Summary
NUT carcinoma (NC) represents a rare subset of highly aggressive poorly differentiated carcinomas characterized by rearrangement of the NUT (aka NUTM1, nuclear protein in testis) gene, most commonly fused to BRD4. Originally described as a mediastinal/thymic malignancy, NC has been reported at a variety of anatomic regions including the upper and lower aerodigestive tract. To date, only seven NC cases of probable salivary gland origin have been reported. We herein describe three new cases (all affecting the parotid gland) in two women (39 and 55 years old) and one man (35-years old). Histological examination showed poorly differentiated neoplasms composed of poorly cohesive small to medium-sized cells with variable squamoid cell component that was focal and abrupt. Immunohistochemistry showed uniform expression of p63 and distinctive punctate expression of the NUT antigen in the tumor cell nuclei. Review of the reported salivary gland NC cases (total: 10) showed a male: female ratio of 1.5:1 and an age range of 12 to 55 years (median: 29). Site of the primary tumor was the parotid (7), sublingual (2) and submandibular (1) glands. All presented as rapidly growing masses treated by surgery followed by adjuvant radio-/chemotherapy. Initial nodal status was positive in 8/10. At last follow-up (1 to 24 months; median: 5), 7/10 patients died of disease at a median of 5.5 months (1-24 months) and only two were disease-free at 7 and 14 months. Of 9 cases with genetic data, the fusion partner was BRD4 (n=7), non-BRD4/3 (n=1) or undetermined (n=1). None of 306 carcinomas spanning the spectrum of salivary carcinoma types screened by NUT IHC was positive. This is the first small series on salivary NC highlighting the importance to include this rare disease in the differential diagnosis of poorly differentiated salivary gland carcinomas and in cases of presumable poorly differentiated carcinoma of unknown origin.
Keywords: salivary glands, carcinoma, NUT carcinoma, midline carcinoma, poorly differentiated carcinoma, squamous cell carcinoma, BRD4, FISH
Introduction
NUT carcinoma (NC) represents a rare subset of highly aggressive poorly differentiated epithelial neoplasms defined by rearrangement of the NUT gene, which is most commonly fused to BRD4 to form the BRD4-NUT oncogene.1 Originally described in 1991 as a mediastinal/thymic malignancy2, NC has been recently reported from a variety of anatomic regions including in particular the upper and lower aerodigestive tract, but rarely intra-abdominal organs as well.3-7 Affected patients are mainly young adults but the age range varies greatly from the new-born to the elderly.3-7 Although originally considered strictly a neoplasm related to midline structures, recent case reports described this rare disease in lateralized organs such as the lungs and parotid gland. For this reason, the World Health Organization (WHO) classification of tumors has changed the name from NUT midline carcinoma to NUT carcinoma.8-10 A thorough review of the English literature uncovered a total of 7 single case reports of NC of probable or possible salivary gland origin.11-17 We herein describe three new NC cases originating in the parotid gland and review previously reported cases.
Materials and Methods
The three NC cases were retrieved from the consultation files of three of the authors (A.A., I.F. and C.F.). They have been diagnosed in the years 2010, 2015 and 2017. Immunohistochemistry (IHC) was performed on 3-μm sections cut from paraffin blocks using a fully automated system (“Benchmark XT System”, Ventana Medical Systems Inc, 1910 Innovation Park Drive, Tucson, Arizona, USA) and the following antibodies: pankeratin (clone AE1/AE3, 1:40, Zytomed, Berlin, Germany), CK7 (OV-TL, 1:1000, Biogenex), p63 (SSI6, 1: 100, DCS), S100 protein (polyclonal, 1:2500, Dako), CD56 (clone MRQ-42, 1:100, CELL MARQUE), chromogranin A (clone LK2H10, 1:500, Beckman-Coulter GmbH), synaptophysin (clone SY38, 1:50, Dako), SMARCB1 (MRQ-27, 1:50, Zytomed), and anti-NUT antibody (clone C52B1, 1:45, Cell Signaling). Interpretation of results of the NUT IHC was based on published data showing distinctive granular (punctate or dusty) nuclear immunoreactivity present in > 50% of neoplastic cell nuclei.18 Epstein Barr virus (EBV) in-situ hybridization (EBER 1/2 probes, ZytoVision, Bremerhaven, Germany) was performed according to the manufacturer guidelines. Positive and negative controls were used throughout. Normal testicular tissue was used as a positive control for the NUT IHC.5,6
Fish
To detect gene translocation involving the NUT gene locus, fluorescence in-situ hybridization (FISH) was performed on sections cut from formalin-fixed paraffin-embedded tissue blocks using the ZytoLight SPEC NUT Dual Color Break Apart Probe (ZytoVision, Bremerhaven, Germany) with standard protocols according to the manufacturer's instructions. The presence of two pairs of fused green and orange signals was considered normal findings. On the other hand, translocation-positive nuclei showed one fused orange/green signal and one separate orange and green signal. 200 nuclei were counted in four different areas of each tumor. Eighty percent positive interpretable nuclei were defined as positive for a rearrangement.
To identify the BRD4-NUT chromosomal translocation by FISH, BRD4 and NUT dual color split-apart FISH on formalin-fixed, paraffin embedded sections was performed as described4, using the following probes for NUT split-apart: NUT 5′ centromeric probes, RP11-368L15 and RP11-1084A12 (biotin labeled, red) and NUT 3′ telomeric probes, RP11-1H8 and RP11-64o3 (digoxigenin labeled, green); and the following probes for BRD4 split-apart: BRD4 5′ centromeric probes, RP11-207i16 and RP11-3055m5 (biotin labeled, red), and BRD4 3′ telomeric probes, RP11-319O10 and RP11-681D10 (digoxigenin labeled, green).
Case 3 was in addition evaluated by conventional karyotyping studies. Chromosome metaphases of tumor cells were obtained from short term primary cultures as previously described.19 Chromosomes were GTG-banded, and the karyotype was established according to the ISCN rules (2016).
Immunohistochemical screening for NUT expression in salivary gland carcinomas
A total of 306 salivary gland carcinomas were stained with the monoclonal anti-NUT antibody (281 using tissue microarrays and the remainder on conventional slides). Histological subtypes according to the most recent WHO classification20 were summarized in Table 1.
Table 1. Summary of the salivary gland tumors tested for NUT immunoexpression in this study.
| Tumor type | +/Total number of cases tested |
|---|---|
| Adenoid cystic carcinoma (7 high-grade) | 0/50 |
| Mucoepidermoid carcinoma | 0/41 |
| Salivary duct carcinoma* | 0/62 |
| high-grade adenocarcinoma/NOS* | 0/35 |
| Acinic cell carcinoma | 0/32 |
| Poorly differentiated SCC** | 0/28 |
| Myoepithelial carcinoma | 0/17 |
| polymorphous carcinoma*** | 0/13 |
| Undifferentiated/rhabdoid carcinoma | 0/05 |
| Basal cell adenocarcinoma | 0/07 |
| Oncocytic carcinoma | 0/04 |
| Epithelial myoepithelial carcinoma | 0/04 |
| Primary malignant mixed tumor | 0/04 |
| Poorly differentiated neuroendocrine carcinoma | 0/02 |
| Cystadenocarcinoma | 0/02 |
| Total | 0/306 |
= this group includes also some carcinoma ex pleomorphic adenoma cases.
= this group includes tumors without detectable other primary at time of clinical workup but many likely represent metastases from others, e.g. previous skin carcinomas, occult primaries, etc.
=all cases belong to the old category of polymorphous low-grade adenocarcinoma (PLGA).
Results
Clinical history
Case 1
A 39 year-old female without significant past medical or family history presented with a rapidly enlarging, infiltrative tumor of the right parotid gland and right-sided lymphadenopathy, which was excised by radical parotidectomy and right levels II-III neck dissection at her local hospital. This was diagnosed locally as likely high-grade mucoepidermoid carcinoma. The patient underwent adjuvant chemoradiotherapy (66Gy 33# with weekly cisplatin). Approximately three months after excision, imaging showed disseminated metastatic disease to the bone (to sacrum, third lumbar vertebra, left neck of femur and rib), lymph nodes and lungs. She went on to receive palliative radiotherapy to the lumbosacral spine (30 Gy 10#). She was referred to our center to discuss further potential treatment options. Pathology review showed NUT carcinoma. The patient was given palliative radiotherapy, and three months later commenced on an investigational targeted therapy, but died of progressive disease four months after this.
Case 2
A 35-year-old man underwent total parotidectomy and neck dissection for an infiltrative parotid mass. Histological evaluation (performed abroad) was reported as undifferentiated large cell carcinoma of the parotid corresponding to pT3 pN2b, grade 3, R0. Postoperative investigations (three months later) showed multiple osseous metastases. A biopsy was obtained from the pelvic bone. The initial blocks/slides could not be retrieved for review. Palliative chemotherapy was initiated.
Case 3
A 55-year-old female noted a right parotid mass that rapidly grows to a size of 9 cm in greatest dimension. She underwent superficial parotidectomy in another institution. Three months later, she presented with a recurrence at the parotidectomy scar and underwent total parotidectomy with facial nerve excision and a radical neck dissection (at this time a pT3pN1) complemented with chemo- and radiotherapy. Six months after initial diagnosis, she developed lung, liver and bone metastasis and died of her progressive disease seven months after primary surgery.
Pathological findings
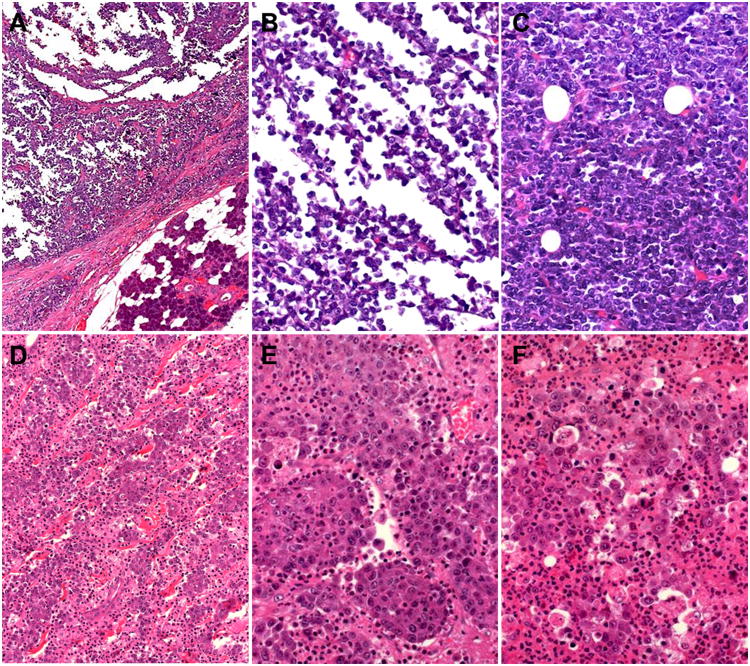
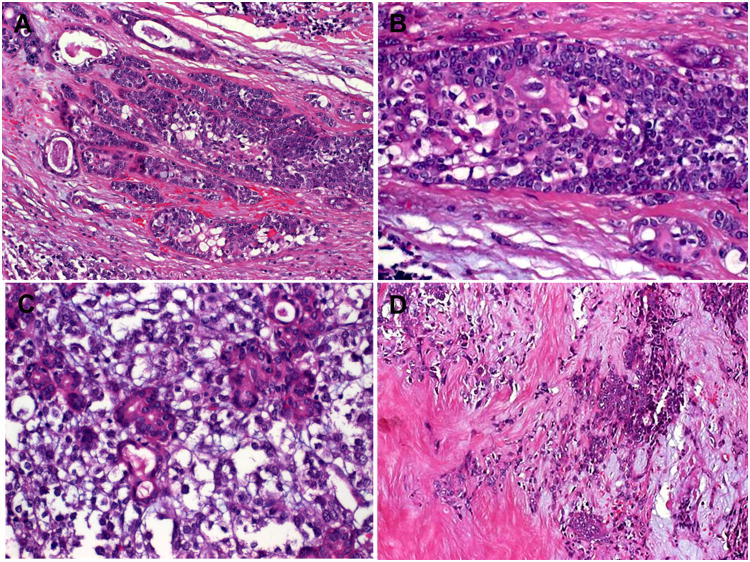
Pathological features were essentially comparable and are described herein together. All tumors were located within the salivary gland parenchyma (Fig. 1A). They were disposed in cords, nests and/or diffuse poorly cohesive sheets of undifferentiated but relatively uniform small to medium-sized polygonal cells (Fig. 1B-E). The tumor cell nuclei showed variable chromatin condensation with inconspicuous (Fig. 1C) to variably recognizable nucleoli (Fig. 1F) and were surrounded by a moderate rim of pale-eosinophilic to clear cytoplasm. Superficial resemblance to poorly differentiated squamous cell carcinoma was most evident in Case 1 (Fig. 1D, E). The latter showed acutely inflamed stroma with prominent stromal and intraepithelial granulocytosis (Fig. 1F). Prominent single cell necrosis/apoptosis and extensive areas of necrosis were frequent. Islands of abrupt squamous differentiation were noted in all three cases including the bone metastasis from case 2 (Fig. 2A, B). The background stroma varied from edematous slightly myxoid to fibrous. Entrapment of native ductules was a prominent feature in all cases (Fig. 2A, C), occasionally with entrapment of single goblet cells, likely of ductal origin (Fig. 2A). Other unusual features included variable myxohyaline stromal changes with entrapped basaloid neoplastic nests that might be mistaken for preexisting pleomorphic or basal cell adenoma (Fig. 2D).
Figure 1.
Salivary NUT carcinomas were centred within salivary parenchyma (A) and displayed poorly cohesive sheets of small to medium-sized cells arranged into pseudoalveolar (B), solid (C), corded (D) or nested (E) pattern. The nucleoli ranged from inconspicuous (C) to prominent (F), note extensive granulocytosis in F (images A,B,C from Case 3; D,E,F from Case 1).
Figure 2.
Foci of abrupt (clear cell) keratinization were seen in all three cases. Potentially misleading features included florid ductular proliferations (A, C) with entrapped of rare goblet cells (A; mid-lower field), (D) and myxohyaline stromal changes reminiscent of pleomorphic adenoma (D). Images A-D from Case 3.
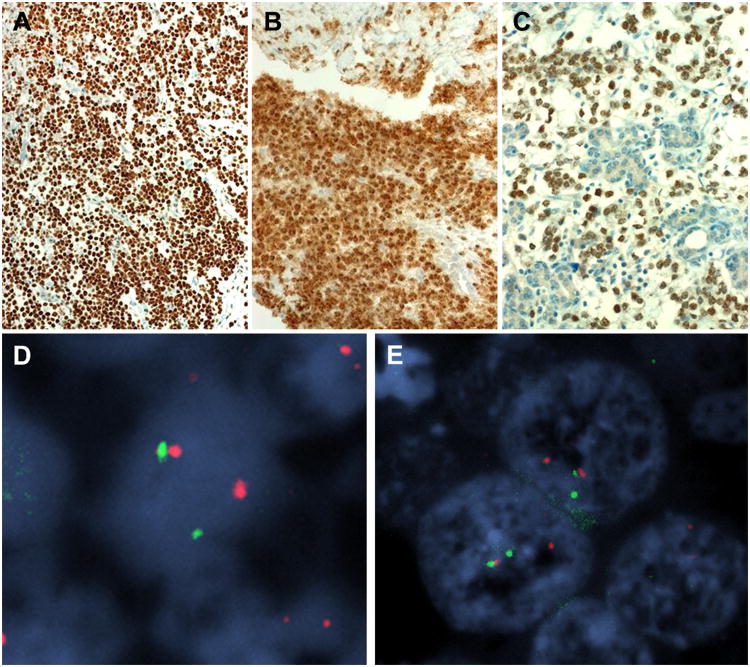
Immunohistochemistry showed uniform expression of p63 (Fig. 3A), variable reactivity for pancytokeratin and lack of neuroendocrine marker expression. In areas lacking strong pancytokeratin expression, the latter highlighted the foci of abrupt squamous differentiation. All three tumors showed distinctive punctate nuclear expression of the NUT protein (Fig. 3B, C). EBER ISH was negative in all cases.
Figure 3.
Immunohistochemistry showed consistent expression of p63 (A; Case 3) and NUT protein (B; Case 2). The NUT immunostain highlighted the neoplastic cells amid native salivary tissue (C; Case 3). FISH analysis using the NUT probe (D) and BRD4 probe (E) showed break-apart signals indicating a NUT/BRD4 translocation (image from Case 1).
FISH analysis using the NUT probe showed break apart signals in >90% of the neoplastic cell nuclei in all three cases (Fig. 3D). BRD4 was the fusion partner in case 1 (determined by FISH (Fig. 3E) and Case 3 (conventional karyotyping studies revealed a 43∼46, XX, t(15;19)(q14;p13) karyotype corresponding to a NUT/BRD4 translocation). In Case 2, the fusion partner could not be determined due to failure of FISH testing as a result of poor tissue preservation.
NUT IHC in unselected salivary gland carcinoma types
None of 306 carcinomas across the spectrum of salivary gland carcinoma types screened with the NUT IHC was positive (table 1).
Clinicopathologic features of all known salivary NCs
Including our three cases, a total of 10 salivary NC cases have been reported (Table 2).11-17 They affected mainly males (M: F ratio = 1.5: 1) with an age range of 12 to 55 years (median: 29). Nine patients were under 40 years at the time of diagnosis and 3/10 cases were children. Site of origin of the primary tumor was the parotid (n=7), the sublingual (n=2) and the submandibular (n=1) glands. Regarding laterality, six tumors were on the right and three on the left side (one of unknown laterality). All tumors presented as rapidly growing masses. Regional lymph nodes were initially involved in 8/10 cases. Treatment was surgery followed by adjuvant radio-/chemotherapy in all cases. The majority of patients developed rapid postoperative disseminated disease. At last follow-up (1 to 24 months; median: 5), 7/10 patients died of disease at a median of 5.5 months (1-24 months). One patient was alive with metastatic disease under palliative treatment while only two patients were alive without evidence of disease at 7 and 14 months.
Table 2.
Clinicopathologic features of reported NUT carcinomas of the salivary glands (n=10).
| No | Author/year | Age/Gender | Site | Size cm | Symptoms/duration | initial nodal/M status | Treatment | MTS/duration | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | den Bakker et al, 2009 | 15 M | Right Parotid gland | 1.5 (6 cm at surgery 8 mo later) | Mass | N+M0 | Surgery + aRCT | No | ANED (7 mo) |
| 2 | Ziai et al, 2010 | 15 M | left Submandibular gland | 2.5 | Mass and pain, 3 mo | N+M0 | Surgery + aRT | No | ANED (14 mo) |
| 3 | Park et al, 2014 | 12 M | Right parotid gland | 2.4 | Mass | N+M0 | Surgery + aRCT | 2 recurrences within 12 mo | DOD (24 mo) |
| 4 | Vulsteke et al, 2016 | 32M | Left parotid gland | 3.8 | Rapidly growing mass | N0M0 | Surgery + aRT, then CT for MTS | disseminated bone MTS (1 mo) | DOD (4 mo) |
| 5 | Andreasen et al, 2016 | 40 F | Right sublingual gland | 4 | Swelling, meal-related pain (1mo) | N0M0 | surgery, palliative RCT | wide-spread bone + cervical nodes (1 mo) | DOD (5.5 mo) |
| 6 | Klijanienko et al, 2017 | 21 F | Left parotid gland | 5 | Mass | N+M0 | surgery, RT | subcutaneous & liver (2 mo) | DOD (2 mo) |
| 7 | Seim et al, 2016 | 26 M | Right sublingual gland | 3 | Pain, swelling, otalgia | N+M0 | surgery, palliative RCT | extensive lung+ bone MTS (2 wks) | DOD (1 mo) |
| 8 | Current series | 39 F | Right Parotid gland | 3.6 | Rapidly growing mass | pN2b M0 | Surgery + aRCT BET inhibitor | disseminated bone, nodes & lung (3 mo) | DOD (7 mo) |
| 9 | Current series | 35 M | Parotid gland | NA | Mass | N+M0 | Surgery, then palliative CT | Bone (postoperative) | AWD |
| 10 | Current series | 55F | Right parotid gland | 9 | Mass | N+M0 | Surgery, then RCT | live, lung & bone (6 mo) | DOD (7 mo) |
a=adjuvant; ANED=alive with no evidence of disease; AWD=alive with disease; CT=chemotherapy; DOD= died of disease; F=female; M= male; mo=month; MTS= metastasis; RCT= radiochemotherapy; RT=radiotherapy.
All eight tumors stained with the anti-NUT antibody showed positive nuclear staining and all 9 cases assessed by genetic methods (FISH and/or karyotyping) showed NUT rearrangement. The fusion partner was BRD4 (n=7), non-BRD4/3 (n=1) or unknown due to FISH failure (n=1). As illustrated in table 3, NC of salivary glands showed frequent expression of pancytokeratin (8 of 9 cases) and consistently expressed p63 (9 of 9 cases) similar to their non-salivary counterparts.4,5 Neuroendocrine markers were largely negative except for focal minor expression of synaptophysin (2 of 9 cases) and variable reactivity for CD56 (2 of 9 cases) observed in two cases each, respectively. All cases have been consistently negative for EBV ISH. Our cases were stained with SMARCB1; all three showed intact expression. The original diagnosis was stated in 9 cases (Table 3). A diagnosis of undifferentiated or poorly differentiated SCC/carcinoma/tumor was rendered or suggested in 6 cases. The remaining three cases were interpreted as probably representing high-grade variants of myoepithelial carcinoma, adenoid cystic carcinoma or mucoepidermoid carcinoma, respectively.
Table 3.
Histopathological, immunohistochemical and molecular findings in salivary NUT carcinomas (n=10).
| Case | Histological pattern | unusual features | Original diagnosis | Pan-CK | p63 | CK7 | SYN | Ch-A | CD56 | INI1 | NUT IHC | Genotype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Poorly cohesive basaloid cells, squamoid islands | Foci of cartilage | Poorly differentiated carcinoma (FNA) | + | + | NA | - | - | + | NA | + | NUT-BRD4 |
| 2 | Poorly cohesive basaloid cells, squamoid islands | No | Nots specified | + | + | NA | - | - | - | NA | NA | NUT-variant (other than BRD4/BRD3) |
| 3 | Basaloid with desmoplasia, no squamous islands | No | Poorly differentiated SCC | + | + | NA | - | - | - | NA | + | NA |
| 4 | Poorly cohesive large cells | No | Myoepithelial carcinoma with neuroendocrine features | + | + | NA | +F | NA | + | NA | + | NUT-BRD4 |
| 5 | Poorly cohesive basaloid cells, squamoid islands | No | Poorly differentiated basaloid SCC | + | + | + | <1% | - | - | NA | + | NUT-BRD4 |
| 6 | Poorly cohesive basaloid cells | No | Poorly differentiated AdCC (FNA) | + | + | NA | - | - | - | NA | + | NUT-BRD4 |
| 7 | Poorly cohesive large cells | No | Undifferentiated carcinoma | NA | NA | NA | NA | NA | NA | NA | NA | NUT-BRD4 |
| 8 | Poorly cohesive large cells, squamoid islands | Prominent granulocytes | High-grade tumor, favor MEC | + | + | + | - | - | - | intact | + | NUT-BRD4 |
| 9 | Poorly cohesive basaloid cells, squamoid islands | No | Undifferentiated large cell carcinoma | + | + | NA | - | - | - | intact | + | NUT, partner unknown (FISH failed) |
| 10 | Poorly cohesive basaloid cells, squamoid islands | No | Undifferentiated tumor | - | + | - | - | - | - | intact | + | NUT-BRD4 |
AdCC, adenoid cystic carcinoma; Pan-CK, pancytokeratin; Ch-A, chromogranin A; FNA, fine needle aspiration; F, focal; IHC, immunohistochemistry; NA, not available; MEC, mucoepidermoid carcinoma; SSC, squamous cell carcinoma; SYN, synaptophysin.
Discussion
Since its first description as a mediastinal (midline) malignancy2, NC has been reported from diverse sites with about one third-to-one half of cases originating in the head and neck area.3,5,6 More than half of head and neck cases affected the sinonasal cavities.5,6 While this rare disease probably is still significantly under-recognized, even at these characteristic (mediastinal and sinonasal) sites, NC originating at unusual sites such as the salivary glands is likely under-recognized as well, as reflected by the initial diagnostic suggestions in reported cases. NC was not considered in the vast majority of cases and the diagnosis was mainly made after molecular testing. This is mainly due to the superficial similarity of this neoplasm to conventional squamous cell carcinoma (SCC) or other poorly differentiated carcinomas and the uniform reactivity for p63 as a marker of squamous cell differentiation. Thus, diagnosis of salivary NC is only feasible if NC is included in the differential diagnosis and NUT IHC and/or molecular testing performed.
Although NC is considered a variant of SCC by some authors, its relationship to conventional SCC is unclear. Lack of other molecular alterations in NC in contrast to the highly heterogeneous molecular profile of conventional SCC argues for the BRD-NUT fusion being the sole driver of this rare disease.2,3 NC frequently displays predominantly undifferentiated phenotype by conventional histology, likely as a consequence of global transcriptional repression and blockade of cell differentiation resulting from the underlying genetic aberration.21 In contrast to conventional SCC of head and neck, the prognosis of NC is poor with the majority of patients succumbing to their disease within a few months of diagnosis.22,23 This is mainly the consequence of aggressive nature of NC with a high rate of regional and distant disease dissemination usually shortly after diagnosis and to the lack of response to conventional chemotherapeutic regimens. Accordingly, recognition of NC is mandatory for providing important prognostic information specific to this entity and is essential for guiding and optimizing patient´s therapy.22,23 In addition, emerging new therapeutic strategies targeting the underlying gene fusion (bromodomain inhibitors and histone deacetylase inhibitors) might be a promising option for this orphan disease in the future.24,25 Thus recognition of NC is encouraged for a better characterization of this lethal disease.
The frequency of NC among salivary gland cancer is unknown. In the sinonasal tract, NC accounted for 10% of poorly differentiated/undifferentiated carcinomas.26 Lack of NUT immunoreactivity among 306 salivary gland carcinomas across the spectrum of salivary carcinoma types screened in this study suggests the rarity of NC among consecutive salivary gland carcinomas which is in line with the rarity of this entity in other organs as well. However, as NUT rearrangements are not expected among specific differentiated subtypes of salivary gland carcinomas other than poorly differentiated SCC and undifferentiated carcinomas, the exact frequency of NC among poorly differentiated/undifferentiated primary salivary gland carcinomas remains to be assessed in future studies. Thus, it is recommended to include NUT IHC in the workup of undifferentiated carcinomas and in SCC in the salivary glands with features suggestive of NC as reported above. However, blind screening of salivary gland carcinomas that are fitting the well characterized carcinoma types seems to be useless. The NUT IHC is considered sufficient for diagnosis based on the high specificity (almost 100%) and sensitivity (87%) of the antibody.5,6,18 While genetic testing is generally not necessary for diagnosis, it is recommended to determine the fusion partner for better understanding and characterization of this rare aggressive disease. There is preliminary evidence suggesting prognostic difference between BRD4-NUT and NUT-variant carcinomas with the latter possibly associated with longer overall survival.4
Salivary NUT carcinoma should be distinguished from a variety of primary and metastatic neoplasms including poorly differentiated SCC and high-grade or “dedifferentiated” variants of several salivary gland carcinoma types including myoepithelial carcinoma, mucoepidermoid carcinoma, basal cell adenocarcinoma, epithelial myoepithelial carcinoma and others.27 While most of these salivary gland carcinoma entities can be excluded by well-established diagnostic criteria, some secondary morphological features such as entrapment of reactive or distorted native ducts and duct epithelia (including goblet cells) might be a source of confusion. Furthermore, heterologous cartilaginous differentiation as reported in a previous salivary NC should be distinguished from carcinoma ex pleomorphic adenoma.11 Likewise, presence of cells with voluminous clear cytoplasm (abrupt squamous differentiation) should not be mistaken for sebaceous differentiation. Based on these differential diagnostic considerations and given the heterogeneous and non-specific NUT carcinoma morphology, inclusion of NC in the differential diagnosis of poorly differentiated and unclassified salivary gland malignancies and use of the NUT immunohistochemistry in their work-up is encouraged.
In summary, this is the first small series on salivary NC highlighting the importance to include this rare disease in the differential diagnosis of poorly differentiated salivary gland carcinomas, in particular in cases of poorly differentiated SCC of presumable salivary gland origin or of unknown origin and in high-grade myoepithelial carcinomas.
Footnotes
Conflict of interest: none
References
- 1.French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–7. [PubMed] [Google Scholar]
- 2.Kubonishi I, Takehara N, Iwata J, et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res. 1991;51:3327–3328. [PubMed] [Google Scholar]
- 3.French C. NUT midline carcinoma. Nat Rev Cancer. 2014;14:149–150. doi: 10.1038/nrc3659. [DOI] [PubMed] [Google Scholar]
- 4.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22:4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 5.Stelow EB, French CA. Carcinomas of the upper aerodigestive tract with rearrangement of the nuclear protein of the testis (NUT) gene (NUT midline carcinomas) Adv Anat Pathol. 2009;16:92–6. doi: 10.1097/PAP.0b013e31819923e4. [DOI] [PubMed] [Google Scholar]
- 6.Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216–21. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shehata BM, Steelman CK, Abramowsky CR, Olson TA, French CA, Saxe DF, Ricketts RR, Katzenstein HM. NUT midline carcinoma in a newborn with multiorgan disseminated tumor and a 2-year-old with a pancreatic/hepatic primary. Pediatr Dev Pathol. 2010;13:481–5. doi: 10.2350/09-10-0727-CR.1. [DOI] [PubMed] [Google Scholar]
- 8.French CA, Bishop J, Lewis JS, Muller S, Westra WH. Tumours of the nasal cavity, paranasal sinuses and skull base: NUT carcinoma. In: El-Naggar A, Chan J, Grandis J, Takashi T, Slootweg P, editors. WHO Classification of Tumors of Head and Neck. 4th. Lyon: IARC Press; 2017. pp. 20–21. [Google Scholar]
- 9.French CA, den Bakker MA. Thymus: NUT carcinoma. In: Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th. Lyon: IARC Press; 2015. pp. 229–231. [Google Scholar]
- 10.Chirieac LR, French CA, Sholl L, Yatabi Y. Lung: Other and unclassified carcinomas. In: Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th. Lyon: IARC Press; 2015. pp. 97–98. [Google Scholar]
- 11.den Bakker MA, Beverloo BH, van den Heuvel-Eibrink MM, Meeuwis CA, Tan LM, Johnson LA, French CA, van Leenders GJ. NUT midline carcinoma of the parotid gland with mesenchymal differentiation. Am J Surg Pathol. 2009;33:1253–8. doi: 10.1097/PAS.0b013e3181abe120. [DOI] [PubMed] [Google Scholar]
- 12.Ziai J, French CA, Zambrano E. NUT gene rearrangement in a poorly-differentiated carcinoma of the submandibular gland. Head Neck Pathol. 2010;4:163–8. doi: 10.1007/s12105-010-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HS, Bae YS, Yoon SO, Lim BJ, Hong HJ, Ro JY, Hong SW. Usefulness of Nuclear Protein in Testis (NUT) Immunohistochemistry in the Cytodiagnosis of NUT Midline Carcinoma: A Brief Case Report. Korean J Pathol. 2014;48:335–8. doi: 10.4132/KoreanJPathol.2014.48.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vulsteke C, Lurquin E, Debiec-Rychter M, Gheysens O, Nuyts S, Schoenaers J, Politis C, Mebis J, Hauben E, Clement PM. First evidence of treatment efficacy in metastatic carcinoma of the parotid gland with BRD4/NUT translocation. J Chemother. 2016;28:242–6. doi: 10.1179/1973947815Y.0000000046. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen S, French CA, Josiassen M, Hahn CH, Kiss K. NUT Carcinoma of the Sublingual Gland. Head Neck Pathol. 2016;10:362–6. doi: 10.1007/s12105-015-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klijanienko J, Le Tourneau C, Rodriguez J, Caly M, Theocharis S. Cytological features of NUT midline carcinoma arising in sino-nasal tract and parotid gland: Report of two new cases and review of the literature. Diagn Cytopathol. 2016;44:753–6. doi: 10.1002/dc.23506. [DOI] [PubMed] [Google Scholar]
- 17.Seim NB, Philips RHW, Schoenfield L, Teknos TN, Rocco JW, Agrawal A, Ozer E, Carrau RL, Kang SY, Old MO. NUT Midline Carcinoma of the Sublingual Gland: Clinical Presentation and Review. Head Neck Pathol. 2017 Mar 27; doi: 10.1007/s12105-017-0809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–91. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martins C, Fonseca I, Roque L, Pereira T, Ribeiro C, Bullerdiek J, Soares J. PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol. 2005;18:1048–55. doi: 10.1038/modpathol.3800386. [DOI] [PubMed] [Google Scholar]
- 20.El-Naggar AK, Chan JKC, Grandis JR, et al. WHO Classification of Head and Neck Tumours. 4th. Lyon: IARC Press; 2017. [Google Scholar]
- 21.Stelow EB, Bellizzi AM, Taneja K, Mills SE, Legallo RD, Kutok JL, Aster JC, French CA. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–34. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 22.Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18:5773–5779. doi: 10.1158/1078-0432.CCR-12-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chau NG, Hurwitz S, Mitchell CM, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer. 2016;122:3632–3640. doi: 10.1002/cncr.30242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.French CA. The importance of diagnosing NUT midline carcinoma. Head Neck Pathol. 2013;7:11–16. doi: 10.1007/s12105-013-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang W, French CA, Cameron MJ, Han Y, Liu H. Clinicopathological significance of NUT rearrangements in poorly differentiated malignant tumors of the upper respiratory tract. Int J Surg Pathol. 2013;21:102–10. d. doi: 10.1177/1066896912451651. [DOI] [PubMed] [Google Scholar]
- 26.Kakkar A, Antony VM, Irugu DVK, Adhikari N, Jain D. NUT Midline Carcinoma: A Series of Five Cases, Including One with Unusual Clinical Course. Head Neck Pathol. 2017 Sep 25; doi: 10.1007/s12105-017-0858-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagao T. “Dedifferentiation” and high-grade transformation in salivary gland carcinomas. Head Neck Pathol. 2013;7(1):S37–47. doi: 10.1007/s12105-013-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]