Abstract
Studies in humans suggest that stronger incentive motivational responses to Pavlovian food cues may drive over-consumption leading to and maintaining obesity, particularly in susceptible individuals. However, whether this enhanced incentive motivation emerges as a consequence of obesity or rather precedes obesity is unknown. Moreover, while human imaging studies have provided important information about differences in striatal responsiveness between susceptible and non-susceptible individuals, the neural mechanisms mediating these behavioral differences are unknown. The Nucleus Accumbens (NAc) mediates cue-triggered reward seeking and activity in the NAc is enhanced in obesity-susceptible populations. Therefore here, we used selectively-bred obesity-prone and obesity-resistant rats to examine intrinsic differences in incentive motivation, and the role of NAc AMPARs in the expression of these behaviors prior to obesity. We found that obesity-prone rats exhibit robust cue-triggered food-seeking (Pavlovian-to-instrumental transfer, PIT). Using intra-NAc infusion of AMPAR antagonists, we show that this behavior is selectively mediated by CP-AMPARs in the NAc core. Additionally, biochemical data suggest that this is due in part to experience-induced increases in CP-AMPAR surface expression in the NAc of obesity-prone rats. In contrast, in obesity-resistant rats PIT was weak and unreliable and training did not increase NAc AMPAR surface expression. Collectively, these data show that food cues acquire greater incentive motivational control in obesity-susceptible populations prior to the development of obesity. This provides support to the idea that enhanced intrinsic incentive motivation may be a contributing factor, rather than a consequence of obesity. In addition, these data demonstrate a novel role for experience-induced up-regulation of NAc CP-AMPARs in PIT, pointing to potential mechanistic parallels between the processes leading to addiction and to obesity.
Keywords: Addiction, Motivation, Striatum, AMPA receptor, PIT, Glutamate plasticity
1. Introduction
The rise in global obesity has prompted a closer examination of the psychological and neurobiological processes that influence over-eating and enhanced motivation to consume palatable foods. Studies in humans support the idea that cravings triggered by stimuli associated with food (i.e., food cues) may contribute to food-seeking and over-eating leading to obesity (Burger and Stice, 2014; Stoeckel et al., 2008 see Stice et al., 2013 for review). For example, food cues induce feelings of hunger, bias food choice, and increase the amount of food consumed (Fedoroff et al., 1997; Jansen et al., 2008; Watson et al., 2014). These cues include sensory properties of food itself, like the crunching sensation of digging your hand into a bag of potato chips, as well as distal cues like packaging, and branding logos (Bouton, 2011). The ability for food cues to trigger cravings is not unique to obese populations, but rather individuals that struggle to maintain a healthy weight are more sensitive to these motivational properties of food cues (Fedoroff et al., 1997; Ferriday and Brunstrom, 2011; Jansen et al., 2008; Lehner et al., 2017; see Small, 2009 for review). This suggests that brain regions mediating incentive motivation, such as the NAc (Berridge et al, 2009, 2010; Cartoni et al., 2016; Holmes et al., 2010), differ functionally between obesity-susceptible vs. -resistant populations, thereby contributing to overconsumption in susceptible individuals (Burger and Stice, 2014; Stoeckel et al., 2008; Tomasi and Volkow, 2013). This has prompted vibrant discussion about the degree to which these neurobehavioral differences seen in susceptible individuals are similar vs. different to those driving drug-seeking in addiction (Berridge et al., 2010; Ferrario, 2017; Long et al., 2015; Michaud et al., 2017; Stice et al., 2013; Volkow et al., 2013).
A central question that arises in this discussion is whether enhanced neurobehavioral responses to food cues emerge as a consequence of weight gain, or whether there are intrinsic differences in the motivational responses to food cues that precede weight gain. In support of pre-existing differences, we recently found that outbred rats subsequently identified as susceptible to diet-induced obesity display greater cue-triggered approach (an indicator of incentive motivation) prior to diet manipulation and weight gain (Robinson et al., 2015). However, identification of susceptible and resistant rats in outbred populations requires the introduction of high-fat diets and weight gain which can themselves alter neural function and behavior (Baladi et al., 2012; Brown et al., 2017; Dingess et al., 2017; Hryhorczuk et al., 2016; Oginsky et al., 2016a). This limitation can, however, be overcome by using established rat lines that were selectively bred for their propensity or resistance to diet-induced obesity (Levin et al., 1997; Vollbrecht et al., 2015). Thus, by using these obesity-prone and obesity-resistant rats in the current study we can know a priori who is susceptible and resistant to obesity without introducing a high-fat diet or weight gain. This allows us to examine intrinsic neurobehavioral differences that precede obesity.
Recent studies from our group have shown that NAc function is enhanced in these selectively-bred obesity-prone vs. obesity-resistant rats. For example, basal intrinsic excitability of medium spiny neurons within the NAc is enhanced in adult obesity-prone vs. obesity-resistant rats, in the absence of any diet manipulation (Oginsky et al., 2016b). Furthermore, consumption of a sugary, fatty “junk-food” diet increases the expression and function of NAc calcium-permeable AMPA receptors (CP-AMPARs) in obesity-prone, but not obesity-resistant rats (Oginsky et al., 2016a). This up-regulation of CP-AMPARs is interesting in part because these receptors mediate the “incubation of cocaine-seeking” (Wolf, 2016; Wolf and Ferrario, 2010), consistent with the role of the NAc in incentive motivational processes (Berridge et al, 2009, 2010; Cartoni et al., 2016). However, whether cue-triggered food-seeking (i.e., Pavlovian-to-instrumental transfer; PIT) is stronger in obesity-prone vs. obesity-resistant rats prior to obesity is unknown. Moreover, while NAc AMPAR-mediated transmission has been indirectly implicated in the expression of PIT (Corbit and Balleine, 2011; Crombag et al., 2008), to date no studies have directly examined the role of endogenous NAc AMPAR-mediated transmission in this behavior. Therefore here, we used PIT, a well-established measure of incentive motivation, to determine whether cue-triggered food-seeking is stronger in obesity-prone vs. obesity-resistant rats. We then examined whether experience during training leading up to PIT testing alters NAc AMPAR expression. Lastly, we determined the role of NAc AMPARs in the expression this behavior.
2. Materials and methods
2.1. Subjects
Obesity-prone (OP) and obesity-resistant rats (OR), originally established by Barry Levin (Levin et al., 1997), were bred in house. Breeding was maintained on a Poiley Rotation System using 12 breeding pairs for each line. Breeders were originally purchased from Taconic. Adult males ranging from 65 to 85 days old at the start of the experiment were used (OP N = 49; OR N = 58; ns for individual experiment are given in results below). Rats were group housed and maintained on a reverse light-dark schedule (12/12); experiments were conducted during the dark phase. All procedures were approved by The University of Michigan Committee on the Use and Care of Animals. For characterization and validation of obesity phenotypes in these lines (see, Vollbrecht et al., 2015). Additional details for all procedures and housing can be found at: https://sites.google.com/a/umich.edu/ferrario-lab-public-protocols/
2.2. Behavioral procedures
Procedures were adapted from (Delamater et al., 2017a; Holland and Gallagher, 2003) see Fig. 1A. Rats were food restricted to 85–90% of their free-feeding bodyweight throughout. They were first trained to press one lever (active, fixed ratio 1: FR1) to earn food pellets (45 mg, Bioserv, #F0021; 0.75 protein, 0.5 fat, 2.36 carbohydrate kCal/g); a second lever (inactive) was present throughout, but had no programmed consequence (40-min sessions). After reaching the acquisition criterion (50 pellets earned within a single session), rats were then switched to a variable interval (VI) reinforcement schedule that was made leaner across training (8, 20-min sessions: 2, VI10”; 2, VI30”; 4, VI60”). Lever responses and food cup entries were recorded throughout. Next, rats underwent Pavlovian conditioning in which one auditory cue (CS+, 2-min) was paired with pellet delivery and a second auditory cue (CS−, 2-min) was presented an equal number of times, but was never paired with pellets (8, 60-min sessions; 4 trials/CS/session; CSs: tone and white-noise CS+/CS− counterbalanced). During CS + trials 4 pellets were delivered on a VI30” schedule (range: 15–45 sec). A variable 5-min inter-trial-interval (ITI; range 3–7 min) was used. Levers were unavailable throughout Pavlovian conditioning, and pellet delivery was not contingent upon any response. Food cup entries were recorded throughout. Rats were given an instrumental “reminder” session one day prior to PIT testing. During PIT testing, both levers were available for the entire duration of the test session (40 min), but pellet deliveries were omitted (see Fig. 2A). After 10 min, each CS was presented 4 times in a quasi-random order with a 2-min fixed ITI. Lever responses and food cup entries were recorded throughout. In addition, videos were made during PIT testing sessions following intra-cranial infusions.
Fig. 1.
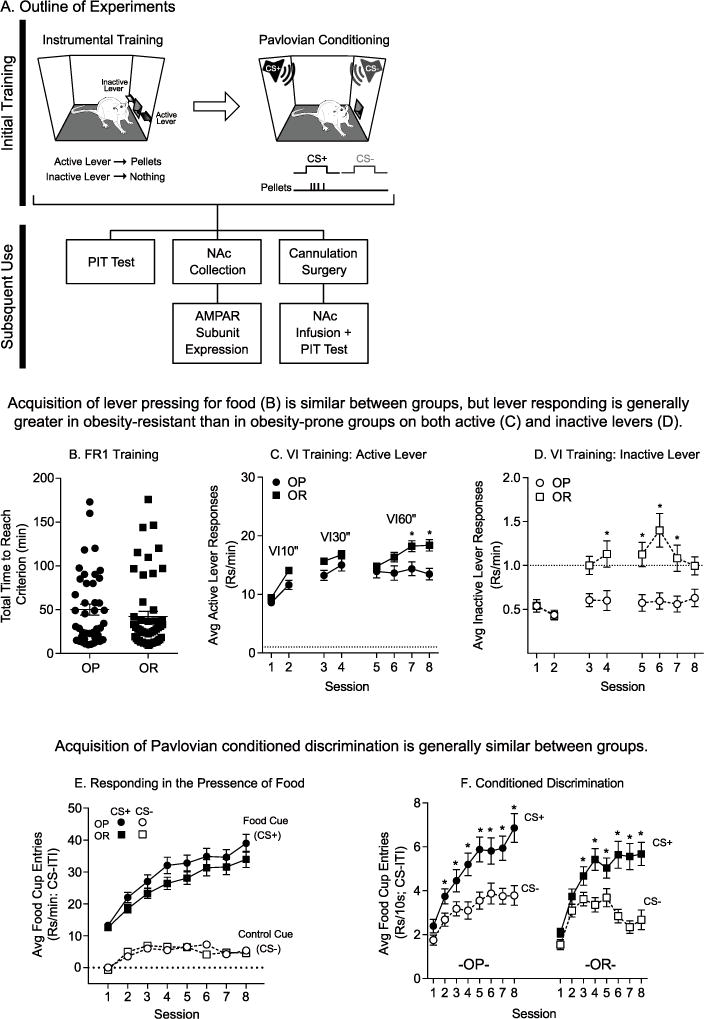
Acquisition of instrumental and Pavlovian responding was similar between obesity-prone and obesity-resistant groups. A) Schematic of training and timeline of studies. All rats received identical training, first undergoing instrumental training and then Pavlovian conditioning. Following training, rats were either tested for PIT (OP n = 20; OR n = 19), used to assess NAc AMPAR expression levels (OP-Trained n = 10; OP-Control n = 6; OR-Trained n = 10; OR-Control n = 6), or cannulated and subsequently tested for PIT following intra-NAc AMPAR blockade (OP: Vehicle n = 11; CNQX n = 8; NASPM n = 8; OR: Vehicle n = 22; CNQX n = 20, NASPM n = 19). B) The total time to reach the acquisition criterion during FR1 training was similar between groups. Values for each subject (circles, squares) and the mean (horizontal line) are shown. C) Average active lever responding during VI training. The average rate of active lever responding increased across sessions in both groups, but the rate of responding was greater in obesity-resistant rats in the final 2 sessions of VI training. D) Average inactive lever responding during VI training. The average rate of inactive lever responding was very low compared to active responding (note difference in scale of y-axis between panels D and C). However, rates of responding were greater in obesity-resistant vs. obesity-prone groups. E) Average food cup entries above baseline (CS-ITI; dotted line = ITI) during Pavlovian conditioning. Rates of responding during the entire CS period were greater during CS+ (closed symbols) vs. CS− (open symbols) presentations, and rates of entries were similar between groups. F) Average food cup entries during the first 10 sec of each CS presentation. Both groups rapidly acquire the discrimination, preferentially responding during the CS+ vs. CS−, although discrimination emerged one session earlier in the obesity-prone vs. obesity-resistant group. * = Sidak’s post-test, p < .05. All data shown as average ± SEM unless otherwise noted.
Fig. 2.
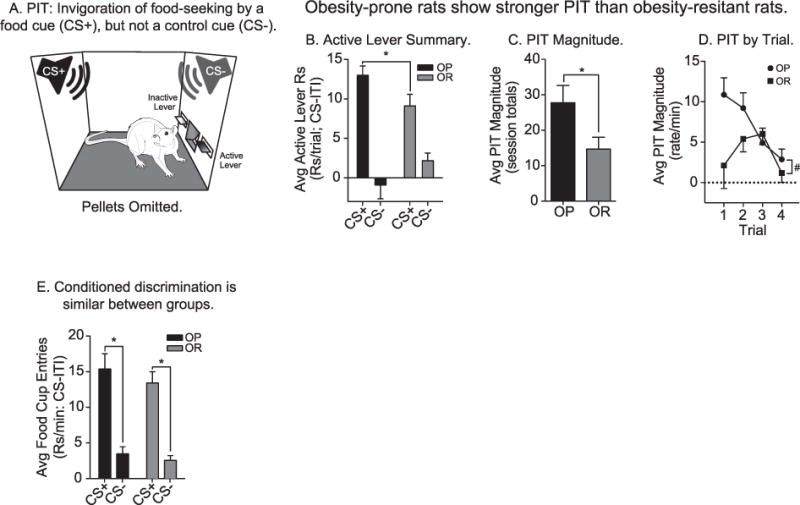
PIT testing: Obesity-prone rats show stronger PIT than obesity-resistant rats. A) Schematic of Pavlovian-to-instrumental transfer test, a measure of cue-triggered food-seeking and incentive motivation (OP n = 20; OR n = 19). B) Average active lever responding above baseline in the presence of the CS+ and CS−. The average rate of responding on the active lever was greater during CS+ vs. CS− in both groups. However, the obesity-prone group responded more vigorously during CS+ presentations than the obesity-resistant group. C) The average magnitude of PIT (i.e., the difference in active lever responding during CS+ vs. CS− presentations) was greater in obesity-prone vs. obesity-resistant rats. D) The average magnitude of PIT across trials was greater in the obesity-prone vs. the obesity-resistant group. The obesity-prone group exhibited classic, robust PIT, which is most prominent in early trials, but slowly declines across repeated CS presentations, whereas the obesity-resistant group showed weak PIT that was variable, and short-lived. E) Conditioned discrimination during PIT testing was present in both groups, with rats preferentially entering the food cup during CS+ vs. CS− presentations. The rate of responding during CS+ and CS− presentations and the magnitude of discrimination was similar between the groups. All data shown as average rate of responding above baseline (ITI) ± SEM; * = p < .05; # = Main effect of group, p < .05.
2.3. BS3 crosslinking and Western blotting
Surface vs. intracellular expression of AMPAR subunits was determined in a separate set of rats using established procedures (Dingess et al., 2017; Oginsky et al., 2016a). Comparisons were made between Trained and untrained Control groups. For the Trained groups, NAc tissue was collected 24 hr after the final instrumental reminder session. This time point corresponded to the time when PIT testing would have occurred. For untrained Control groups, rats were food restricted, handled, and co-housed with their Trained counterparts. Tissue was rapidly extracted on ice, chopped (400 μm), and incubated in ACSF containing BS3 (5 mM) for 30 min (4 °C). Glycine (100 mM) was added to quench the crosslinking reaction after 10 min of BS3 incubation. Samples were centrifuged for 2 min at 14,000 RPM (4 °C). The pellet was re-suspended in ice cold lysis buffer containing: 25 mM HEPES, 500 mM NaCl, 2 mM EDTA, 1 mM DTT, 1 mM PMSF 20 mM NaF; 1:100 EDTA-free Protease Inhibitor Cocktail (Sigma-Aldrige; 11836170001); and 0.1% Nonidet P-40 [v/v]; pH 7.4) and homogenized by sonication. Samples were stored at −80 °C until surface and intracellular GluA1 (Thermo-Scientific; PA1-37776; 1:1000 in TBS) and GluA2 (EMD Millipore; AB1768-I; 1:4000 in TBS-T and 5% milk) protein expression levels were determined using SDS-PAGE and Western blotting as previously described (Boudreau et al., 2012). Bands of interest were quantified using Image J (NIH).
2.4. Post-training surgery and intra-NAc infusions
To assess effects of NAc core AMPAR blockade on the expression of PIT, a separate set of rats was trained as described above, and bilateral guide cannulae were implanted above the NAc core (Plastics Ones: C316G; AP: +1.4 mm, ML: ±2.2 mm relative to bregma; DV: −5.5 mm from skull) under isoflurane anesthesia (2.5–5%). Carprofen was administered pre-operatively and again 24 hr later (2.5 mg/kg, s.c.). Food restriction was lifted prior to surgery and re-applied after recovery (7 days). Next, rats were given 2 instrumental and 2 Pavlovian reminder sessions identical to pre-surgical training. Bilateral infusions of Vehicle, the general AMPAR antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 0.3μg/0.5 μl; 2.58 mM) or the CP-AMPAR selective antagonist 1-Naphthylacetyl spermine trihydrochloride (NASPM: 20μg/0.5 μl; 83.35 mM) were administered prior to PIT testing using a within-subject design. Vehicle solutions were artificial cerebro-spinal fluid (ACSF) for CNQX and 6% Dimethyl sulfoxide (DMSO) in ACSF for NASPM. In pilot studies we determined that PIT behavior was stable across three repeated vehicle infusions and re-training sessions, but became variable with additional infusions. Therefore, we limited each rat to a maximum of 3 infusions, with half of the rats receiving ACSF and half of the rats receiving 6% DMSO in ACSF in the vehicle condition. Importantly, we did not see any behavioral differences between rats receiving DMSO or ACSF vehicle infusions. A maximal dose of CNQX was used in the current study and is based on previous studies (Bell et al., 2000; Ferrario et al., 2010; Pierce et al., 1996). Rats were tested in each condition using a counterbalanced design (3 infusion tests per rat). Infusions were delivered at a rate of 0.25μl/min, and the injectors were left in place for 1 additional minute to allow for diffusion. Rats were then left undisturbed for 5 additional minutes before being moved to operant chambers for testing. After each test, rats were left undisturbed in their home cage for a 24 hr wash out period and were then given reminder sessions as described above. Thus, infusions were separated by 5 days. Cannulae placements and injection sites were confirmed using established histological procedures and anatomical landmarks (Paxinos and Watson, 2007). Analysis of placements was conducted blinded and all data from rats with placements outside of the NAc or with excessive tissue scarring were excluded from all analyses (excluded: OP n = 4; OR n = 7).
2.5. Statistics
Statistical analyses were performed using Graphpad Prism (Version 7.0c) and included: unpaired and paired t-tests, and one-way and two-way RM ANOVAs. Sidak’s multiple comparisons were used for post-hoc and planned comparisons.
3. Results
3.1. Behavior during instrumental and Pavlovian training is similar between groups
To assess incentive motivation in the form of cue-triggered food-seeking (i.e., PIT), rats first underwent instrumental and Pavlovian conditioning in separate sessions (see methods). Three cohorts of rats were used for behavioral, biochemical, and pharmacological studies (Fig. 1A). Behavior during training did not differ significantly across cohorts and therefore the data have been collapsed for ease of presentation in Fig. 1 (total rats trained: OP N = 43; OR N = 52). Prior to food-restriction, obesity-prone rats were heavier than obesity-resistant rats, as expected from previous studies (Vollbrecht et al., 2015; data not shown: OP: 465 ± 13.05; OR: 414 ± 8.38; unpaired two-tailed t-test: t(105) = 3.39, p < .01; note this includes untrained Control rats). This difference is within normal variance for adult Sprague Dawley rats and is representative of the tails of expected weight distributions for males of this age (Lillie et al., 1996). During FR1 training, obesity-prone and obesity-resistant rats reached the acquisition criterion within a similar timeframe (Fig. 1B: unpaired two-tailed t-test, p = .32; OP: 1.79 sessions ±0.14; OR: 1.53 sessions ±0.13). When rats were transitioned to a VI schedule of reinforcement, active lever responding increased as a function VI length, as expected (Fig. 1C: two-way RM ANOVA; main effect of session, F(7,644) = 36.91, p < .01). Additionally, although both groups preferentially responded on the active lever, both active and inactive lever responding was higher in obesity-resistant vs. obesity-prone groups (Fig. 1C: main effect of group, F(1,92) = 6.72, p = .01; session × group inter-action, F(7,644) = 3.23, p < .01; Fig. 1D: two-way RM ANOVA; main effect of group, F(1,92) = 12.05, p < .01; session × group interaction, F(7,644) = 5.29, p < .01).
Next, rats underwent Pavlovian conditioning in which pellet delivery was paired with a CS+ (i.e., food cue), but never with a CS− (i.e., control cue). Data in Fig. 1E show the average rate of food cup entries during CS presentations (note that food cup entries during the CS+ were recorded in the presence of food). In both groups, the number of food cup entries above baseline (ITI responding; dotted line) were greater during CS + vs. CS− presentations and this difference increased across training (Fig. 1E: two-way RM ANOVA; OP: main effect of CS, F(1,42) = 209.8, p < .01; main effect of session, F(7,294) = 47.13, p < .01; session × CS interaction, F(7,294) = 31.00, p < .01; OR: main effect of CS, F(1,51) = 243.2, p < .01; main effect of session, F(7,357) = 41.38, p < .01; session × CS interaction, F(7,357) = 28.18, p < .01). Although CS+ responding here was measured in the presence of food, these data are consistent with the development of conditioned discrimination between the CS+ and CS−. In a subset of the rats, food cup entry data were recorded in 10-sec bins during Pavlovian training (OP n = 23; OR n = 32). Pellet delivery never occurred during the first 10 sec of the CS+. This therefore allowed us to evaluate food cup entries uncontaminated by the pellet and provided a clean measure of conditioned responding (Fig. 1F). As expected, rats acquired clear conditioned discrimination that increased across sessions, with both groups preferentially entering the food cup during the first 10 sec of the CS+ vs. the CS− (Fig. 1F: OP: two-way RM ANOVA; main effect of CS, F(1,22) = 21.86, p < .01; session × CS interaction, F(7,154) = 4.36, p < .01; OR: two-way RM ANOVA; main effect of CS, F(1,31) = 39.16, p < .01; session × CS interaction, F(7,217) = 11.64, p < .01). This conditioned anticipatory discrimination emerged one session earlier in OPs than in ORs. Finally, the rate of entries into the food cup did not differ significantly between groups (Fig E: CS+: two-way RM ANOVA; no main effect of group, p = .15; CS−: no main effect of group, p = .86; Fig. 1F: CS+: two-way RM ANOVA; no effect of group, p = .60; CS−: two-way RM ANOVA; no effect of group, p = .87).
3.2. Obesity-prone rats show robust Pavlovian-to-instrumental-transfer
After training, rats were tested for cue-triggered food-seeking (i.e., PIT; Fig. 2A; OP n = 20; OR n = 19), a classic measure of incentive motivation (Berridge and Robinson, 2003; Wyvell and Berridge, 2000). Pellets were omitted during testing, and the degree to which the CS+ invigorated active lever pressing above baseline relative to the CS− provided a clean measure of the motivational influence of the food cue (Cartoni et al., 2016; Holmes et al., 2010; Lovibond, 1983; Morse and Skinner, 1958; Rescorla and Lolordo, 1965). Obesity-prone rats exhibited significantly greater PIT than obesity-resistant rats, making more active lever responses during CS+ presentations than obesity-resistant rats (Fig. 2B: two-way RM ANOVA; main effect of CS, F(1,37) = 52.80, p < .01; group × CS interaction, F(1,37) = 5.95, p = .02). In addition, the magnitude of PIT (i.e., the difference in active lever responding elicited by the CS+ vs. CS−) was significantly greater in obesity-prone vs. obesity-resistant groups (Fig. 2C: unpaired two-tailed t-test: t(37) = 2.27, p = .03). This difference was also apparent when the magnitude of PIT was examined across trials (Fig. 2D: two-way RM ANOVA; main effect of group, F(1,37) = 5.14, p = .03). Specifically, obesity-prone rats showed a classic pattern of robust CS+ triggered responding during early trials that slowly declined across testing; whereas PIT in obesity-resistant rats was variable and short lived. In contrast, conditioned discrimination, (i.e., the difference in food cup entries elicited by the CS+ vs. CS−) was similar between groups (Fig. 2E: two-way RM ANOVA; main effect of CS, F(1,37) = 92.25, p < .01; no effect of group, p = .67; no group × CS interaction p = .18). Thus, although the CS+ acquired the same predictive significance in both groups, the ability of the CS+ to invigorate food-seeking behavior was stronger in obesity-prone rats.
3.3. Initial training experience is sufficient to increase CP-AMPAR surface expression in obesity-prone, but not obesity-resistant rats
As stated above, NAc CP-AMPARs mediate the “incubation of cocaine-seeking” (Conrad et al., 2008; Lee et al., 2013; Loweth et al., 2014; Ma et al., 2014; Wolf, 2016), and CP-AMPAR up-regulation occurs more readily in obesity-prone vs. obesity-resistant rats (Oginsky et al., 2016a). However, whether CP-AMPARs are involved in PIT is unknown. Therefore, we next determined whether experience during initial training differentially alters surface and intracellular expression of NAc AMPAR subunits in obesity-prone vs. obesity-resistant rats (OP-Trained n = 10; OP-Control n = 6; OR-Trained n = 10; OR-Control, n = 6). Expression of GluA1 and GluA2 differed between untrained Control groups, with greater GluA1 surface expression and lower GluA2 intracellular expression in obesity-resistant vs. obesity-prone control groups (Supplemental Fig. 1: Surface GluA1: unpaired two-tailed t-test: t(9) = 4.42, p < .01; Intracellular GluA2: unpaired two-tailed t-test; t(10) = 4.96, p < .01). Given these differences in the control groups, comparisons were made between Trained and Control groups within obesity-prone and obesity-resistant groups (Fig. 3).
Fig. 3.
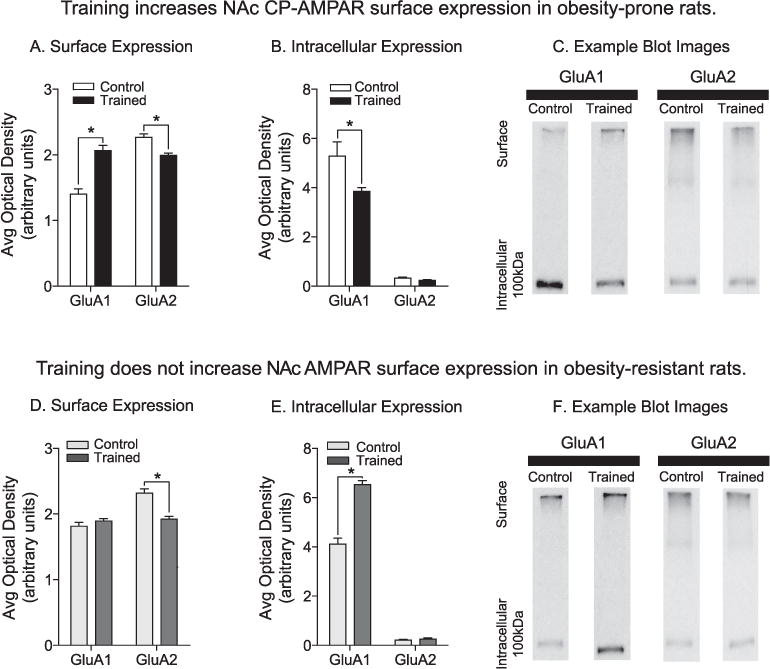
Experience during training increases NAc CP-AMPAR surface expression in obesity-prone, but not obesity-resistant rats. A) Average surface expression of GluA1 and GluA2 subunits in obesity-prone groups: Relative to the Control group (white bars), surface GluA1 expression was increased, whereas surface GluA2 expression was decreased following training (black bars). This pattern is consistent with an increase in GluA2-lacking CP-AMPARs. B) Average intracellular expression of GluA1 and GluA2 subunits in obesity-prone groups. Intracellular GluA1 levels were decreased following training in obesity-prone rats. C) Representative images of GluA1 and GluA2 expression in crosslinked NAc tissue from obesity-prone groups. D) Average surface expression of GluA1 and GluA2 subunits in obesity-resistant rats. GluA1 surface expression did not differ between Control (light gray bars) and Trained (dark gray bars) groups, but GluA2 surface expression was decreased in the Trained vs. Control groups. E) Average intracellular expression of GluA1 and GluA2 subunits in obesity-resistant rats. Intracellular GluA1 expression was increased following training, with no differences observed in intracellular GluA2 expression. F) Representative images of GluA1 and GluA2 expression in crosslinked NAc tissue from obesity-resistant groups. OP-Trained n = 10; OP-Control n = 6; OR-Trained n = 10; OR-Control n = 6; * = p < .05.
In obesity-prone rats, experience during training increased GluA1 surface expression and decreased GluA2 surface expression compared to Controls (Fig. 3A: two-way RM ANOVA; main effect of training: F(1,26) = 11.17, p < .01; subunit × training interaction: F(1,26) = 65.46, p < .01). This was accompanied by a reduction in intracellular GluA1 in Trained vs. Control groups (Fig. 3B: two-way RM ANOVA; main effect of training, F(1,26) = 10.35, p < .01; subunit × training interaction, F(1,26) = 7.92, p < .01). This surface increase in GluA1 expression along with reductions in GluA2 surface expression suggests an increase in GluA2-lacking, CP-AMPARs (Conrad et al., 2008; Oginsky et al., 2016a; Wenthold et al., 1996). In contrast, in obesity-resistant groups training did not alter GluA1 surface expression, but instead produced a significant reduction in GluA2 surface expression (Fig. 3D: two-way RM ANOVA: main effect of training, F(1,25) = 14.61, p < .01; subunit × training interaction, F(1,25) = 33.07, p < .01). On the intracellular level, training in obesity-resistant groups increased GluA1, without altering GluA2 expression (Fig. 3E: two-way RM ANOVA: main effect of training, F(1,25) = 110.10, p < .01; subunit × training interaction, F(1,25) = 102.60, p < .01). This pattern in obesity-resistant rats is not typical of CP-AMPAR increases, but may suggest an intracellular accumulation of GluA1-containing AMPARs (see also section 4.3).
3.4. Blockade of CP-AMPARs in the NAc core prevents the expression of PIT in obesity-prone rats
Given that obesity-prone rats exhibit robust PIT (Fig. 2) and that PIT relies on excitatory transmission in the NAc, we next determined whether AMPAR blockade in the NAc core would prevent the expression of PIT. Although obesity-resistant rats had not displayed robust PIT, nor were increases in GluA1 or GluA2 surface expression found after training, obesity-resistant rats were none-the-less included in these studies because it is still possible that reductions in GluA2 surface expression seen in obesity-resistant rats could alter AMPAR-mediated transmission. Intra-NAc infusions were conducted using a counterbalanced, within-subjects design, however, not all animals are represented in each condition due to unsuccessful bilateral infusions (OP: Vehicle, n = 11; CNQX n = 8; NASPM n = 8; OR: Vehicle n = 22; CNQX n = 20, NASPM n = 19). Importantly, there were no statistical differences between behavior during post-operative vs. pre-operative instrumental and Pavlovian sessions and no significant order effects of infusion were found (data not shown). However, active lever responding during the first 10 min of testing (prior to cue presentation) and during the ITI under Vehicle conditions was significantly higher in obesity-resistant vs. obesity-prone rats (data not shown: pre-cue period OP vs. OR: unpaired two-tailed t-test: t(31) = 4.24, p < .01; ITI OP vs. OR: unpaired two-tailed t-test: t(31) = 2.89, p < .01). Due to these differences in baseline responding, the effects of AMPAR blockade on PIT were evaluated within each group separately (Figs. 4 and 5). Importantly, this did not impede the ability to assess PIT and effects of antagonists, as PIT is defined by differences in responding above baseline in the presence of the CS+ vs. the CS−.
Fig. 4.
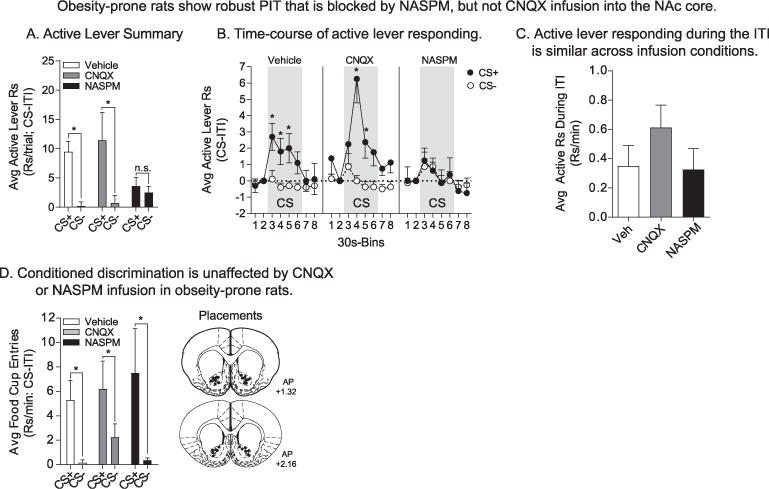
Infusion of NASPM into the NAc core blocks the expression of PIT in obesity-prone rats without altering conditioned discrimination. A) The average rate of active lever responding above baseline during the first CS+ and CS− presentation of PIT testing following infusion. Obesity-prone rats showed robust PIT following Vehicle infusion (n = 11), with presentation of the CS+ but not the CS− eliciting increases in active lever responding (white bars). Infusion of CNQX (0.3μg/0.5μl/hemisphere; 2.58 mM; n = 8) did not alter the expression of PIT (gray bars), but infusion of NASPM (20μg/0.5μl/hemisphere; 83.35 mM; n = 8) blocked PIT by reducing responding during CS+ presentations to levels similar to responding during the CS− (black bars). B) Time-course of active lever responding. Following Vehicle infusion, CS+ presentation elicited an immediate increase in the average rate of active lever responding, whereas the CS− did not. CNQX infusion produced a slight delay in the onset of responding following CS+ presentation, but did not eliminate the expression of PIT. In contrast, NASPM infusion selectively blocked CS+ triggered active lever responding throughout the entire CS presentation period. C) Active lever responding during the ITI was similar across infusion conditions. D) Average food cup entries during CS+ and CS− presentation. Conditioned discrimination was unaffected by infusion conditions. Following Vehicle, CNQX, and NASPM infusions food cup entries were greater during CS+ vs. CS− presentation. Infusion placements are shown at the right; * = p < .05, CS + vs. CS−.
Fig. 5.
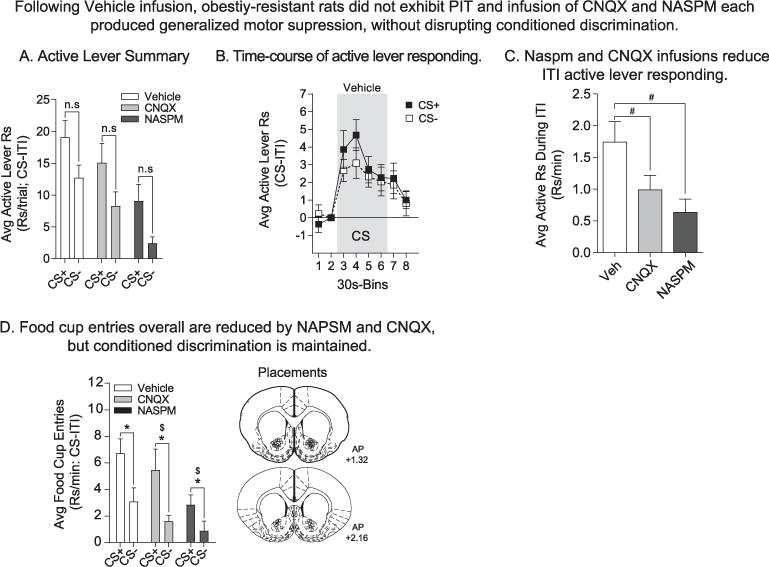
In obesity-resistant rats, PIT was absent following all three infusion conditions, but conditioned discrimination was maintained throughout. A) The average rate of active lever responding above baseline during the first CS+ vs. CS− presentation did not differ significantly following Vehicle (n = 22), CNQX (0.3μg/0.5μl/hemisphere; n = 20), or NASPM (20μg/0.5μl/hemisphere; n = 19) infusion. B) Time-course of active lever responding following Vehicle infusion. The average rate of active lever responding was increased by both CS+ and CS− presentation. Thus, PIT was absent in the obesity-resistant group and a non-specific responding to stimulus presentation was observed. C) Active lever responding during the ITI was significantly reduced by infusion of CNQX and NASPM, consistent with a generalized motoric depression by AMPAR blockade. * = Sidak’s post-test, p < .05. D) Average food cup entries during CS+ and CS− presentation. Conditioned discrimination was present under all three infusion conditions. Rats preferentially entered the food cup during CS+ vs. CS− presentation. In addition, overall rates of entry were reduced by infusion of CNQX and NASPM. * = Main effect of CS, p < .05; $ = Main effect of drug; # = Main effect of drug. Infusion placements are shown at the right.
Total active lever responding during the first CS+ and CS− presentation following Vehicle, CNQX, and NASPM infusion in obesity-prone rats is shown in Fig. 4A, and the time-course of this responding is shown in Fig. 4B (30 sec bins). Effects of infusion on the first presentation of each cue were examined to avoid potential confounds of extinction from repeated CS presentation (see Fig. 2D) and to examine behavior most proximal to drug infusion. Importantly, the order of CS+ vs. CS− presentation was counter-balanced across infusion conditions. Consistent with behavior in intact rats, obesity-prone rats exhibited PIT following Vehicle infusion (Fig. 4A: Sidak’s planned comparison: CS+ vs. CS−: Vehicle, t(22) = 3.40, p < .01). Analysis of the time-course of their behavior shows that the CS+ elicited robust active lever responding that began immediately at CS+ onset, whereas active lever responding was unaltered by presentation of the CS− (Fig. 4B left: Vehicle: two-way RM ANOVA: main effect of CS, F(1,9) = 16.56, p < .01; CS+, closed symbols; CS−, open symbols). Infusion of CNQX did not block the expression of PIT, whereas infusion of NASPM did (Fig. 4A: Sidak’s planned comparison: CS+ vs. CS−: CNQX, t(22) = 3.27, p = .01; NASPM, t(22) = 0.36, p = .97). Furthermore, when the rate of active lever responding was compared between Vehicle and drug infusion conditions, NASPM, but not CNQX, significantly reduced responding during the CS+ with no effect on CS− responding (Fig. 4A: Sidak’s planned comparison: Vehicle vs. CNQX: CS+, p = .81; CS−, p = .99; Vehicle vs. NASPM: CS+, t(32) = 3.15, p < .01; CS−, p .40). This blockade by NASPM was consistent throughout CS+ presentation (Fig. 4B right: two-way RM ANOVA; NASPM: no effect of CS, p = .98, no CS × time interaction p = .92). Although CNQX infusion did not produce robust effects on PIT (Fig. 4A and B middle), the onset of responding was delayed compared to Vehicle conditions, with significant increases in lever pressing only emerging 30 sec after CS+ onset (Fig. 4B middle; two-way RM ANOVA: CNQX: main effect of CS, F(1,7) = 10.75, p = .01). Importantly, infusion of either antagonist did not produce any general motoric effects; active lever responding during the first 10 min of testing (Supplemental Fig. 2A: two-way ANOVA: no effect of drug, p = .77) and during the ITI between CS presentations (Fig. 4C; one-way ANOVA: no effect of drug, p = .96) was similar across infusion conditions. In addition, inspection of videos did not reveal any overt motor effects on behavior. Furthermore, NASPM infusion did not alter conditioned discrimination in approach to the food cup during CS+ vs. CS− presentations (Fig. 4D: two-way RM ANOVA: main effect of CS, F(1,23) = 11.69, p < .01; no effect of drug, p = .65), demonstrating that the effect of NASPM infusion was specific to the expression of PIT. This also confirms that the effect of NASPM is not the result of a loss of discrimination between the cues in obesity-prone rats, but rather is due to an attenuation of the ability of the CS+ to invigorate food-seeking (i.e., incentive motivation).
The pattern of behavior in obesity-resistant rats was very different from that seen in the obesity-prone group. First, obesity-resistant rats did not exhibit robust PIT following infusion of Vehicle, CNQX, or NASPM and differences in the magnitude of responding during either cue did not reach statistical significance (Fig. 5A: Sidak’s planned comparison: CS+ vs. CS−: Vehicle, t(59) = 2.19, p = .09; CNQX, t(59) = 2.23, p = .09; NASPM, t(59) = 2.18, p = .10). The time-course of active lever responding following Vehicle infusion is shown in Fig. 5B. Active lever responding was elevated at the onset of both the CS+ and CS− (Fig. 5B: two-way RM ANOVA: main effect of time, F(7,147) = 13.38, p < .01; no effect of CS, p = .50). Furthermore, compared to Vehicle conditions, both CNQX and NASPM infusion reduced overall rates of active lever responding during CS presentations (Fig. 5A: two-way RM ANOVA: main effect of drug, F(2,59) = 8.29, p < .01), during the ITI (Fig. 5C: one-way ANOVA: main effect of drug, F(2,59) = 4.84, p = .01) and during the 10 min pre-cue period (Supplemental Fig. 2: two-way ANOVA: main effect of drug, F(2,59) = 7.94, p < .01). Thus, in obesity-resistant rats infusion of either antagonist resulted in general motor suppressant effects. These motor effects were also apparent when entries into the food cup were examined (Fig. 5D: two-way RM ANOVA: main effect of drug, F(2,59) = 3.38, p = .04), but were not visually apparent in video recordings. Despite these general effects, the number of entries during the CS+ were significantly greater than during the CS−, regardless of infusion condition (Fig. 5D: two-way RM ANOVA: main effect of drug, F(1,59) = 22.28, p < .01). Thus, although obesity-resistant rats discriminate between the CS+ and CS−, both cues increased active lever responding. The latter is not indicative of invigoration by the food cue (CS+) per se, but rather of a generalized effect of stimuli on responding.
In sum, the data above demonstrate that obesity-prone rats exhibit more reliable, robust PIT that is mediated by NAc CP-AMPARs, and that training produces an increase in surface expression of GluA1-containing AMPARs in this group. In contrast, in obesity-resistant rats the expression of PIT was relatively weak and variable, despite reliable discrimination between the CS+ vs. CS−, and there was no clear evidence for AMPAR increases. Furthermore, in both groups, conditioned discrimination in approach to the food cup was unaffected by NAc AMPAR blockade, supporting a selective role for NAc CP-AMPARS in the transfer of Pavlovian incentive motivation to food-seeking behavior.
4. Discussion
Studies in humans suggest that in obesity-susceptible individuals, stronger motivational responses elicited by food cues drive over-consumption that lead to and maintain obesity (see introduction) and may share neurobehavioral features with drug addiction (Berridge et al., 2010; Dagher, 2009; Ferrario, 2017). However, to date only one preclinical study has examined potential intrinsic differences in cue-triggered motivation in models of susceptibility to obesity (Robinson et al., 2015), and the underlying mechanisms are poorly understood. Differences in motivational responses to food cues may arise from alterations in NAc function, as cue-triggered food- and drug-seeking require NAc excitatory transmission (Corbit and Balleine, 2011; Di Ciano and Everitt, 2004; Fuchs et al., 2004). Here, we found that obesity-prone rats exhibited robust PIT (i.e., incentive motivation) that was mediated by NAc core CP-AMPARs. Additionally, biochemical data suggest that this is due in part to experience-induced increases in NAc CP-AMPAR surface expression. In contrast, obesity-resistant rats displayed weak and variable PIT that was not associated with CP-AMPAR up-regulation. These data demonstrate that incentive motivational responses to food cues are stronger in obesity-prone rats prior to obesity, and establish a novel role for the up-regulation of NAc CP-AMPARs in this form of incentive motivation. Together these data substantiate the idea that enhanced cue-triggered food “craving” is a feature of susceptibility to obesity that may lead to over-eating and weight gain.
4.1. Obesity-prone rats display robust PIT
In two separate cohorts, we found that obesity-prone rats exhibited robust PIT, where presentation of the food cue (CS+), but not the control cue (CS−), selectively invigorated food-seeking in the absence of food itself (Figs. 2 and 4). In contrast, the magnitude of PIT was weak to absent in obesity-resistant rats (Figs. 2 and 5). These differences in PIT expression are not explained by differences in learning, as acquisition of the instrumental and Pavlovian tasks was similar between groups (Fig. 1). Moreover, both groups showed clear conditioned discrimination between the cues during testing, preferentially approaching the food cup during CS+, but not CS− presentations (Figs. 2E, 4D and 5D). Thus, weaker PIT in obesity-resistant groups is not due to an inability to understand the predictive significance of each cue.
We previously found that the magnitude of conditioned approach was greater in outbred rats subsequently identified as susceptible to obesity compared to resistant rats (Robinson et al., 2015). However, here we did not find group differences in conditioned discrimination. This is likely due to the use of food restriction in the current study, which is sufficient to eliminate differences in approach in outbred rats (see, Robinson et al., 2015 for discussion). Additionally, several procedural differences may also contribute (e.g., use of a prolonged CS+ and the inclusion of a CS− here, but not in our previous study; see, Silva and Timberlake, 1997 for discussion). Nonetheless, incentive motivation in the form of CS+ driven food-seeking (PIT) was more robust in obesity-prone rats. This is consistent with enhanced cue-triggered motivation found previously in outbred populations that are susceptible to weight gain (Robinson et al., 2015).
4.2. Experience-induced increases GluA1 surface expression in obesity-prone rats
The expression of PIT relies on activation of the NAc (Corbit and Balleine, 2011), although the role of NAc AMPARs in PIT has not previously been examined. Here, we found that NAc GluA1 surface expression was increased, while GluA2 surface expression was decreased following training in obesity-prone, but not obesity-resistant rats (Fig. 3). This is consistent with an increase in GluA2-lacking CP-AMPARs (i.e., GluA1/1 or GluA1/3 containing AMPARs) and with the role of CP-AMAR up-regulation in the “incubation of cocaine craving” effect (Ferrario et al., 2010; Scheyer et al., 2016; Wolf, 2016). In contrast, in obesity-resistant rats increases in intracellular GluA1 without changes in GluA2 expression were found, suggesting a possible accumulation of intracellular CP-AMPARs. CP-AMPARs can be rapidly recruited to synapses to enhance neurotransmission (Clem and Huganir, 2010). Thus, it is possible that the intracellular accumulation of GluA1 in obesity-resistant rats represents an internal pool of CP-AMPARs, but that either their recruitment and/or retention at the synapse are insufficient for their accumulation at the surface in obesity-resistant rats (see Ferrario et al., 2011 for discussion of retention of CP− vs. Non-CP AMPARs at synaptic sites).
We also found greater GluA1 surface and lower GluA2 intracellular expression in obesity-resistant vs. obesity-prone untrained control groups. This was surprising because we previously reported similar basal NAc GluA1 surface expression between obesity-prone and obesity-resistant rats (Oginsky et al., 2016a). However, in our previous study rats were fed ad lib, whereas in the current experiment rats were mildly food deprived. Indeed, recent studies have shown that food restriction itself is sufficient to produce modest increases in NAc GluA1 expression (Ouyang et al., 2017; Peng et al., 2015). Therefore, the basal differences found here may have arisen from the differential impact of food restriction in obesity-prone vs. obesity-resistant groups. This raises the intriguing possibility that dieting may produce undesired effects in obesity-susceptible populations that will be investigated in future studies. While interesting, this difference between control groups does not interfere with the primary objective of this experiment, which was to assess the effects of training on NAc AMPAR expression in these selectively-bred lines of rats.
4.3. NAc CP-AMPARs mediate enhanced incentive motivation in obesity-prone rats
Consistent with biochemical data, infusing the CP-AMPAR antagonist NASPM blocked the expression of PIT in obesity-prone rats (Fig. 4). Importantly, NASPM did not affect active lever responding during any other phase of testing and left conditioned discrimination intact. Thus, the effect of NASPM was selective to the expression of PIT. CP-AMPAR mediated enhancements in incentive motivation in obesity-prone rats is similar to alterations that drive withdrawal-dependent increases in cocaine-seeking, a key feature of addiction (Conrad et al., 2008; Wolf and Tseng, 2012). This is consistent with the overlap in neural systems underlying incentive motivational responses to food and drug-associated cues, but also raises questions about the degree to which recruitment of NAc CP-AMPARs represents aberrant vs. normal plasticity. In support of aberrant plasticity, the “incubation” of cocaine, but not sucrose craving is associated with increases in NAc CP-AMPAR expression and function in outbred rats (Conrad et al., 2008; Counotte et al., 2014). However, arguing against aberrant plasticity, the expression of PIT is absent in transgenic mice in which synaptic insertion of GluA1-containing AMPARs throughout the brain is prevented (Crombag et al., 2008). This latter study suggests that the recruitment of CP-AMPARs may be part of normal plasticity underlying incentive motivation.
Surprisingly, infusion of the general AMPAR antagonist CNQX did not block the expression of PIT in obesity-prone rats. Although speculative, this may be due to the fact that CNQX is a competitive antagonist, whereas NASPM is not. Thus, the efficacy of CNQX, but not NASPM, is reduced by the presence of glutamate. In addition, the affinity and efficacy of CNQX are altered by AMPAR auxiliary subunits, which likely differ between AMPAR populations (Kawai, 1991; Kott et al., 2009; Maclean and Bowie, 2011; Menuz et al., 2007). Thus, in cases when CP-AMPARs dominate synapses, CNQX may be less effective at blocking AMPAR-transmission. Regardless of these possibilities, the selective loss of PIT following NAc CP-AMPAR blockade in obesity-prone rats is consistent with the up-regulation of these receptors following training (see above) and with the role of these receptors in enhanced incentive motivation for other reinforcers like cocaine (Huang et al., 2015; Wolf, 2016; Wolf and Tseng, 2012).
In the obesity-resistant group, the expression PIT following Vehicle infusion was weak, with both the CS+ and CS− invigorating active lever responding (Figs. 2 and 5). Given the absence of reliable PIT, it is not surprising that neither NASPM nor CNQX had any selective effects on behavior in this group. Instead, we found that infusion of either drug produced a general suppression of lever responding and food cup entries during all phases of testing. Although the mechanistic reason for this effect is unclear, one would expect sufficient blockade of excitatory transmission in the NAc to produce a reduction in general behavioral output. In addition, it is worthwhile to note that at no time did obesity-resistant rats appear lethargic or uncoordinated in their movements (based on videos recorded during testing). However, similar to obesity-prone rats, conditioned discrimination persisted following infusion of either drug. While behavioral dissociations between conditioned discrimination and PIT responding have been established (Delamater, 1996; Delamater et al., 2017b; Lichtenberg et al., 2017), to our knowledge, results here are the first to demonstrate receptor-mediated dissociations between these two behaviors.
4.4. Conclusions
In sum, enhanced incentive motivation in obesity-prone rats is mediated by NAc CP-AMPARs. These neurobehavioral differences may render obesity-susceptible populations more sensitive to the motivational influence of food cues, producing more intense, focused, “wanting” that may limit the ability to divert behavior towards healthier alternatives. These data also demonstrate that in addition to mediating the intensification of cocaine-seeking (Huang et al., 2015; Wolf, 2016), NAc CP-AMPARs also mediate the expression of PIT for a food cue. This raises important questions about whether CP-AMPAR up-regulation represents aberrant vs. normal neural processes that underlie cue-triggered reward seeking behaviors, and the degree to which susceptibility to obesity shares features of addiction.
Supplementary Material
Acknowledgments
This work was supported by NIDDK R01-DK106188 to CRF. RCD was supported by NIDA T32-DA007281 and NIDDK 1F31-DK111194-01. We thank Drs. Travis Brown and Terry Robinson for helpful comments.
Financial disclosure
Dr. Ferrario received funding for this research from the NIDDK, grant number R01-DK106188. Ms. Derman received funding for this research from the NIDA, grant number T32-DA007281 and from the NIDDK, grant number 1F31-DK111194-01.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.neuropharm.2017.12.039.
Footnotes
Both authors of this paper declare having no financial conflicts of interest.
References
- Baladi MG, Daws LC, France CP. You are what you eat: influence of type and amount of food consumed on central dopamine systems and the behavioral effects of direct- and indirect-acting dopamine receptor agonists. Neuropharmacology. 2012;63(1):76–86. doi: 10.1016/j.neuropharm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharm.: Official pub American College Neuropsychophar. 2000;23(3):335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;2:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Milovanovic M, Conrad KL, Nelson C, Ferrario CR, Wolf ME. A protein cross-linking assay for measuring cell surface expression of glutamate receptor subunits in the rodent brain after in vivo treatments. Curr Protoc Neurosci. 2012:1–19. doi: 10.1002/0471142301.ns0530s59. Chapter Unit 5 - Subsection 30. http://europepmc.org/articles/pmc3356776. [DOI] [PMC free article] [PubMed]
- Bouton ME. Learning and the persistence of appetite: extinction and the motivation to eat and overeat. Physiol Behav. 2011;103(1):51–58. doi: 10.1016/j.physbeh.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Brown RM, Kupchik YM, Spencer S, Garcia-Keller C, Spanswick DC, Lawrence AJ, et al. Addiction-like synaptic impairments in diet-induced obesity. Biol Psychiatry. 2017;81(9):797–806. doi: 10.1016/j.biopsych.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KS, Stice E. Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. Neuroimage. 2014;99:122–128. doi: 10.1016/j.neuroimage.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni E, Balleine B, Baldassarre G. Appetitive Pavlovian-instrumental transfer: a review. Neurosci Biobehav Rev. 2016;71:829–848. doi: 10.1016/j.neubiorev.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science (New York, NY) 2010;330(6007):1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci : Official J Soci Neurosci. 2011;31(33):11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Schiefer C, Shaham Y, O’Donnell P. Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats. Psychopharmacology. 2014;231(8):1675–1684. doi: 10.1007/s00213-013-3294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Holland PC, Gallagher M, Huganir RL. A role for alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid GluR1 phosphorylation in the modulatory effects of appetitive reward cues on goal-directed behavior. Eur J Neurosci. 2008;27(12):3284–3291. doi: 10.1111/j.1460-9568.2008.06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A. The neurobiology of appetite: hunger as addiction. Int J Obes. 2009;33(Suppl 2):S30–S33. doi: 10.1038/ijo.2009.69. https://doi.org/10.1038/ijo.2009.69, 2009 Jun. [DOI] [PubMed] [Google Scholar]
- Delamater A. Effects of several extinction treatments upon the integrity of Pavlovian stimulus-outcome associations. Anim Learn Behav. 1996;24(4):13. [Google Scholar]
- Delamater AR, Derman RC, Harris JA. Superior ambiguous occasion setting with visual than temporal feature stimuli. J Exp Psychol Anim Learn Cogn. 2017a;43(1):72–87. doi: 10.1037/xan0000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR, Schneider K, Derman RC. Extinction of specific stimulus-outcome (S-O) associations in Pavlovian learning with an extended CS procedure. J Exp Psychol Anim Learn Cogn. 2017b;4(10) doi: 10.1037/xan0000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci.: Official J Soc Neurosci. 2004;24(32):7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingess PM, Darling RA, Derman RC, Wulff SS, Hunter ML, Ferrario CR, et al. Structural and functional plasticity within the nucleus accumbens and prefrontal cortex associated with time-dependent increases in food cue-seeking behavior. Neuropsychopharmacol.: Official pub American College-Neuropsychopharmacol. 2017;12(10):57. doi: 10.1038/npp.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff IC, Polivy J, Herman CP. The effect of pre-exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite. 1997;28(1):33–47. doi: 10.1006/appe.1996.0057. [DOI] [PubMed] [Google Scholar]
- Ferrario CR. Food addiction and obesity. Neuropsychopharmacol.: Official pub American College Neuropsychopharmacol. 2017;42(1):361. doi: 10.1038/npp.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacol.: Official pub American College Neuropsychopharmacol. 2010;35(3):818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61(7):1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM. ‘I just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. Int J Obes (2005) 2011;35(1):142–149. doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004;176(3–4):459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17(8):1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holmes NM, Marchand AR, Coutureau E. Pavlovian to instrumental transfer: a neurobehavioural perspective. Neurosci Biobehav Rev. 2010;34(8):1277–1295. doi: 10.1016/j.neubiorev.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Hryhorczuk C, Florea M, Rodaros D, Poirier I, Daneault C, Des Rosiers C, et al. Dampened mesolimbic dopamine function and signaling by saturated but not monounsaturated dietary lipids. Neuropsychopharmacol.: Official pub American CollegeNeuropsychopharmacol. 2016;41(3):811–821. doi: 10.1038/npp.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Schluter OM, Dong Y. Silent synapses speak up: updates of the neural rejuvenation hypothesis of drug addiction. Neuroscientist. 2015;21(5):451–459. doi: 10.1177/1073858415579405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Vanreyten A, van Balveren T, Roefs A, Nederkoorn C, Havermans R. Negative affect and cue-induced overeating in non-eating disordered obesity. Appetite. 2008;51(3):556–562. doi: 10.1016/j.appet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kawai N. Spider toxin and pertussis toxin differentiate post- and presynaptic glutamate receptors. Neurosci Res. 1991;12(1):10. doi: 10.1016/0168-0102(91)90095-g. [DOI] [PubMed] [Google Scholar]
- Kott S, Sager C, Tapken D, Werner M, Hollmann M. Comparative analysis of the pharmacology of GluR1 in complex with transmembrane AMPA receptor regulatory proteins gamma2, gamma3, gamma4, and gamma8. Neuroscience. 2009;158(1):78–88. doi: 10.1016/j.neuroscience.2007.12.047. [DOI] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16(11):1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner R, Balsters JH, Burgler A, Hare TA, Wenderoth N. Food-predicting stimuli differentially influence eye movements and goal-directed behavior in normal-weight, overweight, and obese Individuals. Front Psychiatr. 2017;8(230) doi: 10.3389/fpsyt.2017.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273(2 Pt 2):R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- Lichtenberg NT, Pennington ZT, Holley SM, Greenfield VY, Cepeda C, Levine MS, et al. Basolateral amygdala to orbitofrontal cortex projections enable cue-triggered reward expectations. J Neurosci.: Official J Soc-Neurosci. 2017;37(35):8374–8384. doi: 10.1523/JNEUROSCI.0486-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie LE, Temple NJ, Florence LZ. Reference values for young normal Sprague-Dawley rats: weight gain, hematology and clinical chemistry. Hum Exp Toxicol. 1996;15(8):612–616. doi: 10.1177/096032719601500802. [DOI] [PubMed] [Google Scholar]
- Long CG, Blundell JE, Finlayson G. A systematic review of the application and correlates of YFAS-diagnosed ‘food addiction’ in humans: are eating-related ‘addictions’ a cause for concern or empty concepts? Obes Facts. 2015;8(6):386–401. doi: 10.1159/000442403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J Exp Psychol Anim Behav Process. 1983;9(3):225–247. [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2014;76:287–300. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83(6):1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean DM, Bowie D. Transmembrane AMPA receptor regulatory protein regulation of competitive antagonism: a problem of interpretation. J Physiol. 2011;589(Pt 22):5383–5390. doi: 10.1113/jphysiol.2011.219485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuz K, Stroud RM, Nicoll RA, Hays FA. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science (New York, NY) 2007;318(5851):815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- Michaud A, Vainik U, Garcia-Garcia I, Dagher A. Overlapping neural endophenotypes in addiction and obesity. Front Endocrinol. 2017;8(127) doi: 10.3389/fendo.2017.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse WH, Skinner BF. Some factors involved in the stimulus control of operant behavior. J Exp Anal Behav. 1958;1:103–107. doi: 10.1901/jeab.1958.1-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR. Eating ‘Junk-Food’ produces rapid and long-lasting increases in NAc CP-AMPA receptors: implications for enhanced cue-induced motivation and food addiction. Neuropsychopharmacol.: Official pub American College Neuropsychopharmacol. 2016a;41(13):2977–2986. doi: 10.1038/npp.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginsky MF, Maust JD, Corthell JT, Ferrario CR. Enhanced cocaine-induced locomotor sensitization and intrinsic excitability of NAc medium spiny neurons in adult but not in adolescent rats susceptible to diet-induced obesity. Psychopharmacology. 2016b;233(5):773–784. doi: 10.1007/s00213-015-4157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J, Carcea I, Schiavo JK, Jones KT, Rabinowitsch A, Kolaric R, et al. Food restriction induces synaptic incorporation of calcium-permeable AMPA receptors in nucleus accumbens. Eur J Neurosci. 2017;45(6):826–836. doi: 10.1111/ejn.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson CJ. The Rat Brain in Stereotaxic Coordinates. sixth. Academic Press; 2007. [Google Scholar]
- Peng XX, Lister A, Rabinowitsch A, Kolaric R, Cabeza de Vaca S, Ziff EB, et al. Episodic sucrose intake during food restriction increases synaptic abundance of AMPA receptors in nucleus accumbens and augments intake of sucrose following restoration of ad libitum feeding. Neuroscience. 2015;295:58–71. doi: 10.1016/j.neuroscience.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci.: Official J Soci Neurosci. 1996;16(4):1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Lolordo VM. Inhibition of avoidance behavior. J Comp Physiol Psychol. 1965;59:406–412. doi: 10.1037/h0022060. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, et al. Individual differences in Cue-induced motivation and striatal systems in rats susceptible to diet-induced obesity. Neuropsychopharmacol.: Official pub American College Neuropsychopharmacol. 2015;40(9):2113–2123. doi: 10.1038/npp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, et al. AMPA receptor plasticity in accumbens core contributes to incubation of methamphetamine craving. Biol Psychiatry. 2016;80(9):661–670. doi: 10.1016/j.biopsych.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva KM, Timberlake W. A Behavior systems view of conditioned states during long and short CS–US intervals. Learn Motiv. 1997;28(4):465–490. [Google Scholar]
- Small DM. Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes (2005) 2009;33(Suppl 2):S44–S48. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev. 2013;37(9 Pt A):2047–2058. doi: 10.1016/j.neubiorev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol. 2013;48(1):1–19. doi: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. The addictive dimensionality of obesity. Biol Psychiatry. 2013;73(9):811–818. doi: 10.1016/j.biopsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht PJ, Nobile CW, Chadderdon AM, Jutkiewicz EM, Ferrario CR. Pre-existing differences in motivation for food and sensitivity to cocaine-induced locomotion in obesity-prone rats. Physiol Behav. 2015;152(Pt A):151–160. doi: 10.1016/j.physbeh.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Wiers RW, Hommel B, de Wit S. Working for food you don’t desire. Cues interfere with goal-directed food-seeking Appetite. 2014;79:139–148. doi: 10.1016/j.appet.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci.: Official J Soc Neurosci. 1996;16(6):1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17(6):351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35(2):185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5(72) doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci: the official jSoc Neurosci. 2000;20(21):8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


