Abstract
QUantification of Exchange as a function of Saturation Power On the Water Resonance (QUESPOWR) MRI is a new method that can estimate chemical exchange rates. This method acquires a series of OPARACHEE MRI acquisitions with a range of RF powers for the WALTZ16* pulse train, which are applied on the water resonance. A QUESPOWR plot can be generated from the power dependence of the % water signal, which is similar to a QUESP plot that is generated from CEST MRI acquisition methods with RF saturation applied off-resonance from water. A QUESPOWR plot can be quantitatively analyzed using linear fitting methods to provide estimates of average chemical exchange rates. Analyses of the shapes of QUESPOWR plots can also be used to estimate relative differences in average chemical exchange rates and concentrations of biomolecules. The performance of QUESPOWR MRI was assessed via simulations, an in vitro study with iopamidol, and an in vivo study with a mouse model of mammary carcinoma. The results showed that QUESPOWR MRI is especially sensitive to chemical exchange between water and biomolecules that have intermediate to fast chemical exchange rates and chemical shifts that are close to water, which are notoriously difficult to assess with other CEST MRI methods. In addition, in vivo QUESPOWR MRI detected acidic tumor tissues relative to normal tissues that are pH-neutral, and therefore may be a new paradigm for tumor detection with MRI.
Keywords: QUESPOWR, OPARACHEE, QUESP, CEST MRI, Tumor pH
1. Introduction
Chemical Exchange Saturation Transfer (CEST) MRI has evolved during the last 15 years to become a useful contrast mechanism for pre-clinical and clinical studies [1,2]. Endogenous CEST MRI contrast is dependent on the rate of chemical exchange of the proton from a biomolecule to the bulk water, and the chemical exchange rate constant is sensitive to the chemical environment in which the biomolecule resides. Therefore, it is possible to interrogate the tissue environments at the molecular level using CEST MRI techniques. For example, the combination of the concentrations of biomolecules and their chemical exchange rates have been used to detect different tissue environments during assessments of breast cancer vs. fibroglandular tissue [3], glioma vs. normal white matter [4], glioma vs. radiation necrosis [5], ischemic vs. non-ischemic brain tissues [6], and tissues with a reduced redox state [7], bacterial infections [8], and reduced glycosaminoglycan content [9]. CEST MRI has also been used to detect tissues with different endogenous concentrations of metabolites, including glutamate [10] and creatine [11].
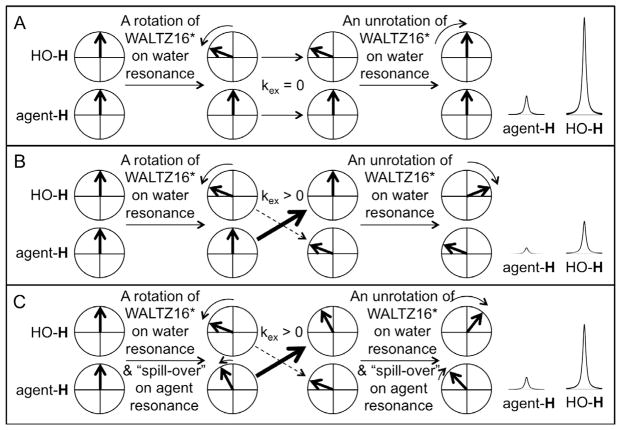
The initial and most common procedure for endogenous CEST MRI applies selective saturation on the MR resonance of a labile proton of a biomolecule, which eliminates the net coherent magnetization from the proton resonance. Subsequent chemical exchange of the biomolecule’s proton with water causes a decrease in the net detectable MR signal from water. An alternative method of generating MRI contrast that is dependent on chemical exchange applies a series of selective, low-power radio frequency (RF) pulses on the bulk water resonance [12]. Known as “On resonance PARamagnetic Agent CHemical Exchange Effects” (OPARACHEE), this method typically uses a WALTZ16* pulse train to perform a combination of rotations and “unrotations” of the net coherent magnetization of water [13], resulting in a net 0° rotation of the water resonance (Fig. 1). If some protons on water molecules undergo chemical exchange with the biomolecule during the WALTZ16* pulse train, then these protons experience only a fraction of the combined rotations and “unrotations” of the WALTZ16* pulse train and therefore experience more than 0° net rotation of the net coherent magnetization, causing a loss of net magnetization for the water resonance (Fig. 1B). Importantly, this method only requires RF pulses on the water resonance so that a series of MR frequencies does not need to be saturated, which greatly accelerates image acquisition.
Fig. 1.
Schematic of the QUESPOWR method. (A) The OPARACHEE MRI protocol with a WALTZ16* pulse train at the water resonance causes no loss in detectable MR signal from water in the absence of chemical exchange. The WALTZ16* pulse train causes a rotation of the water’s net magnetization in the ZY plane (shown as a 60° rotation in the schematic), which is eventually “unrotated” to return the net magnetization to equilibrium. In practice, WALTZ16* performs a combination of rotations and unrotations, but the concept is the same as shown in the schematic. The effects of MR relaxation are assumed to be negligible in this schematic. (B) The same protocol causes a loss in detectable MR signal of the water due to non-zero chemical exchange (kex) during the WALTZ16* pulse train. The chemical exchange occurs stochastically during the pulse train, and only one example of chemical exchange part-way through the pulse train is shown in the schematic. Chemical exchange from the agent to water is emphasized with a thick arrow, while chemical exchange is de-emphasized with a dashed arrow, to indicate the direction of exchange that is important for QUESPOWR. (C) “RF spill-over” of the WALTZ16* pulse train at the MR frequency of the agent causes rotation and “unrotation” of the agent’s net magnetization in the ZY plane. Coupling the agent’s rotation of magnetization with chemical exchange causes only a partial loss in detectable MR signal of the water. Therefore a QUESPOWR MRI acquisition, consisting of a series of OPARACHEE MRI acquisitions over a range of RF powers for the WALTZ16* pulse, shows a RF power-dependence on detectable water signal.
The OPARACHEE MRI method was originally used to detect exogenous paramagnetic contrast agents that have fast chemical exchange rates and large chemical shifts [14–16]. A fast chemical exchange rate causes more water protons to quickly transition to the agent during the relatively short WALTZ16* pulse train. A large chemical shift improves the selectivity of the WALTZ16* pulse train for the water resonance, and also contributes to dephasing of the excited magnetization. Both of these effects cause the exchanging protons to generate a lower net water signal. Therefore, it may seem counter-intuitive to apply OPARACHEE MRI acquisition methods to generate contrast from biomolecules with labile protons that have small chemical shifts or slow chemical exchange rates. However, we hypothesized that OPARACHEE MRI acquisition methods may still generate some image contrast when the chemical shifts are small and the chemical exchange rates are slow.
When using the common endogenous CEST MRI acquisition protocol that saturates at a biomolecule’s MR resonance, the CEST amplitude increases as the saturation power is increased [17]. However, high saturation powers cause off-resonance saturation of water that causes the CEST amplitude to decrease, especially when the exchanging protons have a small chemical shift and slow chemical exchange rate (known as a “spill-over effect”) [18–20]. We hypothesized that the contrast generated by an OPARACHEE MRI acquisition protocol has a similar dependence on the RF power of the WALTZ16* pulse. This method should show increased suppression of water signal as the WALTZ16* pulse power is increased, yet very high RF powers for the WALTZ16* pulse should reduce the suppression of water signal due to a “reverse spill-over effect” (Fig. 1C). Furthermore, the dependence of CEST amplitude on saturation power can be analyzed using the non-linear QUantification of Exchange as a function of Saturation Power (QUESP) method [17], and linear variations of this method [21,22]. Therefore, we also hypothesized that similar analysis methods can be used to fit the power-dependent suppression of the water signal using the OPARACHEE MRI acquisition protocol. To emphasize this point, we have defined our method as “QUantification of Exchange as a function of Saturation Power On the Water Resonance” (QUESPOWR). We refer to a series of OPARACHEE MRI acquisitions with a range of RF powers for the WALTZ16* pulse as a single QUESPOWR MRI acquisition, and we refer to the analysis of this series of acquisitions as a QUESPOWR plot.
2. Theory
2.1. Derivation of the linear analysis methods for QUESP
To understand how to evaluate a QUESPOWR plot, we first derived linear equations that can be used to analyze a QUESP plot. Methods have been previously reported that can analyze the region of the QUESP plot at low power, as listed below. However, analysis methods for the high power region of a QUESP plot have not yet been reported. Therefore, this derivation is useful for understanding the entire range of a QUESP plot.
The Bloch-McConnell Eq. (23) describe the time evolution of a two-pool system under chemical exchange. Eq. (1) is their matrix form, which can be used to assess the magnetization of labile protons of water and biomolecules (for simplicity, we refer to “water” and “biomolecules” for the remainder of this report).
| (1A) |
where
| (1B) |
| (1C) |
| (1D) |
The subscripts w and b denote the pools for water and biomolecules respectively. T1w and T1b are the longitudinal relaxation times (in units of seconds) for the water and biomolecules. The same convention applies for the transverse relaxation time (T2). kex is the chemical exchange rate (in units of Hz) of a proton exchanging from biomolecules to water, Δω is the chemical shift difference between the exchangeable proton of the biomolecules and water (in units of rad/s), ωB1 is the power of the radiofrequency pulse (in rad/s), χ is the normalized concentration of the exchangeable protons in the biomolecules relative to water. The biomolecules may be composed of more than one exchangeable proton resonating at the same frequency (Δω). Thus, χ is equal to Σ nb[b]/nH2O[H2O], where nb and nH2O denote the number of protons in the biomolecules and water, while [b] and [H2O] are their respective concentrations.
Four assumptions were made to simplify our derivation: (1) T2b and T1b are equal to T1w and T2w respectively. This is a valid assumption because the relaxation times of the solute do not have a significant effect on CEST contrast [22–24]. (2) T2w is equal to T1w. Although this assumption may not be accurate in all cases, the final equations of the derivations below do not include these terms, so that these relaxation time constants do not affect the determination of chemical exchange rates. (3) The saturation pulse is on resonance with the water (Δω = 0). (4) The saturation pulse is sufficiently long to allow the system to reach steady state (Eq. (1) equal to zero, resulting in Eq. (2)).
| (2A) |
| (2B) |
Under the assumptions described above, CEST contrast can be considered to be a function of water magnetization with (Eq. (2)) and without (Eq. (3)) the presence of the biomolecules (Eq. (4)).
| (3) |
| (4) |
The analytical solution for the steady state longitudinal magnetization (Mwz) of water with and without biomolecules was obtained by symbolic evaluation of Eqs. (2) and (3) using MUPAD (Mathworks, Inc., Natick, MA). These analytical formulas were inserted into Eq. (4), then the resulting formula was simplified to produce Eq. (5). Eq. (6) was obtained after collecting terms and simplifying Eq. (5). The chemical exchange rate can be determined from Eq. (6) by fitting of the relationship between CEST and RF power in Eq. (6A) to estimate coefficients A, B, and C. These coefficients are then used to solve for kex in a quadratic analysis (Eq. (6E)).
| (5) |
| (6A) |
where
| (6B) |
| (6C) |
| (6D) |
| (6E) |
The previously reported Omega Plot method can be derived from Eq. (5) by assuming that the chemical shift is large (Δω approaches infinity) (Eq. (7)) [21,22]. The non-linear QUESP analysis and the linear Omega Plot are analogous to a non-linear Michaelis–Menton enzyme kinetics analysis and the well-known linear Lineweaver–Burke analysis of enzyme kinetics [25]. Therefore, we refer to an Omega Plot as the LB-QUESP analysis method, where “LB” represents “Lineweaver-Burke”, to distinguish this method from other linear analysis methods derived below.
| (7) |
The chemical exchange rate is then determined by the LB-QUESP method from the slope and y-intercept of Eq. (7) (Eq. (8)).
| (8) |
Conversely, if it is assumed that the chemical shift is small (Δω approaches zero), then Eq. (5) simplifies to Eq. (9). The slope and y-intercept of Eq. (9A) are used to estimate the chemical exchange rate (Eq. (10)).
| (9A) |
| (9B) |
| (10) |
Notably, the assumption that saturation has reached steadystate is not required for this type of analysis method. If saturation is not applied for an infinite time, Eq. (9B) can be modified to include a time-dependent term (Eq. (11)) [17].
| (11A) |
| (11B) |
The parameter β in Eq. (11B) is independent of saturation power. Therefore, the slope and y-intercept can still be used to estimate the chemical exchange rate (Eq. (12)).
| (12) |
We have shown previously [22] that the precision of measuring chemical exchange rates with Eq. (7) is increased if this equation is rearranged to include on both sides. Thus, we rearranged Eqs. (7) and (9) to yield Eq. (13) and (15) respectively. This rearrangement is analogous to the rearrangement of a Lineweaver–Burke equation to create a Hanes-Woolf equation for linear analyses of enzyme kinetics. Therefore, we refer to this rearrangement for our QUESP analysis as a HW-QUESP analysis, where “HW” represents “Hanes-Woolf”.
| (13) |
| (14) |
| (15) |
| (16) |
Eqs. (13) and (14) were used for the analysis of low-power regions of the QUESPOWR and QUESP plot. We refer to this method as LP-HW-QUESP to emphasize that this analysis method is used for the Low Power region of the plot. Similarly, we elected to use Eqs. (15) and (16) to analyze the high power regions of the QUESPOWR and QUESP plots. We refer to this method as HP-HW-QUESP to emphasize that this analysis method is used for the High Power region of the plot. The HW-QUESP analysis method mitigates error from normalizing the % water signal at high power, due to the reciprocal nature of RF power in this equation. In addition, Eq. (5) can be used to analyze both the low-power and high-power regions of the QUESPOWR and QUESP plots, and we refer to this method as the LP-HP-HW-QUESP method.
2.2. Applying the same linear analysis methods to QUESPOWR
The water signal during a WALTZ16* pulse train is dependent on chemical exchange with biomolecules. More specifically, the transfer of a proton from a biomolecule to a water molecule is the critical direction of chemical exchange for QUESPOWR, because the phase of the MR resonance of the biomolecule’s proton at the time of transfer affects the net MR signal of water at the time of MR detection. The transfer of a proton from a water molecule to a biomolecule (and most likely another biomolecule than the biomolecule which transfers a proton to water) only serves to balance the net proton transfer. This important point is emphasized by using a thick arrow in Fig. 1B and C for the proton transfer from a biomolecule to water. Based on this rationale, kex for evaluating QUESPOWR should still be defined as the chemical exchange rate of a proton exchanging from biomolecules to water, as described in Section 2.1 and Eq. (1). Furthermore, the water signal during a WALTZ16* pulse train is also dependent on T1 and T2 relaxation. However, as described in Section 2.1 T2b and T1b can be assumed to be equal to T1w and T2w, respectively. Thus, the designation of “w” and “b” in these terms for relaxation time constants in Eq. (1) is irrelevant. Finally, the chemical shift difference of the proton on a biomolecule vs. water does not depend on the sign of this chemical shift, so that the term Δω in Eq. (1) applies to QUESPOWR regardless of whether protons on the biomolecule or water are saturated. Similarly, Eq. (1) does not depend on the rotation direction of the radiofrequency pulse, ωB1. Therefore, the Bloch-McConnell equations shown in Eq. (1) can be used to describe the evolution of the magnetization of water undergoing chemical exchange with biomolecules during the WALTZ16* pulse train.
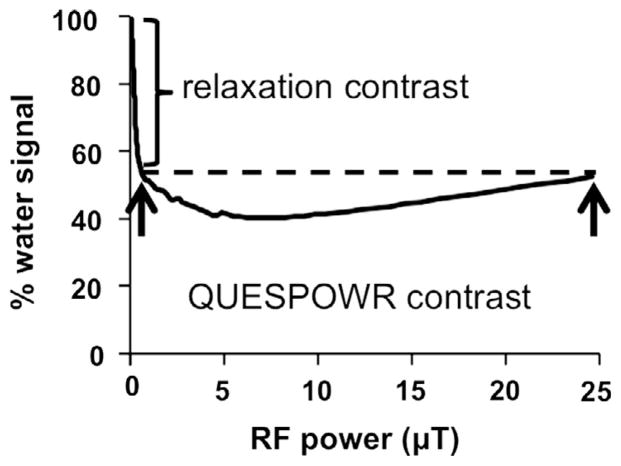
The decrease in water signal caused by T1 and T2 relaxation during the WALTZ16* pulse train is significant when the pulse train has a long total time relative to the T1 or T2 relaxation time constants of the water. Fortunately, this relaxation-dependent decrease in water signal can be evaluated at very low power while the effects of slow chemical exchange are negligible, so that the % water signal that is affected by slow chemical exchange can be separated from the % water signal substantially affected by relaxation (Fig. 2). To separate these effects, we renormalized the % water signal using the water signal at power corresponding to a π/2 tip angle (ωnorm; Eq. (17)).
Fig. 2.
Schematic of the renormalization of QUESPOWR contrast.
| (17A) |
| (17B) |
| (17C) |
| (17D) |
| (17E) |
| (17F) |
This renormalized water signal contrast, ContrastQUESPOWR, is equivalent to in Eq. (4). Therefore, Eqs. (4)–(14) are applicable to the analysis of QUESPOWR plots. The water signal at infinitely high power can also be used for this renormalization, because the effect of slow chemical exchange during the WALTZ16* pulse train is negligible at high power due to the “reverse spill-over effect” as described in Fig. 1. In practice, an infinitely high power cannot be achieved, so we performed a renormalization using the water signal obtained with the highest power that the MRI instrument could safely produce for the WALTZ16* pulses during in vivo studies. This renormalization based on powers that are not infinitely high may cause a systematic error. However, we have previously shown that the HW-QUESP method compensates for inaccurate power levels, so that the LP-HW-QUESP and HP-HW-QUESP methods avoid this potential systematic error [22].
2.3. Graphical analysis of QUESPOWR
The shape of the QUESPOWR acquisition plot can also be used to estimate differences in the chemical exchange rates between samples or tissues. This plot has a minimum % water signal when the derivative of Eq. (5) is zero, which occurs at the RF power defined by Eq. (18). This minimum % water signal depends on the chemical shift of the exchanging protons (Δω). Yet if the average chemical shift of exchanging protons on all biomolecules is assumed to be the same in all tissues, then different RF powers for minimum % water signal in different tissues can indicate different average chemical exchange rates in different tissues. Furthermore, the shape of the QUESPOWR acquisition plot can be relatively flat, especially if the average chemical shift is high. Therefore, the minimum RF power that creates the flat region of this plot should be used to estimate differences in exchange rates between samples or tissues, to avoid overestimating the exchange rates.
| (18) |
Combining Eqs. (5) and (18) shows that the maximum contrast in QUESPOWR plots and QUESP plots occurs when the chemical exchange rate and chemical shift difference between water and the biomolecules are commensurate (Eq. (19)). The LP-HW-QUESP and HP-HW-QUESP methods each analyze portions of these plots on either side of the maximum value described in Eq. (13), while the amount of contrast present in the images with respect to the image acquired for normalization will depend on Eq. (19). Therefore, each analysis method is sensitive to different combinations of chemical exchange rates and chemical shifts. More specifically, LP-HW-QUESP is sensitive to chemical exchange when the exchange rate is less than the chemical shift, while HP-HW-QUESP is sensitive to chemical exchange when the exchange rate is greater than the chemical shift.
| (19) |
3. Methods
3.1. Computational methods
Simulated results for QUESPOWR MRI acquisitions and CEST MRI acquisitions were obtained using the Bloch-McConnell equations (Eq. (20)) [23,25]. This matrix was used as a propagator via the expm command in MATLAB (Mathworks, Inc., Natick, MA) (Eq. (21)) to obtain the state of the magnetization after a single pulse (Eq. (22)). For the WALTZ16* pulse train, the individual propagators were solved and then combined into one propagator (Eq. (23)), which was used to find the magnetization at the end of the pulse train (Eq. (24)).
| (20) |
with
ωw, ωb: precession frequency of the water and the biomolecules, rad/s.
ω: frequency of irradiation, rad/s.
Mw: concentration of water, molarity.
| (21) |
| (22) |
| (23) |
| (24) |
These propagators can be multiplied together because this computational method uses a homogenous form as previously described [26]. This form of the equations is conducive to programming numerical simulations.
The simulations used a 2-pool model representing the chemical exchange between a labile proton at 1260 Hz chemical shift with a chemical exchange rate of 200 Hz and 100 mM concentration. As noted in the results, some simulations were performed with chemical shifts of 150,000 or 800 Hz, and with chemical exchange rates of 800 or 330,000 Hz, to investigate the effects of chemical shifts and exchange rates on QUESPOWR MRI. Simulations were also performed by setting the labile pool offset to 800 Hz and varying the chemical exchange rate between 0 and 10,000 Hz, to test the ability to accurately measure chemical exchange rates with QUESPOWR analysis methods. Other simulations tested the effects of a more dilute labile proton at 10 mM concentration. A set of simulations was performed with a 3-pool model that included a second labile proton with a chemical shift of 800 Hz, chemical exchange rate of 47 Hz, and 1 M concentration, which tested the weighting of QUESPOWR toward fast-exchanging protons. The T1w and T1b relaxation time constants were set to 1.0 s, and the T2w and T2b relaxation time constants were set to 333 ms for all simulations. Simulations were also performed at T2w = 10 ms to verify that the water line width did not affect results.
CEST MRI simulations were performed with a 10 s saturation time. The WALTZ16* pulse train of the QUESPOWR MRI simulations used a 5 ms pulse length, for a total train length of 480 ms. The power was iterated between 0 and 50 μT for both methods. Linear fittings of simulated results were performed using the regress command in MATLAB.
3.2. Chemical solutions
Iopamidol (Isovue®, Bracco Diagnostics, Inc.) was prepared at concentrations of 79mM at pH values of 6.21, 6.43, 6.56, 6.84, 6.95 and 7.26 pH units. A series of chemical solutions was also prepared at pH 6.84 with concentrations of 0, 16, 32, 47, 63 and 79mM. All solutions were buffered using phosphate buffered saline. Each chemical solution was placed in a 5mmNMR tube that was 7 inches long, and the tubes were placed along the long axis of the MRI magnet bore. Samples were maintained at 37.0 ± 0.2 °C using an automated feedback loop between the temperature probe and an air heater (SA Instruments, Inc., Stony Brook, NY).
3.3. In vivo mouse model
All in vivo studies were conducted according to approved procedures of the Institutional Animal Care and Use Committee of the University of Arizona. A model of MCF7 mammary carcinoma was prepared by injecting one million tumor cells in 0.5mL of 50%Matrigel™ into the upper right flank of three 6-week-old female SCIDmice. A slow-releasing estrogen pellet was implanted in the back of the neck. Imaging studies were conducted when the subcutaneous tumor reached 150–2000 mm3. Notably, all imaging was performed before the tumor showed visible signs of necrosis. To prepare a mouse for MRI, the mouse was anesthetized with 1.5–2.5% isoflurane delivered in 1 L/min oxygen gas ventilation, and then secured to a customized cradle. The cradle was inserted into the MRI magnet in a prone position. The breathing rate was monitored and the core body temperature was regulated at 37.0 ± 0.2 °C.
3.4. MRI acquisition methods
A Bruker Biospec MRI scanner with a 7 T magnetic field strength, 20 cm bore, and 78 mm transceiver volume coil was used for all studies. All MR images were obtained using a gradient spoiled FISP imaging sequence with an echo time of 2.3 ms; repetition time of 4.7 ms; 30° excitation angle; 1 mm slice thickness; 250 × 250 μm in-plane resolution; 3.2 × 3.2 cm FOV; centric encoding order; unbalanced “FID” mode; 418.54 ms scan time. For in vitro studies, the WALTZ16* pulse train consisted of a series of 5 ms pulses for a total train of 480 ms. To analyze chemical solutions, the FISP MRI protocol was prepended with a CEST saturation sequence that used an “ultrafast” approach [27]. This approach applied a B0 magnetic field gradient of +5 ppm to −5 ppm across the long axis of a sample, while also applying a WALTZ16* pulse train at the MR frequency of the water resonance without the B0 gradient. A region of the sample at 0 ppm B0 gradient experienced a WALTZ-16* pulse train that was on-resonance with the MR frequency of water, while the water resonance in other regions of the sample at non-zero B0 gradient experienced off-resonance WALTZ-16* effects. These on-resonance and off-resonance WALTZ-16* effects on water signal were then spatially mapped by the FISP MRI acquisition protocol. This approach is considered to be “ultrafast” because all on-resonance and off-resonance effects can be obtained with a single WALTZ16* pulse train, rather than iterating the WALTZ16* resonance frequency relative to a sample with a potentially inhomogeneous water MR frequency. To generate a QUESPOWR MRI acquisition, the WALTZ16* pulse power was scaled from 0.02 μT to 26 μT in 0.1 dB increments for a total of 601 RF powers. The large amount of data ensured that “wiggles” in the QUESPOWR plot were well resolved. These wiggles may be attributed to the effects of T1 relaxation during the WALTZ16* pulse train, as described below. The pulse time for phantoms and in vivo measurements were selected based on experimental conditions that optimized the Contrast-to-Noise Ratio of the images. These timings differed from timings used for other OPARACHEE MRI studies that used paramagnetic agents [12,14]. This result suggests that the optimal WALTZ16* timings may be different for studies with paramagnetic and diamagnetic agents.
CEST spectra of the chemical solutions were also acquired using the ultrafast CEST approach. The same B0 magnetic field gradient from +15 to −15 ppm was applied, while also applying a 3 μT continuous-wave saturation pulse for 5 s at the MR frequency corresponding to the water resonance without the B0 gradient. The B0 gradient caused a region of the sample to have the MR frequency of the agent at the same frequency as the saturation pulse, generating CEST at that sample region. The region of the sample at 0 ppm B0 gradient had a water MR frequency at the same frequency as the saturation pulse, generating direct saturation at that sample region. Other regions of the sample experienced off-resonance effects from the saturation pulse. The combination of these effects created a CEST spectrum with only a single saturation pulse, which was “ultrafast” relative to CEST MRI methods that iterate the frequency of the saturation pulse relative to a sample with a homogenous water MR frequency.
In vivo MRI studies were performed by prepending the FISP MRI acquisition protocol with a WALTZ16* pulse train. Prior to this acquisition protocol, a Fastmap shimming routine was used to optimize the B0 magnetic field homogeneity in the tumor region to ensure that the WALTZ16* pulse train was on resonance with the water in the tumor tissue [28]. Motion was suppressed by respiration gating prior to the WALTZ16* pulse. The QUESPOWR MRI acquisition used WALTZ16* powers that scaled from 0.3 μT to 25 μT in 0.5 dB increments for a total of 81 WALTZ16* powers. The WALTZ16* pulse train consisted of a series of 2 ms pulses for a total train of 192 ms.
3.5. MRI analysis methods
To analyze the results of the ultrafast QUESPOWR MRI acquisition protocol applied to chemical solutions, the amplitude of the water signal was measured along the long axis of each chemical sample. The region along the long axis with the greatest water signal suppression was assumed to be on-resonance with the WALTZ16* pulse train. The water signal with non-zero WALTZ16* pulse power was normalized to the water signal with zero WALTZ16* pulse power, and the resulting % water signal was plotted as a function of RF pulse power. These results were then renormalized using the % water signal with a 90° WALTZ16* pulse for the LP-HW-QUESP, while the signal at the highest power was used to normalize the HP-HW-QUESP method (Eq. (17)). The higher of these two signals was used to renormalize the LP-HP-HW-QUESP method. The renormalized % water signal was then inverted to create a QUESPOWR plot. In some cases, the QUESPOWR plot was median-filtered with a 0.5 dB window to reduce noise and wiggles in the QUESPOWR plots. These QUESPOWR plots were analyzed using variations of the HW-QUESP fitting method. The R2 correlation coefficient was determined for linear and non-linear fittings, and the 95% confidence interval was determined for non-linear fittings, using MATLAB (Mathworks, Natick, MA).
The shapes of the QUESPOWR MRI acquisition results were analyzed to assess relative differences in chemical exchange rates. The % water signal with the minimum RF power that was within 1% of the minimum water signal was identified for this data shape analysis.
The Bloch-McConnell equations were then used to simultaneously fit all CEST spectra to estimate the pH-noncatalyzed and base-catalyzed exchange rates of iopamidol [17,22]. A four-pool model was used for this fitting, to account for the CEST effects of the two inequivalent amide protons and the hydroxyl protons of the agent, and the water pool. The uncatalyzed k0 and base catalyzed kb values were used to estimate the chemical exchange rate constant of any sample at any pH value (Eq. (25)).
| (25) |
The in vivo QUESPOWR MR images were analyzed on a pixelwise basis. As shown in Eq. (10), the analysis of chemical exchange rates is independent of concentration (χ) and T1 relaxation (T1w), so that the analysis did not require additional MR images to account for concentration or T1w effects. Each water signal measured with non-zero RF power was normalized to the water signal with zero RF power. Because only the HP-HW-QUESP analysis method was used in vivo, the % water signal at the highest RF power (25 μT) was used to normalize in vivo results (Eq. (17)). The renormalized % water signal was then inverted to create a QUESPOWR plot, which was median-filtered to reduce noise and wiggles. The QUESPOWR plots were analyzed using the HP-HW-QUESP fitting method to measure the chemical exchange rate at each pixel. Linear variational Bayesian inference with an uninformative prior was used to fit the in vivo imaging results using the Variational Bayesian Linear Model (vblm) package for MATLAB v1.01 as provided by K. H. Brodersen [29].
4. Results
4.1. Simulations of QUESPOWR MRI
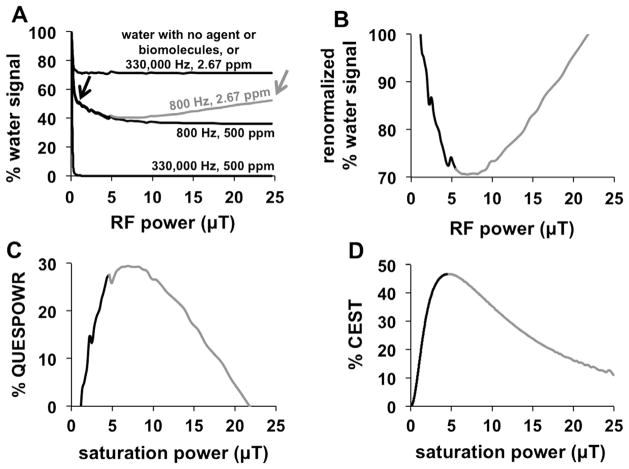
Simulations of a QUESPOWR MRI acquisition (i.e., a series of OPARACHEE MRI acquisitions with varying RF powers) were performed in the presence of labile agents or biomolecules that have large or small chemical shifts of 500 and 2.67 ppm (150,000 and 800 Hz at 7 T), and fast or slow chemical exchange rates of 330,000 or 800 Hz (Fig. 3A). The higher values matched the chemical shifts and exchange rates of a Tm-DOTAM exogenous contrast agent that was previously used to test OPARACHEE MRI [12]. The lower values were estimates of the average chemical shifts and exchange rates of amide, amine, and hydroxyl groups of endogenous biomolecules [30].
Fig. 3.
Simulations of the QUESPOWR method. (A) A QUESPOWR MRI acquisition (a series of OPARACHEE MRI acquisitions with a range of RF powers) was simulated in the presence of agents with different chemical exchange rates and chemical shifts. A single water pool was also simulated, and resulted in an identical QUESPOWR MRI plot as the 330,000 Hz, 2.67 ppm plot. A black arrow is placed at the data point which is used to normalize for the low power LP-HWQUESP method. A gray arrow is placed at the highest power acquired, which is used to normalize for the HP-HW-QUESP method. (B) The % water signal in the presence of an agent with an 800 Hz exchange rate and 2.67 ppm chemical shift was renormalized using the % water signal at 1.17 μT saturation power, (C) which was then inverted to create a % QUESPOWR plot. (D) CEST MRI acquisitions were simulated using an agent with an 800 Hz exchange rate and 2.67 ppm chemical shift, and the % CEST as a function of saturation power was used to create a QUESP plot. The low-power and high-power regions of the QUESPOWR and QUESP plots are shown in black and gray, respectively.
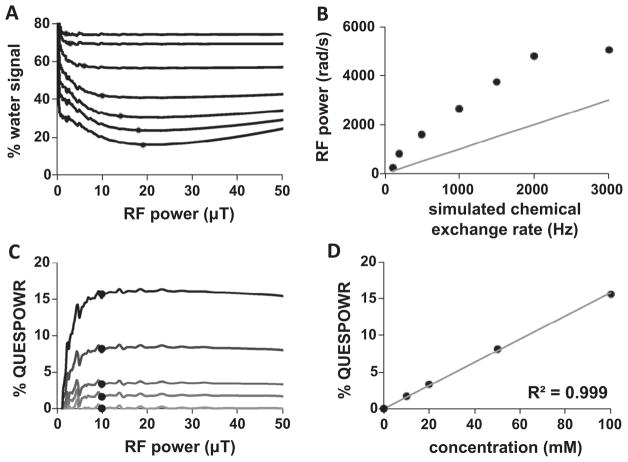
An agent with a fast chemical exchange rate and large chemical shift (330,000 Hz and 500 ppm) caused the simulated water signal to rapidly decrease even when using a low RF power, and have no change in signal as power was further increased. The greatest suppression of water signal was achieved when the RF power caused at least a 90° rotation of the water equilibrium magnetization with each component of the WALTZ16* pulse, which occurred at 1.17 μT for our simulations. No further change in water signal was observed as the RF power was further increased.
An agent with fast chemical exchange and small chemical shift (330,000 Hz and 2.67 ppm) also created a simulated water signal with a rapid decrease when using a low RF power, and with no change in signal as the power was further increased. However, a similar simulation with no agent also produced the same result. This decrease in water signal without agent was attributed to T1 relaxation during the WALTZ16* pulse train. Therefore, OPARACHEE MRI is insensitive to the effects of an agent with fast chemical exchange rate and a small chemical shift.
An agent or biomolecule with a slow chemical exchange rate (800 Hz) caused the simulated water signal to experience an initial rapid signal decrease for 0–1.17 μT RF power, followed by a slow decrease in water signal as the RF power was incremented to intermediate values. High RF powers caused the simulated water signal to increase in the presence of a biomolecule with a small chemical shift (2.67 ppm; shown in gray in Fig. 3A), while an agent with a large chemical shift (500 ppm) maintained the same water signal suppression. A second simulation with T2w and T2b set to 10 ms instead of 333 ms produced identical results (data not shown), which validates that QUESPOWR is not a T2-weighted MR imaging method. Extrapolation of this simulation to infinite RF power caused the water signal to match the level of the water signal without an agent or biomolecule. Overall, these results showed that a QUESPOWR MRI acquisition can generate a decrease in water signal with an agent or biomolecule that has a slow chemical exchange rate.
Importantly, the water signal in the presence of a biomolecule with a slow exchange rate and small chemical shift (800 Hz, 2.67 ppm) shows biphasic behavior with respect to RF power. We generated a QUESPOWR plot by first normalizing to the water signal with RF power that caused a 90° inversion of the water resonance (Fig. 3B), and then inverting this normalized signal (Fig. 3C). Alternatively, a similar plot can be generated by normalizing relative to the signal with the maximum RF power. Arrows are shown in Fig. 3A to highlight points in the plot where signal is constant with only water present. This processing is similar to the process of converting normalized water signals to % CEST measurements (Eq. (4)). This plot is analogous to a QUESP plot for the same biomolecule, which was generated by simulating the effects of directly saturating the biomolecule at different saturation powers (Fig. 3D). Because the biomolecule is lower in concentration than water, the RF spillover onto the biomolecule’s resonance is relatively low when applying RF power on the water resonance during QUESPOWR MRI, relative to the RF spillover onto the water resonance when saturating the biomolecule during QUESP MRI. Consequently, the QUESPOWR plot shows a decrease in % contrast starting at higher RF powers (approximately 7 μT in our simulations) relative to the RF power that causes a decrease in % CEST in the QUESP plot (4.7 μT in our simulations).
The QUESPOWR MRI acquisitions (Fig. 3A) and the QUESPOWR plots (Fig. 3C) show wiggles, especially for simulations with low RF powers, slower exchange rates, and smaller chemical shifts. Simulations were performed with T2w and T2b set to 10 ms that showed identical wiggles (data not shown), suggesting that T2 relaxation is not the source of these wiggles. Therefore, we attribute these wiggles to the effects of T1 relaxation during the WALTZ16* pulse train, or the effects of different flip angles for each WALTZ16* pulse train applied at different powers, or both of these effects. Notably, similar wiggles were observed during a previous evaluation of OPARACHEE MRI that used a 220 ms WALTZ-16* pulse train, which was also significantly long relative to the T1 relaxation time constant of the sample [12].
4.2. QUESP analyses of simulated QUESP plots
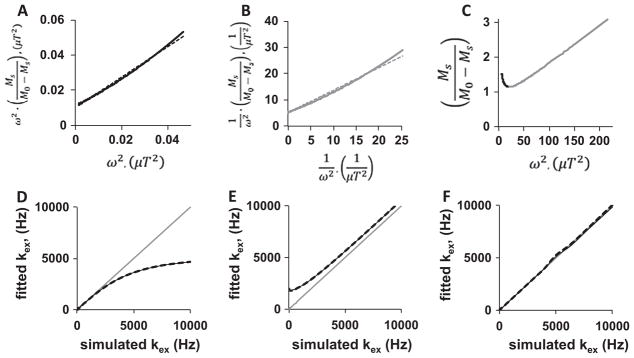
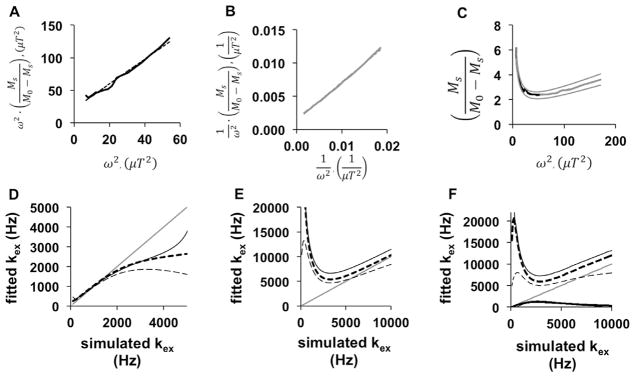
Before analyzing the QUESPOWR plot (Fig. 3C), we assessed methods for analyzing the QUESP plot (Fig. 3D) to investigate the validity of these analysis methods. The portion of the QUESP plot that shows increasing % CEST with increasing saturation power was analyzed using the linear LP-HW-QUESP method, which showed excellent linear fitting (R2 = 0.992; Fig. 4A). We used the HP-HW-QUESP method (Eq. (15)) to fit the portion of the QUESP plot at high saturation power (Fig. 4B), which also showed excellent linear fitting (R2 = 0.995). Finally, the combination of both HW-QUESP methods, termed LP-HP-HW-QUESP (Eq. (5)), was fit to the entire range of the QUESP plot (Fig. 4C). This process resulted in excellent fitting to the QUESP plot as demonstrated by the 95% confidence interval error bars that cannot be discerned from the fit line and simulated data, and a R2 value of 1.000.
Fig. 4.
HW-QUESP analysis of a QUESP plot. (A) A LP-HW-QUESP fitting method (dashed line) was used to evaluate the low-power region of the QUESP plot from Fig. 1D (black solid line). (B) A HP-HW-QUESP fitting method (dashed line) was used to evaluate the high-power region of the QUESP plot (gray solid line). (C) The LP-HP-HW-QUESP fitting method was used to evaluate the low-power (black) and high-power (gray) regions of the QUESP plot. The fitted line and the 95% confidence intervals of the fitting overlap with the simulated results. (D) A comparison of the fitted chemical exchange rate (kex) using the LP-HW-QUESP method, relative to the initially simulated chemical exchange rate, shows that LP-HW-QUESP can accurately estimate slow exchange rates, but cannot accurately estimate fast exchange rates. (E) A similar comparison shows that HP-HW-QUESP can estimate fast exchange rates, but cannot accurately estimate slow exchange rates. (F) A similar comparison shows that LP-HP-HW-QUESP can estimate both low and high chemical exchange rates. Figures D–F show results from simulations with 10 mM of agent (thin black line) and 100 mM of agent (thick dashed line), and these graphs also include a thin gray line with slope = 1 to visualize the differences between initially simulated and fitted chemical exchange rates.
The QUESP fitting method and variations of QUESP are intended to estimate the average chemical exchange rate of an agent. The LP-HW-QUESP fitting method was used to estimate the average chemical exchange rate from the simulated QUESP MRI acquisition results that spanned chemical exchange rates between 0 and 10,000 Hz and with an agent that had a chemical shift of 2.67 ppm (Fig. 4D). These results showed that LP-HW-QUESP accurately estimated slow chemical exchange rates, but underestimated fast chemical exchange rates, which matched previously reported results [17,22]. This underestimation becomes substantial when the exchange rate exceeds 2π times the chemical shift, or at an exchange rate of 5000 Hz for our simulations. These fast chemical exchange rates relative to the chemical shift cause the MR resonance of the agent to coalesce with the MR resonance of water, so that accurate estimates of these fast chemical exchange rate fits cannot be obtained.
We used the HP-HW-QUESP fitting method to estimate the chemical exchange rate from the same simulated results (Fig. 4E). HP-HW-QUESP estimated fast chemical exchange rates with a minor overestimation, but showed a greater overestimation of slow chemical exchange rates. Thus, this method provides more accurate estimates of chemical exchange rates when the MR resonance of the agent coalesces with the MR resonance of water. The combined LP-HP-HW-QUESP fitting method provided the most accurate estimate of all chemical exchange rates (Fig. 4F). This combined method only showed a small error in estimations when the exchange rate causes the agent-water system to transition from a resolved MR resonance to a coalescing resonance for the agent (5000 Hz for our simulations). Therefore, the LP-HP-HW-QUESP method extends the HW-QUESP method to accurately measure all chemical exchange rates when experimental noise is negligible.
4.3. QUESP analyses of simulated QUESPOWR plots
After establishing that LP-, HP-, and LP-HP-HW-QUESP methods (Fig. 4) can analyze a QUESP plot (Fig. 3D), we sought to establish that these methods can also analyze a QUESPOWR plot (Fig. 3C). Using a QUESPOWR plot that was renormalized with the % water signal at 1.17 μT RF power, the LP-HW-QUESP method showed excellent linear fitting to the QUESPOWR plot in a low range of RF powers between 0 and 7.5 μT (R2 = 0.983; Fig. 5A). Alternatively, using the version of the QUESPOWR plot that was renormalized using the % water signal with maximum RF power, the HP-HW-QUESP method fit the portion of the QUESP plot at high saturation power with excellent linear fitting (R2 = 0.998; Fig. 5B). Based on the excellent fitting of both low-power and high-power regions of the QUESPOWR plot, the LP-HP-HW-QUESP method was also expected to fit the entire range of simulated RF powers used to create the QUESPOWR plot (Fig. 5C). The results showed an outstanding fit as expected, with excellent 95% confidence intervals and R2 = 0.981.
Fig. 5.
HW-QUESP analysis of a QUESPOWR plot. (A) A LP-HW-QUESP fitting method (dashed line) was used to evaluate the low-power region of the QUESPOWR plot from Fig. 3C (black solid line). (B) A HP-HW-QUESP fitting method (dashed line) was used to evaluate the high-power region of the QUESPOWR plot (gray solid line). (C) The LP-HP-HW-QUESP fitting method was used to evaluate the low-power (black) and high-power (gray) regions of the QUESPOWR plot. The fitted line overlaps with the simulated results, and the 95% confidence intervals of the fitting are shown as smooth gray lines. (D) A comparison of the fitted chemical exchange rate (kex) using the LP-HW-QUESP method, relative to the initially simulated chemical exchange rate, shows that LP-HW-QUESP can accurately estimate slow exchange rates, but cannot accurately estimate fast exchange rates. (E) A similar comparison shows that HP-HW-QUESP can accurately estimate fast exchange rates, but cannot accurately estimate slow exchange rates. (F) A similar comparison shows that LP-HP-HW-QUESP has a similar performance as the HP-HW-QUESP method. Figures D–F show results from simulations with 10 mM of agent (thin black line), 100 mM of agent (thick dashed line), and 100 mM of agent in the presence of a second agent with a chemical exchange rate of 47 Hz and 1 M concentration (thin dashed line). These graphs also include a thin gray line with slope = 1 to visualize the differences between initially simulated and fitted chemical exchange rates.
We then evaluated the abilities of these methods to accurately estimate slow and fast chemical exchange rates from a QUESPOWR plot. The LP-HW-QUESP method provided excellent estimates of slow exchange rates, regardless of concentration of the agent (Fig. 5D). This analysis method underestimated fast chemical exchange rates, which was also observed when this method was used to analyze the QUESP plot. We also simulated the estimate of chemical exchange rates in the presence of a second endogenous biomolecule with a slow chemical exchange rate of 47 Hz and a high 1 M concentration. This additional biomolecule did not affect the estimate of the chemical exchange rate for the first biomolecule, which suggested that the LP-HW-QUESP analysis of QUESPOWR MRI is weighted toward fast exchanging protons of the biomolecules.
The HP-HW-QUESP method showed a minor overestimation of the exchange rates, with more overestimation for slow exchange rates that create a more resolved MR resonance for the agent. This overestimation was greater for a more dilute solution (~15% overestimation at 10 mM) relative to a more concentrated solution (~4% overestimation at 100 mM; Fig. 5E). Furthermore, the addition of a second endogenous biomolecule with 47 Hz exchange rate and a high 1 M concentration reduced the estimation of the exchange rate of the first biomolecule, showing that HP-HW-QUESP has less weighting toward fast-exchanging species than LP-HW-QUESP.
The combined LP-HP-HW-QUESP method also overestimated the chemical exchange rates, especially for slow exchange rates (Fig. 5F). Therefore, this combined method is strongly weighted toward the performance of the HP-HW-QUESP method. In addition, extracting the exchange rate using the LP-HP-HW-QUESP requires solving a quadratic formula that has two possible solutions (the second solutions are shown as overlapping black lines in Fig. 5F), confounding the estimation of the single average exchange rate from the biomolecule. Therefore, the more simplistic HP-HW-QUESP analysis method is preferred relative to the combined LP-HP-HW-QUESP method.
4.4. QUESPOWR shape analyses of simulated results
The shape of the RF power dependence of the % water signal from the simulated QUESPOWR MRI acquisition protocol was used to estimate differences in chemical exchange rates from the simulated results. We refer to this analysis method as a QUESPOWR acquisition shape analysis. All % water signals were identified that were within 1% of the minimum water signal, and the % water signal at the lowest RF signal was then identified from this group (indicated by circles in Fig. 6A). This minimum RF power was correlated with the simulated chemical exchange rate (Fig. 6B). However, as shown in Eq. (18), this minimum RF power for minimum % water signal was also dependent on the chemical shift of the agent, so that an accurate estimate of the exchange rate can only be made if the chemical shift of the agent is known. A QUESPOWR MRI acquisition cannot measure the chemical shift of the biomolecule or biomolecules that are exchanging protons with water, so that an accurate estimate of exchange rate could not be made from these simulated results. Therefore, the QUESPOWR acquisition shape analysis should only be used to qualitatively estimate relative differences in chemical exchange rates between different tissues with endogenous biomolecules that have the same average chemical shift, rather than be used to estimate absolute values of chemical exchange rates.
Fig. 6.
Analysis methods that depend of the shape of simulated QUESPOWR MRI results. QUESPOWR acquisition shape analysis: (A) The lowest RF power was identified that generated % water signal within 1% of the minimum water signal (circles) in simulated QUESPOWR MRI acquisitions with chemical exchange rates of 100–3000 Hz. (B) This minimum RF power was correlated with the simulated chemical exchange rates, although the absolute measurement of chemical exchange rates was inaccurate. QUESPOWR plot shape analysis: (C) The % QUESPOWR generated with 10 μT RF power (circles) was identified for simulated QUESPOWR plots for samples with 0–100 mM concentrations. (D) This % QUESPOWR is correlated with the simulated concentrations. Figure B includes a thin gray line with a slope of 1 to visualize the differences between initially simulated and fitted chemical exchange rates.
The shape of the QUESPOWR plot provided a similar method for estimating the concentration of exchangeable protons (Fig. 6C). We refer to this analysis method as a QUESPOWR plot shape analysis. The QUESPOWR plots show that a maximum % QUESPOWR contrast was generated at a moderate RF power (10 μT in our simulation, as indicated by circles in Fig. 6C), despite the presence of wiggles in this plot. This maximum % QUESPOWR contrast scaled linearly with concentration (Fig. 6D), which may provide an outstanding method for estimating relative differences in concentrations of exchangeable protons between different tissues.
4.5. QUESP analyses of QUESPOWR plots from experiments with chemical solutions
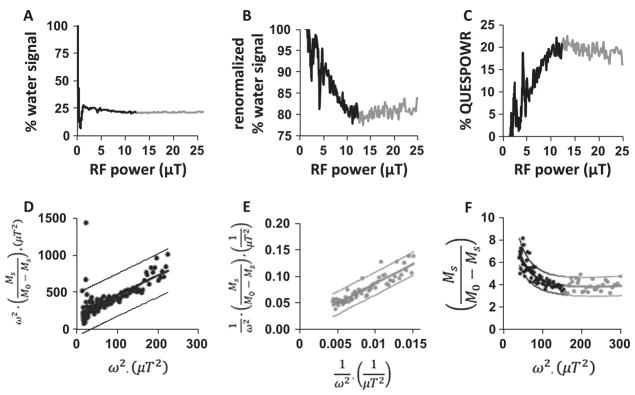
To confirm our simulated results, we performed an experimental study with chemical solutions of iopamidol at pH values ranging from 6.21 to 7.26. The sample with iopamidol at pH 6.84 showed a rapid decrease in water signal at low RF power, and a slower decrease in water signal at intermediate RF power, followed by a slow increase in water signal at high RF power (Fig. 7A). The experimental results also showed a “dip” in % water signal in the transition from low to intermediate RF power. Except for the sharp signal dip, this result matched the simulation of a % water signal in the presence of an agent with a slow chemical exchange rate and small chemical shift (Fig. 3A). These results were normalized using the % water signal obtained with 1.17 μT RF power (Fig. 7B), and then inverted to create a QUESPOWR plot (Fig. 7C). The LP-HW-QUESP method showed excellent linear fitting to the QUESPOWR plot with RF power between 7 μT and 12 μT (Fig. 7D). Similar results were obtained with samples at other pH values.
Fig. 7.
QUESPOWR analyses of chemical solutions. The low-power and high-power regions of the QUESPOWR plots are shown in black and gray, respectively. (A) A QUESPOWR MRI acquisition was obtained with a sample of 79 mM iopamidol at pH 6.84. (B) The % water signal was renormalized using the % water signal at 1.17 μT saturation power, removing the OPARACHEE contrast, (C) which was then inverted to create a % QUESPOWR plot. (D) The LP-HW-QUESP fitting method was used to evaluate the low-power region of the QUESPOWR plot from Fig. 5C. (E) A HP-HW-QUESP fitting method was used to evaluate the high-power region of the QUESPOWR plot. (F) The LP-HP-HW-QUESP fitting method was used to evaluate the low-power and high-power regions of the QUESPOWR plot for a sample at pH 6.43. For panels D–F, the fittings are shown as thick line and the 95% confidence intervals of the fitting are shown as thin lines. 60 data points are used for the HP-HW-QUESP, 60 data points are used for the LP-HW-QUESP plot, and 120 points are used for the LP-HP-HW-QUESP method. As expected, approximately 5% of the data points fell outside of the 95% confidence intervals.
The sample with iopamidol at pH 6.43 was used to test the HP-HW-QUESP fitting method, which showed excellent linear fitting to the QUESPOWR plot with RF power between 12 μT and 25 μT (Fig. 7E). This QUESPOWR plot was renormalized using the percent water signal obtained with maximum RF power. Samples at pH 6.21 and 6.56 showed similar results. However, samples at higher pH values showed an increase in water signal at high RF power that was too shallow to be reliably fit with HP-HW-QUESP in the presence of noise. These experimental results were similar to simulated results, because samples at higher pH have faster chemical exchange rates, and Fig. 3A shows that faster exchange rates generate QUESPOWR water signals that have little or no dependence on RF power when RF power is very high.
The LP-HP-HW-QUESP method also showed good fitting to the QUESPOWR plot with RF power between 7 μT and 25 μT for the sample at pH 6.84 (Fig. 7F). Similar results were obtained for samples at other pH values. The good fitting with LP-HP-HW-QUESP was not unexpected because this method has more parameters for fitting, which inherently causes good fits without necessarily providing accurate results.
These results were used to estimate the average chemical exchange rate of each sample (Table 1). In addition, the Bloch-McConnell equations were fit to experimental CEST spectra of the same samples to estimate the average chemical exchange rate of the fastest exchanging amide at 5.6 ppm. This fastest exchanging amide proton was selected as a ‘gold standard’ measurement because our simulations in Fig. 4D showed that the estimated exchange rate is biased toward biomolecules with the fastest exchange rate. The estimates with LP-HW-QUESP showed good agreement with the estimates from the Bloch-McConnell equations. HP-HW-QUESP showed lower estimated exchange rates at low pH values than the estimates from the Bloch-McConnell equations. This result indicates that the HP-HW-QUESP is more sensitive to measuring the exchange rate of the hydroxyl groups when the exchange rate of the amide proton is comparable to the exchange rate of the hydroxyl groups. This result agreed with simulations in Fig. 4E, which showed that HP-HW-QUESP is more effective at estimating exchange rates when the chemical shift is less than the exchange rate. Estimates with the LP-HP-HW-QUESP method showed a relatively poor correlation with estimates from the Bloch-McConnell equations, and the estimates from LP-HP-HW-QUESP had large 95% confidence intervals. Therefore LP-HP-HW-QUESP generated the most inaccurate estimates of chemical exchange rates. These results and Eq. (25) were used to determine the values of the pH-independent chemical exchange rate, k0, is 1110 Hz, and the base-catalyzed chemical exchange rate, kb, is 7.22 × 109 Hz, setting pKw to 13.6.
Table 1.
The effect of pH on the analyses of experimental QUESPOWR plots.
| pH | Chemical exchange rate (Hz) of a sample of iopamidol at 79 mM
|
||||
|---|---|---|---|---|---|
| LP-HW-QUESPa | HP-HW-QUESPa | LP-HP-HW-QUESPa | QUESPOWR acquisition shapeb | Bloch fittinga | |
| 6.21 | 1235 (1170,1272) | 753 (0,1331) | 0 | 1068 | 1397 (1167,1628) |
| 6.43 | 1121 (1014,1182) | 729 (0,1578) | 1350 (768,1932) | 1401 | 1566 (1311,1821) |
| 6.56 | 1843 (1737,2054) | 2102 (0,2950) | 2748 (2353,3142) | 2136 | 1832 (1539,2125) |
| 6.84 | 1925 (1878,1965) | 0 | 2862 (2261,3463) | 2937 | 2254 (1901,2608) |
| 6.95 | 2446 (2443,2448) | 0 | 2814 (2167,3461) | 3338 | 2923 (2474,3373) |
| 7.26 | 3619 (3412,3950) | 0 | 3259 (2104,4414) | 3311 | 3984 (3382,4586) |
The 95% confidence intervals are reported in parentheses.
Determined from a median-filtered QUESPOWR plot.
We also estimated chemical exchange rates of samples at pH 6.84 that had concentrations from 0 to 79 mM (Table 2). The LP-HW-QUESP method estimated exchange rates for samples at all concentrations, although the sample at lowest concentration produced less accurate estimates presumably due to relatively lower contrast-to-noise in QUESPOWR MR images. The HP-HW-QUESP method only estimated exchange rates for samples at high concentrations. This result again confirmed that HP-HW-QUESP cannot fit the shallow RF-dependent increase in water signal with a highly chemically shifted labile proton and typical image noise, unless a high concentration of sample can improve the contrast-to-noise during QUESPOWR MRI. The LP-HP-HW-QUESP method estimated chemical exchange rates at all concentrations, although these estimates were inaccurate. This combined method estimated a chemical exchange rate for a sample with no agent, further confirming the inaccuracy of this method.
Table 2.
The effect of concentration on the analyses of experimental QUESPOWR plots.
| Conc. (mM) | Chemical exchange rate (Hz) of a sample of iopamidol at pH 6.84 | ||||
|---|---|---|---|---|---|
| LP-HW-QUESPa | HP-HW-QUESPa,b | LP-HP-HW-QUESPa | QUESPOWR plot shape | Bloch fittinga | |
| 0 | 0 | 0 | 815 (−1370,3000) | 2540 | 2254 (1901,2608) |
| 16 | 4009 (3483,5936) | 0 | 2627 (2206,3048) | 2621 | 2254 (1901,2608) |
| 32 | 2478 (2476,2481) | 0 | 1705 (1539,1870) | 2942 | 2254 (1901,2608) |
| 47 | 2266 (2225,2296) | 0 | 1830 (1613,2048) | 2942 | 2254 (1901,2608) |
| 63 | 2547 (2533,2568) | 2654 (0,3364) | 3110 (2100,4120) | 2942 | 2254 (1901,2608) |
| 79 | 2566 (2551,2587) | 2050 (0,2985) | 2939 (2225,3654) | 2942 | 2254 (1901,2608) |
The 95% confidence intervals are reported in parentheses.
Determined from a median-filtered QUESPOWR plot.
4.6. QUESPOWR shape analyses from experiments with chemical solutions
We also assessed the QUESPOWR acquisition shape analysis for evaluating differences in chemical exchange rates with samples of iopamidol at pH values ranging from 6.21 to 7.26. This method identified the minimum RF power that created a % water signal within 1% of the minimum water signal (indicated by circles in Fig. 8A).
Fig. 8.
Analysis methods that depend of the shape of experimental QUESPOWR MRI results. QUESPOWR shape analysis: (A) The lowest RF power was identified that generated % water signal within 1% of the minimum water signal (circles) in QUESPOWR MRI acquisitions of chemical solutions of iopromide at different pH values. (B) This minimum RF power was correlated with the chemical exchange rate of the fastest exchanging amide proton of iopromide, but only for samples with slow chemical exchange rates (as highlighted by the bold line and R2 value of 0.993). QUESPOWR plot shape analysis: (C) The % QUESPOWR generated with 10 μT RF power (circles) was identified for QUESPOWR plots of chemical solutions of samples with 16–79 mM concentrations. (D) This % QUESPOWR is correlated with the experimental concentrations.
To improve the determination of this point, we applied a median filter to the results that reduced noise and wiggles in the QUESPOWR plots. This RF power scaled with the chemical exchange rate of the fastest exchanging proton of iopamidol, which was determined from fitting the Bloch-McConnell equations to CEST spectra (Fig. 8B). The fastest exchanging proton of iopamidol was used for this comparison because simulated results (Fig. 5D) showed that the estimated exchange rate is biased toward the species with a fast chemical exchange rate. This comparison shown in Fig. 8B showed an excellent correlation at slower chemical exchange rates, and a relatively poor correlation at faster exchange rates. This result matched the analysis with HP-HW-QUESP (Table 1), which also failed to adequately evaluate samples dominated by labile pools at high chemical shift.
We then used the QUESPOWR plot shape analysis method to evaluate differences in concentrations of iopamidol between 0 and 79 mM. The % QUESPOWR contrast generated at 10 μT RF power (indicated by circles in Fig. 8C) showed an excellent correlation with concentration (Fig. 8D). This experimental result corroborated our simulated results, further reinforcing that the QUESPOWR plot shape analysis method may provide outstanding evaluations of different concentrations of endogenous labile protons in different tissues.
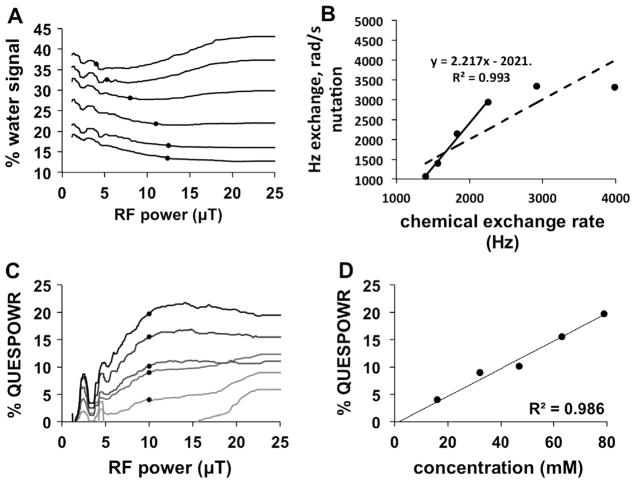
4.7. In vivo QUESPOWR studies
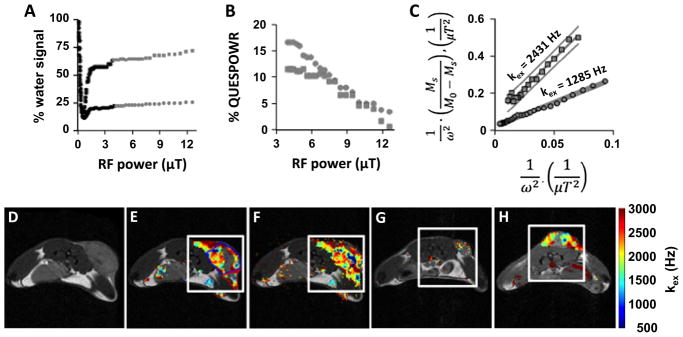
We used QUESPOWR MRI to study a mouse model of MCF7 mammary carcinoma (Fig. 9). A proton-density MR image showed that the subcutaneous tumor region consisted of a round mass with two “wings” that were discernable from normal tissue (Fig. 9D). The QUESPOWR MRI acquisitions showed that the water signal was dependent on RF power for regions-of-interest (ROIs) of the round tumor and one tumor wing (Fig. 9A). However, these results did not show a slow decrease in water signal at intermediate RF powers, and only showed a rapid decrease and “dip” in water signal at low RF power followed by an increase in water signal at high RF power. We were not able to identify the water signal at a low RF power that is used to renormalize the % water signal. Therefore, we used the HP-HW-QUESP method, since this method uses the water signal at highest RF power to renormalize the % water signal. We then created a QUESPOWR plot for both ROIs (Fig. 9B), and analyzed these results with the HP-HW-QUESP method (Fig. 9C). We then performed a similar analysis for each pixel that represented tumor tissue and normal tissues in the image, which resulted in a parametric map of chemical exchange rates (Fig. 9E).
Fig. 9.
QUESPOWR analysis of an in vivo tumor model. The low-power and high-power regions of the QUESPOWR and QUESP plots are shown in black and gray, respectively. (A) A QUESPOWR MRI acquisition was obtained with a subcutaneous tumor model of MCF7 mammary carcinoma. The % water signal was measured for a region-of-interest of the center (circles) and a “wing” (squares) of the tumor. (B) The % water signals for both regions were renormalized using the % water signal at 12.57 μT saturation power, and then inverted to create a % QUESPOWR plot. (C) The HP-HW-QUESP fitting method was used to evaluate the high-power region of the QUESP plot for both tumor regions. (D) A T2-weighted MR image shows the location of the tumor center and “wing” regions. (E) A parametric map of chemical exchange rates estimated from QUESPOWR analysis (panels A–C applied on a pixelwise basis) is overlayed on the anatomical image. A Bayesian inference method was used to avoid overfitting HP-HW-QUESP to the experimental results. (F) The same parametric map of chemical exchange rates was estimated using a least squares fitting method, which overfit HP-HW-QUESP for some pixels, creating spurious results. (G and H) The QUESPOWR analysis method with Bayesian inference was applied to study two additional mice with subcutaneous MCF7 tumors. The rectangle around each tumor indicates the area used for Fastmap shimming.
We used a Bayesian inference method to identify pixels with QUESPOWR plots that could be analyzed with the HP-HW-QUESP method with statistical significance. This method expunged fitted pixels with a poor variational free energy (Fig. 9E). This method avoided overfitting into noise by fitting the noise as well as the parameters of interest. For comparison, a linear least-squares fitting procedure was also used to fit the HP-HW-QUESP method to pixel-wise QUESPOWR plots, expunging pixels with 95% confidence intervals greater than 20% of the fitted kex (Fig. 9F). The linear least squares method clearly produced more spurious results, as evidenced by the fitting of some pixels in imaging regions that represented air. As further evidence of pruning efficiency, the percent of pixels remaining after pruning, not including pixels located over empty space, was 20.93% for the Bayesian inference method (Fig. 9E) and 25.57% for the least-squares fitting method (Fig. 9F). The Bayesian inference method was repeated to analyze two additional mice with subcutaneous MCF7 tumors (Fig. 9G and H), which showed that this procedure was robust. Perhaps most importantly, the great majority of pixels in the final parametric maps of chemical exchange rates were located in the round tumor and tumor wings. The procedure failed to fit QUESPOWR results from almost all pixels in normal tissues.
5. Discussion
This study established that the QUESPOWR MRI acquisition method, the QUESPOWR plot, and linear QUESP analysis methods can estimate chemical exchange rates. In particular, QUESPOWR MRI with HP-HW-QUESP analysis can evaluate biomolecules with small chemical shifts and intermediate-to-fast chemical exchange rates that cause MR coalescence. These types of biomolecules have been especially difficult to evaluate with CEST MRI methods that apply RF saturation off-resonance from the water frequency. These other methods struggle to selectively saturate the off-resonance without also generating saturation “spill over” onto the water resonance. Several clever analysis methods have been developed to account for this “spill over” [18–20]. QUESPOWR MRI takes the opposite approach by applying RF pulses on the water resonance, and employing “reverse spill-over” to interrogate the biomolecules. Therefore, QUESPOWR MRI is a new paradigm for molecular imaging.
QUESPOWR MRI results can be analyzed using several linear fitting methods that evaluate the low-power, high-power, or low & high-power ranges of a QUESPOWR plot. These methods are analogous to the linear fitting methods that can analyze QUESP MRI results. The LP-HW-QUESP fitting method can accurately estimate exchange rates that are slower than the chemical shift difference with water. However, the in vivo study showed a limited dynamic range of the low-power region of a QUESPOWR plot, and this region of the QUESPOWR plot can suffer from wiggles, so that LP-HW-QUESP could not be used to analyze in vivo results. The LP-HP-HW-QUESP method showed poor precision for estimating exchange rates due to the need to fit many parameters when analyzing experimental results that include noise. Therefore, the HP-HW-QUESP is recommended for QUESPOWR plot analyses. This method applies to the range of the QUESPOWR plot that has less noise, which improves the precision when estimating chemical exchange rates. More importantly, HP-HW-QUESP can estimate exchange rates that are faster than the chemical shift difference, which was critical for evaluating the results of in vivo studies with QUESPOWR MRI.
QUESPOWR MRI was able to generate a parametric map of chemical exchange rates for tumor tissue, but was unable to assess normal tissues. We attribute this difference to tumor acidity relative to the neutral extracellular pH (pHe) of normal tissues [31]. The HP-HW-QUESP analysis method is sensitive to biomolecules with fast chemical exchange rates and small chemical shift differences with water. Normal tissues with neutral pHe have fast exchange rates from amide and amine groups at ~2.5–3.5 ppm relative to the water resonance, and also have fast exchange rates from hydroxyl groups at ~0.8–2.0 ppm. The fast exchange from the amide and amine groups at higher chemical shifts reduces the sensitivity of QUESPOWR MRI for analyzing these tissues. Conversely, tumor tissues with acidic pHe have slow exchange rates from amide and amine groups because their chemical exchange with water is base-catalyzed [30]. This slower exchange from these groups at larger chemical shifts allows QUESPOWR MRI to be weighted toward the hydroxyl groups at smaller chemical shifts, providing greater detection sensitivity. In effect, the amides and amines at larger chemical shift “mask” the sensitivity of QUESPOWR MRI for hydroxyl groups at lower chemical shift, and this “masking” is reduced at lower pHe.
This explanation is substantiated by our experimental studies with iopamidol, which has amide and amine groups at very high 4.2 and 5.6-ppm chemical shifts, while also including hydroxyl groups at 0.7–1.2 ppm. The HP-HW-QUESP method could only generate accurate estimates of chemical exchange rates of iopamidol at low pH. Also, these estimates from samples at low pH were lower than the estimated chemical exchange rate for the amide at 5.6 ppm, further substantiating that QUESPOWR MRI with HP-HW-QUESP analysis is weighted toward exchanging species at lower chemical shifts. Together, the experimental results with iopamidol and the in vivo study provide a rationale that QUESPOWR MRI can detect acidic tumors relative to normal tissues, which has potentially profound implications for cancer diagnoses.
The in vivo study with QUESPOWR MRI required several processing steps to reduce experimental noise. Gaussian filtering was applied to spatially smooth the noise, and the pixel-wise QUESPOWR plots were median-filtered to further reduce noise and wiggles. Despite these improvements, the HP-HW-QUESP analyses of pixel-wise QUESPOWR plots showed many spurious results when standard least square linear fitting methods were used, due to overfitting the noisy experimental results. Using a variational Bayes inference method avoided this overfitting, and provided parametric maps of chemical exchange rates without spurious effects. Therefore, the variational Bayes inference fitting method contributes to the methodology of analyzing imaging results that are inherently noisy, and may benefit other CEST MRI or other MRI analysis methods.
QUESPOWR MRI with HP-HW-QUESP linear fitting has some disadvantages. The simulations and in vitro results showed that this fitting method consistently overestimated the average chemical exchange rate by approximately 4–15%. Also, the exchange rate estimates showed a minor yet significant dependence on the concentration of the exchanging protons of the biomolecules. These errors in precision should be taken into account for each biomedical application of the QUESPOWR method. For example, if the estimated exchange rate is to be followed longitudinally, then this systematic error is unimportant because all changes will be measured relative to baseline. As another example, if the absolute quantification of exchange rate is needed with less than 15% error to absolutely quantify tissue pH, then the QUESPOWR method is not appropriate.
B0 inhomogeneity can compromise the application of the on-resonance WALTZ16* pulse train. We used ultrafast CEST MRI methods to study chemical solutions and Fastmap shimming to mitigate B0 inhomogeneities in vivo. Yet additional methods to reduce B0 inhomogeneities should be investigated in future studies. The most important disadvantage of this technique is the use of WALTZ16* pulse trains that have high RF power, which is acceptable for studies with mouse models but is clearly unacceptable for clinical translation. Therefore, future studies are warranted to develop QUESPOWR MRI with lower RF power applied at the water resonance, which will improve clinical translation of this method.
6. Conclusions
The QUESPOWR MRI acquisition method, the QUESPOWR plot, and linear QUESP analysis methods can measure chemical exchange rates to provide potentially important diagnostic information. A methodology was also developed that uses the shape of the QUESPOWR acquisition and QUESPOWR plot to independently estimate chemical exchange rates and concentrations, respectively. The HP-HW-QUESP analysis method can fit results of QUESPOWR MRI and QUESP MRI at high power. This analysis method permitted the evaluation of chemical exchange between water and biomolecules that have fast exchange rates and small chemical shifts, which has confounded the analyses of other CEST MRI methods. HP-HW-QUESP was shown to fit pixel-wise results from in vivo tumor tissues with high specificity relative to normal tissues, which was attributed to the lower extracellular pH of tumor tissues.
Acknowledgments
The authors thank Ms. Christine Howison for technical assistance during in vivo MRI studies. This work was supported by the Phoenix Friends of the University of Arizona Cancer Center (United States), the Better than Ever Program of the University of Arizona Cancer Center, and the National Institutes of Health (NIH, United States) grants 1R01CA167183 and P30CA023074.
References
- 1.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143(1):79–87. doi: 10.1006/jmre.1999.1956. http://dx.doi.org/10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 2.Liu G, Song X, Chan KW, McMahon MT. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 2013;26(7):810–828. doi: 10.1002/nbm.2899. http://dx.doi.org/10.1002/nbm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt B, Zamecnik P, Zaiss M, Rerich E, Schuster L, Bachert P, Schlemmer HP. A new contrast in MR mammography by means of chemical exchange saturation transfer (CEST) imaging at 3 Tesla: preliminary results. Fortschr Rontg Neuen. 2011;183(11):1030–1036. doi: 10.1055/s-0031-1281764. http://dx.doi.org/10.1055/s-0031-1281764. [DOI] [PubMed] [Google Scholar]
- 4.Jones CK, Schlosser MJ, van Zijl PCM, Pomper MG, Golay X, Zhou J. Amide proton transfer imaging of human brain tumors at 3 T. Magn Reson Med. 2006;56:585–592. doi: 10.1002/mrm.20989. http://dx.doi.org/10.1002/mrm.20989. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu DX, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl PC. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med. 2011;17:130–134. doi: 10.1038/nm.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Payen J, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9:1085–1090. doi: 10.1038/nm907. http://dx.doi.org/10.1038/nm.2268. [DOI] [PubMed] [Google Scholar]
- 7.Cai K, Xu HN, Singh A, Moon L, Haris M, Reddy R, Li LZ. Breast cancer redox heterogeneity detectable with chemical exchange saturation transfer (CEST) MRI. Mol Imag Biol. 2014;16(5):670–679. doi: 10.1007/s11307-014-0739-y. http://dx.doi.org/10.1007/s11307-014-0739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu G, Bettegowda C, Qiao Y, Staedtke V, Chan KWY, Bai R, Li Y, Riggins GJ, Kinzler KW, Bulte JWM, McMahon MT, Gilad AA, Vogelstein B, Zhou S, van Zijl PCM. Noninvasive imaging of infection after treatment with tumor-homing bacteria using chemical exchange saturation transfer (CEST) MRI. Magn Reson Med. 2013;70(6):1690–1698. doi: 10.1002/mrm.24955. http://dx.doi.org/10.1002/mrm.24955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci USA. 2008;105(7):2266–2270. doi: 10.1073/pnas.0707666105. http://dx.doi.org/10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R. Magnetic resonance imaging of glutamate. Nat Med. 2012;18(2):302–306. doi: 10.1038/nm.2615. http://dx.doi.org/10.1038/nm.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai K, Singh A, Poptani H, Li W, Yang S, Lu Y, Hariharan H, Zhou XJ, Reddy R. CEST signal at 2 ppm (CEST@2 ppm) from Z-spectral fitting correlates with creatine distribution in brain tumor. NMR Biomed. 2015;28(1):1–8. doi: 10.1002/nbm.3216. http://dx.doi.org/10.1002/nbm.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinogradov E, Zhang S, Lubag A, Balschi JA, Sherry AD, Lenkinski RE. On resonance low B1 pulses for imaging of the effects of PARACEST agents. J Magn Reson. 2005;176:54–63. doi: 10.1016/j.jmr.2005.05.016. http://dx.doi.org/10.1016/j.jmr.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Shaka AJ, Keeler J, Frenkiel T, Freeman R. An improved sequence for broadband decoupling: WALTZ-16. J Magn Reson. 1983;52:335–338. http://dx.doi.org/10.1016/0022-2364(83)90207-X. [Google Scholar]
- 14.Vinogradov E, He H, Lubag A, Balschi JA, Sherry AD, Lenkinski RE. MRI detection of paramagnetic chemical exchange effects in mice kidneys in vivo. Magn Reson Med. 2007;58(4):650–655. doi: 10.1002/mrm.21393. http://dx.doi.org/10.1002/mrm.21393. [DOI] [PubMed] [Google Scholar]
- 15.Suchy M, Ta R, Li AX, Wojciechowski F, Pasternak SH, Bartha R, Hudson RHE. A paramagnetic chemical exchange-based MRI probe metabolized by cathepsin D: design, synthesis and cellular uptake studies. Org Biomol Chem. 2010;8(11):2560–2566. doi: 10.1039/b926639a. http://dx.doi.org/10.1039/b926639a. [DOI] [PubMed] [Google Scholar]
- 16.Li AX, Suchy M, Li C, Gati JS, Meakin S, Hudson RHE, Menon RS, Bartha R. In vivo detection of MRI-PARACEST agents in mouse brain tumors at 9.4 T. Magn Reson Med. 2011;66(1):67–72. doi: 10.1002/mrm.22772. http://dx.doi.org/10.1002/mrm.22772. [DOI] [PubMed] [Google Scholar]
- 17.McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JW, van Zijl PC. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): pH calibration for poly-L-lysine and a starburst dendrimer. Magn Reson Med. 2006;55(4):836–847. doi: 10.1002/mrm.20818. http://dx.doi.org/10.1002/mrm.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun PZ. Simplified quantification of labile proton concentration-weighted chemical exchange rate (k(ws)) with RF saturation time dependent ratiometric analysis (QUESTRA): normalization of relaxation and RF irradiation spillover effects for improved quantitative chemical exchange saturation transfer (CEST) MRI. Magn Reson Med. 2012;67(4):936–942. doi: 10.1002/mrm.23068. http://dx.doi.org/10.1002/mrm.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun PZ, Wang Y, Dai Z, Xiao G, Wu R. Quantitative chemical exchange saturation transfer (qCEST) MRI–RF spillover effect-corrected omega plot for simultaneous determination of labile proton fraction ratio and exchange rate. Contrast Media Mol Imag. 2014;9(4):268–275. doi: 10.1002/cmmi.1569. http://dx.doi.org/10.1002/cmmi.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun PZ. Simultaneous determination of labile proton concentration and exchange rate utilizing optimal RF power: radio frequency power (RFP) dependence of chemical exchange saturation transfer (CEST) MRI. J Magn Reson. 2010;202(2):155–161. doi: 10.1016/j.jmr.2009.10.012. http://dx.doi.org/10.1016/j.jmr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon WT, Ren J, Lubag AJ, Ratnakar J, Vinogradov E, Hancu I, Lenkinski RE, Sherry AD. A concentration-independent method to measure exchange rates in PARACEST agents. Magn Reson Med. 2010;63(3):625–632. doi: 10.1002/mrm.22242. http://dx.doi.org/10.1002/mrm.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randtke EA, Chen LQ, Corrales LR, Pagel MD. The Hanes-Woolf linear QUESP method improves the measurements of fast chemical exchange rates with CEST MRI. Magn Reson Med. 2014;71(4):1603–1612. doi: 10.1002/mrm.24792. http://dx.doi.org/10.1002/mrm.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woessner DE, Zhang S, Merritt ME, Sherry AD. Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn Reson Med. 2005;53:790–799. doi: 10.1002/mrm.20408. http://dx.doi.org/10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 24.Randtke E. ProQuest. UMI Dissertations Publishing, Chemistry and Biochemistry, The University of Arizona; 2013. Development and Evaluation of Exchange Rate Measurement Methods; p. 133. 3611708. < http://hdl.handle.net/10150/314652>. [Google Scholar]
- 25.Ali MM, Liu G, Shah T, Flask CA, Pagel MD. Using two chemical exchange saturation transfer magnetic resonance imaging contrast agents for molecular imaging studies. Acc Chem Res. 2009;42:915–924. doi: 10.1021/ar8002738. http://dx.doi.org/10.1021/ar8002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murase K, Tanki N. Numerical solutions to the time-dependent Bloch equations revisited. Magn Reson Imag. 2011;29(1):126–131. doi: 10.1016/j.mri.2010.07.003. http://dx.doi.org/10.1016/j.mri.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Döpfert J, Zaiss M, Witte C, Schröder L. Ultrafast CEST imaging. J Magn Reson. 2014;243:47–53. doi: 10.1016/j.jmr.2014.03.008. http://dx.doi.org/10.1016/j.jmr.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Gruetter R. Automatic, localized, in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. http://dx.doi.org/10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 29.Bishop CM. Pattern Recognition and Machine Learning. Springer; New York: 2005. p. 486. [Google Scholar]
- 30.Liepinsh E, Otting G. Proton exchange rates from amino acid side chains–implications for image contrast. Magn Reson Med. 1996;35(1):30–42. doi: 10.1002/mrm.1910350106. http://dx.doi.org/10.1002/mrm.1910350106. [DOI] [PubMed] [Google Scholar]
- 31.Chen LQ, Howison CM, Jeffery JJ, Robey IF, Kuo PH, Pagel MD. Evaluations of extracellular pH within in vivo tumors using acidoCEST MRI. Magn Reson Med. 2014;72:1408–1417. doi: 10.1002/mrm.25053. http://dx.doi.org/10.1002/mrm.25053. [DOI] [PMC free article] [PubMed] [Google Scholar]