Abstract
The β-carbonic anhydrase (CA, EC 4.2.1.1) from Leishmania spp. (LdcCA) is effectively inhibited by aromatic/heterocyclic sulphonamides, in the low nanomolar range, but no in vitro antileishmanial activity was detected for such compounds. We formulated some of these sulphonamides as nanoemulsions (NEs) in clove oil, and tested them in vitro against Leishmania infantum MHOM/BR/1974/PP75 and Leishmania amazonensis IFLA/BR/1967/PH8 strains. Interesting inhibitory concentrations IC50 were observed for some of the sulphonamides NEs, with IC50 as low as 3.90 µM (NE-3F) and 2.24 µM (NE-5B) for L. amazonensis and 3.47 µM (NE-5B) for L. infantum. Some of the investigated NEs displayed toxicity for macrophages beyond the parasites. For the same nonoemulsions, a selective index (SI) greater than for Amphotericin B. Haemolytic assay using human red blood cells indicate that the NEs were less cytotoxic than amphotericin B, a widely used antifungal agent. NEs demonstrated to be an excellent strategy for increasing the penetration of these hydrophilic drugs through membranes, with a huge increase of efficacy over the sulphonamide CA inhibitor (CAI) alone.
Keywords: Sulphonamide, Leishmania, nanoemulsions, β-carbonic anhydrase
Introduction
Carbonic anhydrases (CA, EC 4.2.1.1) are widespread enzymes in organisms all over the phylogenetic tree1–5. CAs are metalloenzymes that catalyses the reversible hydration of CO2 to bicarbonate with a proton release6. They are grouped in seven distinct families, named α-, β-, γ-, δ-, ζ-, η-, and θ-Cas, classified according the sequence similarity/divergence3. Due the importance of CAs in cell physiology, their inhibitors possess a range of pharmacologic applications in various fields, such as for antiglaucoma drugs7, diuretics8, antiepileptics9, antiobesity agents10, as well as antitumor agents/diagnostics11. Recently, the potential use of CA inhibitors (CAIs) as anti-infective started to be considered for obtaining antibacterials12–14, antifungals15,16, and antiprotozoan agents17–19, with a novel mechanism of action, in the search of agents devoid the resistance problems common to most classes of clinically used such drugs20.
Leishmaniasis is a parasitic infectious disease caused by several species of Leishmania, an obligate intracellular protozoan parasite of humans that resides and multiplies in macrophages21. It is associated with significant rates of morbidity and mortality in many countries around the world. Leishmaniasis presents three main different clinical forms, visceral, cutaneous, and mucocutaneous22. There is no effective vaccine to prevent human leishmaniasis and the drugs available to chemotherapy have several limitations, as side effects and resistance to classical chemotherapy21,23. Thus, the search for new drug targets is required to develop newer therapies, and CAs are a promising target. The species used in this work, Leishmania (L.) amazonensis and Leishmania (L.) infantum causes visceral Leismaniosis.
We have reported that Trypanosoma cruzi, the aetiological agent of Chagas diseases18, another parasitic protozoan, encodes for an α-CA, called TcCA24, which was inhibited in vitro by many sulphonamide CA inhibitors (CAIs), in the low nanomolar or subnanomolar range24,25. However, in vivo, the growth of the parasite was not inhibited by such sulphonamides24–28. Other protozoa, such as Plasmodium falciparum, encode for CAs belonging to the η-CA class18, whereas Leishmania spp. possess β-CAs29. In earlier works from our groups we have shown that sulphonamides and thiols, well-known classes of CAIs, effectively inhibit in vitro this enzyme (called LdcCA as it has been cloned from the genome of Leishmania donovani chagasi). The sulphonamides showed inhibition constants varying between 50.2 nM and 9.25 µM, whereas some heterocyclic thiols inhibited the enzyme with KIs in the range of 13.4–152 nM29. Some of these thiols were shown to efficiently inhibit the in vivo growth of Leishmania chagasi and L. amazonensis promastigotes, by impairing the flagellar pocket and movement of the parasites and causing their death, whereas the sulphonamides, some of which showed similar inhibitory power in vitro as the thiols, were devoid of any such in vivo effects31. We hypothesised that these differences between the two classes of CAIs are due to the very polar nature of the sulphonamides, which interferes with their penetration through biological membranes of the pathogens in order to inhibit the enzyme, responsible for the pH regulation and probably other physiologic effects30. This is the reason why we investigated the possibility to enhance the bioavailability of the sulphonamide CAIs, by formulating them as nanoemulsions (NEs) in clove oil30,31.
The majority of NEs are dispersions of oil droplets in water with diametre between 20 and 200 nm. A recent study with sulphonamide NEs and Trypanosomas cruzi demonstrated that this represents indeed a good strategy to enhance the penetration of the drugs in the parasites32. Most NEs present small droplet size that allows the Brownian motion of the drops retarding their sedimentation or coalescence. Thus, NEs present kinetic stability30, promoting tissue permeation and penetration of drugs. Their nanometric droplets have large relative surface area, facilitating the contact of the nano-carrier with the biological membranes or tissues, and consequently favouring drug permeation and retention. In this paper, we present the antileishmanial activity of sulphonamide CAIs formulated as NEs in clove oil.
Materials and methods
Materials
Clove oil (Eugenia caryophyllus) was purchased from Ferquima Ltd. (Sao Paulo, Brazil). Pluronic F-127, a nonionic block-copolymer surfactant of (poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide)) (EO100PO66EO100), with MW 12,600, and HLB 22, was purchased from Sigma-Aldrich (St. Louis, MO). Dulbecco’s modified Eagle’s medium (DMEM), resazurin, amphotericin B, and thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was purchased from LGC Biotecnologia (São José, Brazil).
Chemistry
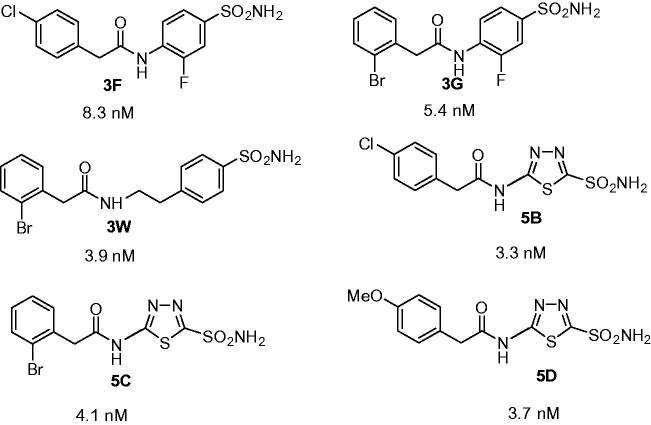
Sulphonamides 3F, 3G, 3W, 5B, 5C and 5D (Figure 1) used in the experiments were reported in an earlier work from our groups25, but they were not tested earlier as LdcCA inhibitors.
Figure 1.
Sulphonamides 3F, 3G, 3W, 5B, 5C and 5D used in the study and their LdcCA inhibitory action (KIs, nM).
CA activity measurements and inhibition studies
An Applied Photophysics (Oxford, UK) stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity33. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Tris (pH 8.4) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 s33. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitors (10 mM) were prepared in distilled-deionised water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3, as reported earlier34–36, and represent the mean from at least three different determinations. All CA isoforms were recombinant ones obtained in-house as reported earlier34–36.
Nanoemulsion preparation
The oil-in-water (O/W) NEs were prepared by high-energy method using an ultrasound processor (Hielscher model UP100H, Hielscher GmbH, Berlin, Germany), according to the method descripted by Senna et al.31. Oil phase was prepared by sulphonamides dissolution in the clove oil. An amount of 5 mg of drug was weighted in a microtube and 1 ml of clove oil was added. The tube was agitated for 1 min for obtaining of the drug solution (5 mg/ml). Aqueous phase was prepared by adding 1 g of Pluronic F127® in 8 g of water. Then 1 ml of oil phase (drug dissolved in clove oil) was added to 9 ml of aqueous phase (Pluronic F127 in water) under constant ultrasound homogenisation (amplitude 80%, continuous cycle no. 1) during 5 min in an ice bath at 5 °C to prevent heating of the dispersion. A transparent NE was obtained at a concentration of 500 µg/ml.
Determination of droplet size
Determination of droplet size and polydispersity index (PDI) were measured, using the dynamic light scattering (DLS) method with a Malvern model 90S NanoSizer® (London, UK). NEs were diluted in distilled water at 1:10 and analysed in a cell with 1 cm optical path at room temperature (25 °C). These analyses were conducted in three runs with 15 readings. The values shown are the mean ± standard deviation of three measurements for each formulation. The PDI reflects the sample quality in the parameter homogeneity of the droplet diameter. PDI results lower than 0.3 were considered satisfactory37.
Leishmania cultures
Leishmania (infantum MHOM/BR/1974/PP75 and L. amazonensis IFLA/BR/1967/PH8 were donated by the Leishmania Type Culture Collection (LTCC) of Oswaldo Cruz Institute/Fiocruz (Rio de Janeiro, Brazil). Parasite cultures were maintained in PBHIL medium supplemented with 10% of FBS at 26 °C38.
RAW 264.7 macrophage cell line culture
RAW 264.7 macrophages were obtained from the National Institute of Metrology, Quality and Technology (Inmetro, Rio de Janeiro, Brazil) and maintained in DMEM medium supplemented with 10% FBS at 37 °C in a 5% controlled CO2 atmosphere. Cell maintenance was performed every 48–72 h, time necessary for cells to achieve confluent monolayers.
Antileishmanial assay
The antileishmanial activity of the sulphonamide NEs was evaluated by the microdilution technique. First, polystyrene 96-well plates were used to serially dilute the samples in a 10% FBS-supplemented PBHIL medium. Amphotericin B and NEs prepared without the sulphonamides were used as positive and negative controls, respectively. L. amazonensis and L. infantum promastigote forms were harvest at late log phase of growth (96 h), washed twice with PBS and resuspended in fresh culture medium to a final concentration of 5 × 106 parasites/ml. Then, 100 µl of each parasite suspensions were added to the plates, and the samples were adjusted to final concentrations ranging from 1 to 128 µM. After 120 h incubation period at 26 °C, parasites viability was assessed by adding 50 µL of resazurin solution (0.005%) as previously described by Rolon et al.39. The minimal inhibitory concentration (MIC) was determined as the lowest concentration capable of inhibiting in vitro growth of the parasites. The 50 and 90% inhibitory concentrations (IC50 and IC90) were calculated by regression analysis using Microsoft Excel 2013 software.
Cytotoxic assay
Sulphonamide NEs cytotoxicity was performed using tetrazolium dye MTT colorimetric assay. RAW 264.7 macrophages were harvest after confluent monolayer achievement. The cells were washed twice with PBS and a cellular suspension of 106 cells/ml was prepared in fresh DMEM culture medium. Aliquots of 100 µl of the cellular suspension were placed into polystyrene 96-well plates, and then incubated at 37 °C in a 5% CO2 atmosphere for 6 h to allow for adherence of macrophages. After this period, the adherent cells were subjected to treatment with several concentrations of the sulphonamide NEs (1–128 µM), and then incubated for additional 48 h. Finally, 20 µl of a MTT solution (5 mg/ml) were added to each well and the plates incubated for 4 h as previously described40. Macrophage viability was determined after formazan crystals solubilisation with DMSO followed by the absorbance measurement at 570 nm using a SpectraMax M5 spectrophotometer (Molecular Devices, Los Angeles, CA). The 50% cytotoxic concentrations were calculated by regression analysis using Microsoft Excel 2013 software.
Selective index determination
The selective index (SI) for promastigote forms of L. amazonensis and L. infantum was calculated by the ratio between the CC50 for RAW 264.7 macrophages and the IC50 for the parasites. Samples with SI values >10 were considered as low cytotoxic agents41.
Haemolytic assay
Haemolytic activity was evaluated as described previously by Ishnava and Shah with a slight modification42. Human erythrocytes from healthy individuals were collected in vacuum tubes containing EDTA as anti-coagulant. The erythrocytes were harvested by centrifugation for 10 m at 2500 rpm at 20 °C, and washed three times in PBS. To the pellet, PBS was added to yield a 10% (v/v) erythrocytes/PBS suspension. The 10% suspension was then diluted 1:10 in PBS. Aliquots of 100 µl of erythrocytes suspension was added, in triplicate, to 100 µl of a two-fold dilution series of sulphonamides NEs and amphotericin B (at concentrations of 128, 64, 32, 16, 8, 4, 2, and 1 µM, respectively, in the same buffer) in microtubes. Negative and positive controls were performed by replacing drug dilution with PBS or with 200 µl of % Triton X-100 for the total haemolysis, respectively. The tubes were incubated for 1 h at 37 °C and then centrifuged for 10 min at 2000 rpm at 20 °C. From the supernatant fluid, 150 µl was transferred to a flat-bottomed microtiter plate, and the absorbance was measured spectrophotometrically at 540 nm. Five concentrations are required for HC50 calculation. The following formula was used to find out the percentage of haemolytic activity: [A540 treated sample − A540 of buffer)/(A540 of Triton X-100 − A540 of buffer)] × 100.
Statistical analysis
The data of the experiments are being carried out through the programme Prism 5.01 GraphPad (GraphPad Software, La Jolla, CA), being considered values statistically significant those with values of the standard deviation (SD), p < .05.
Results and discussion
Carbonic anhydrase inhibition with sulphonamide 3F, 3G, 3W, 5B, 5C, and 5D and preparation of their clove oil NEs
Sulphonamides 3F, 3G, 3W, 5B, 5C, and 5D (Figure 1) were investigated as in vitro inhibitors of LdcCA, the β-CA cloned and characterised earlier by our groups29. As seen from Figure 1, they act as highly efficient LdcCA inhibitors in vitro, with inhibition constants ranging between 3.3 and 8.3 nM. All of them are very effective inhibitors of the protozoan enzyme, making structure-activity relationship difficult to delineate. However, as mentioned above, sulphonamides structurally related to the ones investigated here did not show in vivo anti-leishmanial effects29. This is why we formulated here sulphonamides 1–6 as NEs in clove oil (Figure 2).
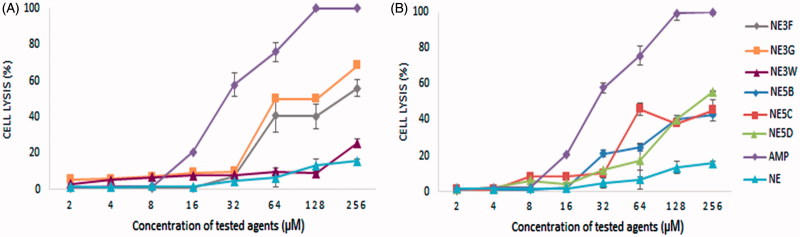
Figure 2.
Haemolysis caused by different agents: amphotericin B (AMP) and nanoemulsions containing inhibitors. (A) NE-3F, NE-3G, NE-3W, AMP, and NEs; B. NE-5B, NE-5C, NE-5D, AMP, and NEs. (B) Haemolysis was determined by an absorbance reading at 540 nm and compared to haemolysis achieved with 1% Triton X-100 (reference for 100% haemolysis). Values are expressed as mean ± standard deviation (n = 3).
Sulphonamides 3F, 3G, 3W, 5B, 5C, and 5D were easily dissolved in clove oil in the concentration of 5 mg/ml. The NEs were produced with 10% of oil phase. NEs were prepared also without the drug in order to evaluate the stability, droplet size and PDI. The NEs obtained were yellow and transparent suggesting that the system was homogeneous with small droplet size (Table 1). Phase separation and precipitation of the drug were not observed, and the NE was considered stable in the concentration of up to 500 µg/ml.
Table 1.
NEs size and polydispersity index.
| Formulation/drug | Drug (mg) | Oil clove (ml) | AP (ml) | Size (nm) | PDI | Stability |
|---|---|---|---|---|---|---|
| NE | – | 1 | 9 | 31.54 ± 0.413 | 0.105 ± 0.012 | Stable |
| NE-3F | 5 | 1 | 9 | 60.12 ± 2.36 | 0.274 ± 0.033 | Stable |
| NE-3G | 5 | 1 | 9 | 100.63 ± 2.05 | 0.262 ± 0.008 | Stable |
| NE-3W | 5 | 1 | 9 | 97.34 ± 2.82 | 0.264 ± 0.15 | Stable |
| NE-5B | 5 | 1 | 9 | 44.83 ± 0.753 | 0.123 ± 0.078 | Stable |
| NE-5C | 5 | 1 | 9 | 53.99 ± 1.12 | 0.233 ± 0.003 | Stable |
| NE-5D | 5 | 1 | 9 | 35.09 ± 0.575 | 0.165 ± 0.019 | Stable |
Drug concentration 500 µg/mL.
Mean ± SD of n = 3 determinations.
AP: aqueous phase containing detergent (Pluronic F127) and water.
NEs without the drug presented an average size of 31.54 nm. The NEs containing the drug presented average sizes between 35 and 100 nm, depending on the drug. The lowest average size was exhibited by NE-5D with a diameter of 35.09 nm. NE-3G and NE-3W exhibited the larger average size values with diameters of 100.63 and 97.34 nm, respectively. These NEs presented PDI below 0.3; indicating that the size distribution is homogeneous and monomodal37. Thus, we conclude that the inclusion method of the drugs in NEs was adequate, producing nanostructured samples with drops below 100 nm and size distribution homogeneous and monomodal.
Anti-Leishmania infantum/amazonensis activity in vivo
The effect of NEs containing the sulphonamides 3F, 3G, 3W, 5B, 5C, and 5D on L. amazonensis and L. infantum promastigotes viability was assessed in vitro. The obtained results are summarised in Tables 2 and 3. All NEs displayed in vitro antileishmanial activity with great variations in the IC50 values, which ranged from 3.47 to 51.7 µM for L. infantum. The IC50 values for L. amazonensis did not vary that much, ranging between 2.24 and 18.26 µM. The best IC50 against these parasites were presented by 5B-NE, followed by 3F-NE (IC50 3.90 µM) for L. amazonensis and NE-3G (IC50 10.72 µM) for L. infantum.
Table 2.
Viability assay of L. amazonensis promastigotes, cytotoxicity test using RAW 267.4 macrophage cells, and determining the selectivity index of nanoemulsions.
|
L. amazonensis |
|||||||
|---|---|---|---|---|---|---|---|
| Nanoemulsions | 3F | 3G | 3W | 5B | 5C | 5D | AMP6 |
| MIC1 | 128 | >128 | 128 | 32 | >28 | >28 | 4 |
| IC502 µM | 3.90 ± 1.96a | 12.01 ± 0.58b | 10.55 ± 4.51b | 2.24 ± 0.178c | 12.41 ± 0.45b | 18.26 ± 5.28d | 0.61 ± 0.01e |
| IC903 µM | 105.58 ± 30.63a | n.d. | 92.74 ± 38.23b | 22.46 ± 6.80c | n.d. | n.d. | 1.23 ± 0.23d |
| CC504 µM | 8.13 ± 1.1a | 6.77 ± 1.7b | 3.21 ± 0.55c | 6.51 ± 1.11b | 8.04 ± 1.33a | 6.75 ± 0.98b | 1.07 ± 1.49d |
| IS505 | 2.06 ± 0.17a | 0.48 ± 0.21c | 0.34 ± 0.12d | 2.12 ± 0.11a | 0.64 ± 0.22c | 0.37 ± 0.09d | 1.78 ± 0.01b |
The means compared by the Student–Newman–Keuls test.
n.d.: not determined.
1MIC: minimum inhibitory concentration.
2IC50: concentration which reduced 50% of the proliferation of L. amazonensis promastigote stage.
3IC90: concentration which reduced 90% of the proliferation of L. amazonensis promastigote stages.
4CC50: concentration in μg·ml−1 cytotoxic for 50% of RAW 264.7 cells.
5SI: selectivity index = CC50/IC50.
6AMP: amphotericin B.
a,b,c,d,eIn the lines: averages with equal letters do not differ statistically (p>.05).
Table 3.
Viability assay of L. infantum promastigotes, cytotoxicity test using RAW 267.4 macrophage cells, and determining the selectivity index of NEs.
|
L. infantum |
|||||||
|---|---|---|---|---|---|---|---|
| Nanoemulsions | 3F | 3G | 3W | 5B | 5C | 5D | AMP6 |
| MIC1 | >128 | >128 | >128 | 64 | >128 | >128 | 4 |
| IC502 µM | 12.00 ± 4.3a | 10.72 ± 2.68a | 12.46 ± 1.35a | 3.47 ± 0.35c | 14.58 ± 0.88a | 51.70 ± 5.18b | 0.67 ± 0.10d |
| IC903 µM | n.d. | n.d. | n.d. | 52.03 ± 8.01a | n.d. | n.d. | 1.01 ± 0.03b |
| CC504 µM | 8.13 ± 1.19a | 6.77 ± 1.07b | 3.21 ± 0.55c | 6.51 ± 1.11b | 8.04 ± 1.33a | 6.75 ± 0.98b | 1.07 ± 1.49d |
| IS505 | 0.66 ± 0.17a | 0.77 ± 0.21a | 0.34 ± 0.12a | 2.01 ± 0.11b | 0.87 ± 0.22a | 0.11 ± 0.09c | 1.59 ± 0.01d |
The means compared by the Student–Newman–Keuls test.
n.d.: not determined.
1MIC: minimum inhibitory concentration.
2IC50: concentration which reduced 50% of the proliferation of L. infantum promastigote stage.
3IC90: concentration which reduced 90% of the proliferation of L. infantum promastigote stages.
4CC50: Concentration in μg·ml−1 cytotoxic for 50% of RAW 264.7 cells.
5SI: selectivity index = CC50/IC50.
6AMP: Amphotericin B.
a,b,c,dIn the lines: averages with equal letters do not differ statistically (p>.05).
IC90 values are summarised in Tables 2 and 3. The inhibitors NE-3F, NE-3W, NE-5B showed IC90 of 105.58 ± 30.63, 92.74 ± 38.23, 22.46 ± 6.80 µM, respectively, for L. amazonensis, and the compound NE-5B induced an IC90 of 52.03 ± 8.01 µM for L. infantum. The inhibitors NE-3F, NE-3W, and NE-5B against L. amazonensis and NE-5B for L. infantum promastigotes were able to inhibit the growth of the parasites. After 48 h in culture medium, no cell growth of L. amazonensis was observed at the concentration of 128 µM of the following inhibitors 3F, 3W in NEs. NEs containing 5B at the concentrations 32 and 64 µM inhibited completely L. amazonensis and L. infantum, respectively. These values correspond the MIC for both Leishmanias (Tables 2 and 3).
It was not possible to determine the MIC and IC90 for promastigotes treated with NEs NE-3G, NE-5C, and NE-5D for L. amazonensis and NE-3F, NE-3G, NE-3W, NE-5C, and NE-5D for L. infantum. Probably the MIC and IC90 for these NEs are above 128 µM (first concentration studied).
On the other hand, the worst compound was NE-5D for L. amazonensis and L. infantum. Only the NE-5B showed IS50 above two for both parasites. Except for compounds NE-5B and NE-3F, all the others NEs containing inhibitors displayed more toxicity for macrophage cell than for parasites (IS50 < 1) (Table 4).
Table 4.
Haemolitic index of nanoemulsions 3F, 3G, 3W, 5B, 5C, and 5D.
| Haemolysis (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| µM | 3F | 3G | 3W | 5B | 5C | 5D | ANF | Nanoemulsion without drug |
| HC50 | 168.28 ± 14.15a | 138.96 ± 32.97a | >256 | >256 | 168.62 ± 18.45a | >256 | 27.93 ± 1.71b | >256 |
| 256 | 55.81 ± 14.50 | 68.51 ± 1.3 | 25.79 ± 2.0 | 43.04 ± 1.1 | 45.32 ± 16.13 | 55.7 ± 10.4 | 100.0 ± 0 | 15.75 ± 1.24 |
| 128 | 40.43 ± 16.73 | 49.9 ± 10.2 | 9.03 ± 0.06 | 40.0 ± 0.93 | 38.01 ± 1.48 | 40.22 ± 2.1 | 100.0 ± 0 | 13.37 ± 3.49 |
| 64 | 40.87 ± 9.36 | 49.86 ± 0.8 | 10.0 ± 0.01 | 24.37 ± 2.9 | 45.89 ± 9.25 | 17.35 ± 9.5 | 75.7 ± 5.24 | 6.71 ± 5.33 |
| 32 | 7.17 ± 0.30 | 10.0 ± 0.04 | 8.06 ± 0.04 | 21.17 ± 2.1 | 10.11 ± 0.18 | 12.15 ± 0.3 | 57.81 ± 6.7 | 4.75 ± 2.66 |
| 16 | 1.10 ± 0.17 | 9.25 ± 0.43 | 7.74 ± 0.43 | 2.11 ± 0.20 | 8.24 ± 0.42 | 4.29 ± 0.50 | 20.81 ± 0.5 | 1.68 ± 0.51 |
| 8 | 1.11 ± 0.24 | 7.32 ± 0.56 | 6.96 ± 0.56 | 1.22 ± 0.38 | 8.21 ± 0.36 | 6.27 ± 0.46 | 2.09 ± 0.15 | 1.81 ± 0.22 |
| 4 | 1.20 ± 0.34 | 6.04 ± 0.07 | 5.13 ± 0.20 | 1.04 ± 0.06 | 1.16 ± 0.28 | 2.02 ± 0.04 | 2.01 ± 0.02 | 1.35 ± 0.37 |
| 2 | 1.00 ± 0.04 | 5.72 ± 0.49 | 3.13 ± 0.07 | 0.95 ± 0.09 | 1.02 ± 0.04 | 1.12 ± 0.20 | 1.18 ± 0.31 | 1.74 ± 0.76 |
The means compared by the Student–Newman–Keuls test.
a,bIn the lines: averages with equal letters do not differ statistically (p > .05).
In parallel, NE cytotoxicity assays were performed on 267.4 macrophage cells. At the concentrations tested, NEs were less cytotoxic than Amphotericin B which is a potent leishmanicide drug with anti-Leishmania effect demonstrated in vitro and in vivo studies against the promastigote and amastigote forms of the Leishmania parasite43. Typically, assays in promastigote forms of the parasite are always present in the initial screening of candidate compounds for leishmanicidal drugs, since they are simple and inexpensive tests44.
Previous work from our group showed that thiols were more effective than sulphonamides in the inhibition of LdcCA from L. donovani chagasi, another Leishmania associated with the visceral form of the disease29.
Several studies have been demonstrated that nano-emulsified carrier systems have potential to solve problems with poor water solubility and poor membrane permeability of some drugs45. Moreover, the toxicity of some drugs can be reduced when they are nano-emulsified with an appropriate carrier46–48. Gupta et al. formulated a nano-emulsified carrier system with copaiba oil to improve the antileishmanial activity and oral bioavailability of amphotericin B46. They produced their NE by mixing a surfactant (TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate), a co-surfactant (phosphatidyl choline (PC)), the oil and the drug, to form an O/W emulsion with particle size of 127 ± 21 nm and PDI of 0.11 ± 0.02. The IC50 value of amphotericin nanoemulsified with copaiba oil and plain amphotericin B against L. donovani was 0.018 ± 0.004 and 0.214 ± 0.06 µg/ml, respectively, demonstrating a nearly 12-fold reduction in the IC50 value. They also reported the haemotoxicity reduction of amphotericin B from 69.8 to 18.2% when formulated in the NE. These results are corroborated by Santos et al. who also observed increase in vivo anti-leishmanial activity and reduction of in vitro haemotoxicity of amphotericin B47. However, they did not use natural oil in NE composition, as their NE was prepared with medium chain triglycerides (MCT), Tween 80, and cholesterol in the oily phase and glycerol and amphotericin B in the aqueous phase, forming particles with 134.8 nm of mean size47. In our study, the compounds that did not inhibit significantly Leishmania spp. in plain solution but started to inhibit the tested parasites with IC50 values ranging from 2.24 to 18.26 µM and from 3.47 to 51.7 µM for L. amazonensis and L. infantum, respectively, when formulated as NEs. The improvement of the anti-leishmanial activity through the production of simple NEs of these sulphonamide CAIs is thus remarkable, whereas its preparation and in the number of components required to formulate the NE are also simplified compared to previously reported procedures46,47.
Haemolytic assay
Human red blood cells provide a handy tool for toxicity studies of the compounds, because they are readily available, their membrane properties are well known, and their lysis is easily monitored by measuring the release of haemoglobin. At the concentration of 256 and 128 µM, amphotericin B (conventional anti-leishmanial therapeutic agent) exerted 100% haemolysis. In comparison, the NE without drug caused only 15.75 and 13.37% haemolysis. Taken together, our results indicate that oil glove present in the NEs have significantly less cytotoxic effects than amphotericin B. In all sulphonamides NEs, at concentrations below 16 µM, a haemolysis of less than 10% was found. These results showed that NEs containing the CAIs are promising for therapeutic drug trials.
The incorporation of sulphonamides in clove oil NE allowed the drugs to meet their intracellular targets, and to perform their anti-leishmanial activity. These inhibitors do not penetrate into the parasite without the NEs (data not shown). These results suggest that 5B-NE present potential activity against amastigotes, since the NE penetrated into macrophages. Other interesting point that could be studied is the synergistic action between clove oil and 5B on the anti-leishmanial activity. Oil clove (Syzygium aromaticum) has been reported to have anti-leishmanial activity due to high eugenol concentration48. The values of IC50 described by Islamuddin et al. against promastigotes and intracellular amastigotes of L. donovani were 21 and 15.24 mg/ml, respectively48. The clove oil caused apoptosis in the L. donovani cell parasite. Although promoting the parasite growth inhibition, the NEs investigated here also reduced the cell viability of macrophages. The macrophages cytotoxicity might be associated principally to the action of sulphonamides, although many such drugs are used for decades without any evidence of such effects7. The oil clove cytotoxicity reported by Islamuddin et al. revealed that there was no toxicity of eugenol-rich oil of S. aromaticum on RAW264.7 cells, even at 200 mg/ml48. Many substances with potential therapeutic applications have been discarded in the past because they were not administrable in a bioavailable form48. This study opens the possibility to formulated NEs containing the CAIs in order to increase the bioavailability of hydrophilic drugs, such as the sulphonamides.
Conclusions
The best IC50 against both parasites were obtained by 5B-NE, followed by 3F-NE (IC50 3.90 µM) for L. amazonensis and 3G-NE (IC50 10.72 µM) for L. infantum NEs have demonstrated potential as a novel vehicle for delivery hydrophophilic drugs such as sulphonamide CAIs improving the bioavailability of the drug and demonstrating a potential use in the treatment of leishmaniasis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Alterio V, Di Fiore A, D’Ambrosio K, et al. . Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68. [DOI] [PubMed] [Google Scholar]
- 2.Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO(2) capture. J Enzyme Inhib Med Chem 2013;28:229–30. [DOI] [PubMed] [Google Scholar]
- 3.Supuran CT, Capasso C. Protozoan carbonic anhydrases In: Supuran CT, Capasso C, eds. Zinc enzyme inhibitors – Volume 1: enzymes from microorganisms. Berlin, Germany: Springer;2017:114–134. [Google Scholar]
- 4.Supuran CT.Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. [DOI] [PubMed] [Google Scholar]
- 5.Lomelino CL, Supuran CT, McKenna R.. Non-classical inhibition of carbonic anhydrase. Int J Mol Sci 2016;17:E1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nocentini A, Vullo D, Del Prete S, et al. . Inhibition of the β-carbonic anhydrase from the dandruff-producing fungus Malassezia globosa with monothiocarbamates. J Enzyme Inhib Med Chem 2017;32:1064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Supuran CT.Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. (b) Supuran CT.carbonic anhydrase activators. Future Med Chem 2018;10: 561,–73. (c) Akocak S, Lolak N, Vullo D, et al. . Synthesis and biological evaluation of histamine Schiff bases as carbonic anhydrase I, II, IV, VII, and IX activators. J Enzyme Inhib Med Chem 2017;32: 1305,–12. (d) Angeli A, Vaiano F, Mari F, et al. . Psychoactive substances belonging to the amphetamine class potently activate brain carbonic anhydrase isoforms VA, VB, VII, and XII. J Enzyme Inhib Med Chem 2017;32: 1253,–9. (e) Licsandru E, Tanc M, Kocsis I, et al. . A class of carbonic anhydrase I - selective activators. J Enzyme Inhib Med Chem 2017;32: 37–46. [Google Scholar]
- 8.(a) Supuran CT.How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016; 31:345–60. (b) Casey JR, Morgan PE, Vullo D, et al. . Carbonic anhydrase inhibitors. Design of selective, membrane-impermeant inhibitors targeting the human tumor-associated isozyme IX. J Med Chem 2004;47: 2337–47. [DOI] [PubMed] [Google Scholar]
- 9.(a) Supuran CT.Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012; 27:759–72. (b) Supuran CT.Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7: E48. [DOI] [PubMed] [Google Scholar]
- 10.Scozzafava A, Supuran CT, Carta F.. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Patents 2013;23:725–35. [DOI] [PubMed] [Google Scholar]
- 11.Nocentini A, Carta F, Ceruso M, et al. . Click-tailed coumarins with potent and selective inhibitory action against the tumor-associated carbonic anhydrases IX and XII. Bioorg Med Chem 2015;23:6955–66. [DOI] [PubMed] [Google Scholar]
- 12.Capasso C, Supuran CT.. Sulfa and trimethoprim-like drugs–antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem 2014;29:379–87. [DOI] [PubMed] [Google Scholar]
- 13.Supuran CT, Capasso C.. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. J Enzyme Inhib Med Chem 2016;31:1254–60. [DOI] [PubMed] [Google Scholar]
- 14.Capasso C, Supuran CT.. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32. [DOI] [PubMed] [Google Scholar]
- 15.Capasso C, Supuran CT.. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin Ther Targets 2015;19:1689–704. [DOI] [PubMed] [Google Scholar]
- 16.(a) Diaz JR, Fernández Baldo M, Echeverría G, et al. . A substituted sulfonamide and its Co (II), Cu (II), and Zn (II) complexes as potential antifungal agents. J Enzyme Inhib Med Chem 2016;31:51–62. (b) Kusuzaki K, Matsubara T, Murata H, et al. . Natural extracellular nanovesicles and photodynamic molecules: is there a future for drug delivery? J Enzyme Inhib Med Chem 2017;32: 908,–16. (c) Ward C, Langdon SP, Mullen P, et al. . New strategies for targeting the hypoxic tumour microenvironment in breast cancer. Cancer Treat Rev 2013;39: 171–9. [Google Scholar]
- 17.Vermelho AB, Capaci GR, Rodrigues IA, et al. . Carbonic anhydrases from Trypanosoma and Leishmania as anti-protozoan drug targets. Bioorg Med Chem 2017;25:1543–55. [DOI] [PubMed] [Google Scholar]
- 18.Prete SD, Vullo D, Fisher GM, et al. . Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum—The η-carbonic anhydrases. Bioorg Med Chem Lett 2014;24:4389–96. [DOI] [PubMed] [Google Scholar]
- 19.(a) Supuran CT.Inhibition of carbonic anhydrase from Trypanosoma cruzi for the management of Chagas disease: an underexplored therapeutic opportunity. Future Med Chem 2016;8:311–24. (b) Fabrizi F, Mincione F, Somma T, et al. . A new approach to antiglaucoma drugs: carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J Enzyme Inhib Med Chem 2012;27: 138,–47. (c) Şentürk M, Gülçin İ, Beydemir Ş, et al. . In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77: 494,–9. (d) Supuran CT, Mincione F, Scozzafava A, et al. . Carbonic anhydrase inhibitors—part 52. Metal complexes of heterocyclic sulfonamides: a new class of strong topical intraocular pressure-lowering agents in rabbits. Eur J Med Chem 1998;33: 247–54. [Google Scholar]
- 20.Capasso C, Supuran CT.. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr Med Chem 2015;22:2130–9. [DOI] [PubMed] [Google Scholar]
- 21.Moraes ARDP, Tavares GD, Rocha FJS, et al. . Effects of nanoemulsions prepared with essential oils of copaiba- and andiroba against Leishmania infantum and Leishmania amazonensis infections. Exp Parasitol 2018;187:12–21. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes Rodrigues JC, Concepcion JL, Rodrigues C, et al. . In vitro activities of ER-119884 and E5700, two potent squalene synthase inhibitors, against Leishmania amazonensis: antiproliferative, biochemical, and ultrastructural effects. Antimicrob Agents Chemother 2008;52:4098–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dagnino APA, Mesquita CS, Dorneles GP, et al. . Phloroglucinol derivatives from Hypericum species trigger mitochondrial dysfunction in Leishmania amazonensis. Parasitology 2018;27:1–11. [DOI] [PubMed] [Google Scholar]
- 24.Pan P, Vermelho AB, Capaci Rodrigues G, et al. . Cloning, characterization, and sulfonamide and thiol inhibition studies of an α-carbonic anhydrase from Trypanosoma cruzi, the causative agent of Chagas disease. J Med Chem 2013;56:1761–71. [DOI] [PubMed] [Google Scholar]
- 25.Güzel-Akdemir Ö, Akdemir A, Pan P, et al. . A class of sulfonamides with strong inhibitory action against the α-carbonic anhydrase from Trypanosoma cruzi. J Med Chem 2013;56:5773–81. [DOI] [PubMed] [Google Scholar]
- 26.Menezes Dda R, Calvet CM, Rodrigues GC, et al. . Hydroxamic acid derivatives: a promising scaffold for rational compound optimization in Chagas disease. J Enzyme Inhib Med Chem 2016;31:964–73. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues GC, Feijó DF, Bozza MT, et al. . Design, synthesis, and evaluation of hydroxamic acid derivatives as promising agents for the management of Chagas disease. J Med Chem 2014;57:298–308. [DOI] [PubMed] [Google Scholar]
- 28.Pan P, Vermelho AB, Scozzafava A, et al. . Anion inhibition studies of the α-carbonic anhydrase from the protozoan pathogen Trypanosoma cruzi, the causative agent of Chagas disease. Bioorg Med Chem 2013;21:4472–6. [DOI] [PubMed] [Google Scholar]
- 29.Syrjänen L, Vermelho AB, Rodrigues Ide A, et al. . Cloning, characterization, and inhibition studies of a β-carbonic anhydrase from Leishmania donovani chagasi, the protozoan parasite responsible for leishmaniasis. J Med Chem 2013;56:7372–81. [DOI] [PubMed] [Google Scholar]
- 30.de Campos VE, Ricci-Júnior E, Mansur CR.. Nanoemulsions as delivery systems for lipophilic drugs. J Nanosci Nanotechnol 2012;12:2881–90. [DOI] [PubMed] [Google Scholar]
- 31.Senna JP, Ricci-Júnior E, Mansur CRE.. Development and evaluation of nanoemulsions containing phthalocyanines for use in photodynamic cancer therapy. J Nanosci Nanotechno 2015;15:4205–14. [DOI] [PubMed] [Google Scholar]
- 32.Vermelho AB, Cardoso VS, Ricci Junior E, et al. . Nanoemulsions of sulfonamide carbonic anhydrase inhibitors strongly inhibit the growth of Trypanosoma cruzi. J Enzyme Inhib Med Chem 2018;33:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalifah RG.The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73. [PubMed] [Google Scholar]
- 34.(a) Briganti F, Pierattelli R, Scozzafava A, Supuran CT, Carbonic anhydrase inhibitors. Part 37. Novel classes of carbonic anhydrase inhibitors and their interaction with the native and cobalt-substituted enzyme: kinetic and spectroscopic investigations. Eur J Med Chem 1996;3:1001–10. (b) Supuran CT, Nicolae A, Popescu A.. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;31: 431,–8. (c) Pacchiano F, Aggarwal M, Avvaru BS, et al. . Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb) 2010;46: 8371,–3. (d) Tars K, Vullo D, Kazaks K, et al. . Sulfocoumarins (1,2-benzoxathiine-2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J Med Chem 2013;56: 293–300. [Google Scholar]
- 35.(a) Supuran CT, Clare BW.. Carbonic anhydrase inhibitors. Part 57. Quantum chemical QSAR of a group of 1,3,4-thiadiazole and 1,3,4-thiadiazoline disulfonamides with carbonic anhydrase inhibitory properties. Eur J Med Chem 1999;34:41–50. (b) Mastrolorenzo A, Rusconi S, Scozzafava A, et al. . Inhibitors of HIV-1 protease: current state of the art 10 years after their introduction. From antiretroviral drugs to antifungal, antibacterial and antitumor agents based on aspartic protease inhibitors. Curr Med Chem 2007;14: 2734–48. [DOI] [PubMed] [Google Scholar]
- 36.(a) Puccetti L, Fasolis G, Vullo D, et al. . Carbonic anhydrase inhibitors. Inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with Schiff’s bases incorporating chromone and aromatic sulfonamide moieties, and their zinc complexes. Bioorg Med Chem Lett 2005;15:3096–101. (b) Garaj V, Puccetti L, Fasolis G, et al. . Carbonic anhydrase inhibitors: synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II and IX with sulfonamides incorporating 1,2,4-triazine moieties. Bioorg Med Chem Lett 2004;14: 5427–33. [DOI] [PubMed] [Google Scholar]
- 37.Zetasizer Nano. User manual. Malvern: Malvern Instruments. Man0485, n. 1.1, 2004. Available from: http://www.malvern.com/en/support/resource-center/usermanuals/MAN0485EN.aspx. [last access 15 Jan 2018]. [Google Scholar]
- 38.Rodrigues IA, Silva BA, Santos ALS, et al. . A new experimental culture medium for cultivation of Leishmania amazonensis: its efficacy for the continuous in vitro growth and differentiation of infective promastigote forms. Parasitol Res 2010;106:1249–52. [DOI] [PubMed] [Google Scholar]
- 39.Rolon M, Vega C, Escario JA, Gómez-Barrio A.. Development of resazurin microtiter assay for drug sensibility testing of Trypanosoma cruzi epimastigotes. Parasitol Res 2006;99:103–7. [DOI] [PubMed] [Google Scholar]
- 40.Mosmann T.Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;983:55–63. [DOI] [PubMed] [Google Scholar]
- 41.Katsuno K, Burrows JN, Duncan K, et al. . Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat Rev Drug Discov 2015;14:751–8. [DOI] [PubMed] [Google Scholar]
- 42.Ishnava KB, Shah PP.. Anticariogenic and hemolytic activity of selected seed protein extracts in vitro conditions. J Dent 2014;11:576. [PMC free article] [PubMed] [Google Scholar]
- 43.Pham TT, Loiseau PM, Barratt G.. Strategies for the design of orally bioavailable antileishmanial treatments. Int J Pharm 2013;454:539–52. [DOI] [PubMed] [Google Scholar]
- 44.Chattopadhyay A, Jafurulla M.. A novel mechanism for an old drug: amphotericin B in the treatment of visceral leishmaniasis. Biochem Biophys Res Commun 2011;416:7–12. [DOI] [PubMed] [Google Scholar]
- 45.Lo´pez-Arencibia A, Garci´a VD, Marti´n-Navarro CM, et al. . In vitro activities of hexaazatrinaphthylenes against Leishmania spp. Antimicrob Agents Chemother 2015;59:2867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta PK, Jaiswal AK, Asthana S, et al. . Synergistic enhancement of parasiticidal activity of amphotericin B using copaiba oil in nanoemulsified carrier for oral delivery: an approach for non-toxic chemotherapy. Br J Pharmacol 2015;172:3596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos DCMD, de Souza MLS, Teixeira EM, et al. . A new nanoemulsion formulation improves antileishmanial activity and reduces toxicity of amphotericin B. J Drug Target 2018;26:357–64. [DOI] [PubMed] [Google Scholar]
- 48.Islamuddin M, Sahal D, Afrin F.. Apoptosis-like death in Leishmania donovani promastigotes induced by eugenol-rich oil of Syzygium aromaticum. J Med Microbiol 2014;63:74–85. [DOI] [PubMed] [Google Scholar]