Abstract
We sought to develop a myeloablative chemotherapeutic regimen to secure consistent engraftment of T-cell depleted (TCD) hematopoietic stem cell transplants (HSCT) without the need for total body irradiation, thereby reducing toxicity, while maintaining low rates of GvHD and without increasing relapse. We investigated the myeloablative combination of busulfan and melphalan, with the immunosuppressive agents fludarabine and rabbit anti-thymocyte gloubin (r-ATG) as cytoreduction prior to a T-cell depleted HSCT. No post-transplant immunosuppression was administered.
Between April 2001 and May 2008, 102 patients (median age 55 years) with a diagnosis of primary or secondary myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) underwent cytoreduction with Bu/Mel/Flu, followed by TCD grafts. T-cell depletion was accomplished by CD34+-selection followed by E-rosette depletion for peripheral blood stem cell grafts, and soybean agglutination followed by E-rosette depletion for bone marrow grafts. Donors included matched and mismatched, related and unrelated donors. Risk stratification was by ASBMT risk categorization for patients with primary disease. For patients with secondary/treatment-related MDS/AML, patients in complete remission (CR) 1 or refractory anemia (RA) were classified as intermediate-risk, and all other patients high-risk.
Neutrophil engraftment occurred at a median of 11 days in 100/101 evaluable patients. The cumulative incidences of grade II–IV acute and chronic GvHD at 1 year were 8.8% and 5.9%, respectively. Overall- and disease-free survivals (DFS) at 5 years were 50.0% and 46.1% respectively, and the cumulative incidence of relapse and treatment related mortality were 23.5% and 28.4% respectively. Stratification by risk group demonstrated superior DFS for low-risk patients (61.5% at 5 years) compared to intermediate or high-risk (34.2, 40.0% respectively, p=0.021). For patients with AML, those in CR1 had superior 5-year DFS compared to those in ≥CR2 (60%, 30.6% respectively, p=0.01), without a significant difference in incidence of relapse (17.1%, 30.6% respectively, p=0.209). There were no differences in DFS for other patient, donor, or disease characteristics.
In summary, cytoreduction with Bu/Mel/Flu and r-ATG secured consistent engraftment of TCD transplants. The incidences of acute/chronic GvHD and disease relapse were low, with favorable outcomes in this patient population with high-risk myeloid malignancies.
Keywords: HSCT, T-cell depleted, chemotherapy-only, myeloid malignancies
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the treatment of choice for a number of hematologic malignancies1,2. Graft-versus-host disease (GvHD) remains one of the major complications of transplantation3,4 and T-cell depletion (TCD) offers an effective approach to its prevention5. Initial reports of TCD transplantation from different institutions used methods that achieved variable degrees of TCD, sometimes with additional pharmacologic immune suppression and described increased risks of graft failure and higher relapse rates6,7. Several more recent reports, however, have described successful methods of TCD HSCT for hematologic malignancies8–10, primarily with the use of total body irradiation (TBI)-based cytoreductive regimens. The low risk of graft rejection and GvHD has been achieved without compromising the anti-leukemic effect of the allografts11,12.
In an attempt to reduce the toxicity and long-term complications of these transplants for younger patients and to make the TCD approach available to an aging population needing allo-HSCT, we designed a chemotherapy-only conditioning regimen. We sought to determine if the combination of busulfan (Bu), melphalan (Mel), fludarabine (Flu), and anti-thymocyte globulin (ATG) would provide adequate immune suppression for consistent engraftment of the TCD graft, while reducing short and long-term regimenrelated toxicity and supporting disease-free survival.
PATIENTS AND METHODS
Patients and Diseases
A total of 102 patients with myeloid malignancies and various treatment backgrounds were treated on (n=80) or according to (n=22) a Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review and Privacy Board-approved phase II trial (NCT01119066) from April 2001 to May 2008 after obtaining informed consent. (Table 1). Reasons for treatment off-protocol included protocol ineligibility because of active infections (n=6), organ insufficiency (n=6), progressive disease (n=4), or treatment prior to opening of protocol (n=6).
Table 1.
Patient characteristics
| Characteristic | No. | % |
|---|---|---|
|
| ||
| Age | ||
| Median (Range), years | 55.1 (1.9–71.3) | |
| ≤20 | 17 | 16.7 |
| 21–49 | 21 | 20.6 |
| 50–59 | 37 | 36.3 |
| ≥60 | 27 | 26.5 |
|
| ||
| Gender | ||
| Male | 50 | 49.0 |
| Female | 52 | 51.0 |
|
| ||
| Disease/Stage at HSCT | ||
| De novo AML | 25 | |
| CR1 | 9 | 24.5 |
| CR2 | 10 | |
| CR3 | 2 | |
| refractory | 4 | |
| Primary MDS | 45 | |
| CR | 16 | 44.1 |
| RA | 22 | |
| RAEB-1 | 5 | |
| RAEB-2, refractory | 2 | |
| Secondary/treatment-related MDS or AML | 32 | |
| CR1/RA | 22 | 31.4 |
| Other (CR2, >RA) | 10 | |
|
| ||
| ASBMT risk category | ||
| Low | 39 | 38.2 |
| Intermediate | 38 | 37.3 |
| High | 25 | 24.5 |
|
| ||
| Primary disease (for patients with 2° MDS/AML) | ||
| Breast cancer | 9 | |
| Lymphoma (HD/NHL) | 7/2 | |
| Sarcoma | 7 | |
| Other hematologic malignancies | 5 | |
| Neuroblastoma | 1 | |
| Gestational trophoblastic disease | 1 | |
|
| ||
| Cytogenetics | ||
| Normal | 31 | |
| monosomy 7/7q- | 25 | |
| monosomy 5/5q- | 11 | |
| trisomy 8 | 11 | |
| 11q23 abnormalities | 9 | |
| t(8;21) or inv(16) | 3 | |
| t(15;17) | 3 | |
| Other cytogenetic abnormalities | 32 | |
| Hyperdiploid | 3 | |
| Complex karyotype | 10 | |
Eligibility included a diagnosis of primary MDS, AML, or secondary/treatment-related MDS/AML and the availability of a suitable donor. Related donors were required to be at least haplotype-matched to the patient, and unrelated donors no more than single HLA mismatch at the HLA A, B, or DRB1 locus. Other inclusion criteria included Karnofsky or Lansky performance status ≥70, no active infection or extramedullary disease, and satisfactory organ function13.
Eligible patients with de novo AML included patients in complete-remission (CR)-1 with intermediate or high-risk cytogenetics, any patient in ≥CR2, or patients with relapsed or refractory disease. Patients with primary MDS with high-risk cytogenetic features, transfusion dependence, or refractory-anemia (RA) with excess blasts (RAEB) were also eligible, as were all patients with secondary/treatment-related MDS or AML. ASBMT criteria determined risk group stratification based on disease status at transplant for primary MDS and AML. For secondary MDS/AML, we classified patients in CR1 or RA as intermediate risk, and high risk for all other stages. Remission status was assessed by morphologic and cytogenetic data. Routine monitoring for minimal residual disease was not performed during this time period. HLA matching for A, B, C, DRB1, and DQB1 loci was established using DNA sequence-specific oligonucleotide probes. Unrelated donors were identified and recruited via the National Marrow Donor Program (NMDP) registry.
Preparative Regimen and Graft
Cytoreduction consisted of intravenous busulfan [0.8 mg/kg/dose for patients <4 years of age or 1 mg/kg/dose for patients ≥4 years] for 6 doses, followed by pharmacologic adjustment of the final 4–6 doses (total 10–12 doses) on days −9 to −7; melphalan 70 mg/m2/dose × 2 doses on days −7 and −6; fludarabine 25 mg/m2/dose × 5 doses on days −6 to −2; and rabbit anti-thymocyte globulin (r-ATG; Thymoglobulin®) 2.5 mg/kg × 2 doses on days −3 and −2. First-dose pharmacokinetic studies for busulfan were performed at the Fred Hutchinson Cancer Research Center using high-performance liquid chromatography-mass spectrometry. The busulfan dose was adjusted to reach a concentration steady-state (CSS) level of 850–1000 ng/mL, with the desired level of approximately 900 ng/mL. Equine-ATG (e-ATG) 15 mg/kg/day for up to 2 doses could be used for patients with intolerance to r-ATG.
Peripheral blood stem cells (PBSCs) were harvested after G-CSF mobilization according to NMDP guidelines for unrelated donors, or G-CSF at 5 mcg/kg every 12 hours for 5–6 days for related donors. Targeted cell dose was 109 MNC/kg (3 × 106 CD34+/kg) of recipient actual weight. CD34+ cells were positively selected using the ISOLEX 300i Magnetic Cell Selection System (Baxter Health Care Corporation, Dearfield, IL, USA), followed by sheep red blood cell (sRBC)-rosette depletion of remaining T cells as previously described13,14. This achieved an approximate 5-log10 depletion of CD3+ cells14. Five donors elected bone marrow (BM) harvesting; T-cell depletion for BM grafts was accomplished by sequential soybean lectin agglutination and sRBC-rosette depletion (SBA-E-) of T-cells15,16. Transplants were infused intravenously 24–48 hours after completing fludarabine.
GvHD Evaluation and Management
GvHD was diagnosed clinically and confirmed by biopsy whenever possible. All patients were evaluated for acute GvHD weekly from engraftment or day 14, whichever was earlier, through 100 days post-transplant. Scoring was based on the Center for International Bone Marrow Transplant Research (CIBMTR) criteria17. Patients surviving >100 days were evaluated for chronic GvHD using the scoring criteria of Sullivan et al.18.
Supportive Care
Patients were managed clinically according to MSKCC standard guidelines13, including prophylactic antimicrobials, transfusion support, and prophylactic antiepileptic medication surrounding busulfan. Patients received G-CSF beginning on day +7 post-HSCT until neutrophil engraftment. Patients were monitored for viral reactivation of EBV and CMV regularly post-transplant. The choice of prophylactic vs. preemptive therapy for these viruses was based on institutional service guidelines at that time.
Engraftment
Engraftment was defined as the first of 3 consecutive days with ANC >500/uL and platelets >20,000/uL without transfusion for 3 consecutive days determined engraftment. Primary graft failure was defined as a failure to recover neutrophil counts by day 28 post-transplant, in the absence of disease. Secondary graft failure was defined as graft failure occurring after initial partial or complete recovery of donor-type hematopoiesis, and was characterized by recurrent pancytopenia with neutrophil counts <0.5 ×109/L, in the absence of disease. Etiologies for primary and secondary rejections or graft failures were evaluated in each case by cytogenetic and/or molecular demonstration of loss of donor-type blood elements; patients with 100% host chimerism were considered to have rejected their grafts, while all other patients were considered to have graft failure. Life-threatening infections comprised organ-localized infections due to viral, fungal, and/or parasitic pathogens13.
Relapse
Relapse was defined as an increase in bone marrow blast count >5%, presence of peripheral blasts, or blasts at any extramedullary site, in the presence of host chimerism. Routine assessment of minimal residual disease using flow cytometry was not performed during this time period. Recurrence of a patient’s leukemia-associated cytogenetic marker in the context of host chimerism also defined relapse, irrespective of the presence of a blast population.
Biostatistics
For all analyses, transplant date was considered time zero, with a data cutoff of October 31, 2016. Overall survival (OS) and disease-free survival (DFS) were defined as the time until death, and relapse or death respectively. Estimates of OS and DFS were calculated using the Kaplan-Meier method, with differences in patient and treatment characteristics accessed using a log-rank test. The cumulative incidence of acute GvHD, chronic GvHD, relapse, TRM, EBV-LPD, and neutrophil and platelet engraftment were estimated using the cumulative incidence method for competing risks, with differences in patient and treatment characteristics assessed using Gray’s test. For both acute and chronic GvHD, all 102 patients were considered at risk at the time of transplant and were included in the analysis. Death or relapse prior to GvHD onset were considered competing risks for both acute and chronic GvHD. For relapse, death prior to disease relapse was considered a competing risk, while for TRM, relapse or non treatment-related death were considered competing risks. For neutrophil and platelet engraftment, death was considered a competing risk, while for EBV-LPD, both death and graft failure were considered competing risks. Donor chimerism at 6 weeks was defined as the chimerism value closest and prior to 6 weeks post-transplant. The difference in relapse incidence based on donor chimerism was assessed via a landmark analysis at 6 weeks. Primary cause of death was determined by previously published criteria19. A p-value <0.05 was considered significant. All statistical analyses were performed using R 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient and donor characteristics
Table 1 details patient and disease characteristics. Patient median age was 55.1 years (range 1.9–71.3) with 64 patients ≥50 years. Diseases included de novo/primary myelodysplastic syndrome (MDS) (n=45), acute myeloid leukemia (AML) (n=25), and secondary/treatment-related MDS or AML (n=32). Based on the modified ASBMT risk group classification schema described above, 39 patients were considered low-risk, 38 intermediate-risk, and 25 high-risk.
Of the 45 patients with primary MDS, 40 patients received chemotherapy before proceeding to HSCT, and 38 were in CR or RA before transplant. For patients with AML, 21 were in CR at the time of transplant, while the other 4 patients had evidence of low-level disease at HSCT (marrow blasts <10% and circulating blasts 1–2%). Primary diseases for the 32 patients with secondary MDS/AML are outlined in Table 1. Twenty-eight patients received chemotherapy for their myeloid malignancy, and 30 patients were in CR or RA status before HSCT.
All patients underwent cytogenetic evaluation, and results are delineated in Table 1. Thirty-one patients had normal cytogenetics, and the remainder had at least one cytogenetic abnormality. Forty-eight patients had at least one leukemia- or MDS-associated abnormality including monosomy 7/7q-, monosomy 5/5q-, trisomy 8, 11q23 abnormalities, t(8;21), inv(16), or t(15;17). Ten patients had a complex karyotype. We defined cytogenetic risk groups as favorable, including normal cytogenetics and other single abnormalities (n=47); intermediate, including trisomy 8 and 11q23 abnormalities (n=12); and high-risk, including partial or complete loss of chromosome 5 or 7, or other multiple cytogenetic abnormalities (n=43).
Donors were related (n=29) or unrelated (n=73), and HLA-matched (n=59) or mismatched (n=43). The degree of mismatch for related donors ranged from 4/8 to 7/8 and from 6/8 to 7/8 for unrelated donors (Table 2).
Table 2.
Transplant characteristics
| Characteristics | No. | % |
|---|---|---|
|
| ||
| Donors | ||
| Matched related | 22 | 21.6 |
| Mismatched related | 7 | 6.9 |
| 7/8 | 5 | |
| 6/8 | 1 | |
| 4/8 | 1 | |
| Matched unrelated | 39 | 38.2 |
| Mismatched unrelated | 34 | 33.3 |
| 7/8 | 28 | |
| 6/8 | 6 | |
|
| ||
| Busulfan dose and adjustment | ||
| CSS, median (range) | 849 ng/mL (450–1535) | |
| Actual dosing, median (range) | 0.9 mg/kg (0.4–1.5) | |
| No adjustment | 59 | 57.8 |
| Increase >20% | 18 | 17.6 |
| Decrease >20% | 25 | 24.5 |
|
| ||
| ATG dose | ||
| r-ATG ×2 doses | 85 | |
| r-ATG ×1 dose | 8 | |
| r-ATG ×1 dose and e-ATG ×1 dose | 4 | |
| e-ATG ×2 doses | 5 | |
|
| ||
| Graft type and cell dose, median (range) | ||
| BM, SBA-E-, no. | 5 | |
| CD34+, ×106/kg | 2.37 (1.73–3.71) | 4.9 |
| CD3+, ×103/kg | 23.8 (9.68–122.86) | |
| PBSC, Isolex CD34+/E-, no. | 97 | |
| CD34+, ×106/kg | 5.87 (0.49–28.8) | 95.1 |
| CD3+, ×103/kg | 1.34 (0–152) | |
Conditioning
All 102 patients completed cytoreduction and had first-dose busulfan levels obtained. Ninety-eight patients received 10 doses of busulfan and 4 patients with advanced disease, treated as per protocol, received 12 doses. The actual CSS for busulfan ranged between 450 and 1535 (median 849) ng/mL. The actual busulfan dose was corrected >20% to 0.4–1.5 mg/kg (median 0.9 mg/kg) for 43 patients to achieve a steady state level of 850–1000 ng/mL (increase in 18 patients and decrease in 25 patients; Table 2). Eighty-five patients received two doses of r-ATG, while 17 patients received modifications due to reactions or intolerance (Table 2).
Grafts
Table 2 details the graft characteristics. For the 97 CD34+-selected/E- PBSC grafts, median cell doses were 5.87 × 106 CD34+ cells/kg (range 1.34–28.8), and 1.34 × 103 CD3+ cells/kg (range 0–152). Six patients received a CD34+ PBSC cell dose of <2 × 106 cells/kg. For the five SBA-E- BM grafts, median cell doses were 2.4 × 106 CD34+ cells/kg (range 1.73–3.71), and 23.8 × 103 CD3+ cells/kg (range 9.68–122.86). Two recipients of BM transplants received grafts of <2 × 106 CD34+ cells/kg
Engraftment
Neutrophil engraftment occurred in 100 patients; 1 patient died before engraftment and 1 patient suffered primary graft failure. Median time to neutrophil engraftment was 11 days (range 9–15 days), with a cumulative incidence of 98% (95% CI 90.8–99.6) at 28 days. Median time to platelet engraftment was 19 days (range 14–116 days), with a cumulative incidence of 75.5% (95% CI 65.8–82.8) at 28 days. Three patients experienced a secondary graft failure on days +29, +82, and +125 after HSCT. Two patients had delayed platelet engraftment until after day 100, which was associated with complicated post-transplant courses involving infections and GvHD.
Of the four patients with primary or secondary graft failure, two had received transplants from fully matched unrelated donors, one patient received a transplant from an unrelated donor with an HLA-A antigen mismatch, and one patient received a transplant from a related donor mismatched at HLA-A and -C antigens. Three patients had busulfan dose adjusted >20% for low initial levels; the fourth had a normal first-dose pharmacokinetic level. For rejection prophylaxis, one patient received 2 doses of equine ATG due to intolerance to r-ATG and the remaining 3 patients both complete doses of rabbit ATG. Grafts for three patients were PBSC with a mean CD34+ cell dose of 6.5 × 106 cells/kg, while one patient received BM graft with a CD34+ cell count of 3.71 × 106 cells/kg. Two patients are long-term survivors following second HSCTs, one recovered counts following 2 doses of immunosuppressive treatment with equine ATG but subsequently died due to GvHD, and the last patient died due to infection while aplastic.
Overall and Disease-Free Survival
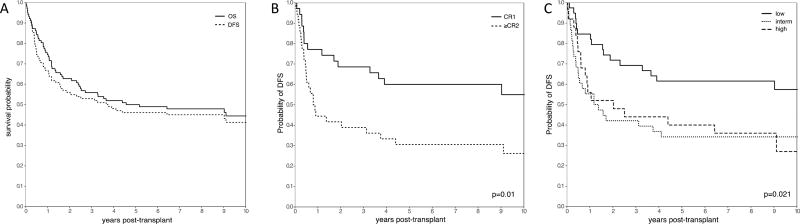
The median follow-up among survivors was 113 months (range 60–180 months). OS was 55.9% (95% CI, 45.7–64.9), 50.0% (40.0–59.2) and 44.5% (34.2–54.2) at 3, 5, and 10 years respectively, while DFS was 52.9% (42.8–62.1), 46.1% (36.2–55.4), and 41.4% (31.2–51.2) (Figure 1A). OS and DFS were similar based on disease, disease status (CR/RA vs other), age, donor type, and cytogenetic risk (Table 3). Additionally, there were no differences in survival between patients who received on- or off- protocol therapy.
Figure 1.
(A) Overall and Disease Free Survival across the entire cohort. (B) Probability of disease-free survival for patients with AML in CR1 vs. ≥CR2. Patients with AML in CR1 had a significantly increased probability of disease free-survival than patients with more advanced disease (p=0.01). (C) Probability of disease-free survival based on ASBMT risk groups. Low risk patients had a significantly higher probability of disease-free survival than intermediate or high risk patients (p=0.021).
Table 3.
Outcomes
| 5 year estimate (95% CI) | p-value | |
|---|---|---|
|
| ||
| Disease-free survival, entire cohort | 46.1 (36.2 – 55.4) | |
|
| ||
| Prognostic factors: | ||
| Disease | ||
| Primary MDS | 46.7 (31.7 – 60.3) | 0.882 |
| Primary AML | 40.0 (21.3 – 58.1) | |
| Secondary/t-MDS/AML | 50.0 (31.9 – 65.7) | |
| Age | ||
| ≤20 | 47.1 (23.0 – 68.0) | 0.399 |
| 21–49 | 52.4 (29.7 – 70.9) | |
| 50–59 | 51.4 (34.4 – 64.9) | |
| ≥60 | 33.3 (16.8 – 50.9) | |
| Donor | ||
| Matched related | 59.1 (36.1 – 76.2) | 0.363 |
| Other | 42.5 (31.6 – 53.0) | |
| ASBMT risk classification | ||
| Low | 61.5 (44.5 – 74.7) | 0.021 |
| Intermediate | 34.2 (19.8 – 49.1) | |
| High | 40.0 (21.3 – 58.1) | |
| Cytogenetic risk group | ||
| Favorable | 46.8 (32.2 – 60.2) | 0.941 |
| Intermediate | 50.0 (20.8 – 73.6) | |
| Poor | 44.2 (29.2 – 58.2) | |
| Remission status, AML patients | ||
| CR1 | 60 (42.0 – 74.0) | 0.01 |
| ≥CR2 | 30.6 (16.6 – 45.7) | |
| Protocol status | ||
| On-protocol | 47.5 (36.3 – 57.9) | 0.518 |
| Off-protocol | 40.9 (20.9 – 60.1) | |
|
| ||
| Cumulative incidence of relapse, entire cohort | 23.5 (15.8 – 32.2) | |
|
| ||
| Cumulative incidence of TRM, entire cohort | 28.4 (20.0 – 37.4) | |
| Age | ||
| <40 | 14.8 (4.5 – 30.9) | 0.113 |
| 40–59 | 29.2 (17.0 – 42.4) | |
| ≥60 | 40.7 (22.0 – 58.7) | |
|
| ||
| Acute GvHD (1-year cumulative incidence) | ||
| Grades II–IV | 8.8 (4.2 – 15.3) | |
| Grades III–IV | 3.9 (1.3 – 9) | |
| Chronic GvHD | 5.9 (2.4 – 11.6) | |
Despite no differences in survival amongst patients who were in CR or RA vs. other, there was a significant difference in outcomes for patients with AML in CR1 (n=35) compared to those patients with AML in ≥CR2 (including relapsed/refractory patients) (n=36), with a 5-year OS of 62.9% (44.8–76.5) and 33.3 (18.8–48.6) respectively (p=0.01). DFS also differed with 5-year estimates of 60% (42.0–74.0) and 30.6 (16.6–45.7) for the AML CR1 and AML ≥CR2 groups respectively (p=0.01; Figure 1B). Interestingly, the corresponding incidences of relapse were not statistically different (17.1%; 6.8–31.4 vs 30.6%, 16.3–46.1; p=0.209), suggesting that there are factors beyond relapse that might contribute to poorer survival in patients with disease in >CR1.
OS and DFS were also significantly different between ASBMT risk groups (p=0.022 and p=0.021 respectively). The OS at 5 years was 64.1% (47.0–76.9) for low-risk patients compared to 39.5% (24.2–54.4) for intermediate-risk and 44.0% (24.5–61.9) for high-risk patients. DFS at 5 years was 61.5% (44.5–74.7) for low-risk patients, 34.2% (19.8–49.1) for intermediate-risk and 40.0% (21.3–58.1) for high-risk patients (Figure 1C). There was no significant difference between survival for patients with good, intermediate, or poor risk cytogenetics (OS p=0.903, DFS p=0.941). Of note, the 11 patients with isolated monosomy 7 or 7q- had a 5-year OS of 63.6% (29.7–84.5) and 5-year DFS of 54.5% (22.9–78.0).
Graft-versus-host disease
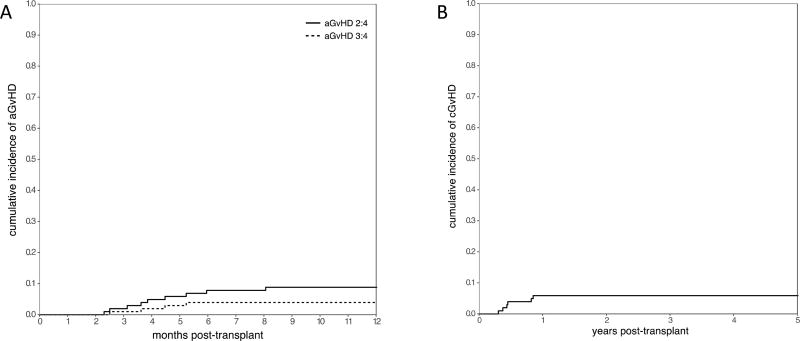
The cumulative incidence of grade II–IV and grade III–IV acute, and chronic GvHD at 1 year was 8.8% (4.3–15.3), 3.9% (1.3–9.0), and 5.9% (2.4–11.6) respectively (Figure 2). Of 91 evaluable patients, 9 developed grade II–IV acute GvHD, including 6 with late acute (Table 3). Organ involvement for acute GvHD included skin in 7 patients, GI in 6, and liver in 2. The median time to onset of grade II–IV acute GvHD was 117 days (range 70–245). There were no differences in the rates of grade II–IV acute GvHD for patients receiving matched-related donor transplants compared to those receiving transplants from other donors. Only 6 of 91 evaluable patients were diagnosed with chronic GvHD at a median of 5.3 months post-HSCT (range 3.7–10.2) including 3 patients with acute/chronic GvHD overlap syndrome and 3 patients with de-novo disease. Chronic GvHD was limited in 3 and extensive in 3 patients. Three patients with isolated acute GvHD, 2 patients with isolated chronic GvHD, and all patients with acute/chronic GvHD overlap ultimately succumbed to complications from treatment. One patient with GvHD later died from relapse after resolution of GvHD.
Figure 2.
Cumulative incidence of acute (A) and chronic (B) GvHD across all patients.
All 12 patients with GvHD (grade II–IV acute or chronic) were recipients of PBSC grafts. Eight of the 12 patients were older than 50 years of age. The median CD3+ cell dose was 1.4 × 103 cells/kg for the entire cohort, whereas it was 0.91 × 103 cells/kg for the patients with grade II–IV acute GvHD and 1.63 × 103 cells/kg for the patients with chronic GvHD. There was no difference in the rates of grade II–IV acute GvHD based on CD3+ cell dose. The median CD34+ cell dose was comparable for the entire cohort, and the acute and chronic GvHD cohorts (5.7, 5.8, and 5.7 × 106 cells/kg respectively).
Relapse
The cumulative incidence of relapse for all 102 patients was 23.5% (15.8–32.2) at 5 years. The cumulative incidence of relapse was 28.0% (12.0–46.6) for patients with primary AML, 26.7% (14.7–40.2) for patients with primary MDS, and 15.6% (5.5–30.4) for patients with secondary MDS/AML at 5 years (p=0.503). There was no significant difference in relapse based on stage of disease at HSCT. Twenty-one of the 24 patients who relapsed died from their disease, 2 of whom achieved remission after chemotherapy but died from other causes, while 3 are still alive and disease-free after a subsequent HSCT.
Mortality
Forty-seven of 102 patients were alive at the time of this analysis. Fifty-five patients died of relapse (n=21) or of non-relapse mortality (n=34). The cumulative incidence of treatment-related mortality (TRM) was 10.8% (5.7–17.7) at 100 days and 28.4% (20.0–37.4) at 5 years. There was trend toward increased TRM based on age with 5-year estimates of 14.8% (4.5–30.9) for patients <40 years old, 29.2% (17.0–42.4) for patients 40–59 years old, and 40.7% (22.0–58.7) for patients ≥60 years old (p=0.113).
Causes of death (COD) from TRM are listed in Table 4, and included infection, GvHD, organ toxicity, and other causes. Infectious deaths (n=16) were most often due to sepsis, Epstein-Barr virus (EBV)-LPD, or pneumonitis. Organ toxicity primarily consisted of pulmonary or hepatic toxicity; there was one case of lethal VOD and one death from other multi-organ failure. Other COD included head trauma, surgical complication, second primary pancreatic cancer, myocardial infarction, and original primary cancer (in a patient who received a transplant for treatment-related myeloid disease).
Table 4.
Causes of death from non-relapse mortality
| No. | |
|---|---|
|
| |
| Infection | 16 |
| Sepsis | 5 |
| Pneumonitis | 5 |
| EBV-LPD | 3 |
| CMV/VZV | 1/1 |
| Fungal | 1 |
|
| |
| GvHD | 6 |
|
| |
| Organ toxicity | 7 |
| Pulmonary/Hepatic | 3/2 |
| Multi-organ failure | 1 |
| VOD | 1 |
|
| |
| Other | 5 |
Immune reconstitution and infections
The absolute CD4+ lymphocyte count reached a level of 200 cells/µL at a median time of 6 months post-transplant for the entire patient cohort (range 1–53 months), with 13% of patients achieving this within one month, 54% within 6 months, and 82% by one year post-transplant. The median time to achieve a PHA proliferative response of 50% of normal was 10 months for the entire patient cohort (range 2–39 months), with 25% of patients recovering by 6 months post-transplant, 54% by one year post-transplant, and 81% by two years post-transplant.
Viremias post-transplant in the entire patient cohort included CMV (n=19), EBV (n=21), HHV6 (n=9), adenovirus (n=5), VZV (n=4), and BK (n=3). Nine patients had infections with 2–4 viruses. None of the 50 CMV seronegative patients developed CMV viremia, including the 11 patients who received grafts from CMV seropositive donors. Approximately half (27/50) of the CMV seropositive patients received prophylactic antiviral therapy against CMV, and only 3 of these patients reactivated CMV. CMV viremia occurred in 3 of the 13 (23%) CMV seropositive patients who received grafts from CMV seropositive donors and in 16 of the 35 (46%) CMV seropositive patients who received grafts from CMV seronegative donors. CMV infections were fatal in 1 of 19 patients. A minority of patients (9 in total) received prophylactic rituximab to prevent EBV reactivation; none of these patients reactivated EBV. EBV infections included EBV viremia in 12 patients and EBV-LPD in 9 patients, with a cumulative incidence of 8.8% at 1-year. Four patients with EBV-LPD succumbed to their infection. One patient died of their VZV infection; no other patients died from their viral infections. No patients with HHV6 viremia had encephalopathy, and all cases of viremia were managed conservatively without antivirals. Four patients had BK hemorrhagic cystitis.
DISCUSSION
Allogeneic hematopoietic stem cell transplantation is a curative approach for myeloid malignancies including MDS and AML2,20–23. The major complications associated with this therapeutic approach have included GvHD24, and the acute and late effects of cytoreductive regimens that have included TBI25–33. In this report we describe a chemotherapy-only cytoreductive approach to successfully engraft T-cell depleted transplants from a variety of donors, including matched and mismatched, related and unrelated, all with a low risk of GvHD, and while maintaining relapse rates comparable to other approaches.
The standard of care for allo-HSCT for myeloid malignancies initially included unmodified HSCT after cytoreduction with TBI and cyclophosphamide (CY)34,35. Over the years, unmodified HSCT after cytoreduction with busulfan and cyclophosphamide36–38 or melphalan39,40 showed results comparable to those with TBI-CY and became part of the standard of care for HSCT for myeloid malignancies, with longterm DFS rates of 27–61% and relapse rates of 11–42%36–40. These approaches, however, suffered from a high risk of GvHD (acute: 24–68%; chronic: 38–65%), especially in the context of grafts from alternative donors.
Several approaches were developed to prevent these complications. Investigators from the Fred Hutchinson Cancer Research Center and the MD Anderson Cancer Center pioneered a new approach of unmodified HSCT with reduced intensity conditioning (RIC) regimens in an effort to reduce TRM41–46, which was successful in reducing TRM in high-risk populations (16–39%), while maintaining similar relapse rates (16–42%), though without improvement in rates of GvHD (acute 25–63%, chronic 37–62%).
GvHD prophylaxis in the form of post-transplant cyclophosphamide was developed by The Johns Hopkins Hospital BMT group. This approach has included RIC, and grafts from haploidentical donors, and it has facilitated consistent engraftment with tolerable rates of GvHD and low toxicity, but with some reports of relatively high rates of relapse47. Subsequently, this group utilized this approach with myeloablative conditioning and matched donors for patients with more aggressive disease and better performance status, with outcomes similar to prior regimens by most measures (DFS: 39–54%; relapse rates: 44%; TRM: 17%; acute GvHD: 43%), but notably lower rates of chronic GvHD (10%)48.
A third approach, developed over many years of investigational studies at our center, is that of T-cell depleted HSCT. Although initial reports of TCD HSCT reported high rates of immunologic rejection6, the addition of ATG to the conditioning regimen was able to overcome this limitation49. Over several decades, we have described our experience with TCD HSCT, from both matched related12,50,51 and unrelated donors11 for patients with AML. The regimens described in our earlier work were solely TBI-based to enable consistent engraftment in the context of T-cell depletion. Rates of relapse (6–23%) and survival (45–56.8%) were comparable to traditional regimens utilizing unmodified grafts, while rates of GvHD were significantly lower (acute grade II-III 0–9%, chronic 0–29%). The use of TBI continued to be problematic, with rates of TRM of 19.4–37.5% in high-risk and older patients. Additionally, TBI has been associated with significant late effects, including the development of cataracts33, diabetes27, and secondary malignancies30. Children are at risk for additional complications26, including impaired growth and final height28,29 as well as multiple endocrine complications including gonadal and thyroid dysfunction32, and these risks have been demonstrated to be lower with the use of busulfan-based cytoreduction28,31 Thus, we sought to develop a chemotherapy-only regimen that reduced the complications of the conditioning from TBI and could achieve sustained engraftment with TCD grafts and cure of the disease.
The novel approach of a chemotherapy-only cytoreductive regimen followed by a TCD HSCT described in this report has accomplished these goals. While utilizing a full spectrum of donor types (related, unrelated, matched, and mismatched), we have (1) secured consistent engraftment with (2) low rates of GvHD, and (3) rates of relapse comparable to that of unmodified transplants, and to that of our previously reported TBI-based TCD HSCTs. Notably this was accomplished with a very low risk of rejection. Additionally, the regimen was well-tolerated across all ages (range 1.9–71.3 years).
We achieved 1-year rates of acute and chronic GvHD similar to our prior studies (8.8% acute grades II–IV, 5.9% chronic GvHD), despite a high percentage of HSCTs using grafts from unrelated and/or mismatched donors, and in an older patient population. The cumulative incidence of relapse associated with this chemotherapy-only cytoreductive regimen and T-cell depleted grafts was 23.5% at 5 years, which is comparable to that of unmodified grafts using high-dose TBI-based cytoreductive regimens36. This rate was not significantly different for patients with primary or secondary disease. Moreover, DFS was not significantly different when assessing for age, donor type, or cytogenetic risk (Table 3). These findings will need to be confirmed in larger numbers of patients.
The cumulative incidence of TRM was 10.8% and 28.4% at 100 days and 5 years respectively, with a trend toward increased mortality in older patients. Causes of non-relapse deaths (Table 4) were primarily infectious, with mortality from direct regimen-related organ toxicity occurring in only 8 of 34 patients.
Delayed immune recovery after transplant has been associated with older age, use of ATG, and TCD. The broad range of patient ages and donor types make comparison with previous reports somewhat difficult. In this study, median time to recovery of CD4+ count of 200/uL was 6.2 months, and 13 months to a PHA of at least 50% of the lower limit of normal. The higher incidence of opportunistic infections such as CMV and EBV in this study compared to recent published reports from this center is likely in part due to a switch in techniques for viral monitoring. The higher rate of viral disease compared with that reported for conventional transplants is similar to that observed in our previous TCD HSCT trials, and likely related to the slower immune recovery. It is notable that this study was performed before the availability of specific adoptive T-cell therapies, now widely used to successfully treat viral disease.
In summary, consistent engraftment of TCD grafts from a wide range of donors was achieved without the use of TBI utilizing a regimen including busulfan/melphalan/fludarabine and rabbit ATG. This myeloablative combination was well-tolerated across a broad spectrum of age groups and maintained low rates of relapse and 100-day TRM similar to previous TCD clinical trials, in addition to low incidences of both acute and chronic GvHD.
Other new chemotherapy-only cytoreductive regimens are now being tested on institutional protocols with the use of alternative melphalan- and thiotepa-based chemotherapy regimens with the addition of clofarabine or fludarabine, in adults and children, with malignant or non-malignant hematologic disorders. Other future directions include: (1) the use of agents such as treosulfan instead of busulfan to further reduce toxicity; (2) the use of prophylactic cellular therapy with CMV, EBV, or other specific cytotoxic T-cells for the prevention or preemptive treatment of infections; and (3) the possible use of agents to improve immune reconstitution such as interleukin-752.
Highlights.
Chemotherapy-only cytoreduction can successfully engraft T-cell depleted HSCTs.
The incidences of acute and chronic GvHD were <10% despite >40% mismatched donors.
This regimen attained 5-year OS of 50% in patients with myeloid malignancies.
Acknowledgments
Supported in part by P01 CA23766 and P30 CA008748 from the National Cancer Institute, National Institutes of Health, The Aubrey Fund, The Tow Foundation, The Laura Rosenberg Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–61. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greinix HT, Nachbaur D, Krieger O, et al. Factors affecting long-term outcome after allogeneic haematopoietic stem cell transplantation for acute myelogenous leukaemia: a retrospective study of 172 adult patients reported to the Austrian Stem Cell Transplantation Registry. Br J Haematol. 2002;117:914–23. doi: 10.1046/j.1365-2141.2002.03532.x. [DOI] [PubMed] [Google Scholar]
- 4.Beutler E, McMillan R. Bone marrow transplantation in acute leukemia. Blood Cells. 1982;8:485–500. [PubMed] [Google Scholar]
- 5.Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol. 2014;11:536–47. doi: 10.1038/nrclinonc.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kernan NA, Bordignon C, Heller G, et al. Graft failure after T-cell-depleted human leukocyte antigen identical marrow transplants for leukemia: I. Analysis of risk factors and results of secondary transplants. Blood. 1989;74:2227–36. [PubMed] [Google Scholar]
- 7.Martin PJ, Hansen JA, Torok-Storb B, et al. Effects of treating marrow with a CD3-specific immunotoxin for prevention of acute graft-versus-host disease. Bone Marrow Transplant. 1988;3:437–44. [PubMed] [Google Scholar]
- 8.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19:898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg JD, Linker A, Kuk D, et al. T cell-depleted stem cell transplantation for adults with highrisk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:208–13. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30:3194–201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:1335–42. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–90. [PubMed] [Google Scholar]
- 13.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–9. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins NH, Fernandez JM, Bleau S, et al. Comparison of bone marrow and G-CSF mobilized peripheral blood progenitor cells from single normal donors before and after T cell depletion. Cytotherapy. 1999;1:223. [Google Scholar]
- 15.Collins NH, Carabasi MH, Bleau S, et al. New technology for the depletion of T cells from soybean lectin agglutinated, HLA-matched bone marrow grafts for leukemia: initial laboratory and clinical results. Prog Clin Biol Res. 1992;377:427–39. [PubMed] [Google Scholar]
- 16.Verdonck LF, Dekker AW, van Heugten H, et al. Depletion of T cells from bone marrow grafts with soybean agglutinin and sheep red blood cells for prevention of graft-versus-host disease. Haematol Blood Transfus. 1987;30:563–6. doi: 10.1007/978-3-642-71213-5_101. [DOI] [PubMed] [Google Scholar]
- 17.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–9. [PubMed] [Google Scholar]
- 19.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13:1469–76. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 20.Castro-Malaspina H, Harris RE, Gajewski J, et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood. 2002;99:1943–51. doi: 10.1182/blood.v99.6.1943. [DOI] [PubMed] [Google Scholar]
- 21.Kurosawa S, Yamaguchi T, Miyawaki S, et al. A Markov decision analysis of allogeneic hematopoietic cell transplantation versus chemotherapy in patients with acute myeloid leukemia in first remission. Blood. 2011;117:2113–20. doi: 10.1182/blood-2010-05-285502. [DOI] [PubMed] [Google Scholar]
- 22.Woodard P, Carpenter PA, Davies SM, et al. Unrelated donor bone marrow transplantation for myelodysplastic syndrome in children. Biol Blood Marrow Transplant. 2011;17:723–8. doi: 10.1016/j.bbmt.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woods WG, Neudorf S, Gold S, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97:56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard F, Auquier P, Herrmann I, et al. Health status of childhood leukemia survivors who received hematopoietic cell transplantation after BU or TBI: an LEA study. Bone Marrow Transplant. 2014;49:709–16. doi: 10.1038/bmt.2014.3. [DOI] [PubMed] [Google Scholar]
- 26.Boulad F, Sands S, Sklar C. Late complications after bone marrow transplantation in children and adolescents. Curr Probl Pediatr. 1998;28:273–97. doi: 10.1016/s0045-9380(98)80030-3. [DOI] [PubMed] [Google Scholar]
- 27.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–72. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernard F, Bordigoni P, Simeoni MC, et al. Height growth during adolescence and final height after haematopoietic SCT for childhood acute leukaemia: the impact of a conditioning regimen with BU or TBI. Bone Marrow Transplant. 2009;43:637–42. doi: 10.1038/bmt.2008.370. [DOI] [PubMed] [Google Scholar]
- 29.Chemaitilly W, Boulad F, Heller G, et al. Final height in pediatric patients after hyperfractionated total body irradiation and stem cell transplantation. Bone Marrow Transplant. 2007;40:29–35. doi: 10.1038/sj.bmt.1705694. [DOI] [PubMed] [Google Scholar]
- 30.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 31.Dai QY, Souillet G, Bertrand Y, et al. Antileukemic and long-term effects of two regimens with or without TBI in allogeneic bone marrow transplantation for childhood acute lymphoblastic leukemia. Bone Marrow Transplant. 2004;34:667–73. doi: 10.1038/sj.bmt.1704605. [DOI] [PubMed] [Google Scholar]
- 32.Sklar C, Boulad F, Small T, et al. Endocrine complications of pediatric stem cell transplantation. Front Biosci. 2001;6:G17–22. doi: 10.2741/a714. [DOI] [PubMed] [Google Scholar]
- 33.Tichelli A, Gratwohl A, Egger T, et al. Cataract formation after bone marrow transplantation. Ann Intern Med. 1993;119:1175–80. doi: 10.7326/0003-4819-119-12-199312150-00004. [DOI] [PubMed] [Google Scholar]
- 34.Thomas ED, Buckner CD, Clift RA, et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission. N Engl J Med. 1979;301:597–9. doi: 10.1056/NEJM197909133011109. [DOI] [PubMed] [Google Scholar]
- 35.Thomas ED, Clift RA, Hersman J, et al. Marrow transplantation for acute nonlymphoblastic leukemic in first remission using fractionated or single-dose irradiation. Int J Radiat Oncol Biol Phys. 1982;8:817–21. doi: 10.1016/0360-3016(82)90083-9. [DOI] [PubMed] [Google Scholar]
- 36.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122:3863–70. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deeg HJ, Storer B, Slattery JT, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100:1201–7. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 38.Litzow MR, Perez WS, Klein JP, et al. Comparison of outcome following allogeneic bone marrow transplantation with cyclophosphamide-total body irradiation versus busulphan-cyclophosphamide conditioning regimens for acute myelogenous leukaemia in first remission. Br J Haematol. 2002;119:1115–24. doi: 10.1046/j.1365-2141.2002.03973.x. [DOI] [PubMed] [Google Scholar]
- 39.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–80. [PubMed] [Google Scholar]
- 40.Vey N, De Prijck B, Faucher C, et al. A pilot study of busulfan and melphalan as preparatory regimen prior to allogeneic bone marrow transplantation in refractory or relapsed hematological malignancies. Bone Marrow Transplant. 1996;18:495–9. [PubMed] [Google Scholar]
- 41.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–72. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 42.Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859–67. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hegenbart U, Niederwieser D, Sandmaier BM, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24:444–53. doi: 10.1200/JCO.2005.03.1765. [DOI] [PubMed] [Google Scholar]
- 44.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14:246–55. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura R, Rodriguez R, Palmer J, et al. Reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation with fludarabine and melphalan is associated with durable disease control in myelodysplastic syndrome. Bone Marrow Transplant. 2007;40:843–50. doi: 10.1038/sj.bmt.1705801. [DOI] [PubMed] [Google Scholar]
- 46.Popat U, de Lima MJ, Saliba RM, et al. Long-term outcome of reduced-intensity allogeneic hematopoietic SCT in patients with AML in CR. Bone Marrow Transplant. 2012;47:212–6. doi: 10.1038/bmt.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–30. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aversa F, Terenzi A, Carotti A, et al. Improved outcome with T-cell-depleted bone marrow transplantation for acute leukemia. J Clin Oncol. 1999;17:1545–50. doi: 10.1200/JCO.1999.17.5.1545. [DOI] [PubMed] [Google Scholar]
- 50.Young JW, Papadopoulos EB, Cunningham I, et al. T-cell-depleted allogeneic bone marrow transplantation in adults with acute nonlymphocytic leukemia in first remission. Blood. 1992;79:3380–7. [PubMed] [Google Scholar]
- 51.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant. 2008;14:458–68. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perales MA, Goldberg JD, Yuan J, et al. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120:4882–91. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]