Abstract
A novel series of 2-substituted mercapto-3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinones 1–20 was synthesised and evaluated for in vitro antitumour activity. N-(4-Chlorophenyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio)acetamide (7) and N-(3,4,5 trimethoxybenzyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]propanamide (19) exhibited excellent antitumour properties, with mean growth inhibitory concentration (GI50) of 17.90 and 6.33 µΜ, respectively, compared with those of 5-fluorouracil 5-FU, gefitinib, and erlotinib (mean GI50: 18.60, 3.24, and 7.29 µΜ, respectively). Comparison of the GI50 (µM) values of compounds 7 and 19 versus those of 5-FU, gefitinib, and erlotinib against an in vitro subpanel of tumour cells lines showed that compounds 7 and 19 have activities almost equal to or higher than that of those standard drugs, especially against lung, CNS, and breast cancer cells. However, compounds 5, 10, 14, 15, 16, 17, and 20 exhibited effective antitumour activity against the different cell lines tested, with growth inhibition percentage (MGI%) of 19, 24, 19, 17, 16, 15, and 16, respectively. A modelling study was performed for compounds 7 and 19 by docking them into the EGFR kinase enzyme to study their mode of binding with the putative binding site.
Keywords: Quinazoline, in vitro antitumour evaluation, EGFR, molecular docking
Introduction
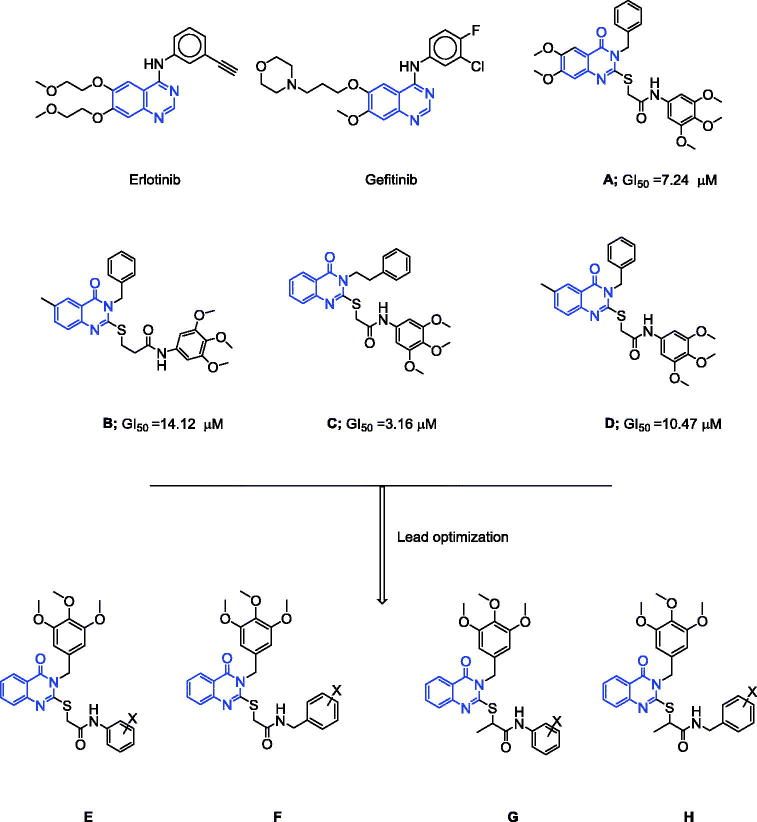
Cancer refers to an abnormal growth of cells, and is the second leading cause of death worldwide1. Several of the current therapeutic agents have numerous side effects caused by their nonselective activity; therefore, the synthesis of safe and selective agents with a high therapeutic index is a vital research area. Quinazolinone nucleus is a characteristic bioactive scaffold present in several critical agents of biological interest2–29. Gefitinib and erlotinib (Figure 1) are known to contain a quinazoline nucleus and are effective in the treatment of breast and non-small cell lung (NSL) cancer via inhibition of epidermal growth factor receptor-tyrosine kinase (EGFR-TK)30,31. EGFR is over-expressed in numerous human tumours such as prostate, ovarian, breast, colon, and renal31–34. In our previously published studies10,11,15,18,19, the 2-mercaptoquinazoline analogue containing trimethoxyphenyl moiety showed significant antitumour activity such as 2-[(3-benzyl-6,7-dimethoxy-4(3H)-quinazolinon-2-yl)thio]-N-(3,4,5-trimethoxyphenyl)acetamide (A; GI50 = 7.24 µM), 2-[(3-benzyl-6-methyl-4(3H)-quinazolinon-2-yl)thio]-N-(3,4,5-trimethoxyphenyl)acetamide (B; GI50 = 14.12 µM), 2-[(3-phenethyl-4(3H)-quinazolinon-2-yl)thio]-N-(3,4,5-trimethoxyphenyl)acetamide (C; GI50 = 3.16 µM), 3-[(3-benzyl-6-methyl-4(3H)-quinazolinon-2-yl)thio]-N-(3,4,5-trimethoxyphenyl) propanamide (D; GI50 =14.12 µM) compared with that of the reference drug 5-fluorouracil (FU; mean GI50 18.60 µM; Figure 1). In this study, we designed several new 2-substituted mercapto-3-(3,4,5-trimethoxybenzyl)quinazolin-4(3H)-ones containing various alkyl, acetamide, and isopropanamide fragments at position 2 of the quinazoline core, with different electronic environments that would affect lipophilicity. The synthesised molecules 2–20 were evaluated for their in vitro antitumour activities at a single dose (10 µM; Figure 1). These hybrids were synthesised with an aim to develop effective and selective antitumour molecules.
Figure 1.
Structures of erlotinib, gefitinib, reported compounds A–D, and designed quinazoline derivatives E–H as antitumour agents.
Experimental
Chemistry
Melting points were recorded on a Barnstead 9100 electrothermal melting apparatus. IR spectra (KBr) were recorded on an FT-IR Perkin-Elmer spectrometer (ν cm−1). 1H and 13C NMR spectra were recorded on Bruker 500 or 700 MHz spectrometers using DMSO-d6 as the solvent. Microanalytical data (C, H, and N) were obtained using a Perkin-Elmer 240 analyser and the proposed structures were within ±0.4% of the theoretical values. Mass spectra were recorded on a Varian TQ 320 GC/MS/MS mass spectrometer. Data of compound 8 were collected on a Bruker APEX-II D8 Venture area diffractometer (Billerica, MA), equipped with graphite monochromatic Mo Kα radiation, λ = 0.71073 Å at 296 (2) K. Cell refinement and data reduction were carried out by Bruker SAINT. SHELXT35,36 was used to solve the structure.
2-Thioxo-3-(3,4,5-trimethoxybenzyl)-2,3-dihydroquinazolin-4(1H)-one (1)
A mixture of 3,4,5-trimethoxybenzyl isothiocyanate (11 mmol, 2.36 g), anthranilic acid (10 mmol, 1.37 g) and triethylamine (15 mmol, 1.51 g), was heated under reflux for 3 h in ethanol (20 ml). The reaction mixture was filtered while hot and the obtained solid was dried.
Yield: 86%; mp: 190–192 °C; IR (KBr, cm−1) ν: 1671 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 13.04 (s, 1H), 7.98 (dd, 1H, J = 7.0 & 1.0 Hz), 7.77–7.70 (m, 1H), 7.42 (d, 1H, J = 8.0 Hz), 7.18 (t, 1H, J = 3.5 & 3.0 Hz), 6.86 (s, 2H), 3.72 (s, 6H), 3.61 (s, 3H); 13C NMR (125 MHz, DMSO-d6): δ 48.9, 55.8, 59.9, 105.2, 115.4, 115.6, 124.5, 127.3, 132.3, 135.5, 136.7, 139.0, 152.6, 159.4, and 175.6; Anal. calcd. for C18H18N2O4S (%): C, 60.32; H, 5.06; N, 7.82. Found: C, 60.29; H, 5.08; N, 7.84; MS: [m/z, 358].
General procedure for the synthesis of compounds 2–13
A mixture of 2-thioxo-3-(3,4,5-trimethoxybenzyl)-2,3-dihydroquinazolin-4(1H)-one (1) (1 mmol, 358 mg) and appropriate alkylhalides or 2-chloro-N-(substituted)acetamides (1 mmol) in 10 ml acetone containing potassium carbonate (2 mmol, 277 mg) was stirred at room temperature for 10–12 h. The reaction mixture was filtered, the solvent removed, and the obtained solid was washed with water and dried.
2-(Methylthio)-3-(3,4,5-trimethoxybenzyl)quinazolin-4(3H)-one (2)
Yield: 93%; mp: 174–175 °C; IR (KBr, cm−1) ν: 1670 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 8.13 (dd, 1H, J = 6.5 & 1.5 Hz), 7.85–7.75 (m, 1H), 7.59 (d, 1H, J = 8.0 Hz), 7.50–7.44 (m, 1H), 6.60 (s, 2H), 5.26 (s, 2H), 3.70 (s, 6H), 3.63 (s, 3H), 2.62 (s, 3H); 13C NMR (125 MHz, DMSO-d6): δ 14.7, 47.0, 55.9, 59.9, 104.5, 118.6, 125.9, 125.9, 126.6, 131.3, 134.8, 136.9, 146.8, 152.9, 157.6, and 160.9; Anal. calcd. for C19H20N2O4S (%): C, 61.27; H, 5.41; N, 7.52. Found: C, 61.31; H, 5.39; N, 7.53; MS: [m/z, 372].
2-((2-Morpholinoethyl)thio)-3-(3,4,5-trimethoxybenzyl)quinazolin-4(3H)-one (3)
Yield: 88%; mp: 150–1152 °C; IR (KBr, cm−1) ν: 1683 (C=O); 1H NMR (500 MHz, CDCl3): δ 8.23 (d, 1H, J = 8.0 Hz), 7.69 (t, 1H, J = 7.5 Hz), 7.52 (d, 1H, J = 8.0 Hz), 7.38 (t, 1H, J = 7.5 Hz), 6.66 (s, 2H), 5.30 (s, 2H), 3.81 (s, 6H), 3.80 (s, 3H), 3.72–3.70 (m, 4H), 3.46–3.43 (m, 2H), 2.75–2.72 (m, 2H), 2.55–2.49 (m, 4H); 13C NMR (125 MHz, CDCl3): δ 29.3, 40.6, 47.6, 53.5, 53.7, 56.1, 57.4, 60.7, 66.8, 66.9, 105.4, 106.5, 119.3, 125.7, 126.0, 127.1, 131.3, 134.4,137.6, 147.3, 152.9, 153.2, 156.5, and 161.9; MS: [m/z, 471].
2-((2-(Piperidin-1-yl)ethyl)thio)-3-(3,4,5-trimethoxybenzyl)quinazolin-4(3H)-one (4)
Yield: 89%; mp: 162–164 °C; IR (KBr, cm−1) ν: 1680 (C=O); 1H NMR (500 MHz, CDCl3): δ 8.18 (d, 1H, J = 7.0 Hz), 7.635 (d, 1H, J = 6.0Hz), 7.49 (d, 1H, J = 7.0 Hz), 7.33 (d, 1H, J = 6.0 Hz), 6.63 (s, 2H), 5.26 (s, 2H), 3.77 (s, 9H), 3.41 (s, 2H), 2.48 (s, 4H), 1.57 (s, 4H), 1.41 (s, 2H); 13C NMR (125 MHz, CDCl3): 60.7, 57.6, 56.1, 54.3, 47.6, 29.4, 25.7, 24.1, 119.2, 126.0, 125.6, 127.0, 131.3, 134.4, 137.7, 147.3, 152.8, 156.5, and 161.9; MS: [m/z, 469].
2-((4-Chlorobenzyl)thio)-3-(3,4,5-trimethoxybenzyl)quinazolin-4(3H)-one (5)
Yield: 91%; mp: 174–175 °C; IR (KBr, cm−1) ν:, 1671 (C=O); 1H NMR (500 MHz, CDCl3): δ 8.26 (dd, 1H, J = 7.0 & 1.0 Hz), 7.75 (t, 1H, J = 7.0 0 Hz), 7.63 (d, 1H, J = 8.0 Hz), 7.43–7.40 (m, 3H), 7.30 (s, 1H), 7.28 (d, 1H, J = 2.0 Hz), 6.62 (s, 2H), 5.29 (s, 2H), 4.53 (s, 2H), 3.83 (s, 3H), 3.78 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 161.9, 155.7, 153.2, 147.2, 137.6, 135.4, 134.5, 133.4, 131.1, 130.6, 128.7, 127.2, 126.0, 125.9, 119.4, 105.1, 60.8, 56.1, 47.6, and 35.8; Anal. calcd. for C25H23ClN2O4S (%):C, 62.17; H, 7.34; N, 5.80. Found: C, 61.22; H, 7.38; N, 5.78. MS: [m/z, 482; M + 1, 483].
2-((4-Oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)acetamide (6)
Yield: 81%; mp: 238–239 °C; IR (KBr, cm−1) ν: 3404 (NH), 1675, 1651 (C=O); 1H NMR (500 MHz, DMSO-d6): 8.30 (s, 1H), 8.12 (s, 1H), 7.81 (d, 1H, J = 5.0 Hz), 7.69 (s, 1H), 7.55 (d, 1H, J = 5.0 Hz), 7.47 (d, 1H, J = 5.5 Hz), 7.240 (d, 1H, J = 2.5 Hz), 6.66 (d, 1H, J = 6.0 Hz), 5.28 (s, 2H), 4.01 (d, 1H, J = 8.5 Hz), 3.73 (s, 6H), 3.66 (s, 3H); 13C NMR (125 MHz, DMSO-d6): δ 168.5, 160.8, 156.6, 152.9, 146.7, 136.9, 134.7, 131.2, 126.6, 126.0, 118.7, 104.6, 59.9, 55.9, 47.2, and 35.7; MS: [m/z, 415].
N-(4-Chlorophenyl)-2-((4-oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)acetamide (7)
Yield: 84%; mp: 250–252 °C; IR (KBr, cm−1) ν: 3295 (NH), 1677, 1655 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 10.47 (s, 1H), 8.10 (dd, 1H, J = 7.0 & 1.0 Hz), 7.70–7.72 (m, 1H), 7.62 (d, 2H, J = 9.0 Hz), 7.48 (d, 1H, J = 8.0 Hz), 7.45–7.40 (m, 1H), 7.30 (d, 2H, J = 8.5 Hz), 6.67 (s, 2H), 5.28 (s, 2H), 4.20 (s, 2H), 3.74 (s, 6H), 3.66 (s, 3H); 13C NMR (125 MHz, DMSO-d6): δ 36.8, 47.2, 55.8, 59.9, 78.5, 78.8, 79.0, 104.7, 118.7, 120.5, 125.7, 125.8, 126.5, 127.0, 128.5, 131.0, 134.6, 137.0, 137.8, 146.6, 152.8, 156.3, 160.8, ανδ 165.6; Anal. calcd. for C26H24ClN3O5S (%): C, 59.37; H, 4.60; N, 7.99. Found: C, 59.32; H, 4.61; N, 7.80. MS: [m/z, 525, M + 1, 526].
N-(4-Fluorophenyl)-2-((4-oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)acetamide (8)
Yield: 83%; mp: 253–255 °C; IR (KBr, cm−1) ν: 3246 (NH), 1677, 1654 (C=O); 1H NMR (500 MHz, CDCl3): δ 9.72 (s, 1H), 8.34 (s, 1H), 7.84–7.28 (m, 5H), 6.96 (d, 2H, J = 5.0 Hz), 6.66 (s, 2H), 5.34 (s, 2H), 4.03 (s, 2H), 3.82 (s, 6H), 3.81 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 166.4, 161.4, 157.7, 153.4, 146.4, 138.0, 135.3, 133.9, 130.4, 127.9, 126.8, 125.0, 121.0, 115.8, 115.6, 105.5, 60.8, 56.2, 48.1, and 36.1; MS: [m/z, 509].
N-(4-Methoxyphenyl)-2-((4-oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)acetamide (9)
Yield: 85%; mp: 210–211 °C; IR (KBr, cm−1) ν: 3260 (NH) 1682, 1662 (C=O); 1H NMR (500MHz, CDCl3): δ 9.52 (s, 1H), 8.32 (d, 1H, J = 7.0 Hz), 7.82 (s, 1H), 7.66 (d, 1H, J = 7.5 Hz), 7.51 (d, 1H, J = 6.5 Hz), 7.34 (d, 2H, J = 8.5 Hz), 6.80 (d, 2H, J = 8.5 Hz), 6.66 (s, 2H), 5.33 (s, 2H), 4.03 (s, 2H), 3.82 (s, 6H), 3.80 (s, 3H), 3.76 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 166.1, 161.4, 157.6, 156.3, 153.4, 146.5, 137.9, 135.2, 131.0, 130.5, 127.8, 126.7, 125.1, 121.0, 119.5, 114.2, 105.4, 60.8, 56.2, 55.4, 48.1, and 36.1; MS: [m/z, 521].
2-((4-Oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)-N-(3,4,5-trimethoxyphenyl)acetamide (10)
Yield: 83%; mp: 230–231 °C; IR (KBr, cm−1) ν: 3335 (NH), 1681, 1652 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 8.27 (s, 1H), 8.15–8.10 (m, 1H), 7.80–7.76 (m, 1H), 7.58–7.53 (m, 1H), 7.46–7.42 (m, 1H), 7.01 (d, 2H, J = 20.5 Hz), 6.67 (d, 2H, J = 20.5 Hz), 5.29 (d, 2H, J = 19.0 Hz), 4.19 (d, 2H, J = 20.5 Hz), 3.78–3.63 (m, 18H); 13C NMR (125 MHz, DMSO-d6): δ 36.8, 47.2, 55.6, 55.8, 59.9, 60.0, 78.5, 78.84, 9.1, 96.8, 104.8, 118.8, 125.8, 126.6, 131.1, 133.5, 134.6, 134.9, 137.0, 146.7, 152.6, 152.9, 156.3, 160.8, and 165.2; MS: [m/z, 581].
2-((4-Oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)-N-(4-sulfamoylbenzyl)acetamide (11)
Yield: 81%; mp: 288–290 °C; IR (KBr, cm−1) ν: 3327, 3236 (NH), 1693 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 10.78 (s, 1H), 8.11–8.10 (m, 1H), 7.80–7.76 (m, 5H), 7.47–7.43 (m, 2H), 7.26 (s, 2H), 6.69 (s, 2H), 5.29 (s, 2H), 4.26 (s, 2H), 3.74 (s, 6H), 3.65 (s, 3H); 13C NMR (125 MHz, DMSO-d6): δ 36.9, 47.3, 55.9, 59.9, 104.7, 118.6, 118.7, 125.7, 126.1, 126.6, 126.7, 131.2, 134.8, 136.9, 138.4, 141.8, 146.6, 152.9, 156.5, 160.8, and 166.3; MS: [m/z, 570].
2-((4-Oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)-N-(3,4,5-trimethoxybenzyl)acetamide (12)
Yield: 84%; mp: 203–205 °C; IR (KBr, cm−1) ν: 3260 (NH), 1682, 1662 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 8.72 (t, 1H, J = 7.5 & 0.5 Hz(, 8.10 (d, 1H, J = 8.0 Hz), 7.73 (t, 1H, J = 7.5 & 0.5 Hz), 7.48–7.44 (m, 2H), 6.65 (s, 2H), 6.55 (s, 2H), 5.28 (s, 2H), 4.25 (d, 2H, J = 6.0 Hz), 4.09 (s, 2H), 3.71 (s, 6H), 3.64 (s, 6H), 3.62 (s, 3H), 3.61 (s, 3H); 13C NMR (125 MHz, DMSO-d6): δ 35.7, 42.9, 47.1, 55.6, 55.8, 59.9, 104.6, 104.7, 118.6, 125.9, 126.0, 126.5, 131.2, 134.7, 136.4, 136.9, 146.6, 152.7, 152.9, 156.5, 160.8, ανδ 166.7; Anal. calcd. for C30H33N3O8S (%):C, 60.49; H, 5.58; N, 7.05. Found: C, 60.51; H, 5.60; N, 7.03; MS: [m/z, 595].
2-((4-Oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)-N-(4-sulfamoylbenzyl)propanamide (13)
Yield: 81%; mp: 278–280 °C; IR (KBr, cm−1) ν: 3308, 3200 (NH), 1676, 1656 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 8.86 (s, 1H), 8.13 (d, 1H, J = 7.0 Hz), 7.82–7.29 (m, 9H), 6.65 (s, 2H), 5.29 (s, 2H), 4.40 (s, 2H), 4.04 (d, 2H, J = 4.0 Hz), 3.70 (s, 6H), 3.64 (s, 3H); 13C NMR (125 MHz, DMSO-d6): δ 35.6, 42.2, 47.2, 55.8, 59.9, 104.6, 118.7, 125.5, 125.9, 126.1, 126.6, 127.2, 131.2, 134.8, 136.9, 142.5, 143.2, 146.6, 152.9, 156.5, 160.1, and 166.9; MS: [m/z, 584].
General procedure for the synthesis of compounds 14–20
A mixture of 2-thioxo-3-(3,4,5-trimethoxybenzyl)-2,3-dihydroquinazolin-4(1H)-one (1) (1 mmol, 358 mg) and appropriate 2-chloro-N-(substituted)propanamides (1 mmol) in 10 ml acetone containing potassium carbonate (2 mmol, 277 mg) was heated under reflux for 6–9 h. The reaction mixture was filtered while hot, the solvent was removed, and the obtained solid was washed with water and dried.
N-(4-Chlorophenyl)-2-((4-oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)propanamide (14)
Yield: 83%; mp: 222–224 °C; IR (KBr, cm−1) ν: 3253 (NH), 1685, 1655 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 10.56 (s, 1H), 8.11 (dd, 1H, J = 7.0 & 1.0 Hz), 7.80 (t, 1H, J = 7.0 Hz), 7.66 (d, 2H, J = 9.0 Hz), 7.54 (d, 1H, J = 8.5 Hz), 7.46 (t, 1H, J = 7.0 Hz), 7.37 (d, 2H, J = 9.0 Hz), 6.64 (s, 2H), 5.24 (s, 2H), 4.76 (q, 1H, J = 9.0 Hz), 3.71 (s, 6H), 3.63 (s, 3H), 1.62 (d, 3H, J = 7.5 Hz); 13C NMR (125 MHz, DMSO-d6): δ 17.3, 46.5, 47.2, 52.0, 55.8, 59.9, 104.6, 118.8, 120.7, 125.7, 126.1, 126.6, 127.0, 128.6, 131.1, 134.8, 136.9, 137.8, 146.7, 152.9, 156.1, 160.7, and 169.5; MS: [m/z, 539, M + 1, 540].
N-(4-Fluorophenyl)-2-((4-oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)propanamide (15)
Yield: 82%; mp: 216–217 °C; IR (KBr, cm−1) ν: 3302 (NH), 1685, 1661 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 10.50 (s, 1H), 8.10 (d, 1H, J = 9.0 Hz), 7.79 (d, 1H, J = 7.5 Hz), 7.65 (q, 2H, J = 5.0 & 4.0 Hz), 7.56 (d, 1H, J = 8.0 Hz), 7.46 (d, 1H, J = 7.5 Hz), 7.13 (t, 2H, J = 9.0 & 8.5 Hz), 6.65 (s, 2H), 5.25 (s, 2H), 4.76 (dd, 1H, J = 7.0 Hz), 3.71 (s, 6H), 3.64 (s, 3H), 1.63 (d, 3H, J = 7.0 Hz); [m/z, 523].
N-(4-Methoxyphenyl)-2-((4-oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)propanamide (16)
Yield: 84%; mp: 202–203 °C; IR (KBr, cm−1) ν: 3275 (NH), 1683, 1654 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 10.29 (s, 1H), 7.81 (s, 1H), 8.11 (s, 1H), 7.57.47 (m, 3H), 6.89 (d, 2H, J = 4.0 Hz), 6.65 (d, 2H, J = 5.0 Hz), 5.25 (s, 2H), 4.76 (dd, 1H, J = 6.5 & 4.5 Hz), 3.71–3.64 (m, 12H), 1.62 (d, 3H, J = 6.5 Hz); 13C NMR (125 MHz, DMSO-d6): δ 17.6, 46.5, 47.2, 55.1, 55.9, 59.9, 104.6, 113.9, 118.8, 120.7, 120.9, 125.8, 126.1, 126.6, 131.1, 131.9, 134.8, 137.0, 146.7, 152.9, 155.4, 156.2, 160.7, and 168.7; MS: [m/z, 535].
2-((4-Oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)-N-(3,4,5-trimethoxyphenyl)propanamide (17)
Yield: 83%; mp: 206–207 °C; IR (KBr, cm−1) ν: 3324 (NH), 1684, 1664(C=O); 1H NMR (500 MHz, CDCl3-DMSO-d6): δ 10.32 (s, 1H), 8.20 (s, 1H), 8.09 (d, 1H, J = 8.0 Hz), 7.76 (d, 1H, J = 8.0 Hz), 7.56 (d, 1H, J = 8.0 Hz), 7.42 (d, 1H, J = 8.0 Hz), 6.98 (s, 2H), 6.63 (s, 2H), 5.23 (s, 2H), 4.76 (dd, 1H, J = 7.5 Hz), 3.72 (s, 12H), 3.65 (s, 3H), 3.62 (s, 3H), 1.62 (d, 3H, J = 7.0 Hz); 13C NMR (125 MHz, CDCl3-DMSO-d6): δ 17.3, 46.4, 47.1, 55.5, 55.7, 59.8, 59.9, 96.8, 96.9, 104.6, 118.8, 125.7, 125.9, 126.5, 131.0, 133.5, 134.5, 134.8, 136.9, 146.7, 152.6, 152.8, 156.1, 158.3, 158.5, 160.7, and 169.0; MS: [m/z, 595].
2-((4-Oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)-N-(4-sulfamoylphenyl)propanamide (18)
Yield: 81%; mp: 218–220 °C; IR (KBr, cm−1) ν: 3360, 3297 (NH), 1687, 1664 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 10.78 (s, 1H), 8.10 (d, 1H, J = 7.0 Hz), 7.87–7.78 (m, 5H), 7.52 (d, 1H, J = 8.5 Hz), 7.46 (t, 1H, J = 7.5 & 8.0 Hz), 7.27 (s, 2H), 6.65 (s, 2H), 5.24 (d, 2H, J = 5.5 Hz), 4.78 (d, 1H, J = 7.0 Hz), 3.72 (s, 6H), 3.65 (s, 3H), 1.63 (d, 3H, J = 7.5 Hz); 13C NMR (125 MHz, DMSO-d6): δ 17.1, 46.6, 47.2, 55.8, 59.9, 104.6, 118.7, 120.7, 125.7, 126.1, 126.4, 126.6, 126.7, 131.1, 134.8, 136.9, 138.6, 139.8, 146.6, 152.9, 156.1, 160.7, and 170.0; [m/z, 584].
2-((4-Oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)-N-(3,4,5-trimethoxybenzyl)propanamide (19)
Yield: 83%; mp: 262–264 °C; IR (KBr, cm−1) ν: 3237 (NH), 1684, 1663 (C=O); 1H NMR (700 MHz, DMSO-d6): δ 8.84–8.77 (m, 2Η), 8.11 (d, 0.5H, J = 5.5 Hz), 7.76 (t, 0.5H, J = 5.5 Hz), 7.51–7.45 (m, 1H), 6.62 (s, 1H), 6.59 (s, 2H), 6.53 (s, 1H), 5.24 (s, 1H), 4.73–4.70 (m, 0.4H), 4.60–4.57 (m, 0.6H), 4.32–4.20 (m, 3H), 3.75–3.60 (m, 18H), 1.59–1.57 (m, 3H); 13C NMR (175 MHz, DMSO-d6): δ 18.4, 21.7, 42.8, 43.1, 46.1, 47.6, 54.8, 55.9, 56.1, 56.2, 60.3, 60.4, 104.6, 104.7, 104.8, 119.2, 126.4, 126.6, 127.0, 131.7, 134.9, 135.1, 135.2, 136.7, 136.8, 137.3, 147.2, 153.2, 153.3, 153.4, 156.6, 161.3, 169.2, and 170.9; MS: [m/z, 609]. Anal. calcd. for C31H35N3O8S (%): C, 61.07; H, 5.79; N, 6.89.Found: C, 61.12; H, 5.81; N, 6.91.
2-((4-Oxo-3-(3,4,5-trimethoxybenzyl)-3,4-dihydroquinazolin-2-yl)thio)-N-(4-sulfamoylbenzyl)propanamide (20)
Yield: 81%; mp: 174–175 °C; IR (KBr, cm−1) ν: 3371, 3253 (NH), 1685, 1663 (C=O); 1H NMR (500 MHz, DMSO-d6): δ 8.93 (s, 1H), 8.11 (d, 1H, J = 1.0 Hz), 7.82–7.80 (m, 1H), 7.64 (d, 2H, J = 8.5 Hz), 7.55–7.49 (m, 2H), 7.38 (d, 2H, J = 8.5 Hz), 7.29 (s, 2H), 6.61 (s, 2H), 5.24 (s, 2H), 4.69 (d, 1H, J = 7.5 Hz), 4.40–4.35 (m, 2H), 3.68 (s, 6H), 3.63 (s, 3H), 1.57 (d, 3H, J = 7.5 Hz); 13C NMR (125 MHz, DMSO-d6): δ 17.8, 42.1, 45.6, 47.1, 55.8, 59.9, 104.4, 118.7, 125.5, 125.7, 125.9, 126.2, 126.5, 127.2, 127.3, 131.2, 134.8, 136.8, 142.5, 143.1, 146.7, 152.8, 156.1, 160.8, and 170.7; MS: [m/z, 598].
X-ray crystallography
Data of compound 8 were collected on a Bruker APEX-II D8 Venture area diffractometer, equipped with graphite monochromatic Mo Kα radiation, λ = 0.71073 Å at 296 (2) K. Cell refinement and data reduction were carried out by Bruker SAINT. SHELXT35,36 was used to solve the structure. The final refinement was carried out by full-matrix least-squares techniques with anisotropic thermal data for non-hydrogen atoms on F. CCDC 1534954 contains the supplementary crystallographic data for this compound and can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Antitumour screening
The antitumour evaluation was performed in nearly 60 human tumour cell lines obtained from nine organs, according to the rules of the Drug Evaluation Branch, NCI, Bethesda, MD37–41.
Docking methodology
All modelling experiments were conducted with MOE 2007.9 of the Chemical Computing Group Inc. (Montreal, Canada)42,43. The starting coordinates of the X-ray crystal structure of the EGFR enzyme in complex with erlotinib (pdb code 1M17) were obtained from the RCSB Protein Data Bank44.
Results and discussion
Chemistry
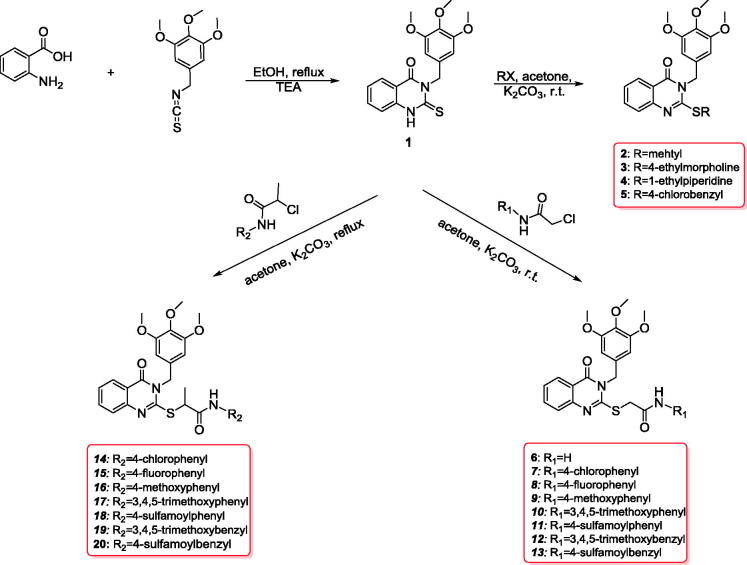
2-thioxo-3-(3,4,5-trimethoxybenzyl)-2,3-dihydroquinazolin-4(1H)-one (1) was obtained at 86% yield by heating 2-aminobenzoic acid with 3,4,5-trimethoxybenzyl isothiocyanate in ethanol containing triethylamine (Scheme 1). The confirmation of compound 1 exists as thione tautomer in the solid-state according to X-ray of quinazoline analogue45,46 due to the dimeric aggregates are connected into layers by C=H···O interactions, involving the bifurcated carbonyl-O atom, and C—H···S interactions45,46.
Scheme 1.
Synthesis of new quinazoline conjugates 1–20.
The thione tautomer was confirmed by presence of singlet signal at 13.04ppm, corresponding to NH group and unique signal at 175.6 ppm related to C=S according to 1H NMR and 13C NMR spectra. Additionally, NMR spectra of compound 1 revealed three characteristic signals related to trimethoxybenzyl group at 59.9, 55.8, 48.9, 5.60, 3.72, and 3.68 ppm. Accordingly, compound 1 was stirred at room temperature with various halides (such as methyl iodide, 4-(2-chloroethyl)morpholine, 1-(2-chloroethyl)piperidine, and 4-chlorobenzylchloride) in acetone containing potassium carbonate to give 2-(substituted alkylthio)-3-(3,4,5-trimethoxybenzyl)quinazolin-4(3H)-ones 2–5 analogues at 88–93% yield (scheme 1). The 1H NMR spectra of compounds 2–5 showed loss of the NH group of the parent compound at 13.04 ppm, and a new signal related to s-alkyl moiety was observed at 4.61–2.62 ppm in the 1H NMR spectra and at 14.7–35.8 ppm in the 13C NMR spectra of these compounds.
Compound 1 was also stirred with various 2-chloro-N-(substituted)acetamides and 2-chloro-N-(substituted)propanamides in acetone containing potassium carbonate to give N-(substituted)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)quinazolinon-2-yl)thio]acetamides 6–13 and N-(substituted)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)quinazolinon-2-yl)thio]propanamides 14–20 at 81–86% yield (Scheme 1).
Compounds 6–13 were confirmed based on their 1H NMR spectra, which showed the presence of singlet signals at 10.78–8.30 ppm and 4.26–4.01 ppm attributable to –SCH2CONH– and –SCH2CONH– groups, respectively, in addition to characteristic signals of trimethoxybenzyl moieties at 5.34–5.28 ppm and 3.82–3.61 ppm. Similarly, 13C NMR spectra showed the presence of signals for –SCH2CONH– at 36.9–35.6 ppm and –SCH2CONH– groups at 168.5–165.2 ppm, accompanied by the characteristic signals of a trimethoxybenzyl moiety at 60.8–47.1 ppm and the carbonyl group of the parent quinazoline moiety at 161.4–160.1 ppm.
Based on the 1H NMR spectra, compounds 14–20 were recognised by the presence of signals for SCH2CONH– at 10.78–8.93 ppm, –SCH(CH3)CONH– groups at 4.78–4.69 ppm, and a typical peak for a SCH(CH3)CONH– moiety at 1.63–1.57 ppm, in addition to the classic signal of a trimethoxybenzyl moiety at 5.25–3.62 ppm. Simultaneously, these compounds were confirmed based on their 13C NMR spectra, which showed signals of –SCH(CH3)CONH–, –SCH(CH3)CONH–, and SCH(CH3)CONH– groups at 45.6–46.6, 17.1–17.8, and 169.0–170.8ppm, respectively, as well as the definitive signals of the trimethoxybenzyl and carbonyl groups of the parent quinazoline moiety at 47.1–59.9 and 160.6–160.8, respectively.
X-ray crystallography
The crystallographic data and refinement information of compound 8 are summarised in Tables S1–S3. The asymmetric unit is comprised of one independent molecule as shown in Figures S1 and S2. All the bond lengths and angles are in normal ranges47. In the crystal structure, the central quinazolin-4(3H)-one plane makes dihedral angles of 62.97° and 68.48° with the trimethoxybenzyl and flurophenyl groups, respectively, in different directions. The crystal packing was formed by three intermolecular interactions between N3=H1N3•••O2, C9=H9A•••O1, and C9=H9B•••O2 with bond lengths 2.07 (3), 2.35, and 2.31 Å and bond angles 158(3)°, 143°, and 144°, respectively.
Antitumour activity
Evaluation of the in vitro antitumour activity of the new synthesised compounds indicated in Table 1 was performed by the National Cancer Institute, Bethesda, MA. A single dose (10 µM) of the test compounds 2–20 was used in the full NCI 60 Human Tumor Cell Line Panel assay37–41.
Table 1.
Percentage growth inhibition (GI %) of in vitro subpanel tumour cell lines at 10 µM concentration.
| % Growth Inhibition (GI %) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subpanel tumour cell lines | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 5-FU |
| Leukaemia | ||||||||||||||||||||
| CCRF-CEM | 12 | – | – | 24 | – | 17 | – | – | – | – | – | – | 21 | 14 | 23 | – | – | 65 | – | 57.1 |
| K-562 | 27 | nt | 13 | 38 | – | 55 | 14 | 21 | – | 21 | nt | – | 37 | 30 | 29 | 15 | 12 | 92 | nt | 42.3 |
| MOLT-4 | 12 | 17 | – | 51 | – | 54 | – | 32 | 11 | 12 | – | – | 38 | 51 | 39 | 22 | – | 82 | – | 43.1 |
| PRMI-8226 | 30 | – | – | 42 | – | 72 | – | 14 | – | – | – | – | 33 | 26 | 17 | 13 | – | 61 | – | 41.4 |
| SR | 17 | – | – | 44 | – | 54 | – | 18 | 34 | 12 | – | – | 39 | 44 | 24 | 37 | 20 | 89 | – | 24.8 |
| Non-small cell lung cancer | ||||||||||||||||||||
| A549/ATCC | – | – | – | 15 | – | 36 | – | – | 38 | – | – | – | 19 | 17 | 15 | 14 | 14 | 65 | 14 | 34.2 |
| HOP-62 | 35 | 13 | – | 11 | – | 46 | 36 | – | 58 | – | – | 16 | 15 | 18 | 16 | 21 | – | 71 | 64 | 47.8 |
| NCI-H226 | 26 | 13 | 12 | 37 | 13 | L | – | 12 | 43 | 34 | – | 12 | 18 | 21 | 23 | 27 | 21 | 30 | 21 | 69.5 |
| HOP-92 | – | – | – | 24 | – | 61 | – | 42 | 61 | – | – | – | 36 | 45 | 40 | 41 | 43 | 56 | 39 | 50.6 |
| NCI-H23 | – | – | – | 15 | 11 | 28 | 38 | – | 23 | – | – | – | 13 | – | 11 | – | – | 49 | – | 39.0 |
| NCI-H322M | – | – | – | – | – | 36 | – | – | 18 | – | – | – | 28 | 19 | 13 | 11 | – | 40 | – | 59.5 |
| NCI-H460 | – | – | – | – | 83 | – | – | 43 | – | – | – | – | – | – | – | – | 87 | 20 | 13.0 | |
| NCI-H522 | 29 | 22 | 32 | 49 | 11 | 60 | 27 | 44 | 67 | 30 | 19 | 17 | 51 | 47 | 40 | 32 | 30 | 90 | 62 | 58.0 |
| Colon cancer | ||||||||||||||||||||
| COLO 205 | – | – | – | 27 | – | – | – | – | – | – | – | – | – | – | – | – | – | 75 | – | 40.2 |
| HCC-2998 | – | nt | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 14 | 21 | >100 |
| HCT-116 | 18 | – | – | 44 | – | 65 | – | 11 | 42 | 14 | – | – | 44 | 35 | 31 | 16 | 24 | 84 | – | 17.8 |
| HCT-15 | – | – | – | 31 | – | 28 | – | 11 | – | – | – | – | 27 | 21 | 24 | 12 | – | 83 | – | 26.5 |
| HT29 | – | – | – | 41 | – | 13 | – | – | – | – | – | – | 13 | 14 | – | – | – | 88 | – | 27.1 |
| KM12 | – | – | – | – | – | 21 | – | – | – | – | – | – | – | – | – | – | – | 83 | – | 40.7 |
| SW-620 | – | – | – | 12 | – | 23 | – | – | – | – | – | – | – | – | – | – | – | 77 | – | 50.1 |
| CNS cancer | ||||||||||||||||||||
| SF-268 | – | – | 14 | – | – | 44 | 13 | 15 | 46 | – | – | 18 | 23 | 20 | 19 | 12 | – | 50 | 18 | 59.0 |
| SF-295 | 23 | – | – | – | – | 38 | – | 15 | – | – | 16 | 12 | – | – | – | 64 | – | 69.1 | ||
| SF-539 | 13 | – | – | – | – | L | – | 22 | 34 | – | – | – | 14 | 16 | 16 | – | – | 75 | 42 | >100 |
| SNB-19 | – | – | – | 19 | – | 37 | – | 26 | 16 | – | – | 21 | 20 | 23 | 18 | 17 | 45 | 33 | 65.9 | |
| SNB-75 | 22 | 15 | 27 | 34 | 12 | 73 | 52 | 34 | L | 27 | 11 | 21 | 26 | 20 | 36 | 34 | 36 | 83 | 77 | 65.9 |
| U251 | – | – | – | – | – | 25 | – | – | 48 | – | – | – | 12 | – | – | – | – | 62 | 14 | 50.3 |
| Melanoma | ||||||||||||||||||||
| LOX IMVI | – | – | – | 13 | – | 44 | – | – | – | – | – | – | 12 | – | – | – | – | 56 | – | 30.4 |
| MALME-3M | – | – | – | – | – | 91 | – | 14 | 18 | 12 | – | – | 17 | – | – | 17 | 13 | 60 | 13 | 58.2 |
| M14 | – | – | – | 14 | – | 26 | – | – | – | – | – | – | 14 | – | – | 11 | – | 94 | – | – |
| MDA-MB-435 | – | – | – | – | – | 31 | – | – | – | – | – | – | – | – | – | – | – | L | – | 36.6 |
| SK-MEL-2 | – | – | – | 25 | – | 19 | – | – | 25 | – | – | – | 19 | 19 | 17 | – | – | L | 15 | 95.5 |
| SK-MEL-28 | – | – | – | – | – | 17 | 12 | – | – | – | – | – | – | – | – | 13 | – | 45 | – | – |
| SK-MEL-5 | 20 | – | – | 15 | – | 51 | 11 | 13 | – | – | – | – | 12 | 16 | 18 | 20 | 18 | 88 | 21 | 33.7 |
| UACC-257 | – | – | – | – | – | 27 | – | – | – | – | – | – | – | – | – | 15 | 13 | 41 | 13 | 19.5 |
| UACC-62 | 25 | – | – | 32 | – | 37 | 15 | 26 | 16 | 23 | 14 | – | 31 | 24 | 32 | 27 | 26 | 64 | 21 | 39.7 |
| Ovarian cancer | ||||||||||||||||||||
| IGROV1 | – | – | – | 24 | – | 59 | – | – | 28 | 11 | – | – | 14 | 23 | 23 | 22 | 24 | 49 | – | 51.2 |
| OVCAR-4 | 34 | – | 30 | 37 | – | 47 | 28 | 18 | 11 | 15 | – | – | 23 | 15 | 30 | 26 | 23 | 42 | 12 | 59.4 |
| OVCAR-5 | – | – | – | – | – | 18 | – | – | 12 | – | – | – | – | – | – | – | – | 32 | – | 44.3 |
| OVCAR-8 | – | – | 15 | 15 | – | 38 | 20 | – | 20 | – | – | 13 | 15 | 16 | 14 | – | 14 | 52 | – | – |
| NCI/ADR-RES | 22 | – | – | 22 | – | 49 | – | 17 | – | – | – | – | 19 | 17 | 14 | – | – | 83 | – | 47.6 |
| SK-OV-3 | – | – | – | – | – | 32 | 13 | – | 49 | – | – | – | – | – | – | – | – | 73 | 43 | 77.5 |
| Renal cancer | ||||||||||||||||||||
| 786-0 | – | – | – | – | – | L | – | 27 | – | – | – | 11 | – | – | – | – | 44 | – | 48.7 | |
| A498 | – | – | – | – | 18 | 59 | – | 13 | 38 | 20 | 12 | – | 36 | 37 | 29 | 29 | – | 79 | – | >100 |
| ACHN | 19 | – | 13 | 15 | – | 82 | 17 | 20 | 67 | – | – | – | 16 | 19 | 15 | 15 | 18 | 52 | 13 | 39.3 |
| CAKI-1 | 25 | – | – | 22 | – | 29 | – | – | – | – | – | – | 29 | 26 | 14 | 11 | – | 66 | – | 39.4 |
| RXF 393 | – | – | – | – | – | 81 | 22 | 18 | 39 | – | – | – | 23 | 26 | 22 | 26 | – | 47 | 34 | 34.3 |
| SN12C | 20 | – | 12 | 31 | – | 29 | – | 21 | 18 | 12 | – | – | 28 | 18 | 25 | 18 | 16 | 50 | 15 | 54.0 |
| TK-10 | – | – | – | 0 | – | 33 | 16 | – | 33 | – | – | – | – | – | – | – | – | 38 | – | 66.9 |
| UO-31 | 41 | 32 | 16 | 48 | 27 | 83 | 20 | 27 | 20 | 25 | 28 | 13 | 52 | 47 | 46 | 49 | 31 | 53 | 17 | 41.3 |
| Prostate cancer | ||||||||||||||||||||
| PC-3 | 18 | – | – | 41 | 13 | 35 | – | 15 | 13 | – | – | – | 28 | 25 | 25 | 17 | 19 | 51 | – | 58.2 |
| DU-145 | – | – | – | – | – | 34 | – | – | – | – | – | – | – | – | – | – | 42 | – | 35.5 | |
| Breast cancer | ||||||||||||||||||||
| MCF7 | – | – | – | 21 | – | 23 | 13 | – | – | – | – | – | 16 | – | 13 | 11 | 18 | 85 | 17 | 11.5 |
| MDA-MB-231/ATCC | 25 | 14 | 11 | 36 | 13 | 51 | 14 | 27 | 42 | 19 | – | 19 | 36 | 35 | 37 | 37 | 30 | 54 | 35 | 78.1 |
| HS 578T | – | – | 13 | – | 93 | 13 | – | 52 | – | – | – | 20 | 14 | 12 | 20 | – | 43 | 22 | >100 | |
| BT-549 | – | – | – | – | 25 | – | 11 | 35 | – | – | – | 19 | – | – | 12 | – | 52 | 30 | 37.8 | |
| T-47D | 14 | – | 15 | 36 | – | 55 | – | 16 | 23 | – | 11 | – | 15 | 23 | 32 | 24 | – | 91 | 36 | 56.7 |
| MDA-MB-468 | 30 | – | – | 24 | – | 53 | 15 | – | 28 | 16 | – | 12 | – | 12 | 15 | 48 | 38 | 99 | 61 | – |
| MGI% | 11 | 2 | 4 | 19 | 2 | 47 | 7 | 10 | 24 | 7 | 2 | 1 | 19 | 17 | 16 | 15 | 10 | 65 | 16 | |
| PCE | 24/57 | 7/55 | 13/57 | 36/57 | 8/57 | 55/57 | 20/57 | 26/57 | 38/57 | 17/57 | 6/56 | 9/57 | 44/57 | 37/57 | 38/57 | 36/57 | 23/57 | 57/57 | 29/56 | 55/59 |
PCE: positive cytotoxic effect; the ratio between the number of cell lines with percentage growth inhibition >10% and total number of cell lines. MGI%: mean growth inhibition percentage; nt = not tested; L= >100%
The in vitro screening of compounds 2–20 at 10 µM showed that compounds 2, 4, 5, 7–11, and 14–20 exhibited remarkable antitumour activities against the tested cell lines with positive cytotoxic effects (PCE) of 24/57, 13/57, 36/57, 55/57, 20/57, 26/57, 38/57, 17/57, 44/57, 37/57, 38/57, 36/57, 57/57, 23/57, and 29/56, respectively, compared with that of 5-FU (55/59) (Table 1). Conversely, compounds 3, 6, 12, and 13 showed weak activities against the tested cell lines with PCE of 7/55, 8/57, 6/56, and 9/57, respectively (Table 1).
2-(Substituted alkylthio)-3-(3,4,5-trimethoxybenzyl)quinazolin-4(3H)-ones 2–5 and 2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamide (6) showed variable antitumour activities with MGI % of 2–19 (Table 1).
N-(Substituted)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamides 7–13 showed mild to potent antitumour activities with MGI % ranging from 7 to 47, while N-(substituted)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)quinazolinon-2-yl)thio]propanamides 14–20 showed potent antitumour activities with MGI % ranging from 10 to 65 (Table 1).
Compounds 3, 4, 6, 8, 11, 12, and 13 showed selective activity against different cancer cell lines. Compounds 3, 4, 11, 12, and 13 showed selective activity against the NCI-H522 cancer cell line, with a range of growth inhibition percentage (RGI %) of 17–32, while compounds 3, 6, 11, and 12 had selectivity against the UO-31 cancer cell line with RGI % of 25–32. The SNB-75 cancer cell line was sensitive to compounds 4, 11, and 12 with RGI % of 21–27, whereas the MDA-MB-468 cancer cell line was sensitive to compounds 11 and 13 with RGI % of 16–19. The A498 cancer cell line was sensitive to compounds 6 and 11 with RGI % of 18–20, while the K-562, NCI-H226, UACC-62, and MDA-MB-231/ATCC cancer cell lines were susceptible to compound 11 with RGI % of 19–34. The MOLT-4, OVCAR-4, and SF-268 cancer cell lines were susceptible to compounds 3, 4, and 13 with RGI % of 17–30. The prostate cancer cell line PC-3 showed selective sensitivity to compounds 2, 5, 7, and 14–18 with RGI % of 18–51; whereas compounds 7 and 19 showed selective activities with RGI % of 34–42 against the DU-145 prostate cell line (Table 1).
Furthermore, compounds 2, 5, 7, 9, 10, and 14–20 showed potent activity against leukaemia, NSL cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, and breast cancer cell lines with RGI % of 12–92, 16 –>100, 18–88, 13 –>100, 16 –>100, 16–83, 16 –>100, and 14–99, respectively (Table 1).
The MGI% data revealed that compounds 7 and 19 were the most active, with antitumour activity against numerous cell lines belonging to diverse tumour subpanels (Table 1). Therefore, these compounds were tested against a panel of 57 tumour cell lines at a 5-log dose range37–41 and the median growth inhibitory (GI50), total growth inhibitory (TGI), and median lethal (LC50) concentrations were calculated for each cell line (Table 2).
Table 2.
Median growth inhibitory (GI50, μM), total growth inhibitory (TGI, μM), and median lethal (LC50, μM) concentrations of compounds 7 and 19 on in vitro subpanel tumour cell lines.
| Subpanel tumour cell lines |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compd. | Activity | Leukaemia | NSC lung cancer | Colon cancer | CNS cancer | Melanoma | Ovarian cancer | Renal cancer | Prostate cancer | Breast cancer | MG-MIDa |
| 7 | GI50 | 68.28 | 8.11 | 30.82 | 4.33 | 12.26 | 8.86 | 5.76 | 17.40 | 5.30 | 17.90 |
| TGI | b | 40.56 | 85.81 | 15.28 | 57.06 | 37.30 | 30.41 | b | 30.46 | 55.20 | |
| LC50 | b | 80.22 | 89.76 | 46.10 | 78.12 | 80.95 | 64.80 | b | 84.75 | 80.52 | |
| 19 | GI50 | 4.57 | 8.95 | 5.47 | 4.62 | 5.25 | 8.07 | 6.62 | 9.03 | 4.47 | 6.33 |
| TGI | 93.44 | 63.45 | 73.35 | 63.85 | 49.74 | 56.50 | 65.06 | b | 70.50 | 70.65 | |
| LC50 | b | 96.36 | 88.55 | 64.62 | b | 92.75 | b | b | b | 93.58 | |
| 5-FU | GI50 | 15.10 | b | 8.40 | 72.10 | 70.60 | 61.40 | 45.60 | 22.70 | 76.40 | 18.60 |
| TGI | b | b | b | b | b | b | b | b | b | b | |
| LC50 | b | b | b | b | b | b | b | b | b | b | |
Full panel mean-graph midpoint (μM).
Compounds showed values >100 μM.
Compounds 7 and 19, compared with 5-FU, exhibited remarkable GI50 activities against leukaemia (68.28, 4.57, and 15.10 µM, respectively), NSL cancer (8.11, 8.95, and 100 µM), colon cancer (30.82, 5.47, and 8.40 µM), CNS cancer (4.33, 4.62, and 72.10 µM), melanoma cancer (12.26, 5.25, and 70.60 µM), ovarian cancer (8.86, 8.07, and 61.40 µM), renal cancer (5.76, 6.62, and 45.60 µM), prostate cancer (17.40, 9.03, and 22.70 µM), and breast cancer (5.30, 4.47, and 76.40 µM) (Table 2).
Additionally, comparing the median GI50 values (µM) of compounds 7 and 19 with those of 5-FU, gefitinib, and erlotinib against an in vitro subpanel of tumour cell lines showed that compounds 7 and 19 had activities almost equal to or higher than these known drugs against most cell lines (Table 3).
Table 3.
GI50 values (μM) of compounds 7 and 19 compared with those of erlotinib, gefitinib, and 5-FU on in vitro subpanel tumour cell lines.
| Subpanel tumour cell lines | GI50 (μM) | ||||
|---|---|---|---|---|---|
| 7 | 19 | Erlotinib | Gefitinib | 5-FU | |
| Leukaemia | |||||
| CCRF-CEM | 4.91 | >100 | 15.84 | 5.01 | 31.62 |
| HL-60(TB( | 3.57 | >100 | 5.01 | 5.01 | 19.95 |
| MOLT-4 | 7.38 | >100 | 5.01 | 3.98 | 12.58 |
| RPMI-8226 | 4.00 | 22.90 | 5.01 | 1.58 | 5.01 |
| SR | 3.01 | 18.05 | 6.30 | 3.16 | 3.98 |
| Non-small cell lung cancer | |||||
| A549/ATCC | 6.33 | 4.66 | 7.94 | 7.94 | 1.99 |
| HOP-62 | 4.36 | 3.10 | 12.58 | 10.00 | 19.95 |
| HOP-92 | 5.70 | 2.26 | 6.30 | 7.94 | >100 |
| NCI-H226 | 14.60 | 4.00 | 6.30 | 15.84 | >100 |
| NCI-H23 | 6.87 | 16.5 | 19.95 | 15.84 | 12.58 |
| NCI-H322M | 23.00 | 19.1 | 0.05 | 0.08 | 19.95 |
| NCI-H460 | 4.19 | 10.4 | 5.01 | 6.30 | 1.00 |
| NCI-H522 | 6.55 | 4.89 | 1.00 | 6.30 | 39.81 |
| Colon cancer | |||||
| COLO 205 | 5.32 | 73.20 | 31.62 | 6.30 | nt |
| HCC-2998 | 13.00 | 26.60 | 79.34 | 10.00 | nt |
| HCT-116 | 3.76 | 4.04 | 5.01 | 7.94 | nt |
| HCT-15 | 2.47 | 17.00 | 3.16 | 5.01 | nt |
| KM12 | 3.98 | 32.30 | 63.09 | 7.94 | nt |
| SW-620 | 4.31 | 31.80 | 5.01 | 7.94 | nt |
| CNS cancer | |||||
| SF-268 | 7.13 | 6.62 | 19.95 | 7.94 | nt |
| SF-295 | 4.36 | 5.55 | 15.84 | 1.99 | nt |
| SF-539 | 2.86 | 2.29 | 12.58 | 10.00 | nt |
| SNB-19 | 6.00 | 6.80 | 3.98 | 12.58 | nt |
| SNB-75 | 2.06 | 1.58 | 12.58 | 6.30 | nt |
| U251 | 5.34 | 3.14 | 19.95 | 10.00 | 79.43 |
| Melanoma | |||||
| LOX IMVI | 7.05 | 13.50 | 5.01 | 7.94 | 6.30 |
| M14 | 2.23 | 16.50 | 6.30 | 5.01 | 50.11 |
| MDA-MB-435 | 1.15 | 22.80 | 15.84 | 3.16 | 10.00 |
| SK-MEL-2 | 2.82 | 15.20 | 12.58 | 12.58 | >100 |
| SK-MEL-28 | 6.69 | 11.40 | 31.62 | 0.31 | 50.11 |
| SK-MEL-5 | 2.85 | 6.38 | 15.84 | 3.98 | 12.58 |
| UACC-257 | 16.30 | 6.04 | 100 | 6.30 | >100 |
| UACC-62 | 2.92 | 6.29 | 1.25 | 5.01 | 12.58 |
| Ovarian cancer | |||||
| IGROV1 | 13.20 | 19.00 | 0.25 | 0.20 | 15.84 |
| OVCAR-3 | 4.23 | 7.86 | 3.16 | 5.01 | 25.11 |
| OVCAR-4 | 10.80 | 3.01 | 19.95 | 7.94 | 79.43 |
| OVCAR-5 | 9.38 | 14.80 | 19.95 | 10.00 | >100 |
| OVCAR-8 | 10.00 | 4.82 | 7.94 | 10.00 | 19.95 |
| NCI/ADR-RES | 3.59 | 8.37 | 6.30 | 12.58 | 39.81 |
| SK-OV-3 | 5.34 | 4.20 | 0.39 | 0.63 | >100 |
| Renal cancer | |||||
| 786-0 | 5.10 | 3.37 | 5.01 | 7.94 | 12.58 |
| A498 | 4.05 | 2.42 | 1.58 | 0.40 | 10.00 |
| ACHN | 8.09 | 3.15 | 0.15 | 0.20 | 10.00 |
| CAKI-1 | 5.46 | 11.30 | 0.10 | 0.16 | 5.01 |
| RXF 393 | 5.31 | 2.59 | 6.30 | 5.01 | 50.11 |
| SN12C | 8.10 | 16.90 | 6.3 | 6.30 | 25.11 |
| TK-10 | 11.00 | 2.63 | 0.10 | 0.10 | >100 |
| UO-31 | 5.90 | 3.75 | 1.99 | 1.25 | 5.01 |
| Prostate cancer | |||||
| PC-3 | 10.80 | 16.70 | 50.11 | 0.79 | 5.11 |
| DU-145 | 7.27 | 18.10 | 1.58 | 2.51 | 50.11 |
| Breast cancer | |||||
| MCF7 | 3.46 | 8.97 | 100 | 10.00 | 1.99 |
| MDA-MB-231/ATCC | 5.36 | 3.08 | 1.99 | 12.58 | >100 |
| HS 578T | 5.24 | 3.95 | 6.30 | 10.00 | >100 |
| BT-549 | 4.90 | 6.42 | 39.81 | 7.94 | 100 |
| T-47D | 5.52 | 2.16 | 3.16 | 6.30 | 79.43 |
| MDA-MB-468 | 2.35 | 7.22 | 0.20 | 0.01 | 31.62 |
nt: not tested.
Structure-activity relationships
Structure activity relationships for antitumour activities with MGI % indicated that (i) 2-benzylmercapto-4(3H)-quinazolinone 5 showed higher antitumour activity (MGI%: 19%) than did the 2-alkylmercapto-4(3H)-quinazolinone derivatives such as compounds 2–4 (MGI%: 2–11%); (ii) N-(substituted phenyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamide analogues 7–11 (MGI%: 7–47%) and N-(substituted)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]propanamide analogues 14–20 are more active than unsubstituted 2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamide (6); (iii) the antitumour activity of N-(substituted)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]propanamide analogues 14–20 is improved compared to that of N-(substituted)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamide analogues 6–13 except compounds 7 and 10; (iv) the structure-activity correlation of N-(substituted)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamide analogues 6–13 revealed that N-(4-chlorophenyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamide (7) (MGI%; 47%) is more active than the corresponding N-(4-flourophenyl)acetamide 8 (MGI%; 7%); similarly, N-(3,4,5-trimethoxyphenyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamide (10) (MGI%; 24%) is more active than the corresponding N-(4-methoxyphenyl)acetamide 9 (MGI%; 10%). In addition, N-(4-methoxyphenyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamide (9) (MGI%: 10%) is more active than the corresponding N-(4-sulfamoylphenyl)acetamide 11 (MGI%: 7%); (v) The less active compounds in this series are N-(3,4,5-trimethoxybenzyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamide (12) (MGI%; 2%) and N-(4-sulfamoylbenzyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]acetamide (13) (MGI%: 1%). Additionally, structure-activity correlation of N-(substituted)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]propanamide analogues 14–20 indicates that: (i) N-(4-chlorophenyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]propanamide (14) (MGI%: 19%) is more active than the corresponding N-(4-flourophenyl)propanamide 16 (MGI%: 17%); (ii) N-(3,4,5-trimethoxyphenyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]propanamide (17) (MGI%: 15%) has the same antitumour activity as the corresponding N-(4-methoxyphenyl)propanamide 16 (MGI%: 16%); (iii) N-(4-methoxyphenyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]propanamide (16) (MGI%: 16%) is more active than the corresponding N-(4-sulfamoylphenyl)propanamide 18 (MGI%: 10%); (iv) N-(3,4,5-trimethoxybenzyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]propanamid (19) (MGI%: 65%) is more active than the corresponding N-(4-sulfamoylbenzyl)-2-[(3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinon-2-yl)thio]propanamid (20) (MGI%: 16%).
Molecular docking results
EGFR are tyrosine kinase enzymes that are overexpressed in numerous tumours such as colon, prostate, breast, ovarian, renal, and NSL cancers31–34,48. The inhibition of tyrosine kinase by quinazoline derivatives such as gefitinib and erlotinib (Figure 1) is well documented30,31. Accordingly, the antitumour activity of the target compounds against colon, prostate, breast, ovarian, renal, and NSL cancers encouraged us to study the molecular docking of the compounds into the putative binding site on EGFR kinase. In this study, the most active compounds 7 (mean GI50: 17.90 µΜ) and 19 (mean GI50:6.33 µΜ) were docked into the putative active site of EGFR kinase, as well as the reference inhibitor erlotinib (mean GI50: 7.29 µΜ)44. All docking calculations were performed using MOE 2007.09 software (MOE of Chemical Computing Group Inc., Montreal, Canada)42.
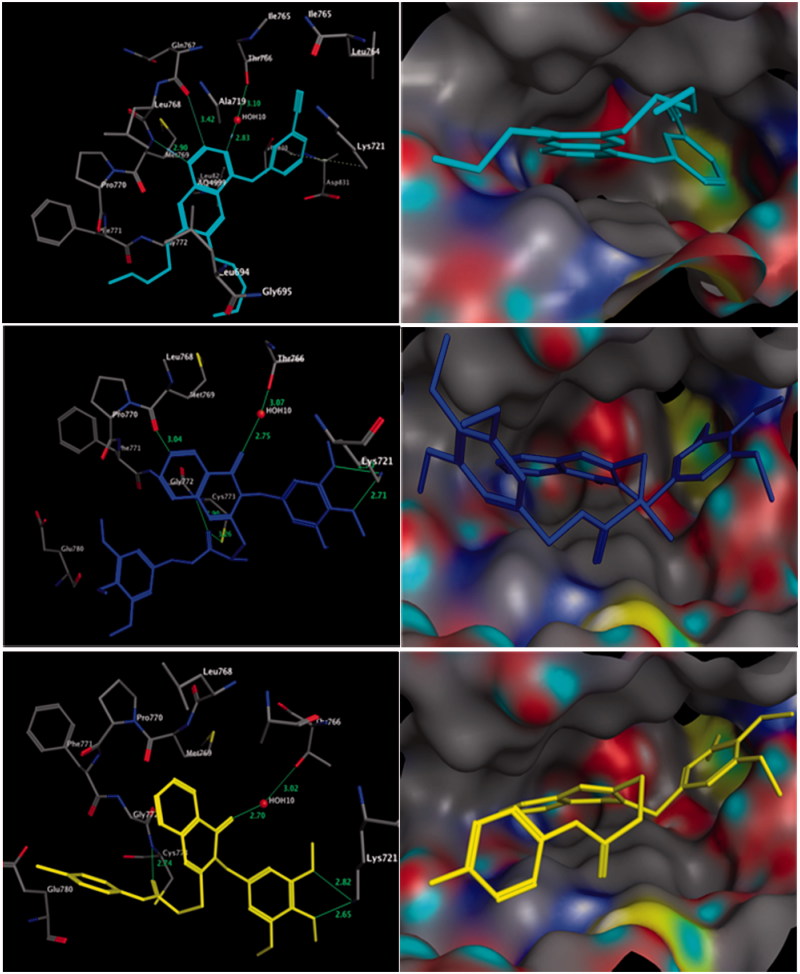
The binding energies of the docked compounds 7, 19, and erlotinib (PDB code; 1M17)44 into the putative binding site of EGFR were −22.11, −25.21, and −26.99 kcal/mol, respectively (Figure 2). The molecular docking of the most active compound 19 revealed that it had similar orientation to erlotinib inside the receptor pocket, as well as additional bonding interactions. The docking results showed six typical and atypical hydrogen bonds with surrounding amino acids as shown in Figure 2. The trimethoxybenzyl fragment at C-3 of the quinazoline core formed bifurcated hydrogen bonds with amino acids Lys721. Moreover, the 4-quinazolinone ring uniquely formed two hydrogen bonds with the distinctive residues Met769 and Thr766, similar to that observed in erlotinib (Figure 2). Additionally, the carbonyl group of the acetanilide fragment of compound 19 formed bifurcated hydrogen bonds with the amino acid residue Cys773 and Gly772 augmenting the recognition within the enzyme binding site (Figure 2 and Table 4).
Figure 2.
Three-dimensional (3D) interactions of erlotinib (upper panel), compounds 19 (middle panel) and 7 (lower panel) with the receptor pocket of EGFR kinase. Hydrogen bonds are shown with a green line.
Table 4.
Results of the docking of compounds 7 and 19 into EGFR (pdb: 1m17), in comparison to the co-crystallised ligand (erlotinib).
| Ligand no. | No. of HBsa | Atoms in H-bonding in the ligand | Atoms in H-bonding in protein | Lengthb (Å) |
|---|---|---|---|---|
| 7 | 4 | O of 3,4,5-timethoxyphenyl | NH of Lys721 | 2.65, 2.82 |
| O of quinazoline-4-one | HOH10 linked to Thr766 | 2.70 | ||
| O of carbonyl anilide | NH of Gly772 | 2.74 | ||
| 19 | 6 | O of 3,4,5-timethoxyphenyl | NH of Lys721 | 2.70, 2.71 |
| O of quinazoline-4-one | HOH10 linked to Thr766 | 2.75 | ||
| O of carbonyl anilide | NH of Gly772 | 2.90 | ||
| O of carbonyl anilide | SH of Cys773 | 3.26 | ||
| Ar-H of quinazoline | O of Pro770 | 3.04 | ||
| Erlotinib | 4 | N1 of quinazoline | NH of Met769 | 2.90 |
| N3 of quinazoline | HOH10 linked to Thr766 | 2.83 | ||
| Ar-H of quinazoline | NH of Leu768 | 3.42 | ||
| 6-ring of anilino group | NH of Lys721 | 4.58 |
HBs: hydrogen bonds;
Length among acceptor and donner atoms in angstrom (Å).
Similar to compound 19, compound 7 binds with four hydrogen bonds. It was found that the trimethoxybenzyl group at C-3 of the quinazoline core was clearly recognised with hydrogen bonding to the amino acid residue Lys721 similar to compound 19, while the quinazoline core was shifted away from the distinctive amino acid residue Met769 (Figure 2). Additionally, two hydrogen bonds with the amino acid residue Gly772 and the distinctive residue Thr766 were found (Figure 2). It is obvious that the molecular docking results can be used to design novel quinazoline derivatives with potential binding to EGFR kinase and antitumour activity (Table 4).
Conclusions
A novel series of 2-substituted mercapto-3-[3,4,5-trimethoxybenzyl]-4(3H)-quinazolinones 1–20, was synthesised and evaluated for in vitro antitumour activity. Compounds 7 and 19 showed strong antitumour activities with mean GI50 values of 17.90 and 6.33 µM, TGI of 55.20 and 70.65 µM, and LC50 of 80.52 and 93.58 µM; these values were compared with the reference drug 5-FU (GI50: 22.60 µM, TGI: 100 µM, and LC50: 100 µM). Comparing the median GI50 (µM) of 5-FU, gefitinib, and erlotinib with that of compounds 7 and 19 showed that compounds 7 and 19 showed antitumour activities almost equal to or higher than that of the known drugs against most subpanel tumour cell lines. A molecular docking study for compounds 7 and 19 into the ATP binding site of EGFR-TK showed similar binding as that of erlotinib.
Supplementary Material
Funding Statement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RG-1435–046.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Wei G, Cui S, Luan W, et al. Natural product-based design, synthesis and biological evaluation of Albiziabioside A derivatives that selectively induce HCT116 cell death. Eur J Med Chem 2016;113:92–101. [DOI] [PubMed] [Google Scholar]
- 2.El-Azab AS, Al-Omar MA, Alaa A-M, et al. Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: molecular docking study. Eur J Med Chem 2010;45:4188–98. [DOI] [PubMed] [Google Scholar]
- 3.Al-Obaid A, Abdel-Hamide S, El-Kashef H, et al. Substituted quinazolines, part 3. Synthesis, in vitro antitumor activity and molecular modeling study of certain 2-thieno-4(3H)-quinazolinone analogs. Eur J Med Chem 2009;44:2379–91. [DOI] [PubMed] [Google Scholar]
- 4.Al-Omary FA, Abou-Zeid LA, Nagi MN, et al. Non-classical antifolates. Part 2: synthesis, biological evaluation, and molecular modeling study of some new 2, 6-substituted-quinazolin-4-ones. Bioorg Med Chem 2010;18:2849–63. [DOI] [PubMed] [Google Scholar]
- 5.El-Azab AS, ElTahir KE.. Synthesis and anticonvulsant evaluation of some new 2,3,8-trisubstituted-4 (3H)-quinazoline derivatives. Bioorg Med Chem Lett 2012;22:327–33. [DOI] [PubMed] [Google Scholar]
- 6.Alafeefy AM, Kadi AA, El‐Azab AS, et al. Synthesis, analgesic and anti-inflammatory evaluation of some new 3H-quinazolin-4-one derivatives. Archiv Der Pharmazie 2008;341:377–85. [DOI] [PubMed] [Google Scholar]
- 7.El-Azab AS, ElTahir KE.. Design and synthesis of novel 7-aminoquinazoline derivatives: antitumor and anticonvulsant activities. Bioorg Med Chem Lett 2012;22:1879–85. [DOI] [PubMed] [Google Scholar]
- 8.El-Azab AS.Synthesis of some new substituted 2-mercaptoquinazoline analogs as potential antimicrobial agents. Phosphorus Sulfur Silicon Relat Elem 2007;182:333–48. [Google Scholar]
- 9.Aziza M, Nassar M, AbdelHamide S, et al. Synthesis and antimicrobial activities of some new 3-heteroaryl-quinazolin-4-ones. Indian J Heterocycl Chem 1996;6:25–30. [Google Scholar]
- 10.Alanazi AM, Alaa A-M, Al-Suwaidan IA, et al. Design, synthesis and biological evaluation of some novel substituted quinazolines as antitumor agents. Eur J Med Chem 2014;79:446–54. [DOI] [PubMed] [Google Scholar]
- 11.Al-Suwaidan IA, Alanazi AM, Alaa A-M, et al. Design, synthesis and biological evaluation of 2-mercapto-3-phenethylquinazoline bearing anilide fragments as potential antitumor agents: molecular docking study. Bioorg Med Chem Lett 2013;23:3935–41. [DOI] [PubMed] [Google Scholar]
- 12.El-Azab AS, Abdel-Hamide SG, Sayed-Ahmed MM, et al. Novel 4 (3H)-quinazolinone analogs: synthesis and anticonvulsant activity. Med Chem Res 2013;22:2815–27. [Google Scholar]
- 13.Alafeefy AM, El-Azab AS, Mohamed MA, et al. Synthesis of some new substituted iodoquinazoline derivatives and their antimicrobial screening. J Saudi Chem Soc 2011;15:319–25. [Google Scholar]
- 14.Alanazi AM, Al-Suwaidan IA, Alaa A-M, et al. Design, synthesis and biological evaluation of some novel substituted 2-mercapto-3-phenethylquinazolines as antitumor agents. Med Chem Res 2013;22:5566–77. [Google Scholar]
- 15.Al-Suwaidan IA, Abdel-Aziz AA-M, Shawer TZ, et al. Synthesis, antitumor activity and molecular docking study of some novel 3-benzyl-4 (3H) quinazolinone analogues. J Enzyme Inhib Med Chem 2016;31:78–89. [DOI] [PubMed] [Google Scholar]
- 16.Alaa A-M, Abou-Zeid LA, ElTahir KEH, et al. Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4 (3H)-quinazolinones. Eur J Med Chem 2016;121:410–21. [DOI] [PubMed] [Google Scholar]
- 17.Alaa A-M, Abou-Zeid LA, ElTahir KEH, et al. Design, synthesis of 2, 3-disubstitued 4 (3H)-quinazolinone derivatives as anti-inflammatory and analgesic agents: COX-1/2 inhibitory activities and molecular docking studies. Bioorg Med Chem 2016;24:3818–28. [DOI] [PubMed] [Google Scholar]
- 18.Alanazi AM, Abdel-Aziz AA, Shawer TZ, et al. Synthesis, antitumor and antimicrobial activity of some new 6-methyl-3-phenyl-4(3H)-quinazolinone analogues: in silico studies. J Enzyme Inhib Med Chem 2016;31:721–35. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed MA, Ayyad RR, Shawer TZ, et al. Synthesis and antitumor evaluation of trimethoxyanilides based on 4 (3H)-quinazolinone scaffolds. Eur J Med Chem 2016;112:106–13. [DOI] [PubMed] [Google Scholar]
- 20.Gawad NMA, Georgey HH, Youssef RM, El-Sayed NA.. Synthesis and antitumor activity of some 2, 3-disubstituted quinazolin-4 (3H)-ones and 4, 6-disubstituted-1, 2, 3, 4-tetrahydroquinazolin-2H-ones. Eur J Med Chem 2010;45:6058–67. [DOI] [PubMed] [Google Scholar]
- 21.Alagarsamy V, Solomon VR, Dhanabal K.. Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H-quinazolin-4-one as analgesic, anti-inflammatory agents. Bioorg Med Chem 2007;15:235–41. [DOI] [PubMed] [Google Scholar]
- 22.Harris NV, Smith C, Bowden K. Antifolate and antibacterial activities of 5-substituted 2,4-diaminoquinazolines. J Med Chem 1990;33:434–44. [DOI] [PubMed] [Google Scholar]
- 23.Kashaw SK, Kashaw V, Mishra P, et al. Synthesis, anticonvulsant and CNS depressant activity of some new bioactive 1-(4-substituted-phenyl)-3-(4-oxo-2-phenyl/ethyl-4H-quinazolin-3-yl)-urea. Eur J Med Chem 2009;44:4335–43. [DOI] [PubMed] [Google Scholar]
- 24.Hennequin LF, Stokes ES, Thomas AP, et al. Novel 4-anilinoquinazolines with C-7 basic side chains: design and structure activity relationship of a series of potent, orally active, VEGF receptor tyrosine kinase inhibitors. J Med Chem 2002;45:1300–12. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Sharma S, Bajaj K, et al. Some new 2, 3, 6-trisubstituted quinazolinones as potent anti-inflammatory, analgesic and COX-II inhibitors. Bioorg Med Chem 2003;11:5293–9. [DOI] [PubMed] [Google Scholar]
- 26.Wissner A, Berger DM, Boschelli DH, et al. 4-Anilino-6,7-dialkoxyquinoline-3-carbonitrile inhibitors of epidermal growth factor receptor kinase and their bioisosteric relationship to the 4-anilino-6,7-dialkoxyquinazoline inhibitors. J Med Chem 2000;43:3244–56. [DOI] [PubMed] [Google Scholar]
- 27.Noolvi MN, Patel HM, Bhardwaj V, Chauhan A.. Synthesis and in vitro antitumor activity of substituted quinazoline and quinoxaline derivatives: search for anticancer agent. Eur J Med Chem 2011;46:2327–46. [DOI] [PubMed] [Google Scholar]
- 28.Paul K, Sharma A, Luxami V.. Synthesis and in vitro antitumor evaluation of primary amine substituted quinazoline linked benzimidazole. Bioorg Med Chem Lett 2014;24:624–9. [DOI] [PubMed] [Google Scholar]
- 29.Smits RA, Adami M, Istyastono EP, et al. Synthesis and QSAR of quinazoline sulfonamides as highly potent human histamine H4 receptor inverse agonists. J Med Chem 2010;53:2390–400. [DOI] [PubMed] [Google Scholar]
- 30.Baselga J, Averbuch SD.. ZD1839 (‘Iressa’) 1, 2 as an anticancer agent. Drugs 2000;60:33–40. [DOI] [PubMed] [Google Scholar]
- 31.Steeghs N, Nortier JW, Gelderblom H.. Small molecule tyrosine kinase inhibitors in the treatment of solid tumors: an update of recent developments. Ann Surg Oncol 2007;14:942–53. [DOI] [PubMed] [Google Scholar]
- 32.Fricker J.Tyrosine kinase inhibitors: the next generation. Lancet Oncol. 2006;7:621. [DOI] [PubMed] [Google Scholar]
- 33.Garofalo S, Rosa R, Bianco R, Tortora G.. EGFR-targeting agents in oncology. Expert Opin Ther Pat 2008;18:889–901. [Google Scholar]
- 34.Cockerill G, Lackey KE.. Small molecule inhibitors of the class 1 receptor tyrosine kinase family. Curr Top Med Chem 2002;2:1001–10. [DOI] [PubMed] [Google Scholar]
- 35.Sheldrick GM.A short history of SHELX. Acta Crystallogr 2008;64:112–22. [DOI] [PubMed] [Google Scholar]
- 36.Sheldrick G. SHELXTL-PC V5. 1. Bruker Analytical X-ray Systems, Madison, WI, USA; 1997. [Google Scholar]
- 37.Grever MR, Schepartz SA, Chabner BA. The National Cancer Institute: cancer drug discovery and development program. Semin Oncol. 1992;19:622–38. [PubMed] [Google Scholar]
- 38.Monks A, Scudiero D, Skehan P, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Nat Cancer Inst 1991;83:757–66. [DOI] [PubMed] [Google Scholar]
- 39.Boyd MR, Paull KD.. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res 1995;34:91–109. [Google Scholar]
- 40.Alley MC, Scudiero DA, Monks A, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 1988;48:589–601. [PubMed] [Google Scholar]
- 41.Shoemaker RH.The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 2006;6:813–23. [DOI] [PubMed] [Google Scholar]
- 42.MOE 2007.9 of Chemical Computing Group. Accessed 15 May 2017. [Google Scholar]
- 43.Al-Suwaidan IA, Alanazi AM, El-Azab AS, et al. Molecular design, synthesis and biological evaluation of cyclic imides bearing benzenesulfonamide fragment as potential COX-2 inhibitors. Part 2. Bioorg Med Chem Lett 2013;23:2601–5. [DOI] [PubMed] [Google Scholar]
- 44.Stamos J, Sliwkowski MX, Eigenbrot C.. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem 2002;277:46265–72. [DOI] [PubMed] [Google Scholar]
- 45.El-Azab AS, Abdel-Aziz A-M, Ng SW, Tiekink ER. 6-Methyl-3-phenyl-2-sulfanyl-idene-1,2,3,4-tetra-hydro-quinazolin-4-one. Acta Crystallogr Sect E Struct Rep Online 2012;68:o862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashim NM, Osman H, Rahim AA, et al. 6-Chloro-3-phenethyl-2-thioxo-2,3-di-hydro-quinazolin-4(1H)-one. Acta Crystallogr Sect E Struct Rep Online 2010;66:o950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen FH, Kennard O, Watson DG, et al. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J Chem Soc Perkin Trans 2 1987;0:S1–S19. [Google Scholar]
- 48.Fricker J.Tyrosine kinase inhibitors: the next generation. Lancet Oncol 2006;7:621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.