Abstract
Consumption of flavan-3-ols, notably (−)-epicatechin (EC), has been highly recommended in complementary and alternative medicine (CAM) due to reports that flavan-3-ols boost antioxidant activity, support vascular function, and prevent cardiovascular disease. To date, in vivo efficacy and mechanisms of action for many CAM therapies, including EC, remain elusive in brain ischemia. In contrast to its purported direct antioxidant role, we hypothesized protection through activation of the endogenous transcriptional factor Nrf2. To screen cellular protection and investigate Nrf2 activation, we adopted a pretreatment paradigm using enriched primary neuronal cultures from mice and washed out EC prior to oxygen glucose deprivation to attenuate direct antioxidant effects. EC protected primary neurons from oxygen glucose deprivation by increasing neuronal viability (40.2 ± 14.1%) and reducing protein oxidation, effects that occurred concomitantly with increased Nrf2-responsive antioxidant protein expression. We also utilized wildtype and Nrf2 C57BL/6 knockout mice in a permanent model of focal brain ischemia to evaluate glial cell regulation and complex sensorimotor functioning. EC-treated wildtype mice displayed a reduction or absence of forelimb motor coordination impairments that were evident in vehicle-treated mice. This protection was associated with reduced anatomical injury (54.5 ± 8.3%) and microglia/macrophage activation/recruitment (56.4 ± 13.0%). The protective effects elicited by EC in both model systems were abolished in tissues and neuronal cultures from Nrf2 knockout mice. Together, these data demonstrate EC protection through Nrf2 and extend the benefits to improved performance on a complex sensorimotor task, highlighting the potential of flavan-3-ols in CAM approaches in minimizing subsequent stroke injury.
Keywords: behavior, cultured neurons, mouse, neuroinflammation, stroke
Introduction
Stroke remains a leading cause of long-term disability and death worldwide, resulting in a total cost of approximately $67 billion annually (http://www.pwc.com/us/en/health-industries/publications/medical-treatment-cost-of-brain-disease.jhtml). With the exception of supportive care, the only viable treatment option is thrombolytic therapy. Although effective when administered within 4.5 h after onset (Maiser et al., 2011), the majority of patients are ineligible due to comorbidities, medicinal contraindications, and/or presentation beyond the therapeutic window. Due to low patient eligibility and the narrow therapeutic window for treatment, the ability of natural compounds to bolster protective systems in the brain prior to stroke is an area of interest.
Complementary and alternative medicinal (CAM) approaches have yielded a convincing body of epidemiological data boasting health benefits associated with the consumption of food sources containing flavan-3-ols. In particular, evidence supports a role for dark chocolate in improving vascular function (Fisher et al., 2006; Sorond et al., 2010; Sudano et al., 2012), reducing the risk for cardiovascular disease and stroke (Hollenberg et al., 2009; Heiss et al., 2010). Recently, benefits were also extended to enhanced cognitive performance (Nehlig, 2013). We have been investigating the naturally occurring flavan-3-ol (−)-epicatechin (EC), enriched in some cocoa, dark chocolate, and green tea, to begin deciphering the ways in which EC prophylaxis might improve stroke outcomes. Despite its demonstrated safety and availability, little is known regarding EC mechanisms of action. A greater understanding of EC and other natural compounds is necessary if these CAM approaches are to be recommended to the general public.
The stoichiometry for direct antioxidation by EC would require relatively high brain bioavailability to protect against stroke-induced oxidation. Because EC undergoes extensive metabolism following oral consumption in humans and rodents (Baba et al., 2001; Ottaviani et al., 2011), this mechanism may not account for neuroprotection. The transcriptional factor Nrf2 is an endogenous regulator of cell survival and is considered a multi-organ protector (Kensler et al., 2007). In response to cellular stress, Nrf2 induces expression of cytoprotective genes that attenuate tissue injury (Wakabayashi et al., 2003; Li et al., 2004). Several lines of evidence from experimental models support a protective role for Nrf2 in various pathological conditions (Ramos-Gomez et al., 2001; Rangasamy et al., 2005). Because activators that induce Nrf2-responsive gene expression have been shown to provide protection against experimental stroke (Kraft et al., 2004; Zhao et al., 2006), the prospect of EC-mediated Nrf2 activation is intriguing.
Our laboratory has previously shown that EC effectively reduced infarct volume and basic neurological deficits when measured 72 h after transient brain ischemia (Shah et al., 2010). However, those studies did not evaluate complex sensorimotor functions after permanent ischemia or the ability of EC to modulate gliosis. Here, we performed a detailed study employing in vivo and in vitro models to further elucidate the role of Nrf2 in EC protection and evaluate outcomes up to 1 week after stroke. The adhesive removal test was selected to evaluate functional outcomes because weakness or loss of forelimb function is more analogous to the clinical setting.
Materials and methods
Animal care
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Florida, in accordance with the Guide for the Care and Use of Laboratory Animals and the guidelines laid down by the National Institutes of Health in the United States regarding the care and use of animals for experimental procedures. Three-month-old male wildtype (WT) and Nrf2 knockout (Nrf2−/−) C57BL/6 mice (Harlan Labs, Indianapolis, IN, USA) were housed in a climate-controlled facility (23 ± 1 °C) on a 12-h reversed light–dark cycle and were provided food and water ad libitum.
EC treatment and pdMCAO
Adult (2–3 months) male WT or Nrf2−/− mice were randomized and administered either methylcellulose vehicle or 5, 10, or 15 mg/kg of freshly prepared EC (Sigma Aldrich, St Louis, MO, USA) by gavage 90 min prior to permanent distal middle cerebral artery occlusion (pdMCAO), as previously described (Wang et al., 2011). The pdMCAO procedure was performed under anesthesia using 4% halothane for induction and 2% halothane (with 1.5/3.0% oxygen/air balance) for maintenance throughout the procedure. Halothane was used because of the documented effects of isoflurane on Nrf2-inducible protein expression (Schmidt et al., 2007), which could confound the interpretation of results. Complete occlusion was confirmed by severance of the vessel. There were no overt signs of stress for any animals participating in these studies.
Adhesive removal test
Due to the relatively mild behavioral deficits produced by pdM-CAO cortical injury, mice were tested for performance on an adhesive removal task. This task requires forelimb strength, sensation, and complex sensorimotor coordination, and impaired animals mimic clinical pathology in which stroke patients often report weakness and/or loss of function in one or more extremities. Adhesive tape was placed on the planar surface of the forepaw and latency to remove was recorded. Mice were trained for 3 days, tested for baseline performance prior to stroke, and post-tested 1 day after stroke.
Histology and immunohistochemistry
Lesion volume and total immunoreactivity were calculated from 16 brain sections collected at even intervals throughout the expanse of the cortical infarct. At 7 days post-stroke, mice were perfused, brains were fixed and cryosectioned, and sections were stained with Cresyl Violet for lesion volume quantification. Immunohistochemistry (IHC) was performed to evaluate reactive gliosis [anti-CD11b, 1 : 1000 (Serotec, Raleigh, NC, USA) or anti-ionized calcium-binding adapter molecule 1 (Iba1), 1 : 1000 (Wako, Richmond, VA, USA) for microglia/macrophages and anti-glial fibrillary acidic protein (GFAP), 1 : 500 (Abcam, Cambridge, MA, USA) for astrocytes] (Leonardo et al., 2010). These antibodies are listed and cited in the Journal of Comparative Neurology database. Primary antibodies were omitted in control experiments to validate antibody selectivity.
Fluoro-Jade staining was also performed as an additional validation tool for identifying cortical neurodegeneration produced by pdMCAO. Briefly, sections were incubated for 5 min in 100% EtOH containing 1% NaOH, followed by 2 min in 70% EtOH and 2 min in distilled water (dH2O). Sections were then incubated for 10 min in 0.6% KMnO4 and for 2 min in dH2O and stained for 10 min in 0.001% Fluoro-Jade B (Thermo Fisher Scientific, Pittsburgh, PA, USA) prepared in 0.1% acetic acid. After staining, sections were rinsed three times for 1 min each in dH2O, dried for 5 min at 40 °C, cleared for 2 min in HistoClear (Thermo Fisher Scientific), and coverslipped with Cytoseal (Thermo Fisher Scientific).
Images were acquired using an Aperio ScanScope XT (Cresyl Violet and IHC) or FL (Fluoro-Jade) slide scanner and quantifications were performed using ImageScope software (Aperio, Vista, CA, USA). The ImageScope algorithm ‘Positive Pixel Count’ was used to quantify pixel numbers corresponding to weak positive, positive, and strong positive signal based on preset criteria. Due to the abundance of quiescent microglia/macrophages and resting astrocytes, strong positive pixels were used to identify staining that reflected activated glial cells. The positive pixel counts were expressed as a ratio of the total cortical area measured, and ipsilateral calculations were then expressed relative to the contralateral hemisphere for each section to control for potential variations in antibody penetrance or tissue processing.
Postnatal-enriched primary neuronal cultures
Mouse cortical neurons were isolated from 0- to 1-day-old WT and Nrf2−/− pups, cultured in serum-free Neurobasal medium supplemented with GlutaMax, L-glutamine, and B27 (Invitrogen, Carlsbad, CA, USA) (Echeverria et al., 2005). Cultures were pretreated with 50 or 100 μM EC for 6 h in Neurobasal/B27 minus antioxidant-supplemented medium prior to oxygen glucose deprivation (OGD), as previously described (Agrawal et al., 2013), with minor modifications. Cultures were rinsed once with the glucose-free, pre-equilibrated medium bubbled with 100% N2 for 10 min and this oxygen-deprived, glucose-free medium was added to cultures and incubated at 5% CO2/95% N2 for 4 h. After OGD, fresh glucose-containing medium was added and cultures were subjected to reoxygenation for 24 h at 37 °C in 5% CO2/95% atmosphere.
Cell viability and toxicity were assessed by MTT (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and LDH (lactate dehydrogenase) assays, respectively (G-Biosciences, St Louis, MO, USA), and live/dead cells were evaluated using calcein AM/ethidium homodimer 1 (EthD-1) staining (Invitrogen). Photomicrographs showing changes in neuronal morphology were captured using a Leica inverted microscope and calcein AM/EthD-1-positive images were merged.
Neuronal protein expression and oxidation
Cells were lysed, protein was extracted, 30 μg of protein per sample was electrophoretically resolved on a 12% SDS-PAGE gel (Bio-Rad, Hercules, CA, USA), and Western blots were performed as previously described (Agrawal et al., 2013) using antibodies generated against heme oxygenase 1 (HO1, 1 : 1000; Enzo Life Sciences, Farmingdale, NY, USA) (Ueda et al., 2008), ferritin light chain (FTL, 1 : 1000; Abnova, CA, USA) (Wu et al., 2011), biliverdin reductase (BVR, 1 : 1000; Enzo Life Sciences) (Maines et al., 1999), and β-actin (1 : 1000; Sigma Aldrich). Proteins were detected using chemiluminescence, and membranes were imaged using a FluorChem E imager (ProteinSimple, Santa Clara, CA, USA). Protein oxidation was measured using the OxyBlot protein oxidation detection kit (EMD Millipore, Billerica, MA, USA), as previously described (Kim & Doré, 2005).
Statistical analyses
All analyses were performed blinded such that experimenters performing surgery, behavioral testing, anatomical measures, and data analysis were unaware of the genotypes and treatments. Data are expressed as mean ± SEM. Statistical significance was set at P < 0.05. Bartlett’s tests showed no significant differences in group variances; therefore, data were evaluated using parametric statistics. Data from experiments evaluating a single factor (treatment) between two groups were analysed by two-tailed, unpaired Student’s t-test. For experiments with more than two groups (dose–response studies), significance was determined using one-way ANOVA to minimize the potential for Type I Error that could result from multiple t-tests. Where main effects occurred, Bonferroni post hoc analyses were then used to evaluate multiple group comparisons. For behavioral experiments, the use of a two-by-two factorial design [treatment (vehicle or EC) and time (pretest or post-test)] required analysis by two-way ANOVA followed by Bonferroni post hoc analyses. All data were analysed using GRAPHPAD PRISM (GraphPad Software Inc., La Jolla, CA, USA) data analysis software.
Results
EC protects enriched primary neuronal cultures from OGD through Nrf2
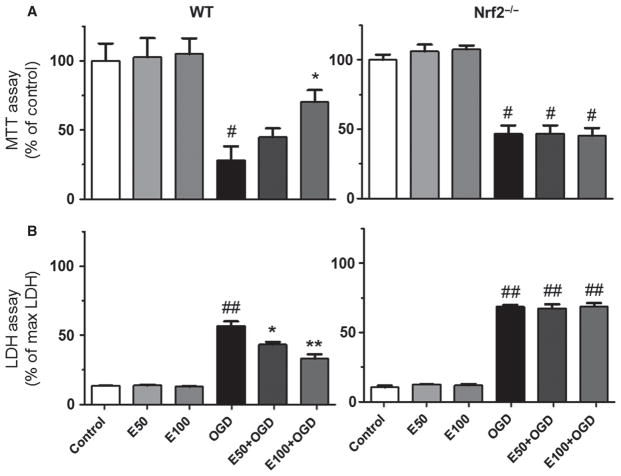
Primary neuronal cultures from WT and Nrf2−/− pups were subjected to OGD (Figs 1 and 2) and pretreated with EC to validate and extend our previous in vitro findings that demonstrated protection. Pretreatment with 50 or 100 μM EC protected WT neurons from OGD, and this protection was essentially abolished in Nrf2−/− cultures. MTT assay (Fig. 1A) showed that cell viability was decreased by 71.7 ± 10.0% after OGD and was increased by 40.2 ± 14.1% in WT cultures pretreated with 100 μM EC (F = 9.364, P = 0.0083). In subsequent experiments with Nrf2−/− cultures, cell viability was decreased by 53.33 ± 6.0% after OGD, whereas EC failed to increase the viability (F = 34.48, P < 0.0001) in the knockout cultures. Similarly, LDH assay (Fig. 1B) showed increased cytotoxicity by 43.3 ± 3.2% after OGD and reductions in OGD-induced cytotoxicity (13.5 ± 2.0 and 23.6 ± 3.1%) in WT cultures after pretreatment with 50 or 100 μM EC (F = 61.93, P < 0.0001). Subsequent experiments in Nrf2−/− cultures showed increased cytotoxicity by 58.27 ± 1.34% after OGD, whereas EC failed to reduce OGD-induced cytotoxicity (F = 302.0, P < 0.0001) in the knockout cultures.
Fig. 1.
EC protects primary neuronal cultures from OGD through Nrf2 activation. WT or Nrf2−/− cells pretreated for 6 h with EC and subjected to 4 h OGD followed by 24 h reoxygenation. (A) MTT assay showed increased viability in WT neurons treated with 100 μM EC, whereas protection was abolished in Nrf2−/− cultures. (B) LDH assay showed reduced cytotoxicity following treatment with 50 or 100 μM EC, whereas protection was negated in Nrf2−/− neurons. *P < 0.05; **P < 0.01; #P < 0.05; ##P < 0.01; #compared with control groups; *compared with OGD.
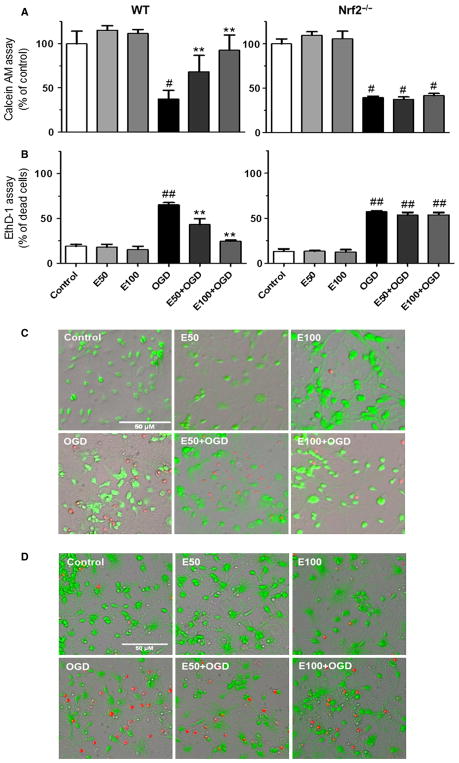
Fig. 2.
EC protects primary neuronal cultures from OGD through Nrf2 activation. WT or Nrf2−/− cells pretreated for 6 h with EC and subjected to 4 h OGD followed by 24 h reoxygenation. (A) Calcein AM assay confirmed the MTT assay, showing a higher percentage of live neurons in WT cultures treated with 50 or 100 μM EC compared with Nrf2−/− cultures. (B) EthD-1 assay confirmed the LDH assay, showing reduced dead neurons in EC-treated WT cultures relative to Nrf2−/− cultures. (C) Micrographs of live (green) and dead (red) cells measured by Calcein AM and EthD-1 staining in WT cultures. (D) Micrographs of live (green) and dead (red) cells measured by Calcein AM and EthD-1 staining in Nrf2−/− cultures. **P < 0.01; #P < 0.05; ##P < 0.01; #compared with control groups; *compared with OGD. Scale bar = 50 μm. [Color version of figure available online].
To validate these initial findings, additional experiments were performed using Calcein AM and EthD-1 assays to provide alternative means of assessing cell viability in this model system. The Calcein AM assay (Fig. 2A) showed that live cells were decreased by 62.54 ± 9.7% after OGD and increased by 30.9 ± 14.2 and 55.4 ± 10.4% in WT cultures pretreated with 50 or 100 μM EC, respectively (F = 2.509, P = 0.0556). In subsequent experiments with Nrf2−/− cultures, the percentage of live cells was decreased by 60.63 ± 1.5% after OGD, whereas EC failed to increase the viability (F = 90.47, P < 0.0001) in the knockout cultures. Similarly, EthD-1 assay (Fig. 2B) showed an increase in dead cells by 46.1 ± 2.6% after OGD and reductions in OGD-induced dead cells (22.0 ± 6.6 and 40.4 ± 1.1%) in WT cultures after pretreatment with 50 or 100 μM EC, respectively (F = 28.06, P < 0.0004). Subsequent experiments in Nrf2−/− cultures showed an increase in dead cells by 43.95 ± 0.87% after OGD, whereas EC failed to reduce OGD-induced dead cells (F = 76.73, P < 0.0001) in the knockout cultures.
EC induces expression of Nrf2-responsive antioxidant proteins
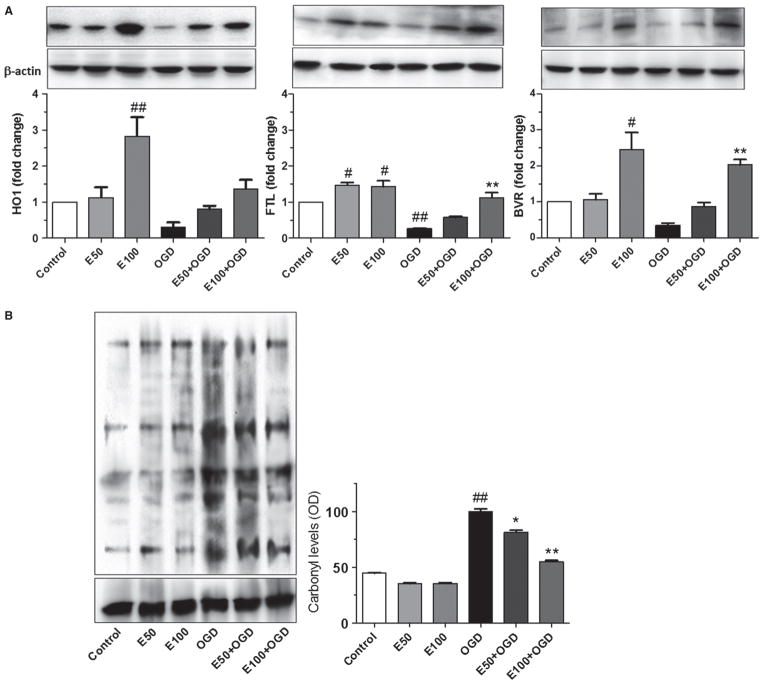
Western blots were performed to assess levels of some Nrf2/ARE-regulated proteins with known cytoprotective roles (Fig. 3A). Results showed a dose-dependent increase in HO1, FTL, and BVR after treatment with 50 or 100 μM EC prior to OGD. The HO1 protein was significantly increased 1.8 ± 0.5 fold compared with control when pretreated with 100 μM EC. HO1 levels were reduced in cells exposed to OGD (0.6 ± 0.1 fold) compared with control and increased to (0.5 ± 0.1 and 1.2 ± 0.2 fold) with 50 or 100 μM EC, respectively (F = 9.87, P = 0.0006). FTL was increased 0.5 ± 0.2 fold compared with control when pretreated with 50 or 100 μM EC. FTL levels were reduced in cells exposed to OGD (0.7 ± 0.1 fold) compared with control and increased to 0.9 ± 0.1 fold with 100 μM EC (F = 26.6, P = 0.0005). Similarly, BVR was increased significantly 1.4 ± 0.5 fold compared with control when pretreated with 100 μM EC. BVR levels were reduced in cells exposed to OGD (0.7 ± 0.1 fold) compared with control and increased (1.7 ± 0.1 fold) with 100 μM EC (F = 12.97, P = 0.0036).
Fig. 3.
EC protection occurs through upregulated expression of Nrf2-responsive proteins and reduced protein oxidation. (A) Western blot shows dose-dependent increase in HO1, FTL, and BVR in WT neurons. (B) OxyBlot assay and quantification shows reduced carbonyl levels in neurons treated with 50 or 100 μM EC relative to untreated cultures. *P < 0.05; **P < 0.01; #P < 0.05; ##P < 0.01; #compared with control; *compared with OGD.
Oxidative protein modifications (Fig. 3B) were also increased in cells exposed to OGD (61.1 ± 2.9%) compared with controls. Interestingly, carbonyl levels were reduced after pretreatment with 50 or 100 μM EC (20.5 ± 2.1 and 49.7 ± 1.4%, respectively) compared with controls (F = 296.4, P < 0.0001).
EC reduces infarct volume and sensorimotor deficits through Nrf2
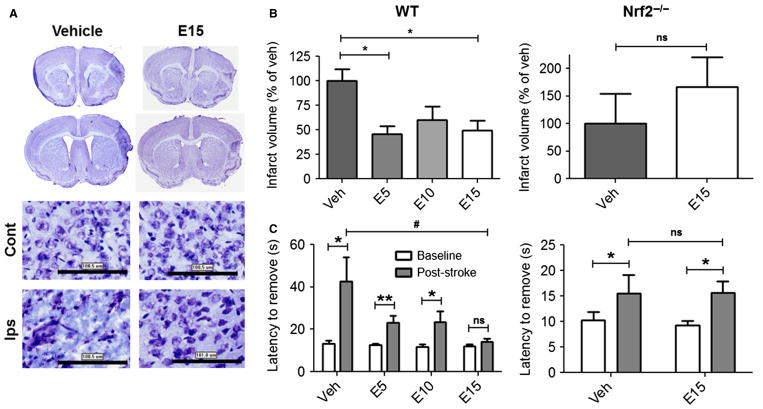
After establishing proof of concept in vitro, we next asked whether protection following preventative, acute oral administration could be extended up to 7 days after stroke in another model of focal cerebral ischemia that lacks reperfusion injury (Fig. 4). Cresyl Violet-stained sections from vehicle-treated mice subjected to pdMCAO showed degenerative morphology within the ipsilateral cortex relative to the contralateral hemisphere. Pretreatment with EC preserved ipsilateral cellular morphology, as it more closely resembled that of the contralateral hemisphere (Fig. 4A). Quantification of infarct volume (Fig. 4B) showed that all EC doses significantly reduced infarct volume in WT mice (5 mg/kg = 54.5 ± 8.3%; 10 mg/kg = 40.1 ± 14.2%; 15 mg/kg = 50.4 ± 10.1%) relative to vehicle (F = 4.412, P = 0.013). In subsequent experiments assessing the optimal dose of 15 mg/kg EC in Nrf2−/− mice, EC failed to reduce infarct volume in these knockout mice (t13 = 0.815, P = 0.215).
Fig. 4.
Nrf2 is required for EC protection from permanent focal ischemia. (A) Cresyl Violet-stained sections show greater degenerative injury after vehicle treatment relative to 15 mg/kg EC. (B) Quantification of infarct volume shows reductions in EC-treated WT mice, whereas no protection was observed in Nrf2−/− mice treated with 15 mg/kg EC. (C) Adhesive removal performance shows reduced deficits in EC-treated WT mice, whereas no protection was observed in Nrf2−/− mice. n = 8; **P < 0.01; *P < 0.05; ns, not significant; Cont, contralateral; Ips, ipsilateral. Scale bars = 100 μm.
Experiments also investigated whether EC promotes functional recovery in WT mice treated with vehicle or EC (Fig. 4C). Results showed main effects of both time (F = 18.46, P < 0.0001) and treatment (F = 4.116, P = 0.011). Subsequent group comparisons showed that the latency to remove adhesive tape was increased 1 day after stroke in vehicle-treated mice compared with pre-surgical baseline scores (42.7 ± 11.4 vs. 13.1 ± 2.0 s, P = 0.025), demonstrating stroke-induced deficits. Deficits were also evident in WT mice after treatment with 5 mg/kg EC (23.3 ± 3.3 vs. 12.5 ± 0.9 s, P = 0.008) or 10 mg/kg (23.5 ± 5.0 vs. 11.6 ± 1.3 s, P = 0.039), yet those that received the 15 mg/kg dose showed no significant deficits (14.0 ± 1.7 s vs. 12.1 ± 0.9 s, P = 0.323). In contrast, the subsequent Nrf2−/− study showed a main effect of time (F = 6.420, P = 0.016) but no effect of treatment (F = 0.035, P = 0.854). Group comparisons confirmed deficits in adhesive removal post-stroke relative to baselines in vehicle-(16.7 ± 3.9 vs. 10.2 ± 1.6 s, P = 0.037) and EC-treated (15.7 ± 2.2 vs. 9.3 ± 0.9 s, P = 0.023) mice, with no differences in the magnitude of post-stroke deficits between vehicle-or EC-treated mice (16.7 ± 3.9 vs. 15.7 ± 2.2 s, P = 0.500).
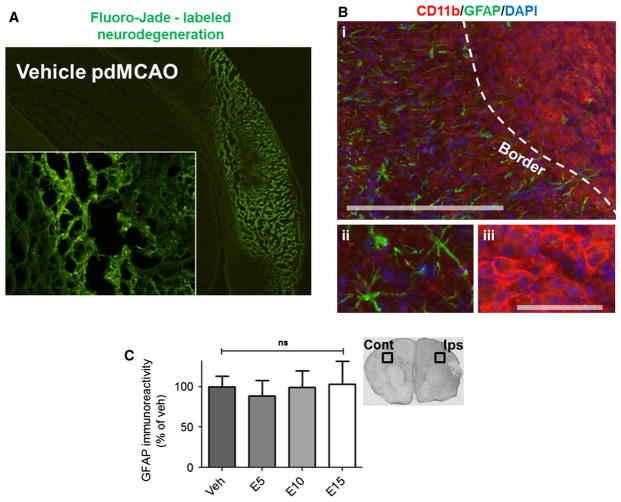
EC does not affect reactive astrogliosis 7 days after pdMCAO
To determine whether EC modulates glial responses to stroke, the gliosis profile was first characterized within the core and peri-infarct zones of vehicle-treated mice 7 days after pdMCAO (Fig. 5A). Fluorescent immunohistochemical double-labeling showed a clear border (i; dashed line) delineating peri-infarct cortex, which displayed an abundance of reactive GFAP-positive astrocytes (ii) from the core infarction area, which was densely populated by CD11b-positive microglia/macrophages (iii) (Fig. 5B). Quantification of GFAP immunoreactivity (Fig. 5C), as measured by DAB staining, showed that despite a ubiquitous astroglial response in vehicle-treated mice, this response was not altered in response to EC treatment (F = 0.090, P = 0.965).
Fig. 5.
EC treatment has no effect on astrogliosis 7 days after permanent focal ischemia. (A) Fluoro-Jade staining shows a vast region of cortical neurodegeneration surrounding the core infarct, which is devoid of neuronal cells. (B) The gliosis profile characterized 7 days after pdMCAO shows the infarction border (i; dashed line), delineating GFAP-positive reactive astrocytes (ii) within the peri-infarct region from CD11b-positive microglia/macrophages (iii) within the core infarction. (C) Quantification of GFAP immunoreactivity visualized with DAB staining showed no differences between vehicle- or EC-treated WT mice. n = 8; ns, not significant; Cont, contralateral; Ips, ipsilateral. Scale bars = 300 μm (i), 100 μm (ii, iii).
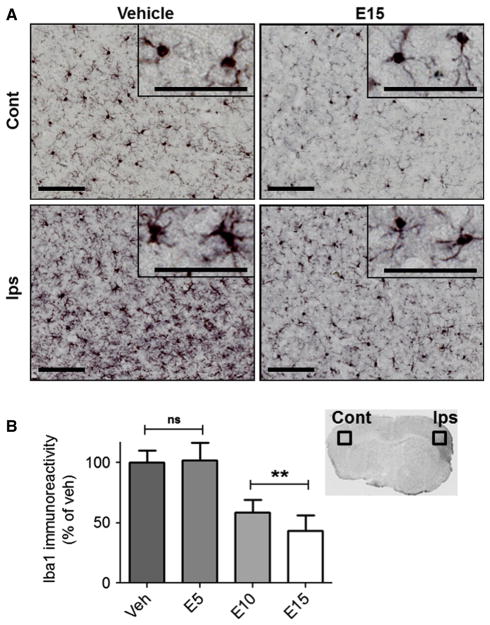
EC reduces microglia/macrophage activation 7 days after pdMCAO
In contrast to GFAP-positive astrocytes, which showed no differences across treatment groups, sections from mice treated with 15 mg/kg EC showed fewer Iba1-positive cells displaying the activated phenotype in the ipsilateral cortex (Fig. 6A). Instead, cellular morphology within the ischemic cortex resembled the quiescent phenotype detected throughout the contralateral hemispheres. Quantification of Iba1 (Fig. 6B) showed significant differences with respect to treatment (F = 5.347, P = 0.005). Specifically, EC reduced Iba1 immunoreactivity relative to vehicle when administered at 10 mg/kg (41.4 ± 10.8%, P = 0.018) or 15 mg/kg (56.4 ± 13.0%, P = 0.008), whereas there were no significant differences between the vehicle and 5 mg/kg groups (100.0 ± 10.0 vs. 102.0 ± 14.5%, P = 0.922).
Fig. 6.
EC treatment reduces microglia/macrophage activation 7 days after permanent focal ischemia. (A) Micrographs show Iba1-positive microglia/macrophages after treatment with vehicle or 15 mg/kg EC. Immunoreactive cells in the contralateral cortex of vehicle-treated mice show a quiescent phenotype compared with activated cells in the ischemic cortex (insets). EC at 15 mg/kg reduced numbers of activated Iba1-positive cells in the ischemic cortex, and the phenotype resembled inactivated contralateral cells (inset). (B) Quantification of Iba1 immunoreactivity shows reductions in WT mice treated with 10 or 15 mg/kg EC relative to vehicle-treated controls, whereas there was no difference in mice treated with 5 mg/kg. n = 8; **P < 0.01; ns, not significant; Cont, contralateral; Ips, ipsilateral; scale bars = 100 μm.
Discussion
Taken together, these data suggest that EC would be beneficial in minimizing stroke injury by reducing oxidative protein damage through the Nrf2 activation pathway, which also leads to dampened microglia/macrophage activation. In vivo experiments demonstrated histological and functional improvements following a range of doses, with 15 mg/kg being the optimal dose in reducing all negative outcomes, including cortical infarction, microglia/macrophage activation, and impaired adhesive removal performance. Furthermore, the absence of protection in Nrf2−/− mice provided direct evidence of a role for Nrf2 in mediating these effects. These results were consistent with experiments in postnatal-enriched primary neuronal cultures, which further demonstrated a protective mechanism through Nrf2 in improving cell viability and reducing cytotoxicity and cell death, which was linked to cytoprotective protein expression and diminished neuronal protein oxidation.
Consistent with benefits identified in epidemiological studies, the administration of naturally occurring polyphenols, including flavan-3-ols, has shown promise in experimental injury models characterized by ischemic neurodegeneration (Choi et al., 2004; Lee et al., 2004; Suzuki et al., 2004; Simonyi et al., 2005; Sutherland et al., 2005; Bu et al., 2007; Leonardo & Doré, 2011). Despite these findings, rigorous investigations using multiple models are needed to elucidate the mechanisms that mediate these benefits, and are also recommended by the Stroke Therapy Academic Industry Roundtable (STAIR, 1999). In the present study, we accomplished this task by evaluating dose–response efficacy, further investigating the in vivo mechanism using Nrf2−/− mice, and extending both the time frame and the translational value of outcome measures. Although we and other laboratories have previously utilized neurological deficit scoring systems and other basic behavioral assays to evaluate functional outcomes shortly after stroke, there is currently a need for additional behavioral paradigms that more closely resemble clinical pathology at extended time points after insult. Notably, these studies adopted the Adhesive Removal Test to determine the functional efficacy of EC. This test reflects impaired forelimb functioning reminiscent of the reported weakness and/or loss of function that patients report in their extremities following stroke. Thus, this measure provides additional means to reproduce pathology similar to that which occurs in the clinical setting. The in vivo doses selected and the response profile observed also highlight the potential for safe, prophylactic use of EC, as the relatively low oral dose of 15 mg/kg effectively improved all outcomes measured, including marked reductions in infarct volume up to 7 days after stroke. Based upon those data, this optimal dose was selected for subsequent investigations with Nrf2−/− mice.
Our findings were also validated in primary neuronal cultures using the OGD model, providing additional evidence that EC protects neuronal cells through Nrf2. To our knowledge, no other laboratories have validated this in vivo mechanism of EC in parallel studies using primary neuronal cultures with the OGD paradigm. To essentially preclude the possibility of direct antioxidant effects in vitro, EC was removed from the media prior to OGD exposure. Therefore, ECs elicitation of neuroprotection in neuronal cells from WT mice suggests activation of neuroprotective pathways as opposed to direct antioxidation. EC activation of Nrf2, in particular, was supported by the demonstrated upregulation of HO1, FTL, and BVR, all of which contain Nrf2-responsive ARE elements within their promoter regions (Leonardo & Doré, 2011), as well as the loss of protection observed in neurons from Nrf2−/− mice. However, we cannot deny that other cellular pathways may have contributed to neuroprotection and the induction of these proteins.
If in vivo EC protection is mediated by Nrf2 activation, as our studies suggest, this raises implications for the potential benefits of EC and EC-enriched extracts in minimizing stroke-induced tissue injury. Notably, Nrf2 induction would allow for biological effects conferred by parent molecules that exhibit low brain bioavailability, which may be the case with EC and other flavan-3-ols after consumption in humans (Manach et al., 2004, 2005). In this scenario, even relatively small amounts of the parent compound can trigger an endogenous switch within the brain that amplifies cellular protection through upregulated protein expression. Indeed, we have previously shown that HO1 plays a role in protecting the brain from permanent focal ischemia (Zeynalov et al., 2009; Shah et al., 2011). Of interest, HO1 also contains the most ARE elements in its promoter region (Wasserman & Fahl, 1997), further suggesting that HO1 is the downstream target mechanism through which EC activation of Nrf2 elicits protection.
In apparent contrast to the major conclusion here, a previous study using the Nrf2 activator sulforaphane reported no improvements using a similar model of photothrombotic ischemia and similar outcome measures (Porritt et al., 2012). Several methodological differences may account for the discrepancy. One key distinction was the use of Nrf2−/− mice here, in contrast to a pharmacological activator validated by Nrf2-regulated gene transcripts as direct measures of Nrf2 induction. In the present study, EC activation of the Nrf2 pathway was also confirmed using the OGD model, and the use of knockout mice allowed for clear interpretations of Nrf2 effects on EC efficacy. A second distinction between the studies was prophylactic treatment with EC versus delayed, post-stroke administration of sulforaphane, which may be an important difference considering the known effects of EC on vascular function (Alfieri et al., 2011). From this perspective, our results may reflect the ability of EC to diminish vascular injury that occurs immediately or shortly after occlusion. However, neither sulforaphane nor EC reduced astrogliosis, which might suggest heightened vessel damage regardless of the treatment administered. This explanation is speculative, however, because vascular effects were not investigated in this study. Alternatively, because Nrf2 induction by activated astrocytes may be a primary mechanism of neuroprotection, astrogliosis may not necessarily signify deleterious processes.
In addition to the timing of administration, the selected endpoints may provide insights into the role of Nrf2 in EC-mediated protection. Histological outcomes were assessed at 7 days in the present report as opposed to 24 and 72 h after photothrombosis. Interestingly, both results are consistent with a previous report that demonstrated efficacy after treatment with the Nrf2 activator tert-butylhydroquinone in WT but not Nrf2−/− mice, and showed worse outcomes in Nrf2−/− mice at 7 days but not 24 h following permanent focal ischemia (Shih et al., 2005). Thus, it appears as though the relationship between Nrf2 activation and neuroprotection is a tenuous one that is related to temporal injury progression and the timing of administration.
While EC clearly prevented neuronal loss and improved functional outcomes, various cell types, including astrocytes, microglia, endothelial cells, or neurons (as demonstrated in cultures) may mediate these effects. Because penumbral expansion is intimately connected with pro-inflammatory signaling by resident immune cells and infiltrating peripheral macrophages (Dirnagl et al., 1999), it is tempting to attribute neuroprotection to reduced microglia/macrophage activation. However, decreased Iba1 immunoreactivity at 7 days is insufficient to establish a mechanism of inflammation-induced penumbral expansion in the absence of an extended time course. Interestingly, although polyphenol-rich green tea extract was able to prevent cell swelling in C6 microglial cultures subjected to OGD, neither EC nor any other individual polyphenol constituent was able to reproduce this effect (Panickar et al., 2009).
Another explanation for EC protection may be through reduced reactive oxygen species (ROS), which could be achieved by dampening immune cell activation. In the case of cerebral ischemia, resident microglia, peripheral macrophages, and neutrophils are present throughout the acute phase of stroke and probably contribute to oxidative stress, particularly within the core and immediate peri-infarct regions. However, the present study was conducted with the major goal of evaluating efficacy 7 days after stroke onset. Because all histology was performed in 7-day-old tissues, a thorough time course evaluating activation of each immune cell type would be necessary to determine the major source(s) of ROS. We noted morphological differences between Iba1-positive cells in the infarct core and those in the peri-infarct regions. Both phenotypes appeared to be activated, but perhaps to different degrees. These phenotypic differences could also reflect different cell populations, as Iba1 is not selective for resident microglia and the amoeboid cells identified throughout the core infarct with CD11b exhibited classic foamy macrophage morphology. Nonetheless, these data do not provide a clear answer as to the definitive cell type involved in ROS generation.
Due to the complexity of the in vivo microenvironment, future studies are necessary to gain a clearer understanding of glial cell modulation by EC and other flavan-3-ols following stroke. In conclusion, we believe that many of the beneficial effects attributed to the consumption of EC-enriched dietary sources can also protect the brain against stroke. Additionally, data suggest that these health benefits are not solely based on antioxidant properties of polyphenols, but may occur in large part through activation of the endogenous Nrf2 cytoprotective pathway.
Acknowledgments
This work was partially supported by R21AT005085 and R21AT005246. The authors thank Matthew Mills, Morgan Bennett, and all other members of the Doré lab. The authors declare no conflicting or competing interests.
Abbreviations
- BVR
biliverdin reductase
- CAM
complementary and alternative medicine
- EC
(−)-epicatechin
- EthD-1
ethidium homodimer 1
- FTL
ferritin light chain
- GFAP
glial fibrillary acidic protein
- HO1
heme oxygenase 1
- Iba1
ionized calcium-binding adapter molecule 1
- LDH
lactate dehydrogenase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Nrf2−/−
Nrf2 knockout
- OGD
oxygen glucose deprivation
- pdMCAO
permanent distal middle cerebral artery occlusion
- WT
wildtype
References
- Agrawal M, Kumar V, Singh AK, Kashyap MP, Khanna VK, Siddiqui MA, Pant AB. trans-Resveratrol protects ischemic PC12 cells by inhibiting the hypoxia associated transcription factors and increasing the levels of antioxidant defense enzymes. ACS Chem Neurosci. 2013;4:285–294. doi: 10.1021/cn300143m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol. 2011;589:4125–4136. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba S, Osakabe N, Natsume M, Muto Y, Takizawa T, Terao J. In vivo comparison of the bioavailability of (+)-catechin, (−)-epicatechin and their mixture in orally administered rats. J Nutr. 2001;131:2885–2891. doi: 10.1093/jn/131.11.2885. [DOI] [PubMed] [Google Scholar]
- Bu Y, Rho S, Kim J, Kim MY, Lee DH, Kim SY, Choi H, Kim H. Neuroprotective effect of tyrosol on transient focal cerebral ischemia in rats. Neurosci Lett. 2007;414:218–221. doi: 10.1016/j.neulet.2006.08.094. [DOI] [PubMed] [Google Scholar]
- Choi YB, Kim YI, Lee KS, Kim BS, Kim DJ. Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res. 2004;1019:47–54. doi: 10.1016/j.brainres.2004.05.079. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Echeverria V, Greenberg DL, Dore S. Expression of prostaglandin E2 synthases in mouse postnatal cortical neurons. Ann NY Acad Sci. 2005;1053:460–471. doi: 10.1111/j.1749-6632.2005.tb00056.x. [DOI] [PubMed] [Google Scholar]
- Fisher ND, Sorond FA, Hollenberg NK. Cocoa flavanols and brain perfusion. J Cardiovasc Pharm. 2006;47(Suppl 2):S210–S214. doi: 10.1097/00005344-200606001-00017. [DOI] [PubMed] [Google Scholar]
- Heiss C, Keen CL, Kelm M. Flavanols and cardiovascular disease prevention. Eur Heart J. 2010;31:2583–2592. doi: 10.1093/eurheartj/ehq332. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK, Fisher ND, McCullough ML. Flavanols, the Kuna, cocoa consumption, and nitric oxide. J Am Soc Hypertens. 2009;3:105–112. doi: 10.1016/j.jash.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kim YS, Doré S. Catalytically inactive heme oxygenase-2 mutant is cytoprotective. Free Radical Bio Med. 2005;39:558–564. doi: 10.1016/j.freeradbiomed.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Bae JH, Lee SR. Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J Neurosci Res. 2004;77:892–900. doi: 10.1002/jnr.20193. [DOI] [PubMed] [Google Scholar]
- Leonardo CC, Doré S. Dietary flavonoids are neuroprotective through Nrf2-coordinated induction of endogenous cytoprotective proteins. Nutr Neurosci. 2011;14:226–236. doi: 10.1179/1476830511Y.0000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo CC, Hall AA, Collier LA, Green SM, Willing AE, Pennypacker KR. Administration of a sigma receptor agonist delays MCAO-induced neurodegeneration and white matter injury. Transl Stroke Res. 2010;1:135–145. doi: 10.1007/s12975-009-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, Slaughter N, Killeen E, Wang X, Huang A, Wang M, Miguel AH, Cho A, Sioutas C, Nel AE. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- Maines MD, Raju VS, Panahian N. Spin trap (N-t-butyl-α-phenylnitrone)- mediated suprainduction of heme oxygenase-1 in kidney ischemia/ reperfusion model: role of the oxygenase in protection against oxidative injury. J Pharmacol Exp Ther. 1999;291:911–919. [PubMed] [Google Scholar]
- Maiser SJ, Georgiadis AL, Suri MF, Vazquez G, Lakshminarayan K, Qureshi AI. Intravenous recombinant tissue plasminogen activator administered after 3 h following onset of ischaemic stroke: a metaanalysis. Int J Stroke. 2011;6:25–32. doi: 10.1111/j.1747-4949.2010.00537.x. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- Nehlig A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Brit J Clin Pharmaco. 2013;75:716–727. doi: 10.1111/j.1365-2125.2012.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani JI, Momma TY, Kuhnle GK, Keen CL, Schroeter H. Structurally related (−)-epicatechin metabolites in humans: assessment using de novo chemically synthesized authentic standards. Free Radical Bio Med. 2011;52:1403–1412. doi: 10.1016/j.freeradbiomed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Polansky MM, Anderson RA. Green tea polyphenols attenuate glial swelling and mitochondrial dysfunction following oxygen-glucose deprivation in cultures. Nutr Neurosci. 2009;12:105–113. doi: 10.1179/147683009X423300. [DOI] [PubMed] [Google Scholar]
- Porritt MJ, Andersson HC, Hou L, Nilsson A, Pekna M, Pekny M, Nilsson M. Photothrombosis-induced infarction of the mouse cerebral cortex is not affected by the Nrf2-activator sulforaphane. PLoS ONE. 2012;7:e41090. doi: 10.1371/journal.pone.0041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Tritschler E, Hoetzel A, Loop T, Humar M, Halverscheid L, Geiger KK, Pannen BH. Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/ reperfusion injury. Ann Surg. 2007;245:931–942. doi: 10.1097/01.sla.0000256891.45790.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Doré S. The flavanol (−)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cerebr Blood F Met. 2010;30:1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Nada SE, Doré S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2- mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol. 2005;31:135–147. doi: 10.1385/MN:31:1-3:135. [DOI] [PubMed] [Google Scholar]
- Sorond FA, Hollenberg NK, Panych LP, Fisher ND. Brain blood flow and velocity: correlations between magnetic resonance imaging and transcranial Doppler sonography. J Ultras Med. 2010;29:1017–1022. doi: 10.7863/jum.2010.29.7.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAIR. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Sudano I, Flammer AJ, Roas S, Enseleit F, Ruschitzka F, Corti R, Noll G. Cocoa, blood pressure, and vascular function. Curr Hypertens Rep. 2012;14:279–284. doi: 10.1007/s11906-012-0281-8. [DOI] [PubMed] [Google Scholar]
- Sutherland BA, Shaw OM, Clarkson AN, Jackson DN, Sammut IA, Appleton I. Neuroprotective effects of (−)-epigallocatechin gallate following hypoxia-ischemia-induced brain damage: novel mechanisms of action. FASEB J. 2005;19:258–260. doi: 10.1096/fj.04-2806fje. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Tabuchi M, Ikeda M, Umegaki K, Tomita T. Protective effects of green tea catechins on cerebral ischemic damage. Med Sci Monitor. 2004;10:BR166–BR174. [PubMed] [Google Scholar]
- Ueda K, Ueyama T, Yoshida K, Kimura H, Ito T, Shimizu Y, Oka M, Tsuruo Y, Ichinose M. Adaptive HNE-Nrf2-HO-1 pathway against oxidative stress is associated with acute gastric mucosal lesions. Am J Physiol-Gastr L. 2008;295:G460–G469. doi: 10.1152/ajpgi.00204.2007. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wang B, Cao W, Biswal S, Doré S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Bao X, Xi G, Keep RF, Thompson BG, Hua Y. Brain injury after intracerebral hemorrhage in spontaneously hypertensive rats. J Neurosurg. 2011;114:1805–1811. doi: 10.3171/2011.1.JNS101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeynalov E, Shah ZA, Li RC, Doré S. Heme oxygenase 1 is associated with ischemic preconditioning-induced protection against brain ischemia. Neurobiol Dis. 2009;35:264–269. doi: 10.1016/j.nbd.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]