Abstract
In explicit sequence learning tasks an improvement in performance (skill) typically occurs after sleep – leading to the recent literature on sleep-dependent motor consolidation. Consolidation can also be facilitated during wakefulness if declarative knowledge for the sequence is reduced through a secondary cognitive task. Accordingly, declarative and procedural consolidation processes appear to mutually interact. Here we used TMS to test the hypothesis that functions in the dorsolateral prefrontal cortex (DLPFC) that support declarative memory formation, indirectly reduce the formation of procedural representations. We hypothesize that disrupting the DLPFC immediately after sequence learning would degrade the retention or consolidation of the sequence within the declarative memory system and thus facilitate consolidation within procedural memory systems, evident as wakeful off-line skill improvement. Inhibitory theta-burst TMS was applied to the left DLPFC (n=10), right DLPFC (n=10) or to an occipital cortical control site (n=10) immediately after training on the serial reaction time task (SRTT). All groups were retested after 8 daytime hours without sleep. TMS of either left or right DLPFC lead to skill improvements on the SRTT. Increase in skill was greater following right DLPFC stimulation than left DLPFC stimulation; there was no improvement in skill for the control group. Across all participants, free-recall of the sequence was inversely related to the improvements in performance on the SRTT. These results support the hypothesis of interference between declarative and procedural consolidation processes, and are discussed in the framework of the interactions between memory systems.
Introduction
Improvements in performance - skill - can result from both declarative and procedural learning (see Hazeltine & Ivry, 2002 for review). The serial reaction time task (SRTT) can be used to probe the representation of skill (see Ivry, 1996 for review; Keele, 1968; Keele, Ivry, Mayr, Hazeltine, & Heuer, 2003; Povel & Collard, 1982) and exposes differences between the neural substrates of declarative and procedural memory systems (Grafton, Hazeltine, & Ivry, 1995; Hazeltine, Grafton, & Ivry, 1997; Willingham, 1997). Individuals with brain lesions provide further evidence that the neural mechanisms underlying declarative and procedural knowledge are distinct (Knowlton, Mangels, & Squire, 1996; Reber, Knowlton, & Squire, 1996; Squire & Knowlton, 1995; see Willingham, 1998 for review). More recently, the SRTT has been used to study memory acquisition and its subsequent consolidation within these two systems (Brown & Robertson, 2007a, 2007b; Robertson, 2007; Willingham, Salidis, & Gabrieli, 2002).
Declarative memory in the SRTT is typically demonstrated by verbal or written recall of the learned sequence; procedural memory is shown by faster performance – improved motor skill (Robertson, 2007). If declarative knowledge of the sequence is acquired, an improvement in motor performance is seen after a period of sleep whereas there is no significant improvement in motor skill after an equivalent period of wakefulness (Walker, Brakefield, Morgan, Hobson, & Stickgold, 2002). Importantly, declarative knowledge has been shown to either reduce or completely inhibit procedural memory consolidation over a period of wakefulness (Brown & Robertson, 2007b; Spencer, Sunm, & Ivry, 2006; Walker et al., 2002). Interestingly, when a secondary cognitive task that interferes with verbal recall of the sequence is performed immediately after the SRTT, to disrupt declarative processing of the sequence, skill improvements are observed without sleep (Brown & Robertson, 2007a). In other words, declarative memory systems appear to inhibit the enhancement of motor skill during wakefulness.
The SRTT has been shown to recruit a network that includes the dorsal premotor cortex, superior parietal cortex, dorsal prefrontal cortex (DLPFC), supplementary motor area, basal ganglia, cerebellum and hippocampus (Grafton et al., 1995; Grafton, Hazeltine, & Ivry, 2002; Hazeltine et al., 1997; Olson et al., 2006; Torriero, Oliveri, Koch, Caltagirone, & Petrosini, 2004; Torriero et al., 2007; van der Graaf, Maguire, Leenders, & de Jong, 2006; Willingham et al., 2002). When the participant is aware of the sequence, learning-related activation is found in the DLPFC, premotor cortex, basal ganglia, and temporal lobe (all of the right hemisphere) and bilaterally in the parieto-occipital junction (Grafton et al., 1995; Hazeltine et al., 1997), although (Willingham et al., 2002) suggest that the increased DLPFC recruitment is not strongly right lateralized. In contrast, sequence learning that occurs without awareness for the sequence’s presence leads to recruitment of the contralateral motor cortex, supplementary motor area and basal ganglia (Grafton et al., 1995; Hazeltine et al., 1997).
In addition there is an abundance of evidence of the DLPFC having a role in conscious executive processes (D'Esposito et al., 1995; Goldman-Rakic, 1995; Miller & Cohen, 2001; Passingham & Sakai, 2004; Petrides, 1991; Rowe & Passingham, 2001; Sakai, Rowe, & Passingham, 2002). The DLPFC also has strong connections to the less accessible structures within the medial temporal lobe (Goldman-Rakic, Selemon, & Schwartz, 1984) known to underlie declarative memory (see Squire, 1992 for review). Accordingly, processing in the DLPFC is likely to be critical for the acquisition or encoding of declarative representation of sequences (Murray & Ranganath, 2007).
Here we aim to demonstrate that engagement of DLPFC in the declarative component of an explicit SRTT inhibits procedural consolidation. We propose that transcranial magnetic stimulation (TMS) of the DLPFC should disrupt its processing of the declarative knowledge of the sequence, and lead to sequence performance improvements - even during wakefulness.
At first glance, improving consolidation by disrupting an area known to support memory is counterintuitive. There are two viable explanations for this prediction. One possibility is that the DLPFC could have direct inhibitory connections with one or more brain areas critical for procedural consolidation. In this direct disinhibition model, disrupting the DLPFC disinhibits procedural memory systems, and thereby facilitates consolidation. Alternatively, declarative memory processes supported by the DLPFC may compete for resources in some other brain area that are necessary for consolidation. The recruitment of such multi-function brain areas by a declarative memory system could be in competition with adjunctive demands of a procedural memory system. In this competition suppression model, disrupting the DLPFC may eliminate or reduce its impact as a resource competitor, leaving more resources to be recruited by the procedural memory system. Thus we propose we should expose wakeful procedural consolidation by disrupting declarative consolidation, but cannot aim to distinguish between these two mechanisms.
Method
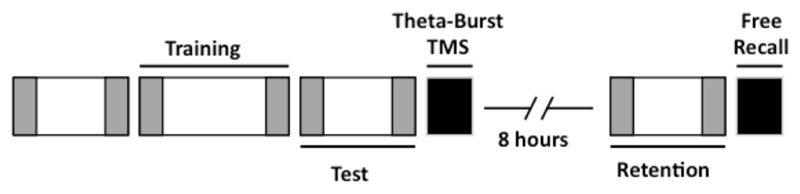
Participants were trained in the morning on the SRTT, using an explicitly cued 12-item sequence. Immediately after, they were exposed to 40 seconds of continuous theta-burst TMS (cTBS) over the left or right DLPFC. Eight hours later participants were re-tested on their retention of the SRTT (Figure 1) and their declarative knowledge of the sequence was probed. Performance was compared with a control group that received cTBS over the occipital cortex (OC). The reduction in mean reaction time (RT) for sequentially-ordered versus randomly-ordered button presses was then compared between test and retention sessions to assess daytime consolidation.
Figure 1. Experimental design.
Participants were trained and tested on the SRTT (9 am). They were given cTBS for 40 seconds, over the left DLPFC, right DLPFC or OC. After 8 hours of wakefulness the participant’s retention of the SRTT was tested and they were also asked to recall the sequence. Changes in reaction time to random (grey boxes) and sequential (white boxes) blocks from training to test were used as a measure of offline improvement. Skill was assessed by the mean difference between reaction times on random and sequence trials, divided by the mean reaction time on random trials.
Participants
Thirty right-handed (self-assessed) participants were randomly assigned to either the left DLPFC (5 women and 5 men; age = 23.1 ± 3.7 years, mean ± SD), right DLPFC (6 women, 4 men; age = 23.3 ± 3.1 years) or the occipital cortex (OC) control group (4 women and 6 men; age = 24.5 ± 4.3 years). Informed written consent was obtained from all participants, and all participants were blind to the hypothesis under investigation. Participants chose to receive either financial compensation or course credits for their participation. All procedures were approved by the University of Birmingham Ethics Committee.
Experimental design
Participants were trained at 9 am and retested at 5pm on the same day. During the morning training period, they practiced a sequence learning task (the SRTT, see below) which consisted of one instructional, one training and one test session (Figure 1). Following the completion of the test session, participants received 40 seconds of cTBS to the left DLPFC, right DLPFC or occipital cortex (OC). Eight hours later participants returned and completed the retention session of the SRTT and a written free-recall test. All participants were questioned about their daytime activity; none reported having slept.
Serial reaction time task (SRTT)
Participants learned a 12-item sequence using their right-hand (2-3-1-4-3-2-4-1-3-4-2-1; Brown and Robertson; 2007b). They viewed a computer monitor displaying a solid black square cue (side 30 mm) appearing at one of four horizontal positions against a white background. These 4 positions corresponded to the four buttons on a custom response pad, upon which the participant’s dominant right hand rested. Once each visual cue had been presented the participants were instructed to press the appropriate button as quickly as possible. Once the correct button had been given the next cue was shown after a delay of 400 ms. Participants were instructed that a change in color of the visual cue from black to blue indicated the onset of the sequence. It has been previously shown that this design engages both declarative and procedural learning (Willingham et al., 2002).
The instructional, test and retention sessions all included 9 repetitions (108 trials) of the sequence preceded and followed by 50 random trials. The morning training session included 15 repetitions (180 trials of the sequence, also preceded and followed by 50 random trials (Figure 1).
Continuous Theta-burst TMS (cTBS)
TMS was delivered using a 70mm diameter figure-eight coil (Rapid2 stimulator; Magstim, Whitland, UK). The coil was placed tangentially to the scalp with the handle pointing posterior-laterally at a 45° angle with respect to the anterior-posterior axis for DLPFC placement and at 0° for OC placement. Theta-burst TMS (TBS) consists of repeating bursts of stimuli. Each burst consists of three stimuli repeating at 50 Hz; bursts are repeating at 5 Hz. Continuous TBS was applied for 40 seconds at 80% of the participant’s active motor threshold (Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005). Active motor threshold was defined as the lowest intensity able to elicit a visible twitch in the right first dorsal interosseus (FDI) muscle in response to 5 out of 10 single pulse stimuli. In comparison to 1Hz repetitive TMS, cTBS seems to have a greater inhibitory affect in terms of magnitude and longevity (Huang et al., 2005; cf. Muellbacher, Ziemann, Boroojerdi, & Hallett, 2000).
Using a 3T Philips scanner, high-resolution T1-weighted images were acquired for both DLPFC groups (1×1×1 mm voxel size, 175 slices in sagittal orientation). The MNI coordinates previously reported by Willingham et al. (2002) lead us to the target location of x=-40/+40, y=28, z=18 for the left and right DLPFC. To relate this standard position to individual participants, each high resolution structural scan was normalized to the MNI brain using an affine (12 parameter) transformation using FLIRT (Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001). The inverse transformation for each participant was then applied to the standard coordinates, to identify the subject-specific target location for each participant. BrainSight (Rogue Research, Montreal, Quebec, Canada) was used to locate this position and place the TMS coil; the coil was positioned so that the electrical gradient traveled dorsal-laterally from the target along the mid-frontal gyrus. The occipital cortex (OC) was located by placing the coil over the left occipital cortex guided by the inion, at a position where most participants observed phosphenes at a stimulation intensity of 70% of maximal output (Franca, Koch, Mochizuki, Huang, & Rothwell, 2006). As cTBS was applied after the SRTT, occipital stimulation could not degrade visual performance during the task.
Free recall
Participants were asked to write down the as much of the sequence as they could using the numbers from 1 to 4 (Brown & Robertson, 2007a). Each number corresponded to a button on the box (index finger button = 1) and to the corresponding position on the screen (left =1). For each reported number to be scored as correct it had to lie within a sequence of at least three correct responses (Brown & Robertson, 2007a; Willingham & Goedert-Eschmann, 1999).
Data Analysis
All incorrect responses and responses of trials that immediately followed an error were removed; however, the inclusion of these “after-error” or “transition” trials does not qualitatively alter the results reported below. Serial reaction times (SRT) were then measured as the time taken to make correct responses in trials that also followed a correct response, within either a sequentially or randomly ordered blocks. For each block any SRT longer than 2.7 standard deviations from a participant’s mean for the block type (sequential or random) was also removed (Brown & Robertson, 2007a). To ensure that carry over effects from the preceding block did not influence results the initial 6 trials from each block were also removed. The mean SRT was then calculated for each participant in the test (morning) and retention (afternoon) sessions (Figure 1). Separate mixed-effects ANOVAs compared block (random vs. sequence), session (test vs. retention) and group (L-DLPFC vs. R-DLPFC vs. OC) for speed (based upon RT) and accuracy (based upon the number of incorrect button presses). Levene tests were used to ensure normality of the data across groups. Paired-sample t-tests compared the group’s changes in reaction times from the test to the retention session, separately for random and sequential responses.
Additionally, to measure change in skill, the relative difference between SRT on random and sequence blocks was recorded. A relative SRT (rSRT) measure was defined as the difference between means for the random and the sequence trials, divided by the mean of the random trials, i.e. (random - sequence)/random. Skill, based upon the rSRT, was therefore indexed between 0 (indicating no learning of the sequence) and 1 (which is approached as the SRT for the sequence trials approaches zero). For each participant skill during the retention session was subtracted by skill during test, resulting in a measure of the change in performance attributable to sequence-specific learning. An ANOVA was used to compare the change in skill between groups (L-DLPFC vs. R-DLPFC vs. OC) with unpaired t-tests used to explore a significant result. Sequence recall was compared between groups with an ANOVA. A Pearson correlation was performed between skill improvements and sequence recall, across all 30 participants.
Results
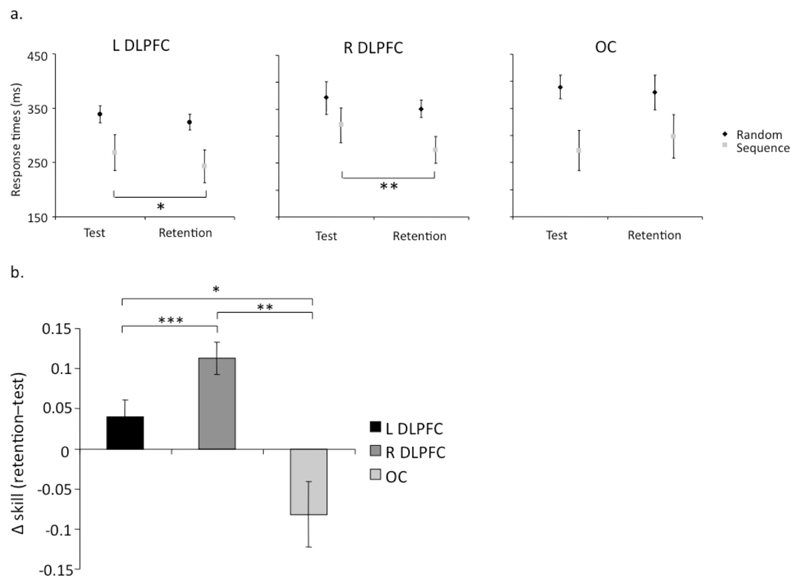
Serial reaction times (SRT) were shorter for sequence blocks than random blocks in the morning test session, showing the expected skill acquisition in all groups (Figure 2a). When tested in the evening retention session, there was a modest reduction in reaction times for the random blocks, in all three groups. However there were clear differences in performance in the sequence blocks between morning and evening, showing differential consolidation across the three groups.
Figure 2. Changes in reaction time and skill.
(a) Participants performance in the test and retention sessions for sequential (grey) and random (black) blocks within the L DLPFC, R DLPFC and OC groups. (b) Change in relative SRT between test and retention sessions. Stimulation of either DLPFC lead to significantly greater consolidation relative to the occipital control site. Additionally right DLPFC stimulation lead to significantly greater improvement than left DLPFC stimulation. Positive values reflect a reduction in RT in retention (i.e. retention - test). In both (a) and (b), * indicates p≤0.05, ** for p≤0.01, and *** for p≤0.001.
To explore these differences, SRTs were analyzed with a mixed-model ANOVA comparing the two block types (random and sequential), the two sessions (test and retention) and the three groups (defined by stimulation site). There was a main effect of block type (F(1,27) = 47.38, p < 0.001), with significantly faster performance occurring on the sequential trials (mean ± SEM: 280 ± 19ms v. 356 ± 12ms). There was a near-significant trend towards faster SRTs in the retention session (mean ± SEM: test = 327 ± 16ms and 309 ± 16ms; F(1,27)=3.68, p=0.06), and trends towards group interactions with session (F(2,27)=2.77, p=0.08) and block type (F(2,27)=2.93, p=0.07). However, as predicted by our hypothesis, there was a reliable three-way interaction between group, session and block type (F(2,27) = 12.27, p < 0.001), consistent with our hypothesis. Paired-sample, two-tailed, t-tests were used to explore the origins of this 3-way interaction.
For the OC group there was no significant change in mean RT from test to retention for either random (mean ± SEM = 8 ± 22 ms; t(9)=0.39, p=0.7) or sequential blocks (mean ± SEM = -29 ± 28 ms; t(9)=1.04, p=0.33). The two groups that received DLPFC stimulation did not change their performance for the random blocks (mean ± SEM: L-DLPFC = 14 ± 11 ms; R-DLPFC = 24 ± 11 ms; t(9)=1.20, p=0.26 and t(9)=2.05, p=0.07, respectively), but improved significantly for sequence blocks (mean ± SEM: L-DLPFC = 25 ± 7 ms, R-DLPFC = 63 ± 14 ms; t(9) = 3.10, p = 0.01 and t(9) = 4.58, p=0.001, respectively).
To assess the effect of stimulation site, a one-way between-subject ANOVA was used to analyze the within-subject change in relative SRT between test and retention sessions (mean change in skill ((random - sequence)/random) ± SEM: L-DLPFC = 0.4 ± 0.2, R-DLPFC = 0.11 ± 0.2, OC = -0.8 ± 0.4; F(2,29) = 11.6, p = 0.0005; Fig. 2b). Tukey post-hoc tests revealed that stimulation of either DLPFC lead to significantly greater consolidation relative to the occipital control site (p=0.015 and p=0.0005, for the left and right DLPFC relative to OC, respectively). Additionally right DLPFC stimulation lead to significantly greater improvement than left DLPFC stimulation (p = 0.04, see Fig. 2b).
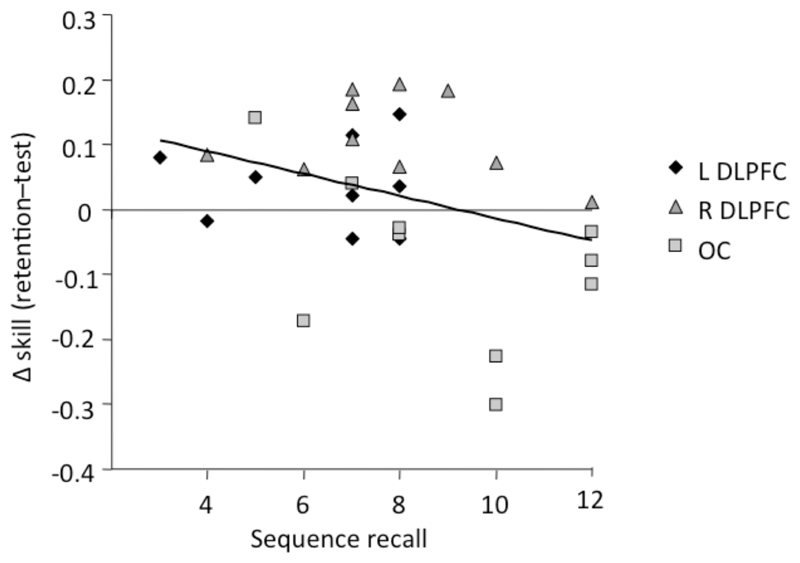
Counter to our hypothesis, there was only a trend towards a reliable difference between the groups on the free-recall test (L-DLPFC = 6.7 ± 2, R-DLPFC = 7.9 ± 2.2, OC = 9 items ± 2.6; ANOVA, F(2,29) = 2.5, p = 0.1). Accordingly, we collapsed data across the 3 groups to investigate whether recall was inversely related to skill change, as predicted by the consolidation competition hypothesis. In accordance with the hypothesis, a negative correlation was found, with an increase in skill being predicted by a reduction in free recall of the sequence (Pearson R = -0.35, p=0.03, one-tailed; Fig. 3).
Figure 3. Correlation between sequence recall and changes in skill.
A Pearson correlation was performed between skill change (retention - test) and sequence recall, across all 30 participants. A negative correlation was found, with an increase in skill being predicted by a reduction in free recall of the sequence (Pearson R = -0.35, p=0.03, one-tailed).
Error rates
There were no significant differences in error rates across the three groups (F(1,27) = 1.6, p = 0.22) or sessions (test vs. retention: F(1,27) = 0.8, p = 0.38). There was a significant effect for block type (sequential vs. random: F(1,27) = 22.8, p < 0.001). Sequential error rates were 5% (±0.01) whereas random were 8% (±0.02). No interactions approached significance (each F(2,27) ≤ 0.13, p ≥ 0.718). Accordingly, the affects of TMS seems to be limited to the efficiency with which the patterns are produced, not their accuracy.
Discussion
We have shown that cTBS over the left or right DLPFC immediately after a sequence learning task results in procedural skill improvement for the task when measured after 8 hours of wakefulness. Stimulation over an occipital control site did not cause any improvement in skill. Furthermore, a negative correlation between free recall of the sequence and our within-subject measure of change in procedural skill suggests that improvement in performance was predicted by reduced ability to recall the sequence. We have thus provided evidence that declarative consolidation competes with procedural consolidation, and disrupting declarative consolidation has facilitated or disinhibited the wakeful consolidation of a motor skill.
Although previous studies have shown small offline improvements in an explicit SRTT task during wakefulness, these are considerably smaller than after a period of sleep, and typically fail to be significantly greater than zero (e.g., Spencer et al., 2006; Walker et al., 2002). In addition Brown and Roberston (2007b) showed that this specific SRTT can result in a decrement in skill after a period of wakefulness (as we saw in our control group), providing a stark contrast with our results elicited following cTBS to the DLPFC. We do not yet know what effect cTBS would have on sleep-dependent consolidation. So while it would be informative to measure post-TBS retention effects after a period of sleep, our conclusion that we have induced wakeful consolidation of a procedural motor skill stands up independent of sleep-related effects.
The complementary difference between our results (i.e., performance improvements following DLPFC disruption) and Robertson and Brown’s (2007b) demonstration that declarative learning of the sequence task leads to performance decrements during the daytime provides clues to the mechanism underlying our performance improvement. These two effects would be most simply accounted for by a competitive consolidation hypothesis in which declarative memory systems functions draw resources away from procedural memory systems. In this model, a declarative learning task would demand significant memory resources and this would lead to a reduction of resources for procedural memory systems. In our case, by disrupting the DLPFC, we have reduced the ability of the declarative memory system to demand general memory resources, allowing additional recruitment of these resources for procedural consolidation.
We have no way of directly identifying the effect of cTBS on neural activity within the DLPFC. While there is no existing literature on its effects on DLPFC, this protocol has been shown to lead to depression of activity in motor cortex over extended durations (Huang et al., 2005). However, it has also been suggested that this protocol could lead to facilitation in motor cortex if it follows a period of activity (Iezzi et al., 2008) or if applied for only brief periods of time (Huang & Rothwell, 2004). Nonetheless, the relevance of the effects of cTBS of the motor cortex to the effects of cTBS of the DLPFC remains ambiguous. Unlike the motor system, where effects on motor evoked potentials are well defined, the ability to record the magnitude of a behavioral event induced by TMS to the DLPFC, or any higher cognitive area for that matter, has yet to be demonstrated.
Interestingly, a clear difference was found between the effects of stimulation of left and right DLPFC, where cTBS over the right DLPFC resulted in greater off-line skill improvement in comparison to cTBS over the left DLPFC. Bilateral DLPFC activation has been reported during sequence learning in the SRTT (Bischoff-Grethe, Goedert, Willingham, & Grafton, 2004; Grafton et al., 1995; Grafton et al., 2002; Hazeltine et al., 1997; Olson et al., 2006; van der Graaf et al., 2006), and greater activation is seen bilaterally during an explicit SRTT compared to an implicit SRTT (Willingham et al., 2002). But our results are more consistent with earlier work (e.g., Grafton et al., 1995; Hazeltine et al., 1997). Specifically, these authors showed that when the SRTT involves a declarative component, activation is greater in the right DLPFC. Our data suggests that the consolidation of an explicit SRTT engages a network involving bilateral DLPFC, since cTBS was effective on left and right hemispheres. However, the right hemispheric stimulation did have a greater effect, suggesting a more pronounced role for R-DLPFC.
In addition, the negative correlation between recall of the sequence and performance improvements suggests that, after a period of wakefulness, degraded declarative knowledge is coupled with an improvement in procedural skill. One conclusion is that cTBS over the DLPFC results in decreased declarative knowledge and this causally and quantitatively leads to increased procedural skill. However the L-DLPFC group showed weaker declarative knowledge of the sequence than the R-DLPFC group, whereas the relative change in procedural skill was reversed between the two groups. Hence there may be hemispheric differences that need to be further explored.
It is also possible that cTBS over either left or right DLPFC resulted in wakeful procedural consolidation through the disruption of declarative knowledge within different, spatially remote, memory systems. Brown and Robertson (2007b) previously showed that learning a word list after an explicit SRTT degraded the ability to freely recall the sequence. This is consistent with the notion that the declarative encoding of the sequence is filtered through an internal monologue, despite the fact that the items were not explicitly associated with a verbal representation. Accordingly, the effect of left DLPFC could be the result of disruption of the linguistic representation of the sequence within the prefrontal cortex of the left hemisphere.
On the other hand, cTBS over the right hemisphere is not consistent with this account; rather cTBS to R-DLPFC may disrupt the development of declarative knowledge within spatial memory systems due to the spatial arrangement of the cues on the screen. Schwarb and Schumacher (2009) recently dissociated learning-related processes from selection-related processes in the right prefrontal cortex using the SRTT. While the region they reported is dorsal to our stimulation target, our finding complements their suggestion that selection processes modulated by sequence knowledge are prominent in the prefrontal cortex of the right hemisphere, when the task is spatial in nature.
There are two potential mechanisms for the recovery of daytime procedural consolidation by disrupting DLPFC, a direct disinhibition model, in which disrupting the DLPFC disinhibits procedural memory systems, facilitating consolidation. Alternatively, a competition suppression model, would suggest that disrupting the DLPFC removes its requirement for some shared cognitive resource, leaving more resources available for the procedural memory system. Given the previous findings of Brown & Robertson (2007a, 2007b), we believe that our data favor the competition suppression model in the explanation of the present results.
Our work makes clear that interactions between declarative and procedural consolidation systems are influenced by the DLPFC, especially in the right hemisphere, and its disruption facilitates the consolidation of procedural skill. Nonetheless, the exact role of the DLPFC in the consolidation of an explicit SRTT remains ambiguous and future work will be necessary to uncover the form and location of the resources for which the declarative and procedural memory systems compete.
Acknowledgements
The authors thank Edwin M. Robertson for fruitful discussion. This work was supported by Welcome Trust Grant to R.C. Miall.
References
- Bischoff-Grethe A, Goedert KM, Willingham DT, Grafton ST. Neural substrates of response-based sequence learning using fMRI. Journal of Cognitive Neuroscience. 2004;16(1):127–138. doi: 10.1162/089892904322755610. [DOI] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Inducing motor skill improvements with a declarative task. Nat Neurosci. 2007a;10(2):148–149. doi: 10.1038/nn1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Off-Line Processing: Reciprocal Interactions between Declarative and Procedural Memories. Journal of Neuroscience. 2007b;27(39):10468–10475. doi: 10.1523/JNEUROSCI.2799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working-memory. Nature. 1995;378(6554):279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Franca M, Koch G, Mochizuki H, Huang YZ, Rothwell JC. Effects of theta burst stimulation protocols on phosphene threshold. Clin Neurophysiol. 2006;117(8):1808–1813. doi: 10.1016/j.clinph.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R. Functional Mapping of Sequence Learning in Normal Humans. Journal of Cognitive Neuroscience. 1995;7(4):497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. Motor sequence learning with the nondominant left hand. A PET functional imaging study. Experimental Brain Research. 2002;146(3):369–378. doi: 10.1007/s00221-002-1181-y. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor-sequence encoding. A PET study. Brain. 1997;120(1):123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Ivry RB. Encyclopedia of the Human Brain. Vol. 3. Elsevier Science; 2002. Motor Skill; pp. 183–200. [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC. The effect of short-duration bursts of high-frequency, low-intensity transcranial magnetic stimulation on the human motor cortex. Clin Neurophysiol. 2004;115(5):1069–1075. doi: 10.1016/j.clinph.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Ivry R. Representational Issues in Motor Learning: Phenomena and Theory. In: Heuer H, Keele SW, editors. Handbook of Perception & Action, volume 2: Motor Skills. Vol. 2. San Diego: Academic Press; 1996. pp. 263–332. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Keele SW. Movement Control in Skilled Motor Performance. Psychological Bulletin. 1968;70(6):387–402. [Google Scholar]
- Keele SW, Ivry R, Mayr U, Hazeltine E, Heuer H. The cognitive and neural architecture of sequence representation. Psychological Review. 2003;110(2):316–339. doi: 10.1037/0033-295x.110.2.316. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273(5280):1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clinical Neurophysiology. 2000;111(6):1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. Journal of Neuroscience. 2007;27(20):5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Rao H, Moore KS, Wang J, Detre JA, Aguirre GK. Using perfusion fMRI to measure continuous changes in neural activity with learning. Brain and Cognition. 2006;60(3):262–271. doi: 10.1016/j.bandc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Passingham D, Sakai K. The prefrontal cortex and working memory: physiology and brain imaging. Current Opinion in Neurobiology. 2004;14(2):163–168. doi: 10.1016/j.conb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Petrides M. Functional specialization within the dorsolateral frontal cortex for serial order memory. Proc Biol Sci. 1991;246(1317):299–306. doi: 10.1098/rspb.1991.0158. [DOI] [PubMed] [Google Scholar]
- Povel DJ, Collard R. Structural Factors in Patterned Finger Tapping. Acta Psychologica. 1982;52(1–2):107–123. doi: 10.1016/0001-6918(82)90029-4. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Knowlton BJ, Squire LR. Dissociable properties of memory systems: Differences in the flexibility of declarative and nondeclarative knowledge. Behavioral Neuroscience. 1996;110(5):861–871. doi: 10.1037//0735-7044.110.5.861. [DOI] [PubMed] [Google Scholar]
- Robertson EM. The serial reaction time task: implicit motor skill learning? J Neurosci. 2007;27(38):10073–10075. doi: 10.1523/JNEUROSCI.2747-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Passingham RE. Working memory for location and time: Activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14(1):77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nature Neuroscience. 2002;5(5):479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Schwarb H, Schumacher EH. Neural evidence of a role for spatial response selection in the learning of spatial sequences. Brain Research. 2009;1247:114–125. doi: 10.1016/j.brainres.2008.09.097. [DOI] [PubMed] [Google Scholar]
- Spencer RM, Sunm M, Ivry RB. Sleep-dependent consolidation of contextual learning. Curr Biol. 2006;16(10):1001–1005. doi: 10.1016/j.cub.2006.03.094. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys and humans. Psychological Review. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Knowlton BJ. Learning about categories in the absence of memory. Proceedings of the National Academy of Sciences, USA. 1995;92(26):12470–12474. doi: 10.1073/pnas.92.26.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Interference of left and right cerebellar rTMS with procedural learning. J Cogn Neurosci. 2004;16(9):1605–1611. doi: 10.1162/0898929042568488. [DOI] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Petrosini L, et al. Cortical networks of procedural learning: evidence from cerebellar damage. Neuropsychologia. 2007;45(6):1208–1214. doi: 10.1016/j.neuropsychologia.2006.10.007. [DOI] [PubMed] [Google Scholar]
- van der Graaf FH, Maguire RP, Leenders KL, de Jong BM. Cerebral activation related to implicit sequence learning in a Double Serial Reaction Time task. Brain Research. 2006;1081(1):179–190. doi: 10.1016/j.brainres.2006.01.103. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron. 2002;35(1):205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Willingham DB. Systems of memory in the human brain. Neuron. 1997;18(1):5–8. doi: 10.1016/s0896-6273(01)80040-4. [DOI] [PubMed] [Google Scholar]
- Willingham DB. A neuropsychological theory of motor skill learning. Psychol Rev. 1998;105(3):558–584. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Goedert-Eschmann K. The Relation Between Implicit and Explicit Learning: Evidence for Parallel Development. Psychological Science. 1999;10(6):531–534. [Google Scholar]
- Willingham DB, Salidis J, Gabrieli JD. Direct comparison of neural systems mediating conscious and unconscious skill learning. Journal of Neurophysiology. 2002;88(3):1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]