Abstract
Purpose
We sought to investigate the potential of D-maltose, D-sorbitol, and D-mannitol as T2 exchange magnetic resonance imaging (MRI) contrast agents. We also sought to compare the in vivo pharmacokinetics of D-maltose with D-glucose with dynamic contrast enhancement (DCE) MRI.
Methods
T1 and T2 relaxation time constants of the saccharides were measured using eight pH values and nine concentrations. The effect of echo spacing in a multiecho acquisition sequence used for the T2 measurement was evaluated for all samples. Finally, performances of D-maltose and D-glucose during T2-weighted DCE-MRI were compared in vivo.
Results
Estimated T2 relaxivities (r2) of D-glucose and D-maltose were highly and nonlinearly dependent on pH and echo spacing, reaching their maximum at pH=7.0 (~0.08mM−1 s−1). The r2 values of D-sorbitol and D-mannitol were estimated to be ~0.02mM−1 s−1 and were invariant to pH and echo spacing for pH ≤7.0. The change in T2 in tumor and muscle tissues remained constant after administration of D-maltose, whereas the change in T2 decreased in tumor and muscle after administration of D-glucose. Therefore, D-maltose has a longer time window for T2-weighted DCE-MRI in tumors.
Conclusion
We have demonstrated that D-maltose can be used as a T2 exchange MRI contrast agent. The larger, sustained T2-weighted contrast from D-maltose relative to D-glucose has practical advantages for tumor diagnoses during T2-weighted DCE-MRI.
Keywords: DCE-MRI, D-maltose, T2ex, tumor imaging
INTRODUCTION
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is often used to identify pathological tissues, such as solid tumors, by dynamically monitoring the change in image contrast after administering a contrast agent (1,2). T1 MRI contrast agents, such as gadolinium (Gd)(III) chelates, are commonly used in DCE-MRI studies because these agents can be detected with a series of T1-weighted MR images that are acquired using routine acquisition protocols (3). Furthermore, Gd(III)-based contrast agents have good detection sensitivity, partly attributed to the fast 107 to 109 Hz chemical exchange rate between a water molecule bound to the agent and the surrounding bulk water. However, metallic Gd(III)-based contrast agents are potentially toxic (4), which has prompted investigations of the use of other MRI contrast agents for DCE-MRI studies.
Nonmetallic MRI contrast agents that are detected by chemical exchange saturation transfer (CEST) have been used for DCE-MRI studies (5). In particular, the hydroxyl protons of D-glucose can generate CEST contrast, and a series of CEST MR images can track the pharmacokinetics of D-glucose uptake and washout in tumor tissues (6–8). Glucose is approved for intravenous administration in patients, which has accelerated the translation of “glucoCEST MRI” for clinical DCE-MRI studies (9,10). Unfortunately, CEST MRI contrast agents have low detection sensitivity relative to relaxation-based MRI contrast agents because of the relatively slow 10 to 104 Hz chemical exchange rate needed to generate a CEST effect (11). This pitfall can be partly compensated by administering D-glucose to subjects at high concentrations; however, a rapid increase in blood glucose level may not be tolerable for all patients. Furthermore, CEST MRI requires the implementation of new pulse sequences that are now only recently emerging for most clinical scanners, currently limiting their broad and rapid dissemination (12).
The hydroxyl protons of D-glucose also exhibit intermediate 104 to 107 Hz chemical exchange rates that can generate MR image contrast based on the T2-exchange (T2ex) mechanism (13,14). T2ex relaxation is a result of proton exchange between an agent and water, which causes the average MR frequency (also known as the MR chemical shift) of the proton to be a time-weighted average value of the MR frequencies of the proton on the agent and water. This time-weighted average is different for each exchanging proton because proton exchange is a stochastic process. The different time-weighted average MR frequency for each proton results in a decay of net coherent magnetization, which manifests as T2 relaxation (15). This improved sensitivity can potentially reduce the amount of glucose to be administered to a subject. The T2ex relaxation mechanism can change the contrast level of T2-weighted MR images, which are routinely obtained in clinical radiology centers using many types of acquisition protocols. Therefore, T2-weighted imaging with glucose is a compelling alternative for acquiring DCE-MRI acquisitions.
As another pitfall, DCE-MRI using D-glucose can be difficult to interpret because the pharmacokinetics of this carbohydrate is confounded by its rapid metabolism in practically all tissues (16). This rapid metabolism is assumed to be responsible for only a ~10-minute change in image contrast after intravenous administration of glucose. This pitfall has led to investigations of other carbohydrates for DCE-MRI, including 3-O-methyl-D-glucose, glucosamine, and N-acetyl-glucosamine, that are not rapidly metabolized by mammalian cells (17,18). These investigations with other carbohydrates have used CEST MRI to detect a dynamic change in image contrast.
The objective of our study was to determine whether carbohydrates other than D-glucose can be used as T2ex contrast agents for DCE-MRI. Mammalian cells do not contain the cellular machinery for binding, transport, and digestion of D-maltose, D-mannitol, and D-sorbitol for energy production (19–22). Therefore, we compared the performances of D-glucose, D-maltose, D-mannitol, and D-sorbitol under multiple pH values, concentrations, and MRI acquisition conditions. Based on these initial studies, we then investigated the utility of D-maltose relative to D-glucose as T2ex contrast agents for in vivo, T2-weighted DCE-MRI.
METHODS
Phantom preparation
Phantoms of D-maltose, D-glucose, D-sorbitol, and D-mannitol were prepared using compounds as purchased from CHEM-IMPEX International Inc. (Bensenville, IL), Sigma-Aldrich (St. Louis, MO), Santa Cruz Biotechnology Inc. (Dallas, TX), and Alfa Aesar (Ward Hill, MA), respectively. Stock solutions (100mM) at eight different pH values (5.0, 5.5, 6.0, 6.5, 7.0, 7.4, 8.0, and 8.5), as measured with a calibrated Orion Star A121 pH meter (Thermo Scientific, Waltham, MA), were prepared using distilled water and 1×phosphate-buffered saline (PBS; Hoefer Inc., Holliston, MA). Sodium azide (3% w/v; Sigma-Aldrich) for preservation was added to each sample including control samples. Stock solutions were diluted to produce seven concentrations (80, 75, 60, 40, 20, 10, and 5 mM), and 0mM 1×PBS was used as a control for each of the aforementioned pH values. A 300-μL volume of each stock was added to nine centrifuge tubes that were taped around a 50-mL tube of distilled water and positioned in a modified cradle. The phantoms were placed in a 7 Tesla (T) BioSpec MRI scanner with a 20-cm bore using a 72-mm quadrature transceiver coil (Bruker BioSpin Inc., Billerica, MA). The temperature was maintained at 37.0±0.2 °C using an automated feedback system between the temperature probe and an air heater (SA Instruments, Stony Brook, NY).
T1 and T2 relaxation time measurements
The T1 relaxation time of each sample was measured using a rapid acquisition with relaxation enhancement (RARE) acquisition sequence and the following parameters: repetition time (TR)=150, 300, 500, 700, 900, 1,200, 2,000, 3,000, 6,000, and 10,000 ms; echo time (TE)=9.07 ms; number of excitations (NEX)=1; RARE factor=2; slice thickness=1.0mm; field of view (FOV)=4cm2; linear encoding order; matrix=128×128; and in-plane spatial resolution=0.31mm2. Total scan time was 25 minutes. The data from these experiments were fit to Equation [1] using least square curve fitting to estimate T1 and M0.
| [1] |
The T2 relaxation time of all samples was measured using a multispin multiecho (MSME) acquisition sequence with variable echo spacing as follows: TR=10,000 ms; NEX=1; linear encoding order; FOV=4cm2; slice thickness =1.0mm; matrix=64×64; number of slices=1; and in-plane spatial resolution=0.625mm2. To ensure good signal-to-noise ratio, seven variable echo spacing experiments were performed for a total time of 600 ms, with repetitions/echo spacing of 40/15, 30/20, 24/25, 20/30, 15/40, 12/50, and 10/60 msec. The data from these experiments were fit to Equation [2] using least square curve fitting to estimate T2 and M0.
| [2] |
Finally, the T1 and T2 relaxivities (r1 and r2, respectively) for all saccharides were measured at each pH and echo spacing fitting the data to Equation [3]:
| [3] |
where Ti,k is the T1 or T2 relaxation time (seconds) at each concentration k, ri is the T1 or T2 relaxivity (mM−1 s−1), [CA]k is the concentration k of the contrast agent, and Ti,0 is the T1 or T2 in the absence of the contrast agent.
In vivo studies
All animals were cared for in compliance with protocols approved by the Institutional Animal Care and Use Committee of the University of Arizona (Tucson, AZ). A549 human lung adenocarcinoma cells were prepared by tripsinization, rinsing once in PBS, and suspended in 50% Matrigel (BD Biosciences, Franklin Lakes, NJ) and 50% PBS. Seven male nude mice, approximately 20 g each, were injected subcutaneously in the right flank with 10×106 cells in 0.1mL of saline. Tumors grew to ~300mm3 before initiating MRI studies with a 7T Bruker Biospec MRI instrument and transceiver coil used for phantom studies. Before the MRI scan, each mouse was anesthetized with 1.5% to 2.5% isoflurane in O2 carrier gas and had a 27G catheter placed in the tail vein. Physiological respiration rate and core body temperature were monitored throughout the MRI session. All animals were imaged while maintaining their temperature at 37.0±0.2 °C using warm air controlled by a temperature feedback system (SA Instruments, Inc., Stony Brook, NY).
A series of T2-weighted DCE-MR images were acquired using a RARE MRI protocol with the following parameters: TR=1,000 ms; echo train length=4; effective TE=30 ms; NEX=2; FOV=6.0cm2; in-plane spatial resolution =0.94mm2; matrix=64×64; and slice thickness =1.0mm, for three slices centered in the tumor. Each individual RARE image was acquired in 32 seconds and repeated 64 times. An initial set of baseline images were acquired for 2 or 10 minutes before intravenous injection of 250 μL of 3.0 mmol/kg of D-maltose solution (Sigma-Aldrich) or before intravenous injection of 250 μL of 5.0 mmol/kg of D-glucose solution (Growcells, Inc., Irvine, CA) over 1 minute, and subsequent infusion of either contrast agent at a rate of 400μL/hour of either agent for the remainder of the scan, resulting in a total acquisition time of 34:10 minutes.
The change in T2 relaxation rate ΔR2(t) during in vivo T2-weighted DCE-MRI experiments was estimated as follows: First, the MRI signal was normalized to the signal before injection as described in Equation [4]:
| [4] |
where S(t) is the MRI signal time t, S(0) is the mean signal for the 2- to 10-minute baseline before injection of D-glucose or D-maltose, TR is the repetition time, R1(t) and R2(t) are the T1 and T2 relaxation rates at time t, whereas R1(0) and R2(0) are the T1 and T2 relaxation rates before injection. Next, assuming that R1(0)=R1(t) and taking the natural logarithm on both sides Equation [4] reduces to Equation [5a], which yields in Equation [5c], where ΔR2(t)=R2(t) – R2(0).
| [5a] |
| [5b] |
| [5c] |
RESULTS
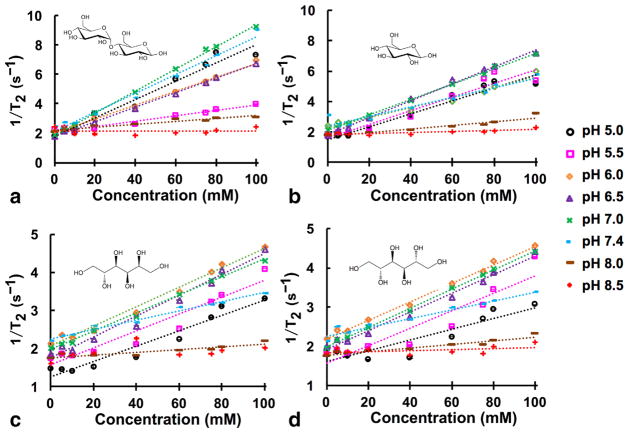
D-glucose and D-maltose showed a significant effect on R2, varying from 2 to 9 s−1 depending on the pH and concentration (Fig. 1; Supporting Table S1). The effect of pH and concentration was less pronounced for D-mannitol and D-sorbitol, with R2 varying from 1.5 to 4.5 s−1. None of the saccharides had a statistically significant effect on T1 relaxation time at the concentrations and pH values used in this study (Supporting Fig. S1; Supporting Table S1).
FIG. 1.
T2 relaxation (s−1) of: (a): D-maltose; (b): D-glucose; (c): D-sorbitol; and (d): D-mannitol as a function of concentration (mM) and pH at 7.0 T and 37 °C. 95% confidence intervals for T2 relaxation at each concentration is shown, with some confidence intervals that are smaller than the data symbol. R2 values are ≥0.99 for all curves fitted with linear regression.
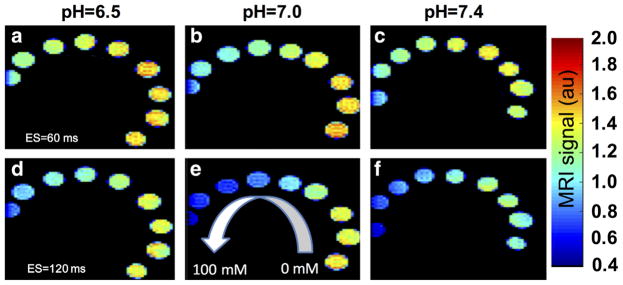
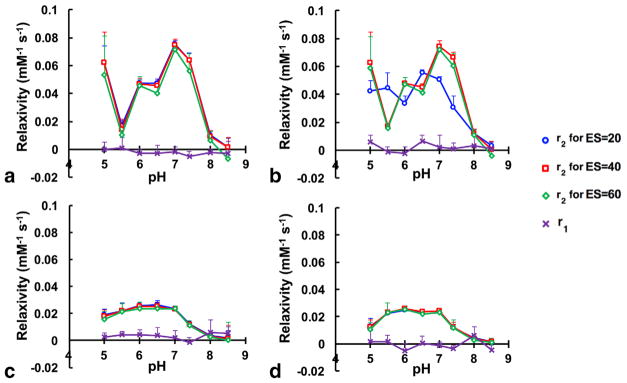
The effects of pH and echo spacing on T2 relaxation were evident in T2-weighted MR images using a spin echo acquisition sequence (Fig. 2). Although only a portion of the tube with 100-mM concentration was in the field of view, the 95% confidence intervals were excellent for determining R1 and R2 values from this tube, showing that the analysis of this tube was precise. Furthermore, Figure 1 does not show systematic error at 100mM, showing that our analysis of the R1 and R2 of this tube was accurate. Therefore, we conducted a systematic study of the effect of echo spacing and pH on the estimated T2 relaxivity (r2) for D-glucose and D-maltose (Fig. 3 and Supporting Figs. S2 and S3; Table 1). Our results indicated that the T2 relaxivities of D-maltose and D-glucose have a nonlinear relationship with pH and exhibit their highest T2 relaxivities (0.08mM−1 s−1) at pH=7.0, whereas their r2 values are not statistically different from zero at pH ≥8.0. Importantly, the measured r2 for D-maltose is independent of echo spacing at all the studied pH values, whereas the r2 of D-glucose is highly dependent on echo spacing, resulting in a 2-fold change in the measured r2 at specific combinations of pH and echo spacing. For comparison, these saccharides had no effect on the T1 of samples with concentrations used in this study.
FIG. 2.
Representative T2-weighted MRI using D-maltose as a contrast agent. D-maltose solutions were prepared at multiple pH values and concentrations, and imaged with a RARE acquisition sequence with echo spacing (ES) of 60 ms (top row) or 120 ms (bottom row) at 7.0 T and 37 °C. The MR signal was normalized to the voxel with the maximum intensity in all six panels.
FIG. 3.
T1 relaxivity (r1) and T2 relaxivity (r2) as a function of pH and echo spacing (ES) of 20, 40, or 60 ms for (a): D-maltose; (b): D-glucose; (c): D-sorbitol; and (d): D-mannitol at 7.0 T and 37 °C. 95% confidence intervals are shown for r1 and r2 at each measured pH.
Table 1.
Effect of pH on T1 (r1) and T2 (r2) Relaxivities of Saccharides at TE=15 ms
| Maltose | ||||
|---|---|---|---|---|
|
| ||||
| pH | r2 (mM−1 s−1) | 95% CIa | r1 (mM−1 s−1) | 95% CIa |
| 5.0 | 0.060 | 0.011 | 0 | 0.006 |
| 5.5 | 0.018 | 0.004 | 0.001 | 0.008 |
| 6.0 | 0.048 | 0.002 | −0.003 | 0.003 |
| 6.5 | 0.049 | 0.002 | −0.003 | 0.006 |
| 7.0 | 0.075 | 0.003 | −0.002 | 0.004 |
| 7.4 | 0.064 | 0.007 | −0.005 | 0.004 |
| 8.0 | 0.01 | 0.002 | −0.002 | 0.002 |
| 8.5 | 0 | 0.006 | −0.003 | 0.009 |
|
| ||||
| Glucose | ||||
|
| ||||
| pH | r2 (mM−1 s−1) | 95% CIa | r1 (mM−1 s−1) | 95% CIa |
|
| ||||
| 5.0 | 0.042 | 0.008 | 0.006 | 0.005 |
| 5.5 | 0.045 | 0.011 | −0.001 | 0.004 |
| 6.0 | 0.033 | 0.005 | −0.002 | 0.005 |
| 6.5 | 0.056 | 0.003 | 0.007 | 0.004 |
| 7.0 | 0.050 | 0.002 | 0.002 | 0.009 |
| 7.4 | 0.032 | 0.007 | 0.001 | 0.004 |
| 8.0 | 0.012 | 0.003 | 0.003 | 0.008 |
| 8.5 | 0.003 | 0.002 | 0.001 | 0.002 |
|
| ||||
| Sorbitol | ||||
|
| ||||
| pH | r2 (mM−1 s−1) | 95% CIa | r1 (mM−1 s−1) | 95% CIa |
|
| ||||
| 5.0 | 0.020 | 0.005 | 0.002 | 0.003 |
| 5.5 | 0.022 | 0.006 | 0.005 | 0.002 |
| 6.0 | 0.026 | 0.003 | 0.004 | 0.004 |
| 6.5 | 0.027 | 0.024 | 0.004 | 0.006 |
| 7.0 | 0.024 | 0.001 | 0.002 | 0.005 |
| 7.4 | 0.012 | 0.001 | −0.001 | 0.004 |
| 8.0 | 0.004 | 0.001 | 0.006 | 0.009 |
| 8.5 | 0.002 | 0.004 | 0.005 | 0.005 |
|
| ||||
| Mannitol | ||||
|
| ||||
| pH | r2 (mM−1 s−1) | 95% CIa | r1 (mM−1 s−1) | 95% CIa |
|
| ||||
| 5.0 | 0.014 | 0.006 | 0.002 | 0.008 |
| 5.5 | 0.022 | 0.007 | 0.002 | 0.005 |
| 6.0 | 0.024 | 0.002 | −0.005 | 0.005 |
| 6.5 | 0.024 | 0.002 | 0.001 | 0.005 |
| 7.0 | 0.024 | 0.001 | −0.001 | 0.003 |
| 7.4 | 0.011 | 0.002 | −0.003 | 0.003 |
| 8.0 | 0.005 | 0.001 | 0.006 | 0.006 |
| 8.5 | 0.001 | 0.002 | −0.005 | 0.004 |
95% confidence intervals shown for data at each corresponding pH value.
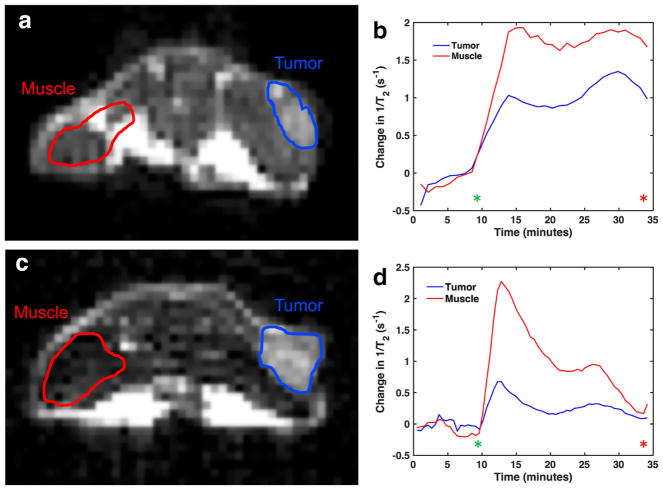
D-glucose and D-maltose were used for in vivo studies based on their higher r2 relaxivities measured in phantoms. We conducted a dynamic MRI study, acquiring T2-weighted images before and during infusion with D-maltose or D-glucose for 34:10 minutes (Fig. 4). We used an echo spacing of 30 ms based on our results with phantoms. The change in R2 (ΔR2) was estimated for muscle and a tumor (Fig. 4). The ΔR2 in the muscle and tumor was significant after injection and infusion of D-glucose, almost returning to its preinjection value after 34:10 minutes. A similar change in ΔR2 was observed after injection and infusion of D-maltose, but this change in R2 did not return to the preinjection value after 30 minutes. This sustained contrast enhancement throughout the DCE-MRI protocol was consistently observed for all 6 mice that were studied with D-maltose (Supporting Fig. S4). Although the ΔR2 showed a difference between tumor and muscle in some mice, the ΔR2 was similar for tumor and muscle in other mice.
FIG. 4.
In vivo DCE-MRI using saccharides as T2ex contrast agents. T2-weighted anatomical images before infusion of (a): D-maltose and (c): D-glucose show the regions of the tumor and muscle that were analyzed. The time-dependent changes in 1/T2 for the tumor and muscle regions for (b): D-maltose infusion and (d): D-glucose infusion show that these saccharides have different pharmacokinetics. The window for infusion of either maltose or glucose is denoted by the start of infusion (green asterisk) and end of infusion (red asterisk).
DISCUSSION
Our study has shown that D-maltose is a T2ex contrast agent. D-sorbitol and D-mannitol also exhibit T2ex relaxation, but at lower r2 relaxivity relative to D-maltose and D-glucose. We have extended the previous studies to show that the r2 relaxivities of both D-maltose and D-glucose are highly and nonlinearly dependent on pH. D-glucose shows dependency on echo spacing near physiological pH. A possible explanation for this observation is based on the mixture of open and closed structural isomers that are under dynamic equilibrium for both D-glucose and D-maltose, thus exposing hydroxyl groups with different exchange rates at different pH values. The efficiency of the T2ex mechanism to create contrast is partly dependent on the echo spacing, which effectively weights the contrast based on the contribution of hydroxyl groups with different exchange rates (23). The lack of dependency on echo spacing for D-maltose shows that T2ex-based DCE-MRI is more robust with D-maltose than with D-glucose. D-mannitol and D-sorbitol also had no dependence on echo spacing because they have less structural degrees of freedom, thus their r2 remains constant at pH ≤7.0 regardless of pH and echo spacing.
Our in vivo T2-weighted DCE-MRI studies showed that D-maltose and D-glucose can be detected in tumor and muscle by T2ex relaxation. More importantly, our in vivo results showed that D-maltose and D-glucose have different pharmacokinetic profiles in muscle and tumor tissues. We purposefully designed our in vivo studies to include an infusion of agent to exploit the differences in metabolism of these saccharides, whereby mammalian cells metabolize D-glucose, but not D-maltose (21,22). We did not only rely on a bolus injection that was used during previous dynamic D-glucose-enhanced MRI studies, which reflect the uptake and clearance of the agent (8,14). The ΔR2(t) attributed to D-maltose remained constant during the last 32 minutes of the experiment in both muscle and tumor tissues, but the ΔR2(t) in muscle and tumor tissue decreased in both tissue types during the 23:30-minute infusion of D-glucose. We propose that the metabolism of D-glucose is responsible for this decrease in image contrast (24,25), whereas the lack of metabolism for D-maltose sustains the contrast.
Our results indicate that D-maltose has advantages relative to D-glucose for T2-weighted DCE-MRI of tumors. The sustained contrast does not require careful timing during the imaging scan session, because any image after ~10 minutes of infusion produced the same amount of contrast. T2-weighted DCE-MRI with D-glucose requires acquisition within the first 10 minutes after initial injection to obtain the greatest contrast. However, dynamic MRI with D-maltose has limitations. D-maltose is not yet approved for clinical use. Similar to D-glucose, D-maltose has a r2 relaxivity that is 10-fold lower than the r2 of Gd- and Fe-based agents requiring administration of large quantities of D-maltose (and D-glucose) to achieve detectable changes in T2 (26,27). The biocompatibility of D-maltose at high concentrations needed for detection will be required to provide a translational pathway to the clinic for this technique.
Our results may be used to support future studies that use spin-lock acquisition methods to detect carbohydrates during DCE-MRI studies (28). A direct comparison of spin-lock and CEST MRI techniques showed that a version of spin-lock MRI applied on water resonance provides more sensitivity for detecting carbohydrates with intermediate chemical exchange rates (29). In general, “on-resonance” acquisition methods that manipulate water resonance to generate contrast based on chemical exchange lack specificity for detecting a single metabolite, relative to “off-resonance” acquisition methods that directly manipulate the resonance of the metabolite. However, the on-resonance spin-lock MRI acquisition method avoids this problem by monitoring the change in contrast before and after the contrast agent is administered to the subject, which can be assigned to the agent with good specificity. Based on the merits of this approach, spin-lock DCE-MRI methods have recently been tested with patients who have glioblastoma (30,31). Spin-lock MRI methods have been used to detect other carbohydrates, including 2-deoxyglucose and 3-Omethyl-glucose, that showed DCE MR image contrast that lasted longer than contrast generated with glucose (32–34). Therefore, our sustained T2-weighted contrast during DCE-MRI results with D-maltose indicate that D-maltose may also be useful for spin-lock DCE-MRI studies.
CONCLUSIONS
Our report confirms previous studies that indicate D-glucose is a suitable T2-weighted DCE-MRI contrast agent. We have also extended these previous studies to characterize D-glucose, D-maltose, D-sorbitol, and D-mannitol with respect to pH and echo spacing. We identified D-maltose as an alternative T2-weighted DCE-MRI agent to D-glucose, which showed sustained uptake and higher contrast in tumor relative to muscle.
Supplementary Material
Acknowledgments
Grant sponsor:NIH; Grant numbers: R01CA169774; P30CA023074, and P50CA95060; Grant sponsor: American Cancer Society; Grant number: Institutional Research Grant number 128749-IRG-16-124-37-IRG.
The authors thank Dr. Sanjay Jain, Johns Hopkins University, for helpful discussions regarding the biology of saccharides.
References
- 1.Jackson A, Buckley D, Parker GJ. Dynamic Contrast-Enhanced Magnetic Resonance Imaging in Oncology. Berlin: Springer; 2005. [Google Scholar]
- 2.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–3298. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- 3.Yankeelov TE, Gore JC. Dynamic contrast enhanced magnetic resonance imaging in oncology: theory, data acquisition, analysis, and examples. Curr Med Imaging Rev. 2007;3:91–107. doi: 10.2174/157340507780619179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647–649. doi: 10.1148/radiol.2423061640. [DOI] [PubMed] [Google Scholar]
- 5.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143:79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 6.Chan KW, McMahon MT, Kato Y, Liu G, Bulte JW, Bhujwalla ZM, Artemov D, van Zijl PC. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med. 2012;68:1764–1773. doi: 10.1002/mrm.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker-Samuel S, Ramasawmy R, Torrealdea F, et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med. 2013;19:1067–1072. doi: 10.1038/nm.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Chan KW, Knutsson L, et al. Dynamic glucose enhanced (DGE) MRI for combined imaging of blood-brain barrier break down and increased blood volume in brain cancer. Magn Reson Med. 2015;74:1556–1563. doi: 10.1002/mrm.25995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Yadav NN, Knutsson L, et al. Dynamic glucose-enhanced (DGE) MRI: translation to human scanning and first results in glioma patients. Tomography. 2015;1:105–114. doi: 10.18383/j.tom.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Weygand J, Hwang KP, Mohamed AS, Ding Y, Fuller CD, Lai SY, Frank SJ, Zhou J. Magnetic resonance imaging of glucose uptake and metabolism in patients with head and neck cancer. Sci Rep. 2016;6:30618. doi: 10.1038/srep30618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherry AD, Wu Y. The importance of water exchange rates in the design of responsive agents for MRI. Curr Opin Chem Biol. 2013;17:167–174. doi: 10.1016/j.cbpa.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones KM, Pollard AC, Pagel MD. Clinical applications of chemical exchange saturation transfer (CEST) MRI. Radiology. 2018;47:11–27. doi: 10.1002/jmri.25838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gore JC, Brown MS, Mizumoto CT, Armitage IM. Influence of glycogen on water proton relaxation times. Magn Reson Med. 1986;3:463–466. doi: 10.1002/mrm.1910030312. [DOI] [PubMed] [Google Scholar]
- 14.Yadav NN, Xu J, Bar-Shir A, Qin Q, Chan KW, Grgac K, Li W, McMahon MT, van Zijl PC. Natural D-glucose as a biodegradable MRI relaxation agent. Magn Reson Med. 2014;72:823–828. doi: 10.1002/mrm.25329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daryaei I, Pagel MD. Double agents and secret agents: the emerging fields of exogenous chemical exchange saturation transfer and T2-exchange magnetic resonance imaging contrast agents for molecular imaging. Res Rep Nucl Med. 2015;15:19–32. doi: 10.2147/RRNM.S81742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Enríquez S, Marín-Hernández A, Gallardo-Pérez JC, Moreno-Sánchez R. Kinetics of transport and phosphorylation of glucose in cancer cells. J Cell Physiol. 2009;221:552–559. doi: 10.1002/jcp.21885. [DOI] [PubMed] [Google Scholar]
- 17.Rivlin M, Tsarfaty I, Navon G. Functional molecular imaging of tumors by chemical exchange saturation transfer MRI of 3-O-Methyl-D-glucose. Magn Reson Med. 2014;72:1375–1380. doi: 10.1002/mrm.25467. [DOI] [PubMed] [Google Scholar]
- 18.Rivlin M, Navon G. Glucosamine and N-acetyl glucosamine as new CEST MRI agents for molecular imaging of tumors. Sci Rep. 2016;6:32648. doi: 10.1038/srep32648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freundlieb S, Ehmann U, Boos W. Facilitated diffusion of p-nitrophenyl-alpha-D-maltohexaoside through the outer membrane of Escherichia coli. Characterization of LamB as a specific and saturable channel for maltooligosaccharides. J Biol Chem. 1988;263:314–320. [PubMed] [Google Scholar]
- 20.Reuss R, Ludwig J, Shirakashi R, Ehrhart F, Zimmermann H, Schneider S, Weber MM, Zimmermann U, Schneider H, Sukhorukov VL. Intracellular delivery of carbohydrates into mammalian cells through swelling-activated pathways. J Membr Biol. 2004;200:67–81. doi: 10.1007/s00232-004-0694-7. [DOI] [PubMed] [Google Scholar]
- 21.Dippel R, Boos W. The maltodextrin system of Escherichia coli: metabolism and transport. J Bacteriol. 2005;187:8322–83231. doi: 10.1128/JB.187.24.8322-8331.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopal S, Berg D, Hagen N, Schriefer EM, Stoll R, Goebel W, Kreft J. Maltose and maltodextrin utilization by Listeria monocytogenes depend on an inducible ABC transporter which is repressed by glucose. PLoS One. 2010;5:e10349. doi: 10.1371/journal.pone.0010349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myint W, Ishima R. Chemical exchange effects during refocusing pulses in constant-time CPMG relaxation dispersion experiments. J Biomol NMR. 2009;45:207–216. doi: 10.1007/s10858-009-9344-9. [DOI] [PubMed] [Google Scholar]
- 24.John SA, Ottolia M, Weiss JN, Ribalet B. Dynamic modulation of intracellular glucose imaged in single cells using a FRET-based glucose nanosensor. Pflugers Arch. 2008;456:307–322. doi: 10.1007/s00424-007-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Enríquez S, Marín-Hernández A, Gallardo-Pérez JC, Moreno-Sánchez R. Kinetics of transport and phosphorylation of glucose in cancer cells. J Cell Physiol. 2009;221:552–559. doi: 10.1002/jcp.21885. [DOI] [PubMed] [Google Scholar]
- 26.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 27.Merbach AS, Helm L, Tóth É. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 28.Schuenke P, Koehler C, Korzowski A, et al. Adiabatically prepared spin-lock approach for T1ρ-based dynamic glucose enhanced MRI at ultrahigh fields. Magn Reson Med. 2017;78:215–225. doi: 10.1002/mrm.26370. [DOI] [PubMed] [Google Scholar]
- 29.Jin T, Autio J, Obata T, Kim SG. Spin-locking versus chemical exchange saturation transfer MRI for investigating chemical exchange process between water and labile metabolite protons. Magn Reson Med. 2011;65:1448–1460. doi: 10.1002/mrm.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuenke P, Paech D, Koehler C, Windschuh J, Bachert P, Ladd ME, Schlemmer HP, Radbruch A, Zaiss M. Fast and quantitative T1ρ-weighted dynamic glucose enhanced MRI. Sci Rep. 2017;7:42093. doi: 10.1038/srep42093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paech D, Schuenke P, Koehler C, et al. T1ρ-weighted dynamic glucose-enhanced MR imaging in the human brain. Radiology. 2017;285:914–922. doi: 10.1148/radiol.2017162351. [DOI] [PubMed] [Google Scholar]
- 32.Jin T, Mehrens H, Hendrich KS, Kim SG. Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. J Cereb Blood Flow Metab. 2014;34:1402–1410. doi: 10.1038/jcbfm.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin T, Mehrens H, Wang P, Kim SG. Chemical exchange-sensitive spin-lock MRI of glucose analog 3-O-methyl-d-glucose in normal and ischemic brain. J Cereb Blood Flow Metab. 2017 Jan 1; doi: 10.1177/0271678X17707419. 271678X17707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zu Z, Spear J, Li H, Xu J, Gore JC. Measurement of regional cerebral glucose uptake by magnetic resonance spin-lock imaging. Magn Reson Imaging. 2014;32:1078–1084. doi: 10.1016/j.mri.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.