Abstract
Background and Purpose
Ginkgo biloba extracts are now prescribed in several countries for their reported health benefits, particularly for medicinal properties in the brain. The standardized Ginkgo extract, EGb761, has been reported to protect neurons against oxidative stress, but the underlying mechanisms are not fully understood.
Methods
To characterize the oral consumption of EGb761 in transient ischemia, we performed the middle cerebral artery occlusion (MCAO) filament model in wild-type and heme oxygenase 1 (HO-1) knockouts. Mice were pretreated for 7 days before the transient occlusion or posttreated acutely during reperfusion; then neurobehavioral scores and infarct volumes were assessed. Furthermore, primary cortical neuronal cultures were used to investigate the contribution of the antioxidant enzyme HO-1 in the EGb761-associated cytoprotection.
Results
Mice that were pretreated with EGb761 had 50.9±5.6% less neurological dysfunction and 48.2±5.3% smaller infarct volumes than vehicle-treated mice; this effect was abolished in HO-1 knockouts. In addition to the prophylactic properties of EGb761, acute posttreatment 5 minutes and 4.5 hours after reperfusion also led to significant reduction in infarct size (P<0.01). After our previous demonstration that EGb761 significantly induced HO-1 levels in a dose- and time-dependent manner in neuronal cultures, here we revealed that this de novo HO-1 induction was required for neuroprotection against free radical damage and excitotoxicity as it was significantly attenuated by the enzyme inhibitor.
Conclusion
These results demonstrate that EGb761 could be used as a preventive or therapeutic agent in cerebral ischemia and suggest that HO-1 contributes, at least in part, to EGb761 neuroprotection.
Keywords: cerebral ischemia, EGb761 Ginkgo biloba extract, heme oxygenase-1, oxidative stress, mice
The most adequate treatment for acute stroke in humans is the immediate canalization of occluded arteries.1 However, reperfusion of the ischemic region may paradoxically exacerbate brain damage via reperfusion injury.2 Immediate mechanisms of ischemia/reperfusion (I/R)-induced brain damage are caused by NMDA-induced excitotoxicity and altered intracellular Ca2+ homeostasis.3 Secondary injury can be caused by the formation of reactive oxygen species (ROS), which mediate oxidative damage, inflammation, and apoptosis.4 Therefore, in addition to other therapeutic approaches,5 one alternative strategy for achieving neuroprotection can be the stimulation of an endogenous antioxidant system.6
Heme oxygenase (HO), the rate-limiting enzyme for the catabolism of the prooxidant heme, produces equimolar quantities of carbon monoxide (CO), iron, and biliverdin, which is immediately reduced to bilirubin by biliverdin reductase.7 Two isoforms of HO exist: an inducible form (HO-1) and a constitutively expressed form (HO-2). These isoenzymes are products of different genes and vary markedly in their tissue distribution and molecular properties. Whereas HO-2 (abundant in brain and testis) regulates normal physiological cell function, HO-1 is induced in response to various noxious stimuli (such as hypoxia and oxidative stress). HO-1 is considered a protective gene in many clinically relevant disease states, including I/R injury, Alzheimer disease, hypertension, atherosclerosis, and diabetes.8,9 Genetic deletion of constitutive HO-2 exacerbates ischemic brain injury.10
One of the most well recognized standardized extracts of Ginkgo biloba leaves, EGb761 (Tanakan), has been shown to have neuroprotective and antioxidant properties6 against various cardiovascular and neurological disorders, such as ischemia, Alzheimer disease, and depression.11,12 Its actions are thought to be mediated mainly via its phenolic and terpenoid compounds. EGb761 has been shown to reduce neuronal death in focal6 and global brain ischemia.12 It also has been used to reduce cognitive dysfunctions associated with dementia, aging, and senility.13
The mechanism(s) of action of EGb761 in ischemia-induced brain injury is unknown, but we postulate that it is mediated at least partially through the HO pathway. Our previous results demonstrated that in primary neuronal cultures, EGb761 induces HO-1 expression in a time- and dose-dependent manner without causing toxicity.14 Furthermore, it has been shown that EGb761 induces phase 2 genes through the Nrf2-antioxidant/electrophilic response element (ARE) signaling pathway15 and that of known proteins, HO-1 has the most ARE elements within its promoter region, making it a unique target for this EGb761-stimulated endogenous pathway.
In this study we examined whether 7 days of pretreatment with EGb761 could dose dependently protect against I/R-induced brain injury and associated neurobehavioral deficits in wild-type (WT) mice. To test whether HO-1 is necessary for EGb761 protection, we repeated these experiments in HO-1 knockout (HO-1−/−) mice. We extended our investigation to determine whether posttreatment with EGb761 could reduce brain injury and, if so, address its therapeutic window. To further understand the mechanism of protection, we examined the specificity and dose-dependent effect of EGb761 on HO-1 and HO-2 induction.
Materials and Methods
Animals
The study was conducted in accordance with the National Institutes of Health guidelines for the use of experimental animals. All experimental procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committee. All mice were obtained from our breeding colonies and genotyped by polymerase chain reaction. Male HO-1−/− and WT C57BL/6 mice (8 to 10 weeks old) were used.
Transient Cerebral Ischemia, Neurological Function, and Infarct Size Determination
Mice were orally administered EGb761 (IPSEN Laboratories) or vehicle (distilled water-PEG 400 [70:30]) once daily for 7 days before induction of middle cerebral artery (MCA) occlusion (MCAO). In a second set of experiments, WT mice were given EGb761 (100 mg/kg p.o.) or vehicle at 5 minutes or 4.5 hours of reperfusion after MCAO. Transient focal cerebral ischemia was induced with an intraluminal filament technique as described previously.16 Briefly, relative cerebral blood flow (CBF) over the parietal cortex was measured by laser-Doppler flowmetry. A nylon filament was inserted into the MCA and maintained for 120 minutes (pre-treatment experiments) or 90 minutes (posttreatment experiments) during which the neck was closed with sutures, anesthesia was discontinued, and the animals were transferred to a temperature-controlled chamber to maintain body temperature at 37.0±0.5°C. After reperfusion, the mice were returned to the temperature-controlled chamber for 6 hours. Twenty-four and 72 hours after reperfusion, mice were scored for neurological function according to a scale in which 0 is no deficit and 4 is maximal deficit. Then, with the mice anesthetized, the brains were removed, sliced coronally into five 2-mm thick sections, and incubated with 1% 2,3,5-triphenyltet-razolium chloride (TTC) in saline for 30 minutes at 37°C to measure infarct volume.16
Measurement of Body Temperature, Blood Gases, Mean Arterial Blood Pressure, and Regional CBF
Physiological parameters (body temperature, PaO2, PaCO2 and mean arterial blood pressure [MABP]) were measured every 15 minutes before and during ischemia and for 1 hour of reperfusion in a separate cohort of mice (n=5/genotype). The regional CBF was determined by [14C]iodoantipyrine (IAP) autoradiography after 1 hour of ischemia in mice pretreated for 7 days with vehicle or EGb761 (n=5 to 8 per genotype), as detailed before.16 Nine autoradiographic images at each of 6 coronal levels (+2, +1, 0, −1, −2, −3 mm from the bregma) were digitized, and regional blood flow was calculated with image analysis software (Inquiry, Loats Associates).
Effect of Ginkgo Biloba Extracts on Protein Expression and Cell Survival After Induced Toxicity in Mouse Primary Neuronal Cultures
All materials used for cell culture were obtained from Invitrogen. Cortical neuronal cells were isolated from 17-day embryos of timed-pregnant mice. Neurons were cultured in serum-free conditions and plated onto 24-well dishes at a density >0.5×106 cells per well in HEPES-buffered, high glucose Neurobasal medium with B27 supplement, as previously described.17 Half of the medium was removed every 4 days and replaced with fresh medium. To compare the effects of EGb761 and individual Ginkgo biloba extract components on HO-1 and HO-2 protein expression, mouse neuronal cultures were treated with vehicle-control, EGb761, bilobalide, or ginkgolides for 8 hours before being harvested for Western blot analysis. To determine whether inhibition of protein synthesis or mRNA synthesis can counter the effect of EGb761 on HO-1 expression, neuronal cells were treated for 8 hours with vehicle-control or EGb761 alone, or with the protein synthesis inhibitor cycloheximide (CHX; Sigma) or the mRNA synthesis inhibitor actinomycin D (ATD; Sigma). Cells were then harvested and homogenized for Western blot analysis. Cells were solubilized with 250 μL of lysis buffer (50 mmol/L Tris-HCl, pH 7.4; 150 mmol/L NaCl; 0.5% Triton X-100), including protease inhibitor cocktail (Sigma), on ice for 30 minutes and centrifuged for 10 minutes at 12 000g. The supernatant was then collected, and protein concentration quantified. Protein was separated on 12% SDS-PAGE gels and transferred to nitrocellulose membranes.17 Blots were stained with Ponceau S Solution (Sigma) to further verify that equal amounts of protein were loaded in each lane. Membranes were blocked for 1 hour at room temperature with 5% skim milk in phosphate-buffered saline (PBS) with 0.1% Tween 20 before incubation at 4°C overnight with polyclonal antibodies to HO-1 (1:2000), HO-2 (1:2000), CP450R (StressGen Inc; 1:2000), and actin (Sigma; 1:5000). Blots were washed and incubated with secondary antibodies for 1 hour at room temperature and developed by enhanced chemiluminescence (Amersham Biosciences). For cell toxicity protocols, neuronal cell cultures (14 days in vitro) were pretreated with different concentrations of EGb761 for 6 hours, and then treated for 18 hours with H2O2 (20 μmol/L), glutamate (30 μmol/L), or vehicle (control) with or without 5 μmol/L HO inhibitor (SnPPIX, Porphyrin Products, Inc). Cell survival was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay.14 Experimental conditions were carried out in quadruplicate and repeated 3 times with different batches of primary cultures.
Luminescence Analysis
Mouse hepatoma cells were stably transformed with pARE-Luc (hepa pARE-luc). pARE-luc is an ARE-dependent reporter plasmid that uses the firefly luciferase gene as a reporter under the control of a minimal promoter of mouse ho1 gene with 3 copies of ARE. Hepa pARE-luc were plated at 10 000 cells per well in 96-well plates and maintained in DMEM containing 10% fetal bovine serum, 10 mg/mL gentamicin (Sigma), and 100 mg/mL geneticin (Invitrogen). On the second day after plating, cells were washed twice with PBS, lysed in 30 μL passive lysis buffer, and shaken for 20 minutes at room temperature. Luciferase assay reagent (50 μL; Promega) was mixed with 10 μL of cell lysate, and fluorescence was read with a luminometer (EG & G Berthold).
Statistical Analysis
Unpaired t test was used to compare vehicle-treated and EGb761-treated groups. Multiple comparisons were analyzed by ANOVA and Tukey test. Data are expressed as means±SEM, with P<0.05 considered to be statistically significant. Regional CBF was determined by 2-way ANOVA followed by Bonferroni multiple comparison tests to find differences between groups in the striatum and cortex after 1 hour of ischemia.
Results
Pre- and Posttreatment With EGb761 Improves Neurological Score and Reduces Infarct Size After I/R Injury
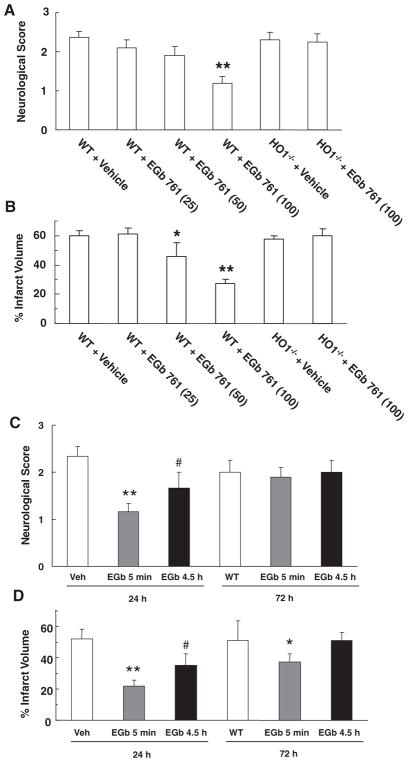
To address the potential of EGb761 to prevent stroke injury, WT and HO1−/− mice were pretreated for 7 days with EGb761 before being subjected to MCAO and reperfusion. WT mice that received 100 mg/kg EGb761 had significantly less neurological dysfunction (P<0.01) than those that received a lower dose or vehicle (Figure 1A). There was no significant difference in neurological function between HO-1−/− mice that received EGb761 and those that did not or between vehicle-treated WT and HO-1−/− mice (Figure 1A). Staining of brain sections with TTC revealed that WT mice pretreated with EGb761 had significantly smaller corrected infarct volumes than did vehicle-treated mice after 24 hours of reperfusion (Figure 1B). The protection was dose dependent, with the greatest effect seen at 100 mg/kg (P<0.01). EGb761 did not have an effect on the infarct size in HO-1−/− mice. Moreover, after identical pretreatment with 100 mg/kg EGb761, WT mice (n=5) had an estimated 44.0% less neurological dysfunction (1.4±0.2 versus 2.5±0.2) and 41.3% smaller infarct size (35.6±5.9 versus 60.7±3.9) than did vehicle-treated controls (n=6) after 72 hours of reperfusion.
Figure 1.
Effect of EGb761 pre- and posttreatment on stroke outcomes. For the pretreatment study, wild-type (WT) and HO-1 knockout (HO-1−/−) mice were pretreated for 7 days with EGb761 and subjected to transient middle cerebral artery occlusion (MCAO) and 24 hours of reperfusion before being assessed for neurological deficit (A) and infarct volume (B). For the posttreatment study, WT mice were subjected to transient MCAO and given 100 mg/kg EGb761 either 5 minutes or 4.5 hours into the reperfusion. Neurological deficit scores (C), and corrected infarct volumes (D) were assessed after 24 or 72 hours of reperfusion. Data are expressed as mean±SEM; n=8 to 12 per group. *P<0.05, **P<0.01 vs vehicle-treated control, #P<0.05 vs the 5-minute exposure.
To address the potential therapeutic role of EGb761 in stroke, mice were posttreated with 100 mg/kg EGb761 at 5 minutes and 4.5 hours after the start of cerebral reperfusion. Posttreatment resulted in significant improvement in neurological score (P<0.01 [5 minutes] and P<0.05 [4.5 hours]) at 24 hours (Figure 1C). However, at 72 hours, no significant difference in neurological function was observed between the groups. In terms of infarct volume, posttreatment with EGb761 at 5 minutes of reperfusion provided significant protection at 24 hours (P<0.01) and 72 hours (P<0.05), but posttreatment at 4.5 hours after reperfusion provided protection only at 24 hours (P<0.05; Figure 1D).
Effect of EGb761 on Physiological Parameters and CBF
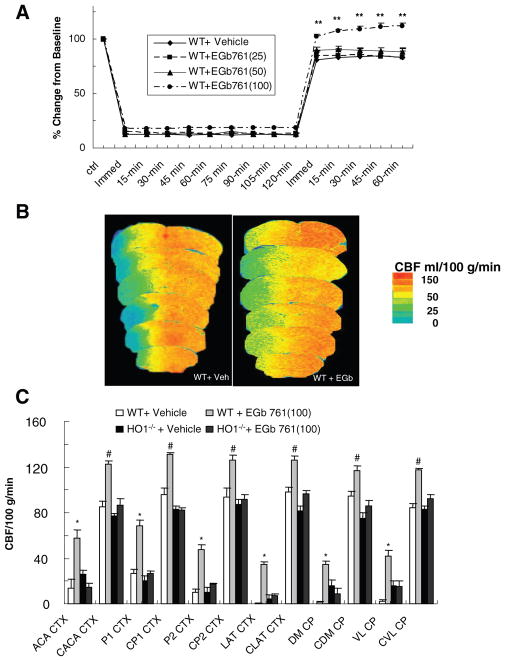
In a separate cohort, we monitored the impact of EGb761 on physiological parameters of mice undergoing MCAO. At the highest dose of EGb761 (100 mg/kg), CBF was significantly higher than that of vehicle-treated mice during the reperfusion period monitored (Figure 2A). No changes in MABP (84 to 73 mm Hg), body temperature (maintained at 37±0.5°C), or blood gas parameters (pH, PaCO2, and PaO2) were observed (Table).
Figure 2.
Effects of EGb761 on cerebral blood flow. Relative cerebral blood flow (CBF, A) was recorded at baseline, at induction of ischemia, and at 15-minute intervals during ischemia and 1 hour of reperfusion. Change in CBF was recorded as a percent of baseline. **P<0.01. In addition, regional CBF was determined by [14C]-IAP autoradiography within 6 regions of contralateral nonischemic cortex, ipsilateral ischemic cortex, and caudate putamen, subdivided into parietal, lateral, and medial areas, at 60 minutes of MCAO. B, [14C]-IAP autoradiographic images of a vehicle-treated wild-type (WT) mouse (left) and a WT mouse that received 100 mg/kg EGb761 (right). The color key indicates that colors toward the red spectrum represent areas of higher flow whereas colors toward the blue spectrum represent areas of lower flow. C, Graph representing mean CBF of each mouse group *P<0.05; #P<0.01. ACA CTX indicates anterior cerebral artery cortex; CACA, contralateral anterior cerebral artery; P1, parietal 1; CP1, contralateral parietal 1; P2, parietal 2; CP2, contralateral parietal 2; LAT CTX, lateral cortex; CLAT CTX, contralateral lateral cortex; DM CP, dorsomedial caudate putamen; CDM CP, contralateral dorsomedial caudate putamen; VL CP, ventrolateral caudate putamen; CVL CP, contralateral ventrolateral caudate putamen.
Table.
Effect of MCAO on Physiological Parameters in Vehicle and EGb761-Pretreated Mice
| Parameter | Vehicle | EGb761 (mg/kg, p.o.) | ||
|---|---|---|---|---|
|
| ||||
| 25 | 50 | 100 | ||
| pH | ||||
| Baseline | 7.34±0.01 | 7.34±0.01 | 7.34±0.01 | 7.35±0.01 |
| 1 hour MCAO | 7.33±0.01 | 7.34±0.01 | 7.33±0.01 | 7.34±0.01 |
| 1 hour reperfusion | 7.33±0.01 | 7.33±0.01 | 7.34±0.02 | 7.34±0.01 |
| PaCO2 | ||||
| Baseline | 39.8±1.0 | 39.9±1.1 | 39.1±1.4 | 38.4±0.7 |
| 1 hour MCAO | 40.2±0.9 | 40.2±1.2 | 39.6±1.3 | 39.3±1.0 |
| 1 hour reperfusion | 40.8±0.7 | 41.0±1.1 | 40.0±1.1 | 39.0±0.9 |
| PaO2 | ||||
| Baseline | 117±4 | 112±5 | 111±7 | 115±5 |
| 1 hour MCAO | 118±5 | 115±6 | 115±7 | 120±6 |
| 1 hour reperfusion | 121±5 | 119±6 | 117±6 | 119±7 |
Values are expressed as mean±SEM, n=5.
Potential differences in vascular responsiveness between WT mice treated with vehicle and those treated with EGb761 were examined by quantifying absolute regional CBF in the different brain regions of the ipsilateral and contralateral hemispheres using the [14C]-IAP protocol (Figure 2B). After 60 minutes of MCAO, the ipsilateral CBF (ml/100 g/min) was significantly higher in mice pretreated for 7 days with EGb761 (100 mg/kg) than in vehicle-treated mice in all regions measured (Figure 2C).
EGb761 Extract Is More Potent Than Its Bilobalide or Ginkgolide Bioactive Constituents for HO-1 Protein Induction
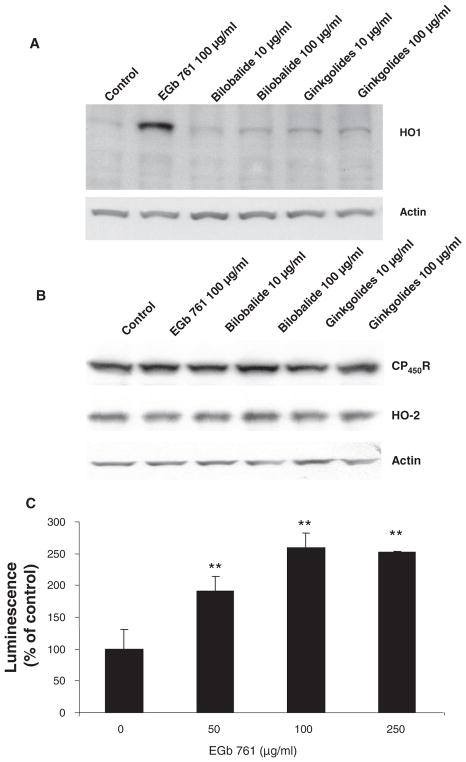
We have reported previously that in mouse primary cortical neurons treated with various concentrations of EGb761 (up to 500 μg/mL), HO-1 induction was evident after 4 and 8 hours of treatment at a concentration as low as 10 μg/mL and increased in a dose-dependent manner.14 Here, to address the effect of various components present in the EGb761 extract, we treated cells with two different doses of the bilobalide and ginkgolide extracts for 8 hours. Western blot analysis clearly indicated that HO-1 was highly induced by EGb761, but at the concentrations tested, the other bioactive constituents did not have as potent an effect (Figure 3A). Interestingly, this result suggests that the multiple components present within EGb761 are more potent together than the individual ones tested here.
Figure 3.
Effect of EGb761, bilobalide, and ginkgolides on HO-1, cytochrome P450 reductase (CP450R), HO-2, and actin protein expression in mouse primary neurons. The cells were treated for 8 hours with the concentrations shown before being harvested and analyzed by Western blot. A, HO-1 protein expression was enhanced by EGb761 and to a lesser extent by bilobalide and ginkgolides. B, The expression of CP450R and HO-2 was unaffected by EGb761, bilobalide, or ginkolides. Actin is shown on the same blot to indicate that loading was similar in all lanes. C, Dose response of Ginkgo biloba extract EGb761 on the minimal HO-1 promoter. Hepa pARE-luc cells were treated for 8 hours with various concentrations of EGb761 before being harvested for luminescence measurement. **P<0.01 when compared with the control group.
No Detectable Effect of EGb761, Bilobalide, or Ginkgolide on the Protein Expression of CP450R, HO-2 in Mouse Primary Neurons
EGb761, bilobalide, and ginkgolide extracts were tested in primary neuronal cultures to determine their effects on expression of HO-2 and cytochrome P450 reductase (CP450R, an electron donor). Western blot analysis showed no obvious changes in the expression of CP450R, HO-2, or actin (used as a loading control; Figure 3B).
EGb761 Increases Response of ARE Elements Present Within the HO-1 Promoter
Hepa pARE-luc cells were treated with various concentrations (0, 50, 100, and 250 μg/mL) of EGb761 for 8 hours. The data show that EGb761 stimulated the minimal HO-1 promoter in a dose-dependent manner to increase the transcription of HO-1 (Figure 3C). The effect peaked at 100 μg/mL treatment; no additional increase was observed at 250 or 500 μg/mL (data not shown).
EGb761 Increases HO-1 Protein Levels and Limits Glutamate-Induced Excitotoxicity in Mouse Primary Neuronal Cultures
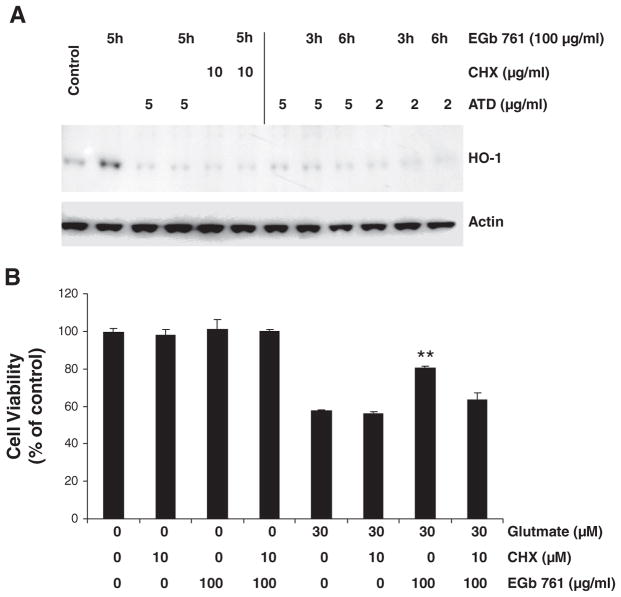
EGb761 substantially induced HO-1 expression in primary neurons after 5 hours of exposure. The protein synthesis inhibitor CHX and the mRNA synthesis inhibitor ATD were able to completely block the HO-1 induction by EGb761, suggesting that the increase in expression represented de novo HO-1 synthesis (Figure 4A). Furthermore, 6 hours of pretreatment with 100 μg/mL EGb761 protected mouse cortical neuronal cells against glutamate-induced excitotoxicity (Figure 4B); this protection was significantly reduced by 10μmol/L CHX.
Figure 4.
Effects of EGb761 on neuronal HO-1 protein expression in mouse primary neuronal cultures. A, Cultured neurons were pretreated for 1 hour with the protein synthesis inhibitor cycloheximide (CHX) or the mRNA inhibitor actinomycin D (ATD) in the concentrations shown before having 100 μg/mL EGb761 added to the culture medium for an additional 3 or 6 hours. The top panel shows the effect of the various drug regimens on HO-1 protein expression. The bottom panel shows actin expression in the same blot to indicate similar protein loading in all lanes. B, Primary neurons cultured for 14 days were pretreated with 10 μmol/L CHX or vehicle for 1 hour before being exposed to 100 μg/mL EGb761 or vehicle for 6 hours. Then cells were rinsed and incubated with fresh medium containing glutamate (30 μmol/L) or vehicle for an additional 18 hours. Each experiment was conducted in quadruplicate and repeated 3 times with different primary culture batches. Cell survival was estimated by the MTT assay and expressed as a percent of control viability. **P<0.01 compared with control groups.
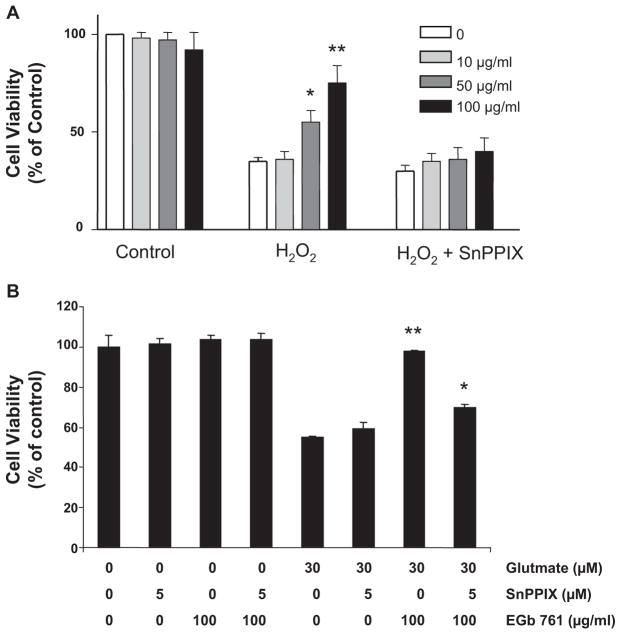
EGb761 Offers Neuroprotection Against H2O2 and Glutamate Toxicity
Six hours of pretreatment with 100 μg/mL EGb761 protected mouse cortical neuronal cells against H2O2-induced oxidative stress (Figure 5A) and against glutamate-induced excitotoxicity (Figure 5B). The HO inhibitor SnPPIX (5 μmol/L) significantly blocked the EGb761 protective effect in both experimental conditions (Figure 5A and 5B).
Figure 5.
EGb761 is neuroprotective against H2O2- and glutamate-induced toxicity. A and B, Primary neurons cultured for 14 days were pretreated for 6 hours with 100 μg/mL EGb761 or vehicle. Cells were exposed to fresh medium containing (A) 20 μmol/L H2O2, (B) 30 μmol/L glutamate, or vehicle (Control) with or without 5 μmol/L SnPPIX for an additional 18 hours. Each experiment was conducted in quadruplicate and repeated 3 times with different primary culture batches. Cell survival was estimated by the MTT assay and expressed as a percent of control viability. *P<0.05. **P<0.01 compared with control groups.
Discussion
Here we showed that the standardized Ginkgo biloba extract EGb761 significantly improved the outcome in mice after cerebral ischemia and reperfusion in terms of neurobehavioral function and infarct size without affecting physiological parameters. Only at the highest dose tested did we observe a small but significant increase in the CBF at 1 hour after reperfusion. Interestingly, all of these changes were lost in the HO-1 knockout mice. Our in vitro results from cell cultures further demonstrate that EGb761 increases de novo HO-1 levels within neurons and leads to cytoprotection. EGb761 pretreatment was able to provide neuroprotection against H2O2-induced oxidative stress injury and glutamate-induced excitotoxicity. Taken together, our results indicate that EGb761 may be beneficial in protecting neurons from insults resulting from I/R injury and that HO-1 likely contributes, at least partially, to this protective effect.
Although strong evidence has indicated that pretreatment with EGb761 is therapeutic against I/R-induced brain injury,18 until now, the mechanism has been unknown. Our current results suggest that the mechanism is through HO-1. Under normal conditions, the protective heat shock protein HO-1 is present in the whole brain, but it is selectively expressed in very few neuronal and non-neuronal cell populations.19 Previous reports have suggested that HO-1 is induced in neurons of the cortex and striatum but not the hippocampus after transient forebrain ischemia.20 Few reports are available to show the relationship between the EGb761 effect on I/R and HO-1. Here we demonstrated that pre- or posttreatment with EGb761 could significantly reduce the resulting infarct size after MCAO in WT mice. This protective effect of pretreatment was absent in HO-1−/− mice, indicating that HO-1 is essential for the actions of EGb761.
Several potential mechanisms of brain injury protection could stem from the action of HO-1. One important pathway is the transcriptional activation of phase 2 genes through a cis-acting element called the ARE.21 Recent reports suggest that EGb761 mediates induction of phase 2 enzymes through this pathway.15 Of phase 2 genes, the HO-1 gene is known to have the most ARE elements within its promoter; the mouse HO-1 gene has two enhancers, both harboring multiple AREs.22 Our results suggest that induction of cell death is inversely correlated with the induction of ARE-dependent expression of antioxidant HO-1 after treatment with EGb761.
There are abundant heme-containing enzymes in mitochondria and endoplasmic reticulum that presumably undergo turnover during ischemic and oxidative stress. The free prooxidant heme that is released from these proteins cannot be recycled and must be degraded. Heme oxygenase catalytic activity degrades the toxic heme to produce various metabolites that at physiological levels could be cytoprotective: CO, iron, and biliverdin/bilurubin. CO is considered to be a neurotransmitter and vasodilator and is capable of modulating guanylyl cyclase.23 Our finding that CBF increases during reperfusion suggests that EGb761 improves blood flow and corroborates a similar finding reported by Krieglstein et al.24 Furthermore, CO has been shown to play an antiinflammatory role in models of heart I/R, spinal cord I/R, organ transplantation, and vascular injury.25,26 By increasing the HO-1 level, EGb761 could increase the CO concentration, enabling cells and tissues to benefit from these biological actions. The cleavage of the heme porphyrin ring produces biliverdin, which is converted to bilirubin; we and others have shown that both are direct antioxidants.17,27 Finally, increases in the physiological iron in cells has been demonstrated to increase ferritin level. EGb761 could then counterbalance the iron-induced oxidative stress by the formation of ferritin, which is cytoprotective.
In cell cultures exposed to glutamate- and H2O2-induced cell death, we found that EGb761 increased HO1 activity in a dose- and time-dependant manner and significantly reduced neuronal cell loss. The results were confirmed with the HO inhibitor SnPPIX, which blocked the protective actions of EGb761 in vitro, again demonstrating that HO activity is essential for EGb761 function. In addition, application of CHX and ATD, inhibitors of protein and mRNA synthesis, respectively, would prevent the synthesis of HO-1. Our results suggest that EGb761 upregulates de novo HO-1 mRNA and protein synthesis. In contrast, no differences in levels of HO-2 or CP450R were observed.
Conclusion
This study suggests that induction of an endogenous protective gene (ie, HO-1 by EGb761) can reduce brain damage and provide significant improvement in neurobehavioral function associated with I/R brain injury. The protection provided by EGb761 would be at least partially through stimulation of ARE elements and increases in HO-1 levels, actions which represent an important signaling cascade that mediates the protective effects. Therefore, Ginkgo biloba extracts might be useful as a preventive therapy or as a postischemic treatment to reduce the damaging effects of stroke. Additional work is actively being pursued to further elucidate the complete cascade by which EGb761 provides the brain with resistance to such hypoxic/ischemic insults.
Acknowledgments
The authors thank Claire Levine for her assistance in preparing this manuscript and all members of the Doré laboratory.
Sources of Funding
This work was supported in part by NIH grants AT001836, AT004025, and by an Ipsen postdoctoral fellowship.
Footnotes
Part of this work was presented at the 2006 Triennial Meeting of the North American Research Conference on Complementary and Integrative Medicine, Edmonton, Canada.
Disclosures
Y.C. is supported by the Ipsen Foundation.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hallenbeck JM, Dutka AJ. Background review and current concepts of reperfusion injury. Arch Neurol. 1990;47:1245–1254. doi: 10.1001/archneur.1990.00530110107027. [DOI] [PubMed] [Google Scholar]
- 3.Xiao ZY, Sun CK, Xiao XW, Lin YZ, Li S, Ma H, Song GR, Cheng R. Effects of Ginkgo biloba extract against excitotoxicity induced by NMDA receptors and mechanism thereof. Zhonghua Yi Xue Za Zhi. 2006;86:2479–2484. [PubMed] [Google Scholar]
- 4.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Gilman S. Pharmacologic management of ischemic stroke: Relevance to stem cell therapy. Exp Neurol. 2006;199:28–36. doi: 10.1016/j.expneurol.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Clark WM, Rinker LG, Lessov NS, Lowery SL, Cipolla MJ. Efficacy of antioxidant therapies in transient focal ischemia in mice. Stroke. 2001;32:1000–1004. doi: 10.1161/01.str.32.4.1000. [DOI] [PubMed] [Google Scholar]
- 7.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290:F563–F571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 8.Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygen-ase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- 9.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Doré S, Sampei K, Goto S, Alkayed NJ, Guastella D, Blackshaw S, Gallagher M, Traystman RJ, Hurn PD, Koehler RC, Snyder SH. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med. 1999;5:656–663. [PMC free article] [PubMed] [Google Scholar]
- 11.Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci. 2003;60:1779–1792. doi: 10.1007/s00018-003-3080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrasekaran K, Mehrabian Z, Spinnewyn B, Chinopoulos C, Drieu K, Fiskum G. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGB 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry. 2003;36(Suppl 1):S89–S94. doi: 10.1055/s-2003-40447. [DOI] [PubMed] [Google Scholar]
- 13.van Dongen M, van Rossum E, Kessels A, Sielhorst H, Knipschild P. Ginkgo for elderly people with dementia and age-associated memory impairment: A randomized clinical trial. J Clin Epidemiol. 2003;56:367–376. doi: 10.1016/s0895-4356(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang H, Pin S, Christen Y, Doré S. Induction of heme oxygenase 1 by Ginkgo biloba in neuronal cultures and potential implications in ischemia. Cell Mol Biol (Noisyle-grand) 2002;48:647–653. [PubMed] [Google Scholar]
- 15.Liu XP, Goldring CEP, Copple IM, Wang HY, Wei W, Kitteringham NR, Park BK. Extract of Ginkgo biloba induces phase 2 genes through keap1-NrF2-ARE signaling pathway. Life Sci. 2007;80:1586–1591. doi: 10.1016/j.lfs.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Saleem S, Li R, Wei G, Doré S. Effects of EP1 receptor on cerebral blood flow in the middle cerebral artery occlusion model of stroke in mice. J Neurosci Res. 2007;85:2433–2440. doi: 10.1002/jnr.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doré S, Takahashi M, Ferris CD, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hrehorovska M, Burda J, Domorakova I, Mechirova E. Effect of Tanakan on postischemic activity of protein synthesis machinery in the rat brain. Gen Physiol Biophys. 2004;23:457–465. [PubMed] [Google Scholar]
- 19.Ewing JF, Maines MD. Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: Heme oxygenase 2 is not a heat shock protein. Proc Natl Acad Sci U S A. 1991;88:5364–5368. doi: 10.1073/pnas.88.12.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takizawa S, Hirabayashi H, Matsushima K, Tokuoka K, Shinohara Y. Induction of heme oxygenase protein protects neurons in cortex and striatum, but not in hippocampus, against transient forebrain ischemia. J Cereb Blood Flow Metab. 1998;18:559–569. doi: 10.1097/00004647-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Doré S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147:53–59. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ, Choi AM. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: Regulation by upstream antioxidant-responsive elements (ARE) Mol Med. 1995;1:827–837. [PMC free article] [PubMed] [Google Scholar]
- 23.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: A putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 24.Krieglstein J, Beck T, Seibert A. Influence of an extract of Ginkgo biloba on cerebral blood flow and metabolism. Life Sci. 1986;39:2327–2334. doi: 10.1016/0024-3205(86)90663-6. [DOI] [PubMed] [Google Scholar]
- 25.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. Faseb J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 26.Fan LH, Wang KZ, Cheng B. Effects of Ginkgo biloba extract on lipid peroxidation and apoptosis after spinal cord ischemia/reperfusion in rabbits. Chin J Traumatol. 2006;9:77–81. [PubMed] [Google Scholar]
- 27.Stocker R, McDonagh AF, Glazer AN, Ames BN. Antioxidant activities of bile pigments: Biliverdin and bilirubin. Methods Enzymol. 1990;186:301–309. doi: 10.1016/0076-6879(90)86123-d. [DOI] [PubMed] [Google Scholar]