Abstract
The current study was carried out to evaluate multicomponent pattern, biological and enzymatic activities of seven Asphodeline taxa root extracts as useful ingredients, due to the fact that these plants are commonly used as traditional food supplements in Turkish regions. The extracts were characterized for free anthraquinones and phenolics to obtain a specific chemical fingerprint useful for quality control. These analyzes were coupled to biological and enzymatic activities in order to obtain comprehensive information of the natural product. Free anthraquinones and phenolics were determined using validated HPLC-PDA methods. Antioxidant properties were determined by different procedures including free radical scavenging, reducing power, phosphomolybdenum and metal chelating assays. Ames assay was performed to evaluate mutagenic/antimutagenic properties. Enzyme inhibitory activities were tested against cholinesterase, tyrosinase, α-amylase and α-glucosidase. From the herein reported results, Asphodeline could be valuable for the production of bioactive products or food supplements for cosmetic and pharmaceutical industries.
Keywords: Asphodeline, anthraquinones, biological activity and bioactive compounds, multicomponent pattern, phenolics
Introduction
Recently, in many industrial fields the easy availability of natural derivatives can be very helpful in order to produce food supplements and/or natural products (also in combination) that can be used to obtain beneficial effects on human health. Many plants, across history and cultures, have been used for medicinal purposes as alternative therapies based on plants in order to avoid drug adverse effects, and over the past years, many articles were reported. To avoid this last drawback and, particularly, to obtain alternative biologically active products, an increased interest in natural products (or natural-like products) was observed1,2. When natural products were used, particular attention must be also paid to standardization process. The methods used in plant material extraction can influence the chemical composition of the resulting extracts and potentially the biological activity3,4. For these reasons, the actual challenge is to fully understand and characterize botanical preparations as “multicomponent pattern” in the context of modern food and health system in which consumers are often uneducated about the use and effective applications of these supplements5. Additionally, the Food and Drug Administration (FDA) specifies certain labeling requirements for foods, supplements, and drugs, and the European Union requires that standardized herbal substances are reported as content of constituents with known therapeutic activity.
For these reasons it is necessary a multidisciplinary approach in order to obtain chemical profiles and biological activities and, particularly, to evaluate possible correlations between these two aspects6–13. As biologically active compounds, generally were considered phenolics, flavonoids, anthraquinones and other secondary plant metabolites (Figure 1) that had show interesting biological activities as potential “markers” to characterize the extract.
Figure 1.
Biologically active compounds considered in this work.
The genus Asphodeline is conventionally used both as medicinal plants in Turkey folk medicine and as vegetables for human nutrition. A. damascena subsp. damascena and A. tenuior subsp. tenuiflora var. tenuiflora are often used to alleviate verrucae and heal lesions. Additionally, A. damascena is also used as salad vegetables. Modern studies show that Asphodeline could provide alimentary value due to the presence of anthraquinones, essential amino acids, and polyphenols14.
Emodine, physcione, rhein and chrisophanol were used as colorants in food, drugs, and cosmetics. Additionally, they attracted attention especially for their anti-microbial, anti-cancer, anti-oxidant, and anti-inflammatory activities and the intake of anthraquinones-rich plants7,8 could be a valid preventive strategy in order to obtain health benefits.
In this paper, and in continuation to our studies on natural products analyzes15–24 and instrument configurations25–28, we report for the first time the free anthraquinones and phenolics pattern (via validated HPLC-PDA procedure), biological activities (ABTS, DPPH, CUPRAC, FRAP, phosphomolibdenum, metal chelating, and Ames assays), enzyme inhibitory activities (against cholinesterase, tyrosinase, α-amylase, and α-glucosidase). Particularly, for the first time was reported the mutagenic/anti-mutagenic evaluation of seven Asphodeline taxa, in particular with the aim to evaluate these extracts as valuable and safe sources for food supplements and/or for bioactive formulations.
Materials and methods
Plant material and methanol extracts
Asphodeline species were collected at flowering stage (May–July) in Turkey regions, and their information and localities are explained below. Voucher specimens were deposited in KONYA Herbarium (Department of Biology, Selcuk University, Konya, Turkey). The roots, air-dried at 45 °C (± 1 °C) for 48 h in the dark to obtain higher phenolics preservation29,30, were finely triturated (5–10 g) and macerated overnight with 250 mL of methanol at room temperature (25 °C ± 1 °C). The concentrated extracts (under vacuum; 40 °C ± 1 °C) were stored at +4 °C (± 1 °C) in the dark until analyzes (extraction yields are reported in Table 1) (en: Endemic).
Table 1.
Yields (%) and total phenolics content, total flavonoids content and radical (DPPH and ABTS) scavenging activities of seven Asphodeline extracts*.
| Asphodeline species (abbreviation) | Yield (%) | Total phenolics content (mg GAEs/g extract)a | Total flavonoids content (mg REs/g extract)b | DPPH scavenging (mg TEs/g extract)c | ABTS scavenging (mg TEs/g extract)c |
|---|---|---|---|---|---|
| A. damascena subsp. damascena (ADD) | 14.84 | 22.54 ± 0.80 | 11.67 ± 0.39 | 24.14 ± 1.37 | 72.01 ± 0.76 |
| A. damascena subsp. gigantea (ADG) | 7.57 | 34.03 ± 0.97 | 23.88 ± 0.39 | 32.52 ± 1.03 | 117.37 ± 1.16 |
| A. damascena subsp. ovoidea (ADO) | 7.57 | 31.34 ± 0.70 | 23.92 ± 0.60 | 32.01 ± 1.16 | 101.70 ± 0.29 |
| A. prismatocarpa (APR) | 10.24 | 27.12 ± 0.45 | 24.21 ± 0.51 | 29.86 ± 1.30 | 89.89 ± 0.72 |
| A. damascena subsp. rugosa (ADR) | 12.93 | 18.61 ± 0.31 | 11.87 ± 0.18 | 23.14 ± 0.98 | 63.41 ± 0.22 |
| A. tenuior subsp. tenuiflora var. tenuiflora (ATT) | 3.98 | 27.57 ± 0.91 | 27.69 ± 1.36 | 35.87 ± 0.93 | 103.11 ± 2.97 |
| A. turcica (ATU) | 12.06 | 26.39 ± 0.24 | 10.33 ± 0.26 | 26.66 ± 1.00 | 78.28 ± 1.50 |
Values expressed are means ± SD of three parallel measurements. aGAEs: gallic acid equivalents; bREs: rutin equivalents; cTEs: trolox equivalents.
A. damascena (Boiss.) Baker subsp. damascena (Boiss.) Baker: Konya, Selcuk University, Alâeddin Keykubat Campus, Bağderesi location, 1170 m, 38° 02’ 51.6” N, 32° 26’ 40” E.
A. damascena (Boiss.) Baker subsp. gigantea E. Tuzlaci: Gaziantep, between Gaziantep and Narli road, Incesu village, 868 m, 37° 13’ 58” N, 37° 18’ 06” E. (en)
A. damascena (Boiss.) Baker subsp. ovoidea E. Tuzlaci: Kahramanmaras, between Kahramanmaras and Goksun road, 650 m, 37° 45’ 50” N, 36° 43’ 52” E. (en)
A. damascena (Boiss.) Baker subsp. rugosa E. Tuzlaci: Kayseri, between Yahyali and Sazak road, 1212 m, 38° 05’ 18”N, 35° 21’ 38” E. (en)
A. prismatocarpa J. Gay ex Baker: Niğde, Çamardi-Mazmili Mountain, Dagdibi village, 1974 m, 37° 39’ 55” N, 35°04’ 45” E. (en)
A. tenuior (Fischer) Ledeb. subsp. tenuiflora (C. Koch) E. Tuzlaci var. tenuiflora (Fischer) Ledeb.: Malatya: between Malatya and Darende road, 1003 m, 38° 30’ 40’’ N, 37° 31’ 19’’ E.
A. turcica Tuzlaci: Antalya, Gebiz, Sanli Beli location, 1299 m 37° 20’ 01” N, 31° 01’ 45” E (en)
HPLC chemicals and reagents
Anthraquinones chemical standards (emodine, rhein, chrisophanol, aloe-emodine, and physcione; all purity ≥99%) were purchased from Extrasynthese (Genay, France). Phenolics as chemical standards (gallic acid, catechin, chlorogenic acid, p-hydroxy-benzoic acid, vanillic acid, epicatechin, syringic acid, 3-hydroxy-benzoic acid, 3-hydroxy-4-methoxybenzaldehyde, p-coumaric acid, rutin, sinapinic acid, t-ferulic acid, naringin, 2,3-dimethoxy benzoic acid, benzoic acid, o-coumaric acid, quercetin dihydrate, t-cinnamic acid, naringenin (all purity >98%)) were purchased from Sigma Aldrich (Milan, Italy). Methanol (HPLC-grade) and formic acid (99%) were obtained from Carlo Erba Reagenti (Milan, Italy). Double-distilled water was obtained using a Millipore Milli-Q Plus water treatment system (Millipore Bedford Corp., Bedford, MA).
Determination of total bioactive components
Total phenolics and flavonoids
The total phenolics content was determined by a reported method31 with slight modification and expressed as gallic acid equivalents (GAEs/g extract), while total flavonoids content was determined by a reported method32 with slight modification and expressed as rutin equivalents (REs/g extract).
Free anthraquinones and phenolics HPLC-PDA pattern
HPLC-PDA free anthraquinones and phenolics pattern was evaluated by validated methods reported in literature8,26,33.
Biological activities evaluation
The activities was evaluated by phosphomolybdenum method34 and expressed as trolox equivalents (TEs/g extract). The reducing power measured using cupric ion reducing (CUPRAC) and ferric ion reducing antioxidant power (FRAP). Metal chelating activity on ferrous ions, determined by the method described by Zengin et al.31, was expressed as EDTA equivalents (EDTAEs/g extract). Acetylcholinesterase (AChE) or butyrylcholinesterase (BChE), α-amylase, α-glucosidase and tyrosinase inhibitory activities were carried out by the method described by Zengin et al.31. Mutagenic and antimutagenic properties were determined by Ames assay35.
Results and discussion
Total bioactive compounds
The total phenolic contents of the Asphodeline extracts were detected by Folin-Ciocalteu assay. Sample solution (0.25 mL) was mixed with diluted Folin-Ciocalteu reagent (1 mL, 1:9, v:v) and shaken. After 3 minutes, Na2CO3 solution (0.75 mL, 1%) was added, and the sample absorbance was read at 760 nm after 2 h of incubation at room temperature (25 °C ± 1 °C). The results are presented in Table 1. The highest phenolic content was found in ADG (34.03 mg GAEs/g extract), followed by ADO (31.34 mg GAEs/g extract) and ATT (27.57 mg GAEs/g extract). The lowest content detected in the ADR (18.61 mg GAEs/g extract).
Flavonoid contents were spectrophotometrically determined. The sample solution (1 mL) was mixed with aluminum trichloride (2%) in methanol. Similarly, blank sample was prepared by adding sample solution (1 mL) to methanol (1 mL) without AlCl3. The sample and blank absorbances were read at 415 nm after 10 minutes of incubation at room temperature (25 °C ± 1 °C). The blank sample absorbance was subtracted from the sample, and the total flavonoids content was expressed as equivalents of rutin (REs). Flavonoid contents of the extracts ranged from 10.33 mg REs/g extracts for ATU to 27.69 mg REs/g extract for ATT (Table 1). From these results, flavonoids constitute a major part of phenolics in Asphodeline extracts tested. The total phenolics and flavonoids content were comparable to our previous report for eight Asphodeline root extracts and the content were found to be 13.5–49.2 mg GAEs/g extract and 10.6–30.9 mg REs/g extract, respectively7. Similar contents were reported for A. lutea14 and A. anatolica34.
Free anthraquinones and phenolics HPLC pattern
Using validated HPLC-PDA assay26 the anthraquinones pattern was obtained for each Asphodeline sample considered in this study. The obtained results (Table 2) show a possible correlation between biological activity and chemical profile. Particularly, we evidenced that aloe-emodine, rheine and chrisophanol abundance was inversely proportional to α-amylase and α-glucosidase inhibitory activities. A higher chrisophanol content brought to an increased chelating power, and with minor exception, also with a higher tyrosinase inhibitory activity. Our obtained results also reveal that ATT extract shows lower phosphomolybdenum, chelating power, AChE, and BChE inhibitory activity, and simultaneously shows lower aloe-emodine, rheine and chrisophanol abundance. In the same way, ATT shows higher emodine and physcione abundances that reveals, coupled to higher total flavonoids, a better and increased DPPH, CUPRAC, α-amylase and α-glucosidase inhibitory activities. These findings were in accordance with just reported papers on other Asphodeline extracts9.
Table 2.
Free anthraquinones and phenolics chemical fingerprint of the seven Asphodeline taxa expressed as total amount (μg/mg extract)*.
| ADD | ADG | ADO | APR | ADR | ATT | ATU | ||
|---|---|---|---|---|---|---|---|---|
| Free Anthraquinones | Aloe-emodine | 24.5 ± 2.0 | 14.6 ± 1.2 | 4.3 ± 0.3 | 24.3 ± 1.9 | 9.4 ± 0.8 | 5.0 ± 0.4 | 15.3 ± 1.2 |
| Rhein | 43.3 ± 3.5 | 71.4 ± 5.7 | 19.4 ± 1.6 | 55.9 ± 4.5 | 26.3 ± 2.1 | 25.5 ± 2.0 | 24.8 ± 2.0 | |
| Emodine | 3.9 ± 0.3 | 1.7 ± 0.1 | 5.5 ± 0.4 | – | 2.3 ± 0.2 | 25.6 ± 2.0 | 0.7 ± 0.1 | |
| Chrisophanol | 470.1 ± 37.6 | 196.1 ± 15.7 | 182.5 ± 14.6 | 794.8 ± 63.6 | 171.7 ± 13.7 | 249.1 ± 19.9 | 129.5 ± 10.4 | |
| Physcione | 47.3 ± 3.8 | 29.7 ± 2.4 | 82.5 ± 6.6 | 46.8 ± 3.7 | 100.7 ± 8.1 | 258.3 ± 20.7 | 15.0 ± 1.2 | |
| Phenolics | Gallic acid | 3.03 ± 0.08 | 11.50 ± 1.57 | 6.77 ± 0.01 | 3.38 ± 1.02 | 9.34 ± 1.01 | 1.08 ± 0.34 | 1.26 ± 0.54 |
| Catechin | BLD | – | – | – | – | 0.10 ± 0.01 | – | |
| Chlorogenic acid | 0.09 ± 0.01 | 0.08 ± 0.03 | – | 0.67 ± 0.14 | 0.26 ± 0.03 | – | – | |
| p-OH-benzoic acid | BLD | 0.08 ± 0.02 | BLD | BLQ | 0.10 ± 0.15 | 0.24 ± 0.10 | – | |
| Vanillic acid | 0.95 ± 0.16 | 1.05 ± 0.32 | 2.04 ± 0.98 | 1.15 ± 0.24 | 3.73 ± 0.84 | 0.66 ± 0.16 | 0.93 ± 0.21 | |
| Epicatechin | BLD | – | BLD | BLQ | BLQ | – | 0.30 ± 0.13 | |
| Syringic acid | 0.08 ± 0.01 | 0.12 ± 0.03 | 0.20 ± 0.04 | 0.82 ± 0.12 | – | – | – | |
| 3-OH benzoic acid | 0.09 ± 0.02 | 0.18 ± 0.02 | 0.27 ± 0.09 | 0.37 ± 0.08 | 0.20 ± 0.03 | – | 0.36 ± 0.11 | |
| 3-OH-4-MeO-benzaldehyde | – | – | – | – | 0.24 ± 0.04 | 0.06 ± 0.01 | 0.08 ± 0.01 | |
| p-coumaric acid | – | – | – | BLQ | – | – | – | |
| Rutin | BLD | – | 0.22 ± 0.10 | BLQ | – | BLD | BLQ | |
| Sinapinic acid | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.12 ± 0.02 | 0.09 ± 0.01 | – | 0.08 ± 0.01 | 0.10 ± 0.02 | |
| t-ferulic acid | – | – | – | – | BLD | – | – | |
| Naringin | 0.15 ± 0.06 | 0.44 ± 0.12 | 0.18 ± 0.01 | – | – | 0.11 ± 0.05 | 0.48 ± 0.11 | |
| 2,3-diMeO-benzoic acid | – | – | BLD | – | 1.01 ± 0.89 | – | – | |
| Benzoic acid | 0.52 ± 0.19 | 1.84 ± 0.98 | 1.44 ± 0.55 | 0.88 ± 0.31 | BLD | – | 0.89 ± 0.31 | |
| o-coumaric acid | – | – | – | – | – | BLD | – | |
| Quercetin dihydrate | – | – | – | – | – | – | – | |
| t-cinnamic acid | – | – | – | – | – | – | – | |
| Naringenin | – | – | – | – | – | – | – | |
*Values expressed are means ± SD of three measurements; BLD: below Limit of Detection; BLQ: below Limit of Quantification.
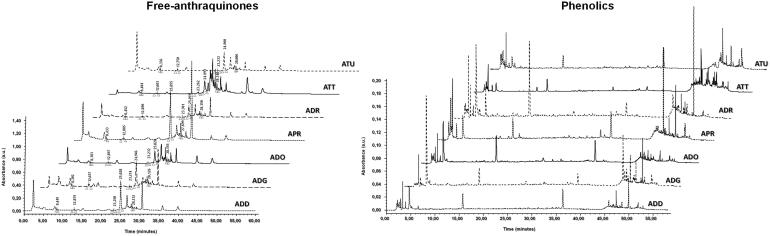
Additionally from the extracts, we can highlight that chrisophanol and physcione are certainly the most abundant anthraquinones present in this taxon, as shown in Figure 2. Among phenolics (see Supplementary materials section S(0).1 for the maximum wavelength, retention times and SST chromatograms), it can be observed that gallic acid, vanillic acid, and benzoic acid are the most representative compounds in these seven Asphodeline spp. Particularly, gallic acid was generally from 2 to 10-folds more concentrated than the others, as reported in Table 2.
Figure 2.
Chromatograms obtained for the free anthraquinones (left) and phenolics (right) pattern in Asphodeline extracts.
In Figure 2 were reported chromatograms at 278 nm obtained from the phenolics chemical fingerprint (see Supplementary materials section S.2 for the chromatograms at 278 nm and marked phenolics). At this wavelength, all considered compounds can be identified, even if each phenolic compound was quantified to its maximum wavelength, as previously reported in literature33. The correct phenolics and anthraquinones identification was carried out using co-elution procedure with references standards, while quantitative analyzes were obtained by external matrix-matched calibration based on HPLC-PDA validated method26,33.
The obtained phenolics profiles could be a valuable starting point in order to justify the observed biological activities. High phenolics content, particularly gallic acid, vanillic acid, and benzoic acid, are responsible for well-known health benefits9,14,36.
Biological activities
Antioxidant properties of Asphodeline extracts were evaluated using different assays including free radical scavenging (ABTS and DPPH), reducing power (CUPRAC and FRAP), phosphomolybdenum and ferrous ion chelating tests.
The DPPH scavenging ability showed the highest value in ATT (35.87 mg TEs/g extract), followed by ADG (32.52 mg TEs/g extract), ADO (32.01 mg TEs/g extract) and APR (29.86 mg TEs/g extract). The weakest activity was observed in ADR with 23.14 mg TEs/g extract. Similarly to DPPH assay, the ABTS scavenging activity of Asphodeline extracts was in the following descending order: ADG > ATT > ADO > APR > ATU > ADD > ADR (Table 1). The radical scavenging activity of Asphodeline extracts showed a similar trend with both total phenolics and flavonoids content. Thus, the phenolics in the extracts could be responsible for the radical scavenging activities. These findings were consistent with several studies on Asphodeline species9,14,32.
The reducing powers of Asphodeline extracts are illustrated in Table 3. ADG, ADO and ATT were more effective in both FRAP and CUPRAC assays compared to other Asphodeline extracts. In addition, ADR had the weakest activity in the assays. This is not surprising since the extract contained significantly lower phenolic content than other extracts. This proved that the phenolics have as effective and potent reductive abilities. Similar approaches were observed for Asphodeline and other plant extracts36.
Table 3.
Phosphomolybdenum assay, reducing power (by CUPRAC and FRAP assays) and metal chelating activities of seven Asphodeline extracts.*
| Asphodeline species | Phosphomolybdenum (mmol TEs/g extract)a | CUPRAC (mg TEs/g extract)a | FRAP (mg TEs/g extract)a | Metal Chelating Activity (mg EDTAEs/g extract)b |
|---|---|---|---|---|
| ADD | 1.32 ± 0.02 | 59.70 ± 1.15 | 45.39 ± 2.15 | 19.90 ± 0.82 |
| ADG | 1.30 ± 0.06 | 73.76 ± 0.70 | 73.66 ± 1.54 | 19.90 ± 0.35 |
| ADO | 1.53 ± 0.07 | 70.58 ± 1.27 | 65.56 ± 3.78 | 22.79 ± 0.60 |
| APR | 1.32 ± 0.07 | 71.86 ± 1.23 | 62.35 ± 1.03 | 22.38 ± 0.58 |
| ADR | 1.28 ± 0.06 | 57.07 ± 1.19 | 43.48 ± 2.40 | 12.91 ± 0.23 |
| ATT | 1.18 ± 0.02 | 79.29 ± 1.86 | 64.34 ± 1.71 | 8.19 ± 0.09 |
| ATU | 1.42 ± 0.01 | 57.87 ± 1.59 | 45.40 ± 0.49 | 19.71 ± 0.03 |
Values expressed are means ± SD of three parallel measurements. aTEs: trolox equivalents; bEDTAEs: ethylenediamine tetraacetic acid equivalents.
The total antioxidant capacities of Asphodeline extracts were evaluated by phosphomolybdenum method. In a descending order can be ranked as ADO (1.53 mmol TEs/g extract) > ATU (1.42 mmol TEs/g extract) > ADD (1.32 mmol TEs/g extract) > APR (1.32 mmol TEs/g extract) > ADG (1.30 mmol TEs/g extract) > ADR (1.28 mmol TEs/g extract) > ATT (1.17 mmol TEs/g extract) (Table 3), which contrast to total phenolic content. According to these results, the observed activity may be explained with the presence of non-phenolic reducing agents such as vitamin C and tocopherol. These findings were supported by several reports37,38. Transition metals (especially iron) play an important role as pro-oxidants of oxidation process including lipid peroxidation. In this sense, metal chelating ability is considered as one of antioxidant mechanism as it reduces the formation hydroxyl and hydroperoxide radical in lipid peroxidation.
Thus, the ferrous ion chelating abilities of the extracts were evaluated and the results are shown in Table 3. ADO possesses the best metal chelating ability with 22.79 mg EDTAEs/g extracts, followed by APR (22.38 mg EDTAEs/g extract), ADG (19.90 mg EDTAEs/g extract), ADD (19.90 mg EDTAEs/g extract) and ATU (19.71 mg EDTAEs/g extract). The order of the extracts was different from that observed for other antioxidant assays. For example, ATT had the highest DPPH scavenging activity among extracts but it exhibited the lowest metal chelating ability (8.19 mg EDTAEs/g extract). Moreover, the extract contained considerable amounts of phenolic compounds.
The observed differences may be explained with the presence of non-phenolic chelators and synergic or antagonistic actions of phytochemicals. Our approaches are in agreement with previous findings39,40, in which negative correlation was observed between metal chelating and other antioxidant activities. In addition, our previous studies showed metal chelating abilities of several Asphodeline species9,14,32.
Enzyme inhibition activities
The treatment and management strategies of several diseases are important subjects in scientific area. The inhibition of key enzymes is one of the most accepted pharmacological approaches41–46. Several synthetic inhibitors, developed for the treatment of several diseases, show side effects such as gastrointestinal disturbances47–49. The side effects of synthetic inhibitors have driven the search for novel and safe inhibitors, especially from natural sources.
The enzyme inhibitory activities of Asphodeline extracts were investigated by spectrophotometric methods against AChE, BChE, tyrosinase, α-amylase and α-glucosidase. The results are summarized in Table 4. In both AChE and BChE inhibitory assays, ADD, ADR, ADO and ATU had the strongest inhibitory activity. The weakest activity for both enzymes was found in ATT. The AChE and BChE inhibitory activities were found to be 0.41–2.09 and 0.36–1.30 mg GALAEs/g extracts, respectively. As regards their tyrosinase inhibitory activity, the following order could be extrapolated: APR > ADR > ATU > ADO > ATT > ADD > ADG. Apparently, the tyrosinase inhibitory activity of ADG (1.45 mg KAEs/g extract) was about 16 folds lower than APR (23.70 mg KAEs/g extract). In our previous study, the activity was reported as 18.57–33 mg KAEs/g extracts9. As reported in Table 4, ATT was the most active on both α-amylase and α-glucosidase with 0.85 mmol ACAEs/g extract and 23.70 mmol ACAEs/g extract, respectively. APR was more effective on these enzymes compared to other extracts. Similarly to our results, these activities were found to be 0.34–1.24 mmol ACAEs/g extract for α-amylase and 1.10–4.99 mmol ACAEs/g extract for α-glucosidase in our first study9. Enzyme inhibitory activities were also reported for A. lutea in another previous study50. These activities were 2.04 mg GALAEs/g extract for AChE, 2.93 mg GALAEs/g extract for BChE, 0.268 mmol ACAEs/g extract for α-amylase, 0.839 mmol ACAEs/g extract for α-glucosidase and 10.31 mg KAEs/g extract for tyrosinase. The differences in the inhibitory activities of Asphodeline species may be due to the different phytochemical composition or the interactions among their components.
Table 4.
Enzyme inhibitory activities of seven Asphodeline extracts.*
| Asphodeline species | Acetylcholine-sterase (mg GALAEs/g extract)a | Butyrylcholine-sterase (mg GALAEs/g extract)a | Tyrosinase (mg KAEs/g extract)b | α-amylase (mmol ACAEs/g extract)c | α-glucosidase (mmol ACAEs/g extract)c |
|---|---|---|---|---|---|
| ADD | 1.61 ± 0.05 | 1.27 ± 0.06 | 10.79 ± 0.73 | 0.79 ± 0.03 | 4.12 ± 0.39 |
| ADG | 1.36 ± 0.10 | 0.41 ± 0.07 | 1.45 ± 0.44 | 0.75 ± 0.02 | 4.79 ± 1.18 |
| ADO | 1.98 ± 0.02 | 0.95 ± 0.02 | 16.98 ± 0.14 | 0.67 ± 0.02 | 10.62 ± 0.26 |
| APR | 1.64 ± 0.03 | 0.82 ± 0.02 | 23.70 ± 0.85 | 0.80 ± 0.02 | 10.50 ± 0.35 |
| ADR | 2.09 ± 0.05 | 0.95 ± 0.03 | 20.69 ± 0.62 | 0.63 ± 0.02 | 4.95 ± 0.11 |
| ATT | 0.41 ± 0.05 | 0.36 ± 0.02 | 14.78 ± 1.28 | 0.85 ± 0.03 | 23.70 ± 0.14 |
| ATU | 1.81 ± 0.02 | 1.30 ± 0.02 | 20.54 ± 0.68 | 0.67 ± 0.03 | 5.17 ± 0.08 |
Values expressed are means ± SD of three parallel measurements. aGALAEs: galantamine equivalents; bKAEs: kojic acid equivalents; cACEs: acarbose equivalents.
Mutagenic/anti-mutagenic evaluation
The results of the preliminary range finding tests for Asphodeline extracts gave no toxic effect to tester strain S. typhimurium TA98 and TA100 at doses of 5000, 2500, and 1000 µg/plate in the presence and absence of S9, respectively. Based on the results of the range finding test, the doses mentioned above were determined as the highest doses. As shown in Table 5, TA98 and TA100 strains did not increase in the number of revertant colonies compared to the negative control when the bacterial strain was treated with Asphodeline extracts at 5000, 2500, and 1000 µg/plate concentrations both with and without metabolic activation enzymes (S9). Asphodeline extracts were not found to be mutagenic for TA98 and TA100 strains. On the contrary, the positive control substances obviously increased revertant colonies in comparison with negative control. Hence, all extracts tested were found to be non-mutagenic at the highest doses on S. typhimurium TA98 and TA100 without metabolic activation in the Ames Assay.
Table 5.
Mutagenic activity expressed as mean number of revertants/plate ± standard deviation of extracts of Asphodeline taxa towards S. typhimurium TA98 and TA100 strains with and without S9.
| Number of His+ Revertants/plate |
|||||
|---|---|---|---|---|---|
| TA98 |
TA100 |
||||
| Samples | Concentration (μg/plate) | S9 (−) | S9 (+) | S9 (−) | S9 (+) |
| *Negative Control | 100 μl/plate | 23 ± 3 | 35 ± 3 | 139 ± 9 | 135 ± 7 |
| ®Positive Control | 637 ± 37 | 2866 ± 31 | 1835 ± 45 | 2632 ± 33 | |
| 0 | 32 ± 4 | 40 ± 4 | 156 ± 24 | 170 ± 11 | |
| ADG | 5000 | 25 ± 5 | 32 ± 3 | 195 ± 1 | 159 ± 8 |
| 2500 | 29 ± 1 | 39 ± 3 | 197 ± 16 | 157 ± 15 | |
| 1000 | 30 ± 1 | 45 ± 3 | 164 ± 17 | 133 ± 1 | |
| ATT | 5000 | 42 ± 6 | 41 ± 7 | 179 ± 4 | 199 ± 8 |
| 2500 | 32 ± 3 | 43 ± 2 | 168 ± 11 | 176 ± 6 | |
| 1000 | 37 ± 3 | 40 ± 2 | 181 ± 12 | 183 ± 8 | |
| ADD | 5000 | 42 ± 5 | 45 ± 0 | 195 ± 11 | 167 ± 8 |
| 2500 | 37 ± 4 | 51 ± 5 | 185 ± 9 | 146 ± 10 | |
| 1000 | 33 ± 2 | 37 ± 5 | 171 ± 7 | 150 ± 13 | |
| ADR | 5000 | 30 ± 1 | 39 ± 3 | 168 ± 9 | 156 ± 13 |
| 2500 | 28 ± 1 | 45 ± 7 | 160 ± 7 | 182 ± 6 | |
| 1000 | 27 ± 3 | 46 ± 2 | 169 ± 7 | 147 ± 4 | |
| ADO | 5000 | 34 ± 0 | 32 ± 3 | 184 ± 4 | 198 ± 4 |
| 2500 | 34 ± 7 | 39 ± 2 | 162 ± 3 | 193 ± 17 | |
| 1000 | 25 ± 3 | 35 ± 3 | 170 ± 10 | 173 ± 12 | |
| ATU | 5000 | 29 ± 6 | 40 ± 2 | 196 ± 8 | 182 ± 4 |
| 2500 | 34 ± 4 | 42 ± 6 | 185 ± 5 | 144 ± 13 | |
| 1000 | 35 ± 0 | 49 ± 7 | 155 ± 9 | 173 ± 2 | |
| APR | 5000 | 27 ± 1 | 34 ± 5 | 182 ± 7 | 116 ± 18 |
| 2500 | 37 ± 3 | 45 ± 2 | 185 ± 2 | 116 ± 18 | |
| 1000 | 28 ± 4 | 35 ± 1 | 188 ± 1 | 121 ± 11 | |
Negative control: DMSO (100 μl/plate) was used for S. typhimurium TA98 and TA100 both in the presence and absence of S9.
®Positive controls: 2-Aminofluorene (7.5 μg/plate) was used as positive indirect mutagen in the presence of S9 mix; 4-nitro-o-phenylenediamine (5 μg/plate) was used as positive direct mutagen in the absence of S9 mix for S. typhimurium TA98 strain; 2-aminoanthracene (5 μg/plate) was used as positive indirect mutagen in the presence of S9 mix; sodium azide (5 μg/plate) was used as positive direct mutagen in the absence of S9 mix for S. typhimurium TA100.
The antimutagenic effect of each extract was assessed from the mean number of revertants/plate, the standard deviation (SD) and the percent inhibition (I%) of the mutagenic activity of 4-NPDA (4-nitro-o-phenylenediamine) and 2-AF (2-aminofluorene) for TA98 strain; SA (sodium azide) and 2-AA (2-aminoanthracene) for TA100 strain on treatment with the three concentrations of the plant extracts. Decreasing colony numbers and inhibition rates for TA98 and TA100 were displayed in Table 6.
Table 6.
Antimutagenicity and inhibition ratios of Asphodeline extracts towards S. typhimurium TA98 and TA100 strains with and without metabolic activation (S9) against direct and indirect mutagens.
| Number of His+ Revertants/plate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| TA 98 |
TA 100 |
||||||||
| Samples | Concentration (μg/plate) | S9 (−) | %I | S9 (+) | %I | S9 (−) | %I | S9 (+) | %I |
| *Negative Control | 100 μl/plate | 36 ± 9 | 37 ± 3 | 162 ± 26 | 127 ± 14 | ||||
| ®Positive Control | 504 ± 38 | 0 | 3622 ± 139 | 0 | 1428 ± 88 | 0 | 4430 ± 181 | 0 | |
| 0 | 27 ± 3 | 32 ± 5 | 160 ± 9 | 139 ± 10 | |||||
| ADG | 5000 | 216 ± 4 | 60 | 292 ± 10 | 93 | 909 ± 46 | 41 | 418 ± 4 | 93 |
| 2500 | 381 ± 6 | 26 | 414 ± 16 | 89 | 975 ± 74 | 36 | 539 ± 2 | 91 | |
| 1000 | 409 ± 25 | 20 | 835 ± 39 | 78 | 1002 ± 82 | 34 | 1190 ± 46 | 76 | |
| ATT | 5000 | 130 ± 7 | 78 | 361 ± 18 | 91 | 963 ± 23 | 37 | 447 ± 2 | 93 |
| 2500 | 368 ± 29 | 29 | 623 ± 33 | 84 | 950 ± 65 | 38 | 523 ± 4 | 91 | |
| 1000 | 431 ± 41 | 15 | 1024 ± 52 | 72 | 1151 ± 56 | 22 | 713 ± 30 | 87 | |
| ADD | 5000 | 358 ± 24 | 52 | 320 ± 24 | 92 | 1006 ± 86 | 33 | 454 ± 8 | 93 |
| 2500 | 397 ± 3 | 22 | 846 ± 19 | 77 | 1250 ± 77 | 14 | 574 ± 33 | 90 | |
| 1000 | 480 ± 16 | 5 | 3147 ± 123 | 13 | 1317 ± 35 | 9 | 1250 ± 57 | 74 | |
| ADR | 5000 | 339 ± 10 | 35 | 593 ± 23 | 84 | 1131 ± 162 | 23 | 461 ± 36 | 92 |
| 2500 | 374 ± 13 | 27 | 3062 ± 45 | 16 | 1144 ± 60 | 22 | 2210 ± 112 | 52 | |
| 1000 | 381 ± 15 | 26 | 3136 ± 59 | 14 | 1260 ± 41 | 13 | 2639 ± 83 | 42 | |
| ADO | 5000 | 299 ± 2 | 43 | 326 ± 24 | 92 | 828 ± 40 | 47 | 599 ± 44 | 89 |
| 2500 | 315 ± 16 | 41 | 531 ± 8 | 86 | 953 ± 20 | 37 | 754 ± 26 | 86 | |
| 1000 | 294 ± 14 | 44 | 2379 ± 41 | 35 | 1010 ± 50 | 33 | 969 ± 16 | 81 | |
| ATU | 5000 | 330 ± 8 | 37 | 369 ± 18 | 91 | 809 ± 37 | 49 | 480 ± 28 | 92 |
| 2500 | 397 ± 1 | 23 | 918 ± 33 | 75 | 839 ± 29 | 46 | 700 ± 14 | 87 | |
| 1000 | 421 ± 16 | 18 | 2505 ± 77 | 31 | 1009 ± 46 | 33 | 1125 ± 106 | 77 | |
| APR | 5000 | 370 ± 19 | 28 | 380 ± 13 | 90 | 777 ± 52 | 51 | 521 ± 14 | 91 |
| 2500 | 401 ± 8 | 22 | 412 ± 21 | 89 | 945 ± 41 | 38 | 783 ± 28 | 85 | |
| 1000 | 455 ± 17 | 10 | 1321 ± 42 | 64 | 1033 ± 53 | 31 | 802 ± 48 | 85 | |
%I: % Inhibition; *Negative control: DMSO (100 μl/plate) was used as negative control for S. typhimurium TA98 and TA100 both in the presence and absence of S9.
®Positive controls: 2-Aminofluorene (7.5 μg/plate) was used as positive indirect mutagen in the presence of S9 mix; 4-nitro-o-phenylenediamine (5 μg/plate) was used as positive direct mutagen in the absence of S9 mix for S. typhimurium TA98 strain; 2-Aminoanthracene (5 μg/plate) was used as positive indirect mutagen in the presence of S9 mix; Sodium azide (5 μg/plate) was used as positive direct mutagen in the absence of S9 mix for S. typhimurium TA100.
According to the results obtained from assays, these extracts of Asphodeline spp. revealed antimutagenicity ratio ranging between moderate to strong activity against 4-NPDA at concentrations of 5000 µg/plate for TA98 strain in the absence of S9 mix. When combined with 4-NPDA, all test doses of the ADO extracts exhibited 43%, 41% and 44% inhibition, respectively, and can be considered strong antimutagenic for TA98 strain. With the addition of metabolic activation enzymes ADG, ATT, APR extracts manifested excellent inhibition ratios against 2-AF at all test doses.
ADG extract showed the highest antimutagenic activity with a ratio of 93% at a dose of 5000 µg/plate against positive mutagen for TA98 strain. ADD, ADO and ATU extracts revealed very strong antimutagenic activity at concentrations of 5000 and 2500 µg/plate (92/77%, 92/86% and 91/75%, respectively). Associated with SA treatment, extracts of APR, ATU, ADO, and ADG were described as strong antimutagenic (51%, 49%, 47%, and 41%, respectively) at a dose of 5000 µg/plate for TA100 in the absence of S9 mix. Also, 2500 and 1000 µg/plate doses of APR, ADO, ADG extracts induced the inhibition greater than 25%, reaching 38/31%, 37/33%, and 36/34% respectively, in the absence of S9 for TA100 and ranking them as moderately antimutagenic (Table 6). Although ATU extract exhibited strong antimutagenic activity with a rate of 46% at a dose of 2500 µg/plate, it revealed moderately antimutagenic action at a concentration of 1000 µg/plate against SA with a ratio of 33% inhibition. In an interesting manner, all the extracts of Asphodeline taxa showed inhibition exceeding 42% and all the concentrations in the range of 5000–1000 µg/plate achieved inhibition ranging between 42 to 93%, making the extracts a very strong antimutagen in the presence of metabolic activation system for TA100 against 2-AA. Against 2-AA in the presence of S9 mix, 5000 µg/plate concentrations of ADG, ATT and ADD extracts showed more than 90% inhibition and the highest concentration attained 93% for all extract ranking them as strongly antimutagenic (Table 6).
Overall, it can be stated from the study that S9 metabolic enzyme system increased the inhibition rate, reaching 93% of mutagenic effects against known chemicals both for TA98 and TA100 strains. These results suggest that extracts of Asphodeline taxa, with high antimutagenic activity in the presence of S9, should be suitable for evaluation concerning CYP450 modulations effects51. As a result, it was determined that Asphodeline taxa, tested in this study, had significant antimutagenic capacities and they could be used in drug and food industries.
Conclusion
In summary, the present work investigated the free-anthraquinones and phenolics chemical fingerprint and biological activities (antioxidant, enzyme inhibitory and mutagenic/antimutagenic activity) of seven Asphodeline root extracts. To the best of our knowledge, these findings have not been reported before for these Asphodeline species with the aim to evaluate these extracts as valuable sources for food supplements and/or for plant-based bioactive formulations.
The analyzed species exhibited notable antioxidant, enzyme inhibitory and anti-mutagenic properties. All extracts did not show any mutagenic effect in Ames test, and are rich sources of phenolics, flavonoids and anthraquinones, which contributed to the observed biological activities. Consequently, the Asphodeline species could be considered as promising sources of natural-functional agents for bioactive formulations.
Glossary
List of abbreviations
- ABTS
2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)
- AChE
acetylcholinesterase
- ADD
A. damascena subsp. damascena
- ADG
A. damascena subsp. gigantea
- ADO
A. damascena subsp. ovoidea
- ADR
A. damascena subsp. rugosa
- APR
A. prismatocarpa
- ATT
A. tenuior subsp. tenuiflora var. tenuiflora
- ATU
A. turcica
- BChE
butyrylcholinesterase
- CUPRAC
cupric ion reducing
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- en
endemic
- FDA
Food and Drug Administration
- FRAP
ferric ion reducing antioxidant power
- GAE
gallic acid equivalents
- HPLC-PDA
high performance liquid chromatography – photodiode array detector
- RE
rutin equivalents
- TE
trolox equivalents.
Acknowledgements
The authors gratefully acknowledge for the financial support given for this research from the Research Foundation of Selcuk University (BAP) and the University “G. d’Annunzio” of Chieti-Pescara.
Disclosure statement
Authors declare no conflict of interests.
References
- 1.Carradori S, D’Ascenzio M, Chimenti P, et al. Selective MAO-B inhibitors: a lesson from natural products . Mol Divers 2014;18:219–43. [DOI] [PubMed] [Google Scholar]
- 2.Lee SY, Baeka N, Nam T-G.. Natural, semisynthetic and synthetic tyrosinase inhibitors. J Enzyme Inhib Med Chem 2016;31:1–13. [DOI] [PubMed] [Google Scholar]
- 3.De Monte C, Carradori S, Granese A, et al. Modern extraction techniques and their impact on the pharmacological profile of Serenoa repens extracts for the treatment of lower urinary tract symptoms. BMC Urol 2014;14:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zengin G, Ceylan R, Guler GO, et al. Enzyme inhibitory effect and antioxidant properties of Astragalus lagurus extracts. Curr Enzyme Inhib 2016;12:177–82. [Google Scholar]
- 5.Ashar BH, Rice TN, Sisson SD.. Medical residents’ knowledge of dietary supplements. South Med J 2008;101:96–1000. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli M.Anthraquinones: analytical techniques as a novel tool to investigate on the triggering of biological targets. Curr Drug Targets 2011;12:366–80. [DOI] [PubMed] [Google Scholar]
- 7.Kremer D, Kosalec I, Locatelli M, et al. Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and F. alnus Mill. bark. Food Chem 2012;131:1174–80. [Google Scholar]
- 8.Kosalec I, Kremer D, Locatelli M, et al. Anthraquinone profile, antioxidant and antimicrobial activity of bark extracts of Rhamnus alaternus, R. fallax, R. intermedia and R. pumila. Food Chem 2013;136:335–41. [DOI] [PubMed] [Google Scholar]
- 9.Zengin G, Locatelli M, Ceylan R, Aktumsek A.. Anthraquinone profile, antioxidant and enzyme inhibitory effect of root extracts of eight Asphodeline taxa from Turkey: can Asphodeline roots be considered as a new source of natural compounds? J Enzyme Inhib Med Chem 2016;31:754–9. [DOI] [PubMed] [Google Scholar]
- 10.Kolayli S, Can Z, Yildiz O, et al. A comparative study of the antihyaluronidase, antiurease, antioxidant, antimicrobial and physicochemical properties of different unifloral degrees of chestnut (Castanea sativa Mill.) honeys. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.1080/14756366.2016.1209494. [DOI] [PubMed] [Google Scholar]
- 11.Uysal A, Zengin G, Mollica A, et al. Chemical and biological insights on Cotoneaster integerrimus: a new (-)-epicatechin source for food and medicinal applications. Phytomedicine 2016;23:979–88. [DOI] [PubMed] [Google Scholar]
- 12.Karioti A, Carta F, Supuran CT.. An update on natural products with carbonic anhydrase inhibitory activity. Curr Pharm Des 2016;22:1570–91. [DOI] [PubMed] [Google Scholar]
- 13.Genovese S, Epifano F, Carlucci G, et al. Quantification of 4′-geranyloxyferulic acid, a new natural colon cancer chemopreventive agent, by HPLC-DAD in grapefruit skin extract. J Pharm Biomed Anal 2010; 53:212–14. [DOI] [PubMed] [Google Scholar]
- 14.Lazarova I, Zengin G, Aktumsek A, et al. HPLC–DAD analysis of phenolic compounds and antioxidant properties of Asphodeline lutea roots from Bulgaria and Turkey. Ind Crops Prod 2014;61:438–41. [Google Scholar]
- 15.Locatelli M, Tammaro F, Menghini L, et al. Anthraquinone profile and chemical fingerprint of Rhamnus saxatilis L. from Italy. Phytochem Lett 2009;2:223–6. [Google Scholar]
- 16.Genovese S, Tammaro F, Menghini L, et al. Comparison of three different extraction methods and HPLC determination of the anthraquinones aloe-emodine, emodine, rheine, chrysophanol and physcione in the bark of Rhamnus alpinus L. (Rhamnaceae). Phytochem Anal 2010;21:261–7. [DOI] [PubMed] [Google Scholar]
- 17.Epifano F, Genovese S, Kremer D, et al. Re-investigation of the anthraquinone pool of Rhamnus spp.: madagascin from the fruits of Rhamnus cathartica and R. intermedia. Nat Prod Commun 2012;7:1029–32. [PubMed] [Google Scholar]
- 18.Genovese S, Epifano F, Curini M, et al. Screening for oxyprenylated anthraquinones in Mediterranean Rhamnus species. Biochem Syst Ecol 2012;43:125–7. [Google Scholar]
- 19.Mollica A, Locatelli M, Stefanucci A, Pinnen F.. Synthesis and bioactivity of secondary metabolites from marine sponges containing dibrominated indolic systems. Molecules 2012;17:6083–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celia C, Trapasso E, Locatelli M, et al. Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblastoma cells. Colloids Surf B Biointerfaces 2013;112:548–53. [DOI] [PubMed] [Google Scholar]
- 21.Epifano F, Fiorito S, Carlucci G, et al. Phytochemistry and pharmacognosy of naturally occurring prenyloxyanthraquinones. Curr Drug Targets 2013;14:959–63. [DOI] [PubMed] [Google Scholar]
- 22.Melucci D, Fedi S, Locatelli M, et al. Application of pyrolysis-gas chromatography-mass spectrometry and multivariate analysis to study bacteria and fungi in biofilms used for bioremediation. Curr Drug Targets 2013;14:1023–33. [DOI] [PubMed] [Google Scholar]
- 23.Genovese S, Epifano F, Carlucci G, et al. HPLC analysis of 4'-geranyloxyferulic and boropinic acids in grapefruits of different geographical origin. Phytochem Lett 2014;8:190–2. [Google Scholar]
- 24.Epifano F, Fiorito S, Locatelli M, et al. Screening for novel plant sources of prenyloxyanthraquinones: Senna alexandrina Mill. and Aloe vera (L.) Burm. F. Nat Prod Res 2015;29:180–4. [DOI] [PubMed] [Google Scholar]
- 25.Locatelli M, Epifano F, Genovese S, et al. Anthraquinone profile, antioxidant and antimicrobial properties of bark extracts of Rhamnus catharticus and R. orbiculatus. Nat Prod Commun 2011;6:1275–80. [PubMed] [Google Scholar]
- 26.Locatelli M, Genovese S, Carlucci G, et al. Development and application of high-performance liquid chromatography for the study of two new oxyprenylated anthraquinones produced by Rhamnus species. J Chromatogr A 2012;1225:113–20. [DOI] [PubMed] [Google Scholar]
- 27.Zaza S, Lucini SM, Sciascia F, et al. Recent advances in the separation and determination of impurities in pharmaceutical products. Instrum Sci Technol 2015;43:182–96. [Google Scholar]
- 28.Locatelli C, Melucci D, Locatelli M.. Toxic metals in herbal medicines. A review. Curr Bioact Compd 2014;10:181–8. [Google Scholar]
- 29.Anwar F, Kalsoom U, Sultana B, et al. Effect of drying method and extraction solvent on the total phenolics and antioxidant activity of cauliflower (Brassica oleracea L.) extracts. Int Food Res J 2013;20:653–9. [Google Scholar]
- 30.Khoddami A, Wilkes MA, Roberts TH.. Techniques for analysis of plant phenolic compounds. Molecules 2013;18:2328–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slinkard K, Singleton VL.. Total phenol analysis: automation and comparison with manual methods. Am J Enol Viticul 1977;28:49–55. [Google Scholar]
- 32.Zengin G, Aktumsek A.. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: an endemic plant to Turkey. Afr J Tradit Complem Altern Med 2014;11:481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zengin G, Menghini L, Malatesta L, et al. Comparative study of biological activities and chemical fingerprint of two wild Turkish species: Asphodeline anatolica and Potentilla speciosa. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.1080/14756366.2016.1178247. [DOI] [PubMed] [Google Scholar]
- 34.Zengin G, Sarikurkcu C, Aktumsek A, et al. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind Crops Prod 2014;53:244–51. [Google Scholar]
- 35.Zengin G, Uysal A, Gunes E, Aktumsek A.. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): a potential source for functional food ingredients and drug formulations. PloS One 2014;9:e113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latocha P, Łata B, Stasiak A.. Phenolics, ascorbate and the antioxidant potential of kiwiberry vs. common kiwifruit: The effect of cultivar and tissue type. J Funct Foods 2015;19:155–63. [Google Scholar]
- 37.Zengin G, Locatelli M, Carradori S, et al. Total phenolics, flavonoids, condensed tannins content of eight Centaurea species and their broad inhibitory activities against cholinesterase, tyrosinase, α-amylase and α-glucosidase. Not Bot Horti Agrobo 2016;44:195–200. [Google Scholar]
- 38.Amessis-Ouchemoukh N, Abu-Reidah IM, Quirantes-Piné R, et al. Phytochemical profiling, in vitro evaluation of total phenolic contents and antioxidant properties of Marrubium vulgare (horehound) leaves of plants growing in Algeria. Ind Crops Prod 2014;61:120–9. [Google Scholar]
- 39.Kalogeropoulos N, Yanni AE, Koutrotsios G, Aloupi M.. Bioactive microconstituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece. Food Chem Toxicol 2013;55:378–85. [DOI] [PubMed] [Google Scholar]
- 40.Sarikurkcu C, Ozer MS, Tepe B, et al. Phenolic composition, antioxidant and enzyme inhibitory activities of acetone, methanol and water extracts of Clinopodium vulgare L. subsp. vulgare L. Ind Crops Prod 2015;76:961–6. [Google Scholar]
- 41.Jamila N, Khairuddean M, Yeong KK, et al. Cholinesterase inhibitory triterpenoids from the bark of Garcinia hombroniana. J Enzyme Inhib Med Chem 2014;30:133–9. [DOI] [PubMed] [Google Scholar]
- 42.Etxeberria U, De la Garza AL, Campión J, et al. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Exp Op Therap Targets 2012;16:269–97. [DOI] [PubMed] [Google Scholar]
- 43.Kim YJ, Uyama H.. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci 2005;62:1707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gülçin İ, Scozzafava A, Supuran CT, et al. Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. 1-5 doi: 10.3109/14756366.2015.1135914. [DOI] [PubMed] [Google Scholar]
- 45.Gocer H, Topal F, Topal M, et al. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J Enzyme Inhib Med Chem 2016;31:441–7. [DOI] [PubMed] [Google Scholar]
- 46.Mollica A, Locatelli M, Macedonio G, et al. Microwave-assisted extraction, HPLC analysis and inhibitory effects on carbonic anhydrase I, II, VA and VII isoforms of fourteen blueberry Italian cultivars. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.1080/14756366.2016.1214951. [DOI] [PubMed] [Google Scholar]
- 47.Yi W, Wu X, Cao R, et al. Biological evaluations of novel vitamin C esters as mushroom tyrosinase inhibitors and antioxidants. Food Chem 2009;117:381–6. [Google Scholar]
- 48.Kumar S, Kumar V, Rana M, Kumar D.. Enzymes inhibitors from plants: an alternate approach to treat diabetes. Pharmacogn Commun 2012;2:18–33. [Google Scholar]
- 49.Murray AP, Faraoni MB, Castro MJ, et al. Natural AChE inhibitors from plants and their contribution to Alzheimer's disease therapy. Curr Neuropharmacol 2013;11:388–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lazarova I, Zengin G, Bender O, et al. A comparative study of Bulgarian and Turkish Asphodeline lutea root extracts: HPLC–UV profiles, enzyme inhibitory potentials and anti-proliferative activities against MCF-7 and MCF-10A cell lines. J Funct Foods 2015;15:254–63. [Google Scholar]
- 51.Del-Toro-Sánchez CL, Bautista-Bautista N, Blasco-Cabal JL, et al. Antimutagenicity of methanolic extracts from Anemopsis californica in relation to their antioxidant activity. J Evid Based Complem Altern Med 2014;2014:273878. [DOI] [PMC free article] [PubMed] [Google Scholar]