liebman JF. Strained Organic Molecules. Academic Press; New York: 1978. [Google Scholar]; b) Thummel RP, editor. Advances in Theoretically Interesting Molecules. JAI Press; Greenwich, CT: 1998. [Google Scholar]; c) Kuck D. Chem Rev. 2006;106:4885–4925. doi: 10.1021/cr050546+. [DOI] [PubMed] [Google Scholar]
- 65.a) Keese R. Chem Rev. 2006;106:4787–4808. doi: 10.1021/cr050545h. [DOI] [PubMed] [Google Scholar]; b) Boudhar A, Charpenay M, Blond G, Suffert J. Angew Chem Int Ed. 2013;52:12786–12798. doi: 10.1002/anie.201304555. [DOI] [PubMed] [Google Scholar]
- 66.Eaton PE. Angew Chem Int Ed Engl. 1992;31:1421–1436. [Google Scholar]
- 67.a) Ternansky RJ, Balogh DW, Paquette LA. J Am Chem Soc. 1982;104:4503–4504. [Google Scholar]; b) Paquette LA, Ternansky RJ, Balogh D, Kentgen G. J Am Chem Soc. 1983;105:5446. [Google Scholar]
- 68.a) Ginsburg D. Acc Chem Res. 1972;5:249–256. [Google Scholar]; b) Wiberg K, Walker FH. J Am Chem Soc. 1982;104:5239–5240. [Google Scholar]
- 69.a) Brooks PR, Bishop R, Craig DC, Scudder ML, Counter JA. J Org Chem. 1993;58:6900–5906. [Google Scholar]; b) Sanström J. Strained Olefins. In: Patai S, editor. Double Bonded Functional Groups, Supplement A3, Part 2. Chap. 21 John Wiley and Sons; New York: 1997. [Google Scholar]; c) Lenoir D, Wattenbach C, Liebman JF. Struct Chem. 2006;17:419–422. [Google Scholar]
- 70.Maier G, Neudert J, Wolf O, Pappusch D, Sekiguchi A, Tanaka M, Matsuo T. J Am Chem Soc. 2002;124:13819–13826. doi: 10.1021/ja020863n. [DOI] [PubMed] [Google Scholar]; b) Kobayashi Y, Nakamoto M, Dr, Inagaki Y, Sekiguchi A. Angew Chem Int Ed. 2013;52:10740–10744. doi: 10.1002/anie.201304770. [DOI] [PubMed] [Google Scholar]
- 71.Myers AG, Sogi M, Lewis MA, Arvedson SP. J Org Chem. 2004;69:2516–2525. doi: 10.1021/jo030368p. [DOI] [PubMed] [Google Scholar]
- 72.Eaton PE, Chakraborty UR. J Am Chem Soc. 1978;100:3634–3645. [Google Scholar]
- 73.Lindström B, Pettersson LJ. CATTECH. 2003;7:130–138. [Google Scholar]
- 74.Santanilla AB, Regalado EL, Pereira T, Shevlin M, Bateman K, Campeau L-C, Schneeweis J, Berritt S, Shi Z-C, Nantermet P, Liu Y, Helmy R, Welch CJ, Vachal P, Davies IW, Cernak T, Dreher SD. Science. 2015;347:49–53. doi: 10.1126/science.1259203. [DOI] [PubMed] [Google Scholar]
- 75.Cava MP, Lakshmikantham MV, Hoffmann R, Williams RM. Tetrahedron. 2011;67:6771–6797. [Google Scholar]
- 76.Wender PA. Acc Chem Res. 2015;48:2169–2170. doi: 10.1021/acs.accounts.5b00332. [DOI] [PubMed] [Google Scholar]
- 77.Berthelot M, translator. Chimie Organique Fondée sur la Synthese. Vol. 2. Mallet-Bachelier; Paris: 1860. p. 811. [Google Scholar]