Abstract
It is often assumed that the human brain only becomes active to support overt behaviour. A new study challenges this concept by showing that multiple neural circuits are engaged even at rest. We highlight two complementary hypotheses which seek to explain the function of this resting activity.
The human brain has a large metabolic demand compared to the rest of the body, consuming ten times more energy than would be predicted based upon its weight. More than 80% of this energy demand supports synaptic transmission, implying that most of the brain’s substantial energy budget supports information processing: allowing us to perceive a visual scene, make a plan, move a limb, or store a memory [1]. One might expect, therefore, than an efficient strategy would be to use the brain only when absolutely necessary. But does the human brain actually rest, idly waiting to process new information from the environment? A number of brain imaging studies suggest that the brain may never truly be at rest: internal and external signals are constantly being processed [2].
One approach to visualising the resting brain has been to compare the pattern of brain activity during a wide range of behaviours against the resting state. This has shown that a consistent network of brain areas decrease their activity during any behaviour (these are task-independent decreases), implying that a resting-state network — one that is engaged during rest — ceases to be engaged while any task is performed [3]. Another approach has used positron emission tomography to directly measure the physiological baseline or resting level of neural activity. This was achieved by calculating the brain’s blood flow and oxygen extraction fraction allowing a resting state network to be identified [4]. Electroencephalographic signatures of spontaneous fluctuations can also be recorded in resting brain activity, confirming their neural, as opposed to haemodynamic origin [5].
Recent work [6] has advanced the analysis of these networks, showing that activation patterns are stable across participants and across recording sessions, and providing an estimate of their signal strengths. This was achieved by converting the low-frequency resting state time-series data — which by definition are uncorrelated with external events — into power spectra, allowing common frequency patterns to be identified without defining any specific temporal relationships. The authors next used a technique called tensor probabilistic independent component analysis (tensor-PICA) to find spectral patterns common across participants, across brain voxels, and across two scanning sessions one week apart. This analysis is ‘model-free’, finding consistent patterns in data without restricting the search to predefined areas of interest or to predefined signals. Finally, by randomly recombining groups of data from across sessions and participants, they were able to measure the mean and variability of these patterns, and estimate the signal strengths. The result was that ten consistent networks appear to be engaged during rest. This confirms that there is a regular set of very low frequency spatiotemporal patterns, and shows that the signal strength changes in these areas at rest is of an equivalent magnitude to that typically evoked by overt behavioural tasks.
Emerging from all these studies — despite their diversity — is a remarkably consistent pattern of brain areas that are engaged during rest. They include, for example, pre- and post-central areas that belong to a functionally defined sensoriomotor network (+ in Figure 1); prestriate and lateral occipital areas within a visual network; and medial prefrontal, posterior cingulate and inferior parietal areas that make up an attentional network [2]. But the functional contribution of these networks during rest remains unknown.
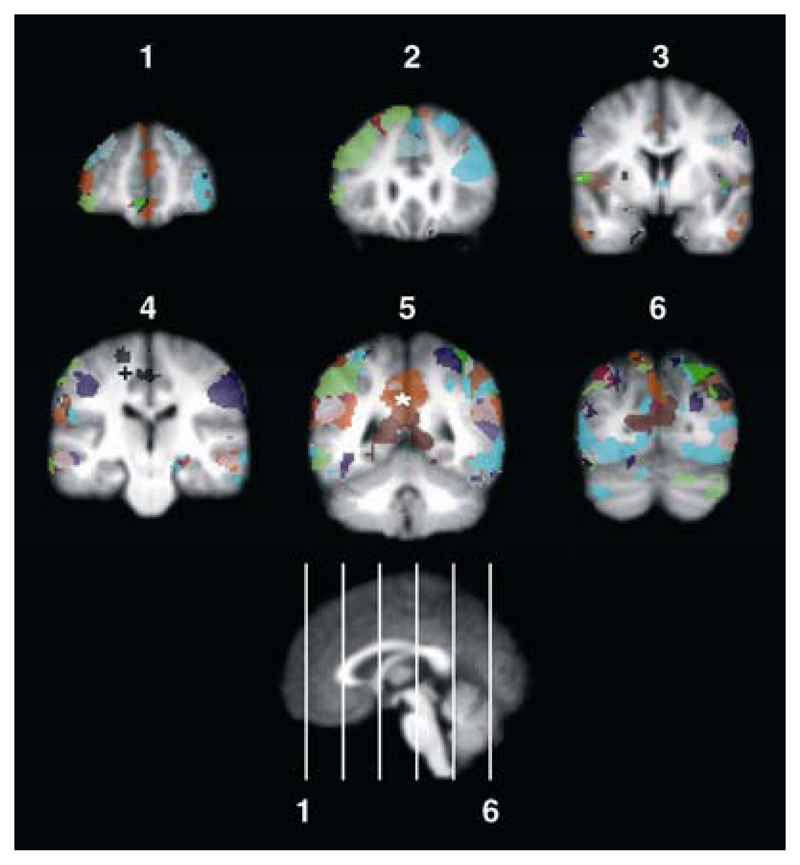
Figure 1. Resting-state networks in the human brain.
This figure colour-codes ten independent components identified by Damoiseaux et al. [6] in resting brain activity, displayed in coronal orientation. Components that include the sensorimotor cortex (+) and the default-mode network (*) are identified. These maps were generated from binary images kindly provided by the authors of the original study, and correspond to the mean tensor-PICA images.
One theory has been proposed to explain the resting state activity. It suggests that a ‘default-mode’ network (* in Figure 1) is engaged in functionally important processing such as continuously monitoring the external world. The posterior cingulate, precuneus, and retrosplenial cortices — areas involved in visuospatial orienting and representation of external space — are engaged at rest, maintaining a background level of attention to detect potentially dangerous external events [2]. This pattern of brain activation then shifts when alternative circuits are engaged to support overt behaviours. So, in this perspective, the brain is envisaged to be constantly monitoring the internal or external environment, processing this information, and temporarily engaging systems to perform overt behaviours before returning to the default mode.
Nonetheless, intrinsic resting brain activity may not be devoted exclusively to default-mode processing. It may also make a vital contribution to the off-line processing and consolidation of memories. Memory consolidation can be expressed behaviourally as performance becoming less susceptible to interference [7] — memory stabilisation — or as performance that improves over time [8] — memory enhancement [9]. Yet off-line processing is by no means limited to these: insights into difficult problem-solving tasks can be gained off-line by forming high-order associations amongst events [10]. Thus, even although we are often ignorant of its presence, off-line processing is responsible for supporting an important, rich and diverse array of behaviours.
Patterns of neural activity seen during a behaviour can be reproduced during sleep [11] and during waking rest [12]. The replay or reprocessing of recent experiences has been observed in both the hippocampus and parietal cortex, areas usually reported within the resting state network [11,13,14]. Thus, the resting state network may in part be devoted to the reprocessing of past experiences to support memory consolidation. This viewpoint suggests that the experiences participants have had even before entering an imaging study may be responsible for engaging parts of the resting network. Future studies might address this possibility by systematically varying participants’ experiences minutes or hours before measuring resting brain activity. This could provide insights into when different components of the network are engaged relative to the initial acquisition of a memory or skill.
Part of the resting state network identified in recent studies is a sensorimotor circuit which includes the primary motor cortex (M1). Activity within this circuit may be responsible for maintaining motor preparedness, allowing rapid responses to unpredictable changes in the environment, consistent with the default mode theory. Nonetheless, some of the resting activity within this circuit is likely to be attributable to memory processing. The engagement of M1 is altered following motor skill learning [15] and M1 makes a critical contribution to the off-line processing of motor skills [16–18]. Furthermore, activity within M1 can reflect past events: its activity during skill acquisition is reproduced, albeit with a different time course, during rest [19]. Similar arguments can be made for many other resting-state circuits.
Off-line memory processing and the default mode theory are not mutually exclusive; in fact, they offer complementary perspectives. The processing of internal signals such as monitoring our own mental state, our intended speech and actions is thought to be an important component of the default processing mode [2]. Interestingly, it appears that the intrinsic components of motor memories are processed off-line during waking rest; whereas extrinsic components require sleep for their enhancement [20]. Thus, the processing of internal, intrinsic information may be a common theme of the resting state network measured in functional magnetic resonance magnetic imaging (fMRI) studies of awake, resting participants.
In summary, converging evidence from multiple studies has convincingly demonstrated that several brain areas — the resting state network — continue to be engaged despite the absence of goal-directed behaviour. Recent work has demonstrated that the resting state network is composed of multiple functional circuits that are consistently engaged across participants and recording sessions. While the function of these circuits remains unknown, one possibility is that these networks are engaged to support the off-line processing of past events for memory consolidation.
References
- 1.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 2.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 3.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 4.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- 8.Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nat Rev Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- 10.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 11.Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 13.Qin YL, McNaughton BL, Skaggs WE, Barnes CA. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Phil Trans R Soc Lond B. 1997;352:1525–1533. doi: 10.1098/rstb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peigneux P, Orban P, Balteau E, Degueldre C, Luxen A, Laureys S, Maquet P. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol. 2006;4:e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- 17.Baraduc P, Lang N, Rothwell J, Wolpert D. Consolidation of dynamic motor learning is not disrupted by rTMS of primary motor cortex. Curr Biol. 2004;14:252–256. doi: 10.1016/j.cub.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Robertson EM, Press DZ, Pascual-Leone A. Off-Line Learning and the Primary Motor Cortex. J Neurosci. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa RM, Cohen D, Nicolelis MAL. Differential Corticostriatal Plasticity during Fast and Slow Motor Skill Learning in Mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 20.Cohen DA, Pascual-Leone A, Press DZ, Robertson EM. Off-line learning of motor skill memory: A double dissociation of goal and movement. Proc Natl Acad Sci USA. 2005;102:18237–18241. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]