Abstract
Elevation of blood glucose results in increased glucose in the fluid that lines the surface of the airways and this is associated with an increased susceptibility to infection with respiratory pathogens. Infection induces an inflammatory response in the lung, but how this is altered by hyperglycemia and how this affects glucose, lactate and cytokine concentrations in the airway surface liquid is not understood. We used Wild Type (WT) and glucokinase heterozygote (GK+/-) mice to investigate the effect of hyperglycemia, with and without LPS-induced inflammatory responses, on airway glucose, lactate, inflammatory cells and cytokines measured in Bronchoalveolar Lavage Fluid (BALF).
We found that glucose and lactate concentrations in BALF were elevated in GK+/- compared to WT mice and that there was a direct correlation between blood glucose and BALF glucose concentrations. LPS challenge increased BALF inflammatory cell numbers and this correlated with decreased glucose and increased lactate concentrations although the effect was less in GK+/- compared to WT mice. All cytokines measured (except IL-2) increased in BALF with LPS challenge. However, concentrations of TNFα, INFγ, IL-1β and IL-2 were less in GK+/- compared to WT mice. This study shows that the normal glucose/lactate environment of the airway surface liquid is altered by hyperglycemia and the inflammatory response. These data indicate that inflammatory cells utilize BALF glucose and that production of lactate and cytokines is compromised in hyperglycemic GK+/- mice.
Keywords: Airway, Glucose, Hyperglycemia, Inflammation, Metabolism
Introduction
There is mounting evidence that raised blood glucose concentrations (hyperglycemia) as a result of diabetes mellitus or severe illness (stress hyperglycemia) are associated with increased susceptibility to respiratory infections, particularly when associated with underlying respiratory disease[1–4]. Glucose concentration in the fluid that lines the luminal surface of the airways (airway surface liquid, ASL) is in normal situations low at ~0.4mM. However, we have shown that elevation of blood glucose leads to increased glucose concentration in ASL (up to ~4.0mM) in nasal and bronchial secretions in patients[1,3,5,6]. Glucose was also elevated in Bronchoalveolar Lavage Fluid (BALF) from the lungs of a mouse model of diabetes (the leptin receptor deficient db/db mouse) compared to wild type[7]. Elevation of glucose was associated with increased Staphylococcus aureus and Pseudomonas aeruginosa in the airway and we have shown that sugars in the ASL can be utilized by these bacteria as a nutrient source for growth[8–10].
Inflammatory stimuli increase the permeability of the epithelium to glucose, elevating glucose diffusion from blood into the ASL[9]. Inflammatory stimuli also increase activation and genesis of inflammatory cells and their recruitment to the airway lumen[7,11]. Thus, glucose in the ASL potentially supplies a local nutrient source for inflammatory cells of the respiratory tract. Hyperglycemia has also been shown to modify inflammatory responses in the lung[12,13]. We hypothesized that the availability of glucose in the ASL and its metabolism by inflammatory cells could contribute to this effect. In addition, if inflammatory cells utilize glucose this would reduce glucose concentration in ASL and affect the concentration of other metabolites such as lactate.
Here, we investigated the relationship between blood glucose and glucose concentration in the BALFs of normal (WT) and hyperglycemic (glucokinase deficient, GK+/-) mice and studied how this relationship, the concentration of lactate, production of cytokines and the inflammatory responses changed when the lungs were challenged with bacterial lipopolysaccharide.
Materials and Methods
Male and female (17 weeks old, average weight 24±3g) heterozygote GK+/- mice on C57/Bl6 background and wild type C57/Bl6 mice were used in the study[14]. Animals were kept in a facility with 12h light/dark cycle at 21±2°C and with 55±15% relative humidity. Water and normal rodent chow were available ad libitum.
WT and GK+/- mice were randomized into treatment groups of 4, 24 and 48 hours’ post LPS challenge versus no challenge (indicated as time 0) using 4-5 animals per group. Experiments were repeated for 0 and 48 hours to obtain additional samples for cytokine analysis and results from these studies were pooled. LPS from P.aeruginosa (Sigma-Aldrich, USA) was previously frozen in 1mg/ml aliquots and diluted to the concentration needed to give 0.0875μg/g mouse in 50μl (based on the average weight of the group) and given by intranasal dosing at time 0. In brief, animals were anaesthetized with isoflurane 4-5% (O2 1.2l/min) prior to administration of the LPS solution to one nostril, which was subsequently inhaled naturally. Mice were then returned to their cages when retaining consciousness.
Body weight was recorded once daily from 0-48 hours after administration of LPS to follow the wellbeing of the animals. Blood was collected from the vena saphena in conscious mice for glucose evaluation after 4 hours of fasting. At 4, 24 or 48 hours after LPS administration, the animals were terminated with an overdose of pentobarbital (0.2ml of 100mg/ml i.p.). The lungs of each animal were subjected to Bronchoalveolar Lavage (BAL). In brief, the trachea was exposed and a catheter inserted into the trachea through a cut between two upper cartilage rings below the larynx. The catheter was secured with a silk suture and three volumes of 0.3ml saline were instilled, gently aspirated, pooled and weighed.
The BAL fluid (BALF) was centrifuged (SorvallRotanta 46R, HettichZentrifugen, Tullingen, Germany, 1200rpm (314g), 10min, 4°C). The pellet was re-suspended in 0.25ml of PBS and the total and differential cell count was performed using SYSMEX XT-1800i Vet. (SYSMEX, Kobe Japan). Cell count is expressed as the amount of cells per ml of recovered BALF. The supernatant was used to measure glucose and lactate on the ABX Pentra 400 (Horiba ABX Medical, Kyoto, Japan) according to the manufacturer’s protocol. Inflammatory cytokines in the supernatant were analyzed by means of an MSD 10-plex (K15048D-1 MSD mouse Pro inflammatory panel (V-plex), Mesoscale Discovery, Maryland, US). At termination, blood from behind the eye was collected in EDTA tubes and blood glucose was measured directly using Accu-check (Roche, Bromma, Sweden). Plasma lactate was assayed using the ABX Pentra 400. All experiments were approved by the local Ethical committee in Gothenburg (184-2012).
Statistical Analysis
Values are reported as the mean±SEM. Statistical analysis was performed using analysis of variance (ANOVA) tests followed by Bonferroni multiple comparison post hoc tests (GraphPad Prism) or Student’st test. P values of <0.05 were considered statistically significant.
Results
Characterization of Airway Glucose Metabolism
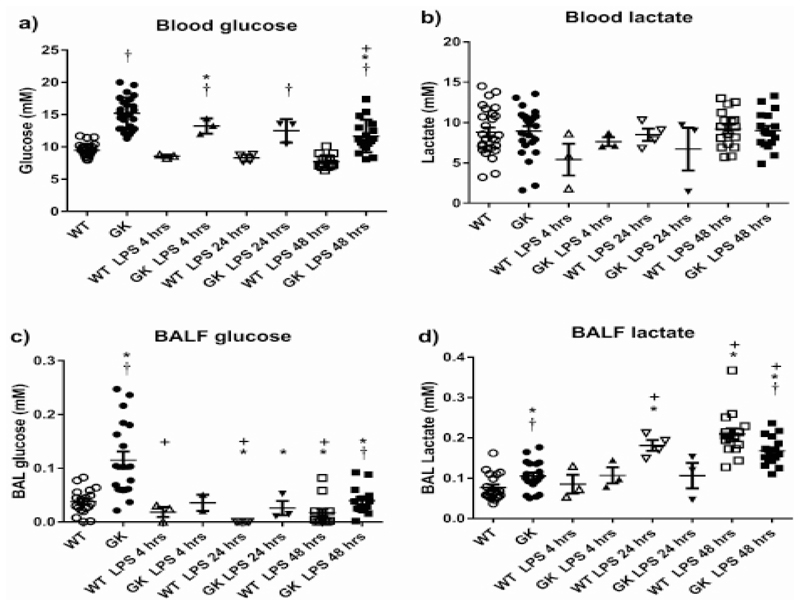
Fasting blood glucose was higher in GK+/- than Wild Type (WT) mice (15.2±4.9 compared to 9.4±0.19mM, p<0.0001, n=25) (Figure 1a). Blood glucose remained higher in GK+/- mice compared to WT 48 hours after treatment with LPS although the concentration of glucose was reduced to 11.7±0.6 and 7.7±0.2mM, respectively (p<0.0001, n=17). There was no significant difference in plasma insulin levels between WT and GK+/- mice (0.59±0.12 and 0.47±0.1ng/ml, respectively, n=9). There were also no significant changes in blood lactate concentration in WT or GK+/- at any of the time points tested (Figure 1b).
Figure 1.
Figure 1a: Blood glucose, Figure 1b: Blood lactate, Figure 1c: BALF glucose and Figure 1d: BALF lactate Concentrations in Wild Type (WT) (open symbols) and hyperglycemic GK+/- mice (black symbols) before (circles) and after treatment with LPS for 4 (triangles), 24 (inverted triangles) and 48 hours (squares). Data are shown as individual measurements. Horizontal bars show mean±SEM. Significantly different from untreated WT control, * p<0.05, significantly different from WT at similar time point, p-<0.05, significantly different from GK, p+<0.05.
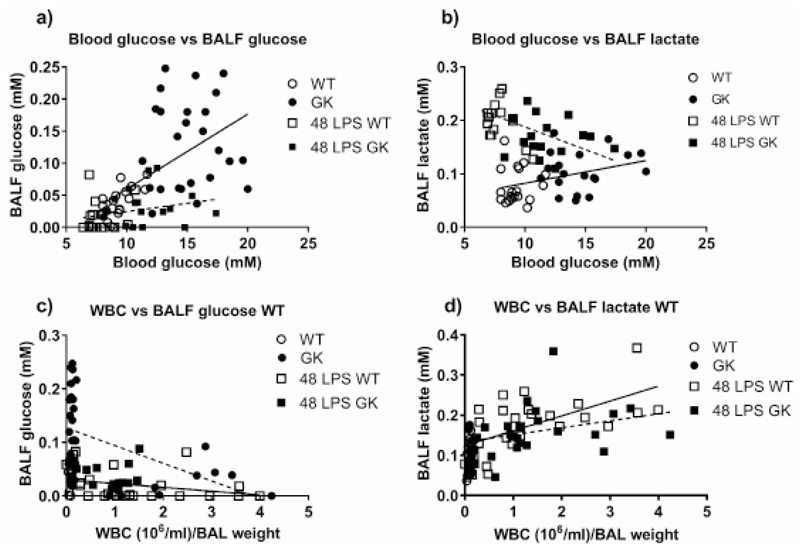
Glucose concentration in the BALF of GK+/- mice was significantly higher than that of WT mice (0.15±0.2 compared to 0.03±0.004mM, p<0.0001, n=19). It remained higher in the GK+/- mice following LPS challenge, but at significantly lower levels for both mouse types (p<0.001 at 24 and 48 hours, n=4 and 14, respectively). We could not detect any measurable glucose in BALF from WT mice at 24 hours (Figure 1c). There was a significant relationship between blood glucose/BALF glucose concentration prior to treatment with LPS and independent of mouse type (p<0.0001, n=44). However, this relationship was lost 48 hours after treatment with LPS (p<0.05, F=6, Df=73) (Figure 2a).
Figure 2.
Figure 2a: Relationship between Blood glucose and BALF glucose, Figure 2b: Blood glucose and BALF lactate, before (circles) and 48 hours after LPS treatment (squares) in WT (open) and GK+/- (black), respectively. Linear regression of the points before LPS treatment (WT/GK) is shown as a solid black line and after LPS treatment (48 LPS WT/GK) as a broken line. Figure 2c: Relationship between total WBC measured in BALF and BALF Glucose, Figure 2d: Total WBC in BALF and BALF lactate before (circles) and 48 hours after LPS treatment (squares) in WT (open) and GK+/- (black), respectively. Linear regression of the points from WT are shown as a solid line and GK+/- as a broken line.
Lactate concentration in the BALF of GK+/- mice was significantly higher than WT in the absence of an inflammatory stimulus (0.1±0.008 compared to 0.07±0.007mM, p<0.05, n=19) (Figure1d). As expected, the concentration of lactate in the BALFs of GK+/- and WT mice increased after treatment with LPS. Less so in the GK+/- mice, only reaching significance after 48 hours (p<0.01, n=16), whereas lactate concentration was significantly increased at 24 and 48 hours in WT mice (p<0.0001, n=3 and n=16, respectively). There were also higher concentrations of lactate in WT compared to GK+/- mice at 48 hours (p<0.01, n=15) whereas the difference at 24 hours was borderline significant (p=0.059, n=4). Thus, less lactate was produced in the lungs of GK+/- mice after an inflammatory stimulus than in WT mice (Figure 1d).
There was no relationship between blood lactate and BALF lactate but there was a small positive relationship between blood glucose and BALF lactate (p<0.01, n=41) at baseline (Figure 2b). This relationship was completely reversed 48 hours after treatment with LPS where lactate in the BALF decreased with increasing blood glucose (p<0.01, n=29) (Figure 2b), similarly for both mouse types.
Impact of Hyperglycemia on Airway Response to Lipopolysaccharide
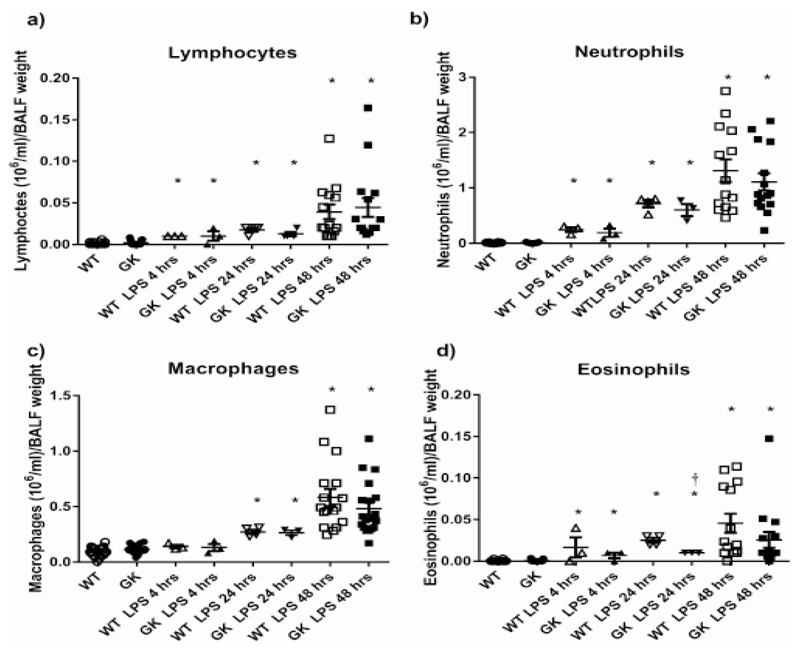
Prior to LPS treatment, GK+/- mice had similar numbers of inflammatory cells (total White Blood Cells (WBC) including lymphocytes, macrophages, neutrophils and eosinophils) in the BALF as compared to WT. Treatment of GK+/- with LPS elicited an increase in inflammatory cells recovered from the BALF which was maximal at 24-48 hours. The absolute numbers of lymphocytes and neutrophils were similarly increased in WT and GK+/- at 4, 24 and 48 hours versus untreated controls. Macrophages in the BALF were not elevated until 24 hours’ post LPS treatment and to a similar extent in WT and GK+/- mice (Figure 3a-c). Interestingly, 24 hours’ post LPS treatment, eosinophils were significantly reduced in GK+/- compared to WT mice (p<0.01, n=3) indicating that the elevation of blood glucose in these mice could influence eosinophil recruitment and/or survival (Figure 3d).
Figure 3.
Figure 3a: Numbers of Lymphocytes, Figure 3b: Neutrophils, Figure 3c: Macrophages and Figure 3d: Eosinophils in BALF from Wild Type (WT) (white symbols) and hyperglycemic GK+/- mice (black symbols) before (circles) and after treatment with LPS for 4 (triangles), 24 (inverted triangles) and 48 hours (squares). Data are shown as individual measurements. Horizontal bars show mean±SEM. Significantly different from untreated control, * p<0.05. Significantly different from WT at similar time point, p+<0.05.
There was a negative correlation of WBC with BALF glucose in WT and GK+/- mice (p<0.05, n=40 and p<0.001, n=47, respectively) (Figure 2c). As white blood cell number increased, glucose in the BALF decreased and this was more pronounced in GK+/- mice. As expected, the number of WBC positively correlated with BALF lactate concentration, similarly in WT and GK+/- mice (p<0.05, n=41 and p<0.01, n=48, respectively) (Figure 2d). We found similar relationships with macrophages and neutrophils, the most numerous cells in BALF, indicating that these were the main source of lactate. There was an indication that the slope of the line was reduced and that less lactate was produced by the inflammatory cells in GK+/- compared to WT mice, however, we were unable to attribute significance to this observation (p=0.08). Interestingly, eosinophils in WT mice showed a positive association with lactate (p<0.0001, n=39) but this was not observed in GK+/- mice (Figure 1d and Figure 3d).
Hyperglycemic Airways and Secretion of Cytokines in Response to Respiratory Challenge
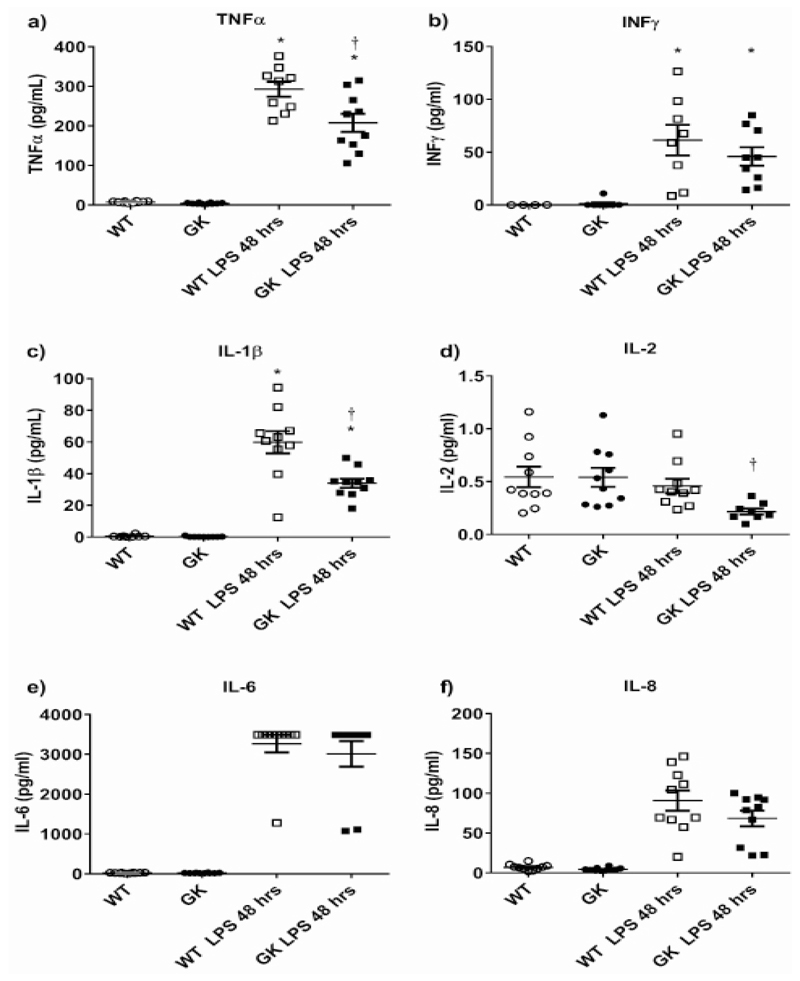
As the greatest differences in the parameters tested were identified at 48 hours’ post LPS challenge, we then investigated cytokine concentration in the BALFs of challenged and unchallenged animals. There was almost no production of cytokines into the BALF of untreated WT and GK+/- mice, consistent with the lack of inflammatory cells. LPS challenge for 48 hours significantly increased the concentration of TNFα, IL-1β, IL-6, IL-8 and INFγ(Figure 4a-f). IL-2 concentrations were lower than other cytokines and showed no elevation with LPS treatment (Figure 4d). There was significantly less IL-2, IL-1β and TNFα in the BALFs of GK+/- compared to WT mice 48 hours after LPS treatment (p<0.01, n=8-10 respectively).
Figure 4.
Figure 4a: Concentration of cytokines TNFα, Figure 4b: INFγ, Figure 4c: IL-1β, Figure 4d: IL-2, Figure 4e: IL-6and Figure 4f: IL-8 present in BALF from wild type (WT) (white symbols) and hyperglycemic GK+/- mice (black symbols) before (circles) and after treatment with LPS for 48 hours (squares). Data are shown as individual measurements. Horizontal bars show mean±SEM. Significantly different from untreated control, * p<0.05. Significantly different from WT at similar time point, p+<0.05.
Discussion
The present study demonstrates increased respiratory glucose concentration in a further hyperglycemic mouse model of human diabetes, in line with previous reports including human disease[1,3,7,10,15]. Also, here, we show a positive and significant correlation of airway glucose levels with increasing blood glucose concentrations, but no impact on inflammatory cells or cytokine release in the unchallenged situation. Exposure to Lipopolysaccharide (LPS) completely changes the blood/BALF glucose relationship. Glucose concentration falls and lactate rises as inflammatory cell numbers increase. We propose that inflammatory cells utilize the available glucose and increase the production of lactate. This effect is altered with hyperglycemia. Lactate concentration is lower and goes in hand with less cytokine production and recruitment of eosinophils. These data indicate a defective/suppressed response in hyperglycemic lungs to respiratory infections.
We have used a mouse model of hyperglycemia (the glucokinase heterozygous knockout model, GK+/-) which exhibit impaired glucose tolerance and diminished insulin secretion in response to glucose resulting in raised blood glucose concentration[16,17].These mice do not exhibit obesity or hyperinsulinemia that is associated with the leptin and leptin receptor deficient models of type II diabetes such as the ob/ob and db/db mouse[18,19].In this mouse, and similar to our findings in vivo and in vitro, raised blood glucose is associated with increased glucose in Bronchoalveolar Lavage Fluid / Airway Surface Liquid (BALF / ASL) and there is a positive relationship between blood glucose concentration and glucose in BALF. It is difficult to calculate the volume of mouse lung ASL and thus the absolute concentration of glucose without using dilution markers. However, a mouse of the weight used in these experiments would likely have a surface area of 70cm2[20,21]. Airway surface liquid has been proposed to be a depth of 8-35 μm depending on the radius of the airway [21,22] and alveolar fluid depths are much smaller at 0.1 μm [23]. A contribution of 50:50 alveolar: airway surface liquid to BALF glucose would give an estimate of 0.48mM glucose in ASL from WT mice and 1.5mM in GK+/- mice. Even if it is assumed that most of the sampling is from the distal lung i.e. 90:10 alveolar: airway, the resultant glucose concentrations are still calculated to be significantly less than corresponding blood glucose in accordance with our previous in vivo and in vitro studies [3,9].
Treatment of mice with LPS induced a robust inflammatory response as indicated by the elevation in inflammatory cells and pro-inflammatory cytokines in the BALF. Inflammatory cell numbers after LPS challenge were similar in GK+/- compared to WT mice except for eosinophils which were reduced. Similarly, eosinophils but not neutrophils, were reduced in ovalbumin challenged lungs of diabetic ob/ob mice[24]. No difference in lung neutrophils was shown in diabetic Zucker rat lungs after tracheal instillation of LPS or in diabetic Wistar rats compared to wildtype [12,13]. Thus, hyperglycemia appears to effect eosinophil recruitment to the lung.
LPS challenge decreased BALF glucose over 48 hours. Although, blood glucose declined with the inflammatory response [25] this was unlikely to be the cause of the change in BALF glucose as the relationship between blood and BALF glucose was lost and BALF glucose concentrations were much lower than expected for the corresponding blood glucose after LPS treatment. There was however, a clear inverse relationship between glucose concentration and number of inflammatory cells/lactate in BALF. A previous study in rats showed that tracheal instillation of LPS increased glucose utilization by White Blood Cells (WBC) in the airways[26]. This was mostly explained by increased numbers of neutrophils which had high glucose utilization and a doubling of glucose utilization by activated macrophages[26]. In our study, neutrophils and macrophages were the predominant cells post LPS treatment. In rats, glucose utilization by activated macrophages and PMNs increased to ~7.5 and 1.75nmols/min/106 cells, respectively. We had approximately 2 x 106 macrophages plus neutrophils in the mouse BALF 48 hours after LPS treatment. If rat and mouse models are similar, then glucose utilization would be ~18nmole/min. WT BALF contained 35nmoles of glucose which would be depleted rapidly by LPS activated inflammatory cells. Thus, utilization of glucose by inflammatory cells would account for the reduced glucose concentration in the BALF after LPS challenge. This hypothesis now needs to be tested to establish if this is indeed the case.
Glucose remained detectable in the BALF (except in WT 24 hours’ post LPS treatment) and glucose concentrations were persistently higher in GK+/- mice. We previously showed that LPS increased glucose permeability across ex vivo mouse trachea and in vitro which would increase glucose diffusion into ASL[9]. We therefore suggest that glucose in ASL is metabolized by inflammatory cells and that increased glucose permeability during the inflammatory response increases glucose diffusion into ASL and this process may help sustain inflammatory cell function. During hyperglycemia, the increased gradient for glucose diffusion would drive even more glucose into ASL resulting in the higher residual BALF glucose we observed after LPS treatment in GK+/- mice.
The increase in inflammatory cells correlated with elevation of pro-inflammatory cytokines and lactate. Lactate is involved in inflammatory cell signaling and is released at sites of inflammation by activated inflammatory cells. Its production is dependent on cellular glucose metabolism by inflammatory cells such as neutrophils[27–29]. However, we also observed a small increase in BALF lactate (but not in cytokines or inflammatory cells) in untreated GK+/- compared to WT mice. We have evidence that hyperglycemia elevates epithelial lactate secretion. This may therefore reflect a contribution from airway epithelial cells to lactate in BALF[8,30–32].
Here we provide evidence that LPS elevated lactate concentration in the BALF of GK+/- mice was reduced compared to WT. Consistent with our results, peritoneal neutrophils from streptozotocin-treated diabetic rats exhibited reduced lactate production[33]. In these neutrophils, the effect was reversed by insulin treatment. Insulin has also been shown to alter other LPS-induced inflammatory responses in diabetic rats[34], yet the GK+/- mice used in this, and two other studies, had normal circulating plasma insulin levels[14,16]. Thus, the role of luminal glucose concentration and insulin on inflammatory cell responses in these mice and in the lung, needs further investigation.
LPS-treated GK+/- mice also exhibited decreased TNFα, IL-1β and IL-2 in the BALF compared to WT. Lower levels of TNFα and IL-1β were reported 4-6 hours after LPS challenge also in diabetic Wistar rats compared to WT[13,35]. Similar to our findings, in one study this was independent of changes in neutrophils although in the other, this was not the case[13,35]. TNFα and IL-1β are secreted by activated macrophages. Whilst, we did not see a significant reduction of macrophages in BALF, there is evidence that macrophage secretory function was impaired in LPS-challenged streptozotocin-induced diabetic mice[36].
IL-2 is secreted by αβT-cells which are involved in endotoxin-induced airway inflammation[37]. We cannot explain why IL-2 was reduced in the GK+/- mice after LPS treatment. Genes involved in IL-2 signaling are implicated in the development of type I diabetes and circulating levels are reduced in this disease[38]. We did not measure plasma IL-2 and so cannot confirm whether this was the case in GK+/- mice. IL-2 is further involved in modifying cytokine secretion from other inflammatory cells. For example, human airway macrophages can be induced to produce TNFα in response to IL-2[39]. In addition, eosinophil degranulation is thought to be triggered by IL-2[38]. Thus, it is possible that the reduction of IL-2 in LPS-treated GK+/- mice leads to the reduction in the secretion of other cytokines and reduced eosinophil numbers at 24 hours.
Conclusion
Taken together, we show that acute LPS challenge in the mouse lung results in a reduction of BALF glucose concentration and elevation of inflammatory cells but that there is altered inflammatory cell responses in the lungs of GK+/- mice. We propose that glucose in the lung lumen is utilized by inflammatory cells during the inflammatory response. We showed previously that hyperglycemia results in elevated ASL glucose and that this promotes the growth of respiratory pathogens in the lung. We also proposed a model for glucose homeostasis across the airway [7,9,30,40]. We now propose a more complex model whereby, during infection, the inflammatory response competes with the airway epithelium and bacteria for glucose in the lung. Hyperglycemia elevates glucose concentration in the lung lumen resulting in a bigger nutrient pool and an altered immune response. Recent findings in the upper airway also showed that glucose in the airway surface liquid may suppress anti-microbial peptide secretion[41,42]. Thus, the balance that favors neutralization of pathogens in normal lungs may shift to favor pathogen evasion/survival in lungs exposed to hyperglycemia. Such changes may underlie the increased susceptibility to infection in patients with respiratory disease and diabetes (COPD/CF).
Acknowledgements
Funded by a Medical Research Council Industrial Collaboration Award with AstraZeneca Gothenburg, Sweden (MR/K012770/1).
Abbreviations
- WT
Wild Type
- GK
Glucokinase
- ASL
Airway Surface Liquid
- BAL
Bronchoalveolar Lavage
- BALF
Bronchoalveolar Lavage Fluid
- LPS
Lipopolysaccharide
- TNF
Tumor Necrosis Factor
- INF
Interferon
- IL
Interleukin
- PBS
Phosphate Buffered Saline
- MSD
Meso Scale Discovery
- WBC
White Blood Cells
- COPD
Chronic Obstructive Pulmonary Disease
- CF
Cystic Fibrosis
Footnotes
Authorship
Deborah L Baines, Emma H Baker, John D Taylor had the original concept for this work. Annika Åstrand, Cecilia Nagorny Holmberg, James P Garnett, Cecilia Wingren and Gaëll Mayer designed the study, acquired and analyzed the data. Deborah L Baines, Annika Åstrand, Cecilia Nagorny Holmberg, Gaëll Mayer and John D Taylor interpreted the data and compiled the work for publication.
References
- 1.Baker EH, Wood DM, Brennan AL, Clark N, Baines DL, et al. Hyperglycaemia and pulmonary infection. Proc Nutr Soc. 2006;65:227–235. doi: 10.1079/pns2006499. [DOI] [PubMed] [Google Scholar]
- 2.Baker EH, Wood DM, Brennan AL, Baines DL, Philips BJ. New insights into the glucose oxidase stick test for cerebrospinal fluid rhinorrhoea. Emerg Med J. 2005;22:556–557. doi: 10.1136/emj.2004.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker EH, Clark N, Brennan AL, Fisher DA, Gyi KM, et al. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J Appl Physiol. 2007;102:1969–1975. doi: 10.1152/japplphysiol.01425.2006. [DOI] [PubMed] [Google Scholar]
- 4.Philips BJ, Redman J, Brennan A, Wood D, Holliman R, et al. Glucose in bronchial aspirates increases the risk of respiratory MRSA in intubated patients. Thorax. 2005;60:761–764. doi: 10.1136/thx.2004.035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood DM, Brennan AL, Philips BJ, Baker EH. Effect of hyperglycaemia on glucose concentration of human nasal secretions. Clin Sci. 2004;106:527–533. doi: 10.1042/CS20030333. [DOI] [PubMed] [Google Scholar]
- 6.Baker E, Akunuri S, Morgan M, Greenaway S, O’Connor B, et al. Effect of airways inflammation and prednisolone treatment on BAL glucose concentrations in asthma and COPD patients. Thorax. 2008:A40. [Google Scholar]
- 7.Garnett JP, Baker EH, Naik S, Lindsay JA, Knight GM, et al. Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax. 2013;68:835–845. doi: 10.1136/thoraxjnl-2012-203178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garnett JP, Gray MA, Tarran R, Brodlie M, Ward C, et al. Elevated paracellular glucose flux across cystic fibrosis airway epithelial monolayers is an important factor for Pseudomonas aeruginosa growth. PLoS One. 2013;8:e76283. doi: 10.1371/journal.pone.0076283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garnett JP, Nguyen TT, Moffatt JD, Pelham ER, Kalsi KK, et al. Proinflammatory Mediators Disrupt Glucose Homeostasis in Airway Surface Liquid. J Immunol. 2012;189:373–380. doi: 10.4049/jimmunol.1200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strand A, Wingren C, Benjamin A, Tregoning JS, Garnett JP, et al. Dapagliflozin-lowered blood glucose reduces respiratory P. aeruginosa infection in diabetic mice. Br J Pharmacol. 2017;174:836–847. doi: 10.1111/bph.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt WR, Zughaier SM, Guentert DE, Shenep MA, Koval M, et al. Hyperglycemia impedes lung bacterial clearance in a murine model of cystic fibrosis-related diabetes. Am J Physiol Lung Cell Mol Physiol. 2014;306:L43–49. doi: 10.1152/ajplung.00224.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright JK, Nwariaku FN, Clark J, Falck JC, Rogers T, et al. Effect of diabetes mellitus on endotoxin-induced lung injury. Arch Surg. 1999;134:1354–1359. doi: 10.1001/archsurg.134.12.1354. [DOI] [PubMed] [Google Scholar]
- 13.Boichot E, Sannomiya P, Escofier N, Germain N, Fortes ZB, et al. Endotoxin-induced acute lung injury in rats. Role of insulin. Pulm Pharmacol Ther. 1999;12:285–290. doi: 10.1006/pupt.1999.0212. [DOI] [PubMed] [Google Scholar]
- 14.Gorman T, Hope DC, Brownlie R, Yu A, Gill D, et al. Effect of high-fat diet on glucose homeostasis and gene expression in glucokinase knockout mice. Diabetes Obes Metab. 2008;10:885–897. doi: 10.1111/j.1463-1326.2007.00819.x. [DOI] [PubMed] [Google Scholar]
- 15.Gill SK, Hui K, Farne H, Garnett JP, Baines DL, et al. Increased airway glucose increases airway bacterial load in hyperglycaemia. Sci Rep. 2016;6 doi: 10.1038/srep27636. 27636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terauchi Y, Kadowaki T. Glucokinase knockout mice. Nihon Rinsho. 1998;56:746–752. [PubMed] [Google Scholar]
- 17.Sakura H, Ashcroft SJ, Terauchi Y, Kadowaki T, Ashcroft FM. Glucose modulation of ATP-sensitive K-currents in wild-type, homozygous and heterozygous glucokinase knock-out mice. Diabetologia. 1998;41:654–659. doi: 10.1007/s001250050964. [DOI] [PubMed] [Google Scholar]
- 18.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 20.Knust J, Ochs M, Gundersen HJ, Nyengaard JR. Stereological estimates of alveolar number and size and capillary length and surface area in mice lungs. Anat Rec Hoboken. 2009;292:113–122. doi: 10.1002/ar.20747. [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Thiagarajah J, Verkman AS. Sodium and chloride concentrations, pH, and depth of airway surface liquid in distal airways. J Gen Physiol. 2003;122:511–519. doi: 10.1085/jgp.200308866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarran R. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc Am Thorac Soc. 2004;1:42–46. doi: 10.1513/pats.2306014. [DOI] [PubMed] [Google Scholar]
- 23.Siebert TA, Rugonyi S. Influence of liquid-layer thickness on pulmonary surfactant spreading and collapse. Biophys J. 2008;95:4549–4559. doi: 10.1529/biophysj.107.127654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, et al. Allergic airway responses in obese mice. Am J Respir Crit Care Med. 2007;176:650–658. doi: 10.1164/rccm.200702-323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harnish MJ, Lange T, Dimitrov S, Born J, Fehm HL. Differential regulation of human blood glucose level by interleukin-2 and -6. Exp Clin Endocrinol Diabetes. 2005;113:43–48. doi: 10.1055/s-2004-830526. [DOI] [PubMed] [Google Scholar]
- 26.De Boisblanc BP, Dobrescu C, Skrepnik N, Nelson S, Spitzer JJ, et al. Compartmentalization of glucose utilization after intravenous vs. intratracheal challenge with LPS. Am J Physiol. 1996;270:L452–458. doi: 10.1152/ajplung.1996.270.3.L452. [DOI] [PubMed] [Google Scholar]
- 27.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalsi KK, Baker EH, Fraser O, Chung YL, Mace OJ, et al. Glucose homeostasis across human airway epithelial cell monolayers: role of diffusion, transport and metabolism. Pflugers Arch. 2009;457:1061–1070. doi: 10.1007/s00424-008-0576-4. [DOI] [PubMed] [Google Scholar]
- 31.Myerburg MM, King JD, Oyster NM, Fitch AC, Magill A, et al. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2010;42:676–684. doi: 10.1165/rcmb.2009-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garnett JP, Kalsi KK, Sobotta M, Bearham J, Carr G, et al. Hyperglycaemia and Pseudomonasaeruginosa acidify cystic fibrosis airway surface liquid by elevating epithelial monocarboxylate transporter 2 dependent lactate-H+ secretion. Sci Rep. 2016;6 doi: 10.1038/srep37955. 37955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alba-Loureiro TC, Hirabara SM, Mendonca JR, Curi R, Pithon-Curi TC. Diabetes causes marked changes in function and metabolism of rat neutrophils. J Endocrinol. 2006;188:295–303. doi: 10.1677/joe.1.06438. [DOI] [PubMed] [Google Scholar]
- 34.Martins JO, Ferracini M, Anger DB, Martins DO, Ribeiro LF, et al. Signaling pathways and mediators in LPS-induced lung inflammation in diabetic rats: role of insulin. Shock. 2010;33:76–82. doi: 10.1097/SHK.0b013e3181a85ec4. [DOI] [PubMed] [Google Scholar]
- 35.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 36.Amano H, Yamamoto H, Senba M, Oishi K, Suzuki S, et al. Impairment of endotoxin-induced macrophage inflammatory protein 2 gene expression in alveolar macrophages in streptozotocin-induced diabetes in mice. Infect Immun. 2000;68:2925–2929. doi: 10.1128/iai.68.5.2925-2929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson R, Rocksen D, Lilliehook B, Jonsson A, Bucht A. Dose-dependent activation of lymphocytes in endotoxin-induced airway inflammation. Infect Immun. 2000;68:6962–6969. doi: 10.1128/iai.68.12.6962-6969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hulme MA, Wasserfall CH, Atkinson MA, Brusko TM. Central role for interleukin-2 in type 1 diabetes. Diabetes. 2012;61:14–22. doi: 10.2337/db11-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strieter RM, Remick DG, Lynch JP, Spengler RN, Kunkel SL. Interleukin-2-induced tumor necrosis factor-alpha (TNF-alpha) gene expression in human alveolar macrophages and blood monocytes. Am Rev Respir Dis. 1989;139:335–342. doi: 10.1164/ajrccm/139.2.335. [DOI] [PubMed] [Google Scholar]
- 40.Garnett JP, Braun D, McCarthy AJ, Farrant MR, Baker EH, et al. Fructose transport-deficient Staphylococcus aureus reveals important role of epithelial glucose transporters in limiting sugar-driven bacterial growth in airway surface liquid. Cell Mol Life Sci. 2014;71:4665–4673. doi: 10.1007/s00018-014-1635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee RJ, Cohen NA. Bitter and sweet taste receptors in the respiratory epithelium in health and disease. J Mol Med Berl. 2014;92:1235–1244. doi: 10.1007/s00109-014-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124:1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]