Abstract
Purpose
This paper presents an overview of different kinds of risk and social network methods and the kinds of research questions each can address.
Recent findings
It also reviews what network research has discovered about how network characteristics are associated with HIV and other infections, risk behaviors, preventive behaviors, and care; and discusses some ways in which network-based public health interventions have been conducted.
Summary
Based on this, risk and social network research and interventions seem both feasible and valuable for addressing the many public health and social problems raised by the widespread use of opioids in the US South.
Keywords: Social networks, risk networks, opioid users, PWUD, phylogenetics, respondent-driven sampling, quasi-networks, HIV, behaviors
Introduction
Why are social and risk networks important?
The Southern opioid epidemic poses many questions that network research may help us resolve. These questions include: 1. why some people take up opioid use and others do not; 2. why some opioid users become injectors; 3. how infections like HIV, hepatitis C, and sexually transmitted infections spread through opioid using sub-communities; 4. how the practice of carrying naloxone to help others who overdose spreads through communities; 5. the processes through which norms towards naloxone change; and 6. how other preventive and harm reduction norms spread through communities at risk. Understanding these processes may help us reduce the current high overdose rates among opioid users, for example.
To understand behaviors like opioid use, opioid injection, or naloxone use, we need to know how messages, norms, emotional support and other forms of social influence spread between individuals and through communities. Furthermore, to the extent to which we can understand how networks form and then are shaped among groups of people, we may be able to discover ways to help communities form safer rather than riskier networks. We also need to study how opioid use itself reshapes users’ and other people’s social connections or support networks.
Thus, there are many mechanisms through which network factors can influence opioid use, likelihood of infectious disease transmission, and overdose risk. Some of the research on this is described below.
To understand who gets infected, and how infections spread through communities, we start with basics: Infections like HIV spread if an infected person engages in high-risk injection or high-risk sex with an uninfected person—who then, in turn, becomes a potential transmitter. Thus, HIV travels between connected members of network pairs (i.e., dyadic networks) which are in turn situated within larger network structures, so a person’s position within a larger community can have an impact on how likely they are to come in contact with infected partners and be exposed to HIV or HCV, as well as how many additional infections are likely to result if they become infected.
Social and risk network research is a way to collect data on these processes, and is described below. In addition, since networks are important shapers of behaviors and of infection transmission, they are also potentially important as tools for interventions. All of this is explained more, and exemplified, later in this paper.
But what is network research?
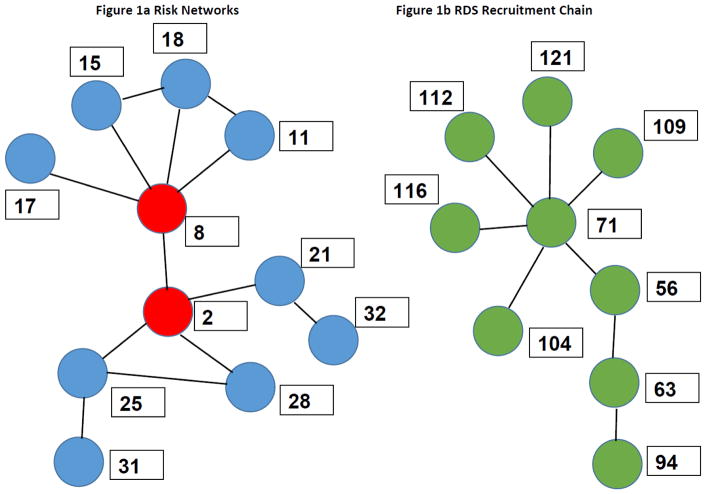
Network research starts with links between two people (See Figure 1, which explains network terminology graphically). If these links involve risk behaviors or the transfer of infected equipment or material, they can be thought of as “risk networks,” and it is tautological (given decades of HIV research) that HIV transmission occurs through such “risk links.” If the networks involve social influence or communication, or other social relationships, they can be thought of as “social links,” and it is well known that social links can sometimes affect risk behaviors, protective behaviors, group norms, health-seeking or adherence-relevant behaviors and much else of interest to HIV and drug use research, prevention and care [1]. The study of risk and social networks, however, does not consist only of the study of such “dyadic” links between two people. More importantly, it also includes the study of “egocentric networks” which consist of all the links of one or more sorts that a given focal participant “Ego” has with her directly connected network members (“Alters”); and also the community networks (sometimes called sociometric networks) that consist of all of Ego’s links with Alters, all of the Alters’ links, and so on ad infinitum. Egocentric networks, then, are useful for studying the risks and influences that Alters have on Ego and, conversely, that Egos have on their Alters; and community networks are useful to understand the spread of infections, behaviors, information, resources or norms through a community.
Figure 1.
Figure 1a. Modified sub-network of the TRIP project data from Athens showing enhanced risk network links among two recently-HIV-infected participants (in red) and 9 HIV-uninfected participants (blue). (Long-term-infected participants have been excluded from this Figure for reasons explained in the text.) In terms of network terminology, participants 2 and 8 form a dyad; and if participant 2 is Ego, participants 8, 21, 28 and 25 are Alters in 17’s egocentric network. The participants in Figure 1 form part of Ego’s connected component within a larger community network that includes long-term positives excluded from Figure 1 plus additional participants and non-participants who are linked to any of those in Figure 1 or, through chain-linkage, at greater network distance from Ego.
Consider now participant 25’s egocentric network (consisting of participants 31, 28, and 2). The egocentric network is sometimes called 25 first ring since its members are at network distance 1 from Ego 25. Ego 25’s second ring then consists of participants 21 and 8 (at distance 2 from Ego 25; and participants 32, 11, 18, 15 and 17 are Ego 232’s third ring.
Figure 1b. By contrast, RDS cannot detect certain characteristics of the community network in Figure 1b since it only collects data on recruitment chains which have dendritic structures that do not fill in other links. For example, in network data, we might learn that person 104 injects drugs with 56, 109, and 112; and that each of these was also linked to 116 and 94—which would indicate many additional paths through which infection could be transmitted.
But how does one study networks? Here, it will be useful to describe several approaches common in the literature.
One sort of data, which we have called “quasi-network data,” is simply obtained by asking a research participant how many network members they have of a given sort—for example, how many people they have had sex with in the last 30 days; or, for a more precise risk quasi-network, how many HIV+ sex partners or how many people who inject drugs (PWID) they have had sex with in the last 30 days. To ascertain their social quasi-network characteristics, one might. For example, ask Ego how many people had urged her to take her antiretroviral medicines in the last 30 days—which is also a measure of the norms of Ego’s contacts [2, 3].
Getting risk and social network data of the kind we have used in many studies (as discussed below) involves not only getting the numbers of such Alters, but also getting enough data so that you can study the nature of each link and the characteristics of each Alter.
To study the community networks of people who use drugs (PWUD), you need to interview and perhaps collect specimens from both Egos and these Alters; and in most designs, use some form of snowball or network sampling design to study the Alters of the Alters and so forth until some set or practical number of steps (network links) away. A critical part of community network studies is to get enough information about the participants so that you can then determine which Alters named in interviews are a given participant whom you interviewed Typically, this takes considerable time to accomplish, although linking software can make this easier (see below). Where ethically feasible, this will usually require getting considerable information about participants’ names, nicknames and contact information so you can study how different participants’ egocentric networks combine to form community networks [4].
In practice, getting data with which to conduct this matching can be difficult [5]. In some localities, drug users have strong norms against giving other people a drug user’s name or contact information. This is in part a protection against police, and in part a result of heavy stigmatization against drug users. Research or intervention projects with good reputations among drug users and strong protections for confidentiality can often nonetheless collect these data—but this requires careful selection, training, and supervision of interviewers, since many interviewers will be reluctant to ask such questions or be unable to establish sufficient rapport with the participants to make it work. Another difficulty posed by network designs is that staff who are appropriate for interviewing or providing services to opioid users might be less able to form rapport with network members with different characteristics (like people who frequently inject amphetamines, men who have sex with men, or sex workers.) This can lead, once word gets out, to under-recruitment of such network members and thus to biased ascertainment of networks.
A related design, Respondent-Driven Sampling (RDS), can be modified to collect partial network data. RDS was not developed as a technique to study networks, but rather as a method to create unbiased estimates of the proportions of given “hidden populations” who are not easily sampled, such as opioid users with specific characteristics like being male, being infected with HIV or engaging in anal sex. (The extent to which RDS succeeds in creating unbiased estimates is unclear.) RDS starts with Egos called “seeds.” Each seed is asked quasi-network questions like how many people he or she knows who engage in some relatively rare characteristic or behavior like non-medical use of prescription opioids, sex trading or attending group sex events. They are also given a set number of coupons and asked to give them to people they know who (for example) have used opioids in a given time period. When Alters come in with those coupons, they are interviewed, and receive coupons with which to recruit people they know who engage in the target behavior. The quasi-network data on numbers of Alters respondents reported knowing are later used to statistically adjust the sample that is recruited to estimate the population proportions with given characteristics. Social and risk network data can be collected on the relationships of recruits to seeds (but not usually vice versa) by asking each Alter who brings a coupon in about his or her relationship with the seed who gave him or her the coupon. (This assumes that the earlier participant is the one who gave the Alter the coupon—which may not be the case if the coupon passes through several hands.)
A final related type of data that can be used in network studies is based on genotyping infectious agents such as HIV, hepatitis C or bacterial STIs. These data can be used to study transmission chains of the infectious agent. Phylogenetic studies have been very helpful in describing the geographical dimensions of infection chains and, in addition, the history of viral transmission across geographic boundaries [6–9]. These issues cannot be measured from network data since social links or risky behaviors between Alters and Egos often do not result in transmissions. Molecular analysis can reconstruct the viral genealogy as a proxy of the transmission chain and use phylodynamic methods to estimate historical dimensions of infection.
The relationship of phylogenetic and network data is quite complicated. First, phylogenetics studies relationships among pathogens collected from infected individuals, so phylogenetic data contain no information about uninfected participants within the phylogenetic transmission chains and no information about how or if uninfected people fit into the risk or social networks of the infected. This greatly restricts the use of phylogenetic data in studies of how social and risk networks affect infection dynamics at either the community or individual level. Another complication is that standard phylogenetic analysis studies the probabilities that a given collection of specimens had a given set of pathways among them. No information is available, however, on how many other unstudied people may have been infected as intermediaries on the computed infection path. Thus, efforts to combine these data with risk network data have to cope with considerable potential missing cases in both sorts of data.
Despite these difficulties, however, designs that include both phylogenetic and social/risk network data and analysis seem useful [10–12]. For example, evidence of short phylogenetic distance between two people’s infectious agents can be used along with other matching data in trying to determine risk network links among participants [6–9,].
A case study to illustrate some strengths and weaknesses of quasi-network data and community network data
The Transmission Reduction Intervention Project (TRIP) is a network-based study that used the networks of recently-HIV-infected people to recruit other recently infected people. Since the methods used in the three cities where data were collected during 2013 – 2016 have been described elsewhere [14–16], here we briefly present the methods for TRIP’s Athens site in Greece.
We started with “seeds” who were likely to have been recently-infected who were referred to us by another research project or other sources. Other (longer-term infected) seeds who were HIV+ but not recently infected were matched with these “recent seeds” on risk group, age and gender. All seeds (and Alters we recruited from their networks) were interviewed using a questionnaire that included items about demographics, sexual and injection behaviors, drug treatment, ART use, stigma and access to care. Participants were asked to name people they injected or had sex with in the past six months; people who injected or had sex in their presence in the past six months; and people who injected, used drugs or had sex with people the participants had injected or had sex with. They were also asked to specify places they usually visit to use drugs, have sex, or meet new sex partners. We then attempted to interview all named Alters and to recruit others who injected drugs at the named venues. We carried out this procedure for a minimum of two steps from each seed. Data on links among Alters were carefully studied and additional efforts were made to determine which Alters named by different participants were the same person so we could conduct community network analyses. Since the network links include both direct risk links (in which two people have engaged in risk behaviors together) and also social links that suggest that people might be vulnerable to infection by a virus deriving from the same possibly un-recruited Alter, we refer to the TRIP network as an “enhanced risk network.”
Comparing the numbers of reported injection or sex partners of the recently-infected seeds and their ring members with the numbers of the members of Ring 1 below (Table 1), it is clear that we recruit and identify only a subset of total partners of the seeds. Adding in the members of Ring 2 shows how these numbers change as we begin to consider the community aspects of networks. Since quasi-network data thus include data about partners whom a network study does not recruit, they are more useful for analyses where the total N of partners (or of social influence ties) is the independent variable we want to study.
Table 1.
Comparing quasi-network data and enhanced risk network data in Athens TRIP study
| Network position and HIV status | # they say they injected with in quasi-network data* | # they say they had sex with in quasi- network data* | # of network members we recruited |
|---|---|---|---|
| 45 Recently infected seeds | 220 | 353 | 66 |
| Ring 1: The recruited network members of the seeds | |||
| 66 members of network ring 1 | 570 | 168 | 93 |
| Ring 2: The recruited network members of Ring 1 members | |||
| 93 members of network ring 2 | 718 | 382 | Not attempted |
Note that some members of the injection and sexual quasi-networks may be the same person. Thus, the size of the total risk quasi-network of the 45 recently-infected seeds is at least 353 and no more than 573
There are important research topics for which dyadic, egocentric or community network data are needed. Dyadic data let us use relationship variables as a level of analysis. In earlier dyadic studies, we showed that consistent condom use is less likely in very close relationships and among participants who report that their peers’ norms support condom use among samples of young adults who (a) do not use “hard drugs;” and (b) separately among young adults who do use hard drugs, as well as (c) among PWID. Dyadic data also let us show that, among PWID, consistent condom use is much more likely in partnerships of HIV+ PWID with partners who do not inject drugs. [17–19] Egocentric data let us study how the characteristics of the set of partners are related to characteristics of Ego. (If we only consider these characteristics of Alters from what Ego reports about them, we will sometimes have larger egocentric networks to analyze than in community network studies because some of the partners Ego reports on will not be recruited.) Using data of this kind, Neaigus et al. (1995) [20] showed that having a high-risk member in a new drug injectors’ egocentric network was a more powerful predictor of Ego’s being HIV-infected than any of Ego’s risk behaviors. Friedman et al [21; pp. 210 – 215] used egocentric network data to help explain why women become infected earlier in their injection careers than do men injectors. It should be noted, however, that sometimes the egocentric networks ascertained from community network studies have larger numbers of Alters than those obtained by using only Ego’s self-reports. This was true for some of the most network-linked participants in our study of PWID networks in Brooklyn in the early 1990s because some operators of shooting galleries (where people went to inject drugs away from observation by police or the public) reported having very few partners, but many other PWID reported that these shooting gallery operators were their partners [21].
Community network studies are difficult to conduct, but they can answer questions other designs cannot. In our first community network study in Brooklyn in the 1990s, we found that PWID with a certain community network characteristic—being a member of the “Seidman 2-core” of the largest connected component1 (which essentially means being in the most linked-together part of the community network) were more likely that other PWID to: be HIV+; to show evidence of prior hepatitis B infection; to engage in higher levels of risk behavior; and to be more likely to tell other PWID about ways to protect themselves against HIV infection [22]. It should be noted that RDS data cannot be used to study phenomena of this type since they only collect “dendritic data” and cannot detect “cyclic” patterns. (See Figure 1b.) A later paper showed how community network characteristics and the natural history of HIV can slow HIV transmission through the community [23]. This paper is described below (in #4 in the section after next).
How to elicit names and how to do matching
In community network studies among “hard to reach” populations like PWUD, eliciting information on Alters can be challenging. Most network studies of PWUD rely on participants’ self-report about Alters [4, 24–32]. If the study aims to go beyond egocentric network data and understand the overall connectivity among the sample, it is critical to collect identifying information about Alters from Egos and to collect similar, detailed information about Egos to enable cross-referencing [27, 33]. Typically, participants are asked to recall some variation of their Alters’ names, street names and demographic characteristics, although some studies have asked about Alters’ physical appearance [e.g., 34] and/or variations of their phone numbers [e.g., 35] to assist with matching. Given stigma and legal consequences surrounding self-report of substance use and drug co-using relationships, as well as confidentiality and privacy concerns, the accuracy of such Alter data may be limited. However, recent analyses of network data from rural PWUD revealed that participants who reported relationships with other participants gave Alters’ exact name and age within two years in 75% and 79% of those relationships that they chose to report, respectively [4].
In a process known as entity resolution, study staff cross-reference Alter descriptions with each other and with Ego data to determine when two Egos have named the same Alter and/or when an Ego has named another Ego (a process described in more detail elsewhere [4]). Entity resolution can be complicated by data inaccuracies, the use of nicknames, and the sheer amount of information that has to be cross-referenced. To assist with the process, name cross-referencing algorithms such as fuzzy matching, soundex [36], q-gram [37], and phonex [38] are helpful in generating a list of possible matches that can then be pared down based on comparisons of demographic data. Standard software programs and Microsoft Excel can implement fuzzy matching, but software programs designed specifically for entity resolution in network data like SPIDER [39] and Linkalyzer [40] have a broader suite of algorithms. Depending on sample size and the power of the algorithms, multiple possible matches can be identified for the same reported Alter, although some matches may be questionable due to strong matches on one important characteristic (e.g., name) but poor matching on another (e.g., age). In these cases, involving field staff familiar with the community and participants in reviewing and confirming/disconfirming possible matches can be helpful. This process has been implemented successfully in a longitudinal, rural study of PWUD and is described in detail elsewhere in this Special Issue [41]
A few epidemiologic findings from studies of the networks of people who inject drugs and their communities
To show why network studies are important, this section briefly summarizes a few findings from network studies in which authors of this paper have been involved. These could not have been discovered in the absence of network research. First, here are some findings from the Brooklyn study of the networks of people who inject drugs in the early 1990s.
-
1
Comparison of the Brooklyn networks with those in Colorado Springs (a city in which the HIV-infected participants were all in small networks or were outside of the high-risk Seidman 2-core) suggested that epidemics were unlikely to occur unless members of the 2-core of a large network got infected [25, 27].
-
2
Personal (egocentric) injection network characteristics are at least as important as risk behaviors in predicting HIV infection among new injectors [24]. Unpublished data from Ukraine suggest that having a previously-undiagnosed infected PWID in a nearby recruitment chain location (in RDS) is associated with HIV seroconversion (Yana Sazanova unpublished analyses).
-
3
The combination of community network location and personal network factors like injecting or having sex with older PWID or MSM help explain why African American PWID are more likely to be HIV infected [42] and why women who inject drugs get infected earlier in their injection careers than men [25].
In the TRIP project discussed above, we found that:
-
4
The patterns of interconnected risk networks (their “topology”) is important. Together with the fact that people who become infected with HIV are particularly likely to infect others during their first few months of infection (as compared to after they have been infected longer), these network topologies help us understand the histories of HIV epidemics in different cities. Specifically, they help us understand why epidemics among PWID in cities level off and then decline rather than leveling off for a while and later shooting upwards. The dynamic behind this is that under typical conditions in PWID communities—which have multi-person equipment-sharing networks but only moderate to low rates of network turnover (other than due to death and incarceration)—already-infected PWID whose immune responses have lowered their viral load can become “firewalls” that block potential transmission chains against explosive epidemic spread driven by high-viral-load early infection [23, 43–46]. In Figure 1a, we removed the long-term HIV-positives from the network diagram as part of an analytic effort to develop a “virus eye view” that shows the extent to which the firewall phenomenon is not operative [44, 47]. We can see from the figure that the recent infections of participants 2 and 8 have at least nine uninfected participants they might easily spread to.
In a later study of young adults and drug injectors in the same neighborhood, we found that:
-
5
Using network data from this study, we identified 30 HIV-discordant partnerships. Of these, five were same-sex male partnerships and 25 were opposite-sex partnerships. No subjects tested positive for syphilis or gonorrhea. Two couples were chlamydia-discordant. For HSV-2, 16 couples were double-positive, eight discordant, four double-negative, and two comprised an HSV-2-negative person with a partner with missing herpes data. These findings both showed the frequency of herpes in the New York epidemic and raised questions about why the partnerships with herpes present remained HIV-discordant [48].
-
6
A large proportion of participants attended group sex events. These events commonly included a mixture of heterosexuals, men who have sex with men, women who have sex with women, and people who inject drugs. The data we collected on event participants are not network data—but are another form of data on “mixing patterns” that have similar implications for HIV and other infectious diseases [49].
Studies done in Baltimore among people who use drugs found that:
-
7
PWID who are HIV positive are more likely to have other social network members who are HIV positive than are PWID who are uninfected [50]. These patterns are not simply due to direct transmissions among risk partners. Rather there is differential affiliation of high risk individuals occupying the same risk network and indirect transmission through individuals who are not assessed. These findings highlight the potential value of testing PWID peers of PLWH as well as specific risk partners.
-
8
Social norms are strong predictors of injection risk behaviors and successful behavior change interventions can change injection-related social norms [51, 52]. One network approach to promote harm reduction is to identify individuals based on network structural characteristics like centrality and to train these highly-connected people to promote risk reduction among network members.
-
9
Frequently, PWID acquire resources to purchase drugs with their drug-using network members, and then share drugs and frequently injection equipment with network members.
-
10
In egocentric network studies among injection and non-injection drug users, social network factors have been found to be associated with history of drug overdose, drug treatment entry, cessation of drug use and being HIV positive [53–57]. Similar patterns were found for PWID in an RDS study in Athens [58].
-
11
HIV risk networks are not the only important networks for HIV prevention and care among PWUD (and PWID) samples. Most health care is conducted by informal caregivers, who are social network members. Social support has been found to be strongly associated with ART adherence [59–60]. For individuals who are not virally suppressed, training network members to provide supportive care may improve HIV care outcomes.
-
12
Lesbian and bisexual women PWID have fewer financial, material, and health resources in their social networks, and experience substantial socioeconomic disparities, than heterosexual women who inject drugs. This may increase their engagement in illicit income generation activities that enhance risk for HIV and incarceration, especially sex exchange behaviors [61].
-
13
The interplay between socioeconomic marginalization and social network marginalization among lesbian and bisexual PWID may contribute to their being more likely that heterosexual women PWID to engage in illicit income generation activities that enhance risk for HIV and incarceration, especially sex exchange behaviors.
Using extended risk networks in interventions: The TRIP study
We discussed the TRIP study above when we described advantages and disadvantages of various types of network data. TRIP was also an intervention study. Its main aim was to find ways to recruit recently-infected people so we could refer them to antiretroviral treatment while they were still highly infectious, and so we could warn their network members to use extreme care to avoid risk for several months since there were recently-infected people in their networks. Nikolopoulos et al. [18] showed that, in Athens TRIP, recruiting members of enhanced risk networks of recently infected PWID led to recruiting recently-infected people at higher rates than did tracing the enhanced risk networks of injectors who were out of the recently-infected stage. Findings from Odessa, Ukraine and as-yet unpublished analyses of data from Chicago, IL, find that the TRIP network methods locate more undiagnosed HIV-positives at lower cost than case-finding techniques currently used in those cities [62]. In Chicago, TRIP also effectively identified people with active syphilis infection and with known HIV [63].
What do we know about young opioid users’ networks?
Little research has been conducted on the networks of the current generation of young opioid users in the United States. The paper by Young in this issue, however, presents some network findings from Kentucky. Here, we briefly present data from a study that interviewed young opioid users in New York City—a setting clearly very different from that of Appalachia. Methods for this research have previously been described [64, 65]. A limited amount of quasi-network data from this study are presented in Table 2. Most respondents, regardless of sex or race/ethnicity, knew two or more opioid users in New York City. Four-fifths have injected with five or more people in the last three months. Four-fifths had at least one sexual partner, and about a third had two – five partners. The distributions by race/ethnicity and by sex of all these variables were similar. Although only five participants were infected with HIV, the large size of these quasi-risk networks may indicate a high potential for rapid spread among young opioid users if the virus penetrates into parts of the networks with high network centrality and even moderate risk behaviors.
Table 2.
Composition of the Quasi-Networks of Young Opioid Users in New York City (and of those who recruited non-seeds in the RDS process)
| Total (n = 539) | Female (n = 174) | Male (n = 356) | White (n = 335) | Non-White (n = 207) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2–5 | 5+ | 0 | 1 | 2–5 | 5+ | 0 | 1 | 2–5 | 5+ | 0 | 1 | 2–5 | 5+ | 0 | 1 | 2–5 | 5+ | |
| How many people in New York City do you know personally who use POs | 26 | 44 | 123 | 124 | 2 | 15 | 44 | 43 | 24 | 29 | 77 | 79 | 19 | 29 | 93 | 94 | 7 | 14 | 30 | 30 |
| proportions | .08 | .14 | .39 | .39 | .02 | .14 | .42 | .41 | .11 | .14 | .37 | .38 | .08 | .12 | .40 | .40 | .09 | .17 | .37 | .37 |
| In the past 3 months, how many people have you injected drugs with | 17 | 14 | 80 | 428 | 5 | 4 | 25 | 136 | 11 | 10 | 55 | 289 | 12 | 6 | 53 | 261 | 5 | 8 | 26 | 165 |
| proportions | .03 | ..03 | .15 | .79 | .03 | .02 | .15 | .80 | .03 | .03 | .15 | .79 | .04 | .02 | .16 | .79 | .02 | .04 | .13 | .81 |
| In the last 90 days how many people did you have vaginal; anal; or oral sex with | 68 | 239 | 192 | 40 | 13 | 93 | 58 | 6 | 55 | 144 | 132 | 34 | 42 | 167 | 105 | 18 | 26 | 70 | 86 | 22 |
| proportions | .13 | .44 | .36 | .07 | .08 | .55 | .34 | .04 | .15 | .39 | .36 | .09 | .13 | .50 | .32 | .05 | .13 | .34 | .42 | .11 |
Probability of difference in trend by sex as ascertained by Pearson’s r.
Probability of difference in trend by race/ethnicity as ascertained by Pearson’s r.
Conclusions: Implications for the opioid epidemic in the US
This paper has reviewed why networks are important, summarized some epidemiologic findings about how networks affect disease and prevention, and described some network interventions. What are the take-home messages from this?
Perhaps most important is that social and risk networks exist. Risk and prevention do not only involve behaviors and what agencies can say about them to people at risk. People who use drugs and members of their communities are pro-active agents who can and do attempt to help each other. This means that programs that work with networks to distribute syringes, naloxone or messages throughout communities at risk can be very effective. Programs that mobilize social support to help people adhere to treatment, or that use network recruitment techniques like those TRIP developed to recruit newly-infected or undiagnosed positives for testing and care can also be effective.
In research terms, we know little about the social, sexual and injection networks of opioid users in epicenters of youthful opioid use, as in the southern United States. We also know little about what social forces shape these networks. Research to learn about these issues can help us in developing effective interventions. Some of these methods have been outlined in this article.
Finally, network research is only one aspect of social research that can help us address opioid use and its associated problems. Sociocultural studies to understand and assess the most effective tactics for local intervention will also be helpful. In addition, we are aware of how little we understand about why so many people are using opioids. In part, it is due to dependency that developed from pain medications and also diversion of such medicines—but this does not explain for whom and where pain needed to be alleviated nor why different localities have different openness on the part of youth and adults to taking up voluntary opioid use. Research on these issues, including socio-geographic studies of overdose and of opioid use, might help the field develop a much deeper understanding of drug use, what it means, and how to address it.
Acknowledgments
This research was supported by the United States (US) National Institute on Drug Abuse (NIDA) grants DP1 DA034989, P30DA011041, R01 DA033862, R01 DA024598, R01 DA035146, K01DA041259 and UG3 DA044829.
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
A Seidman k-core is defined as a set of members of the network (plus their links) such that every member of the k-core is linked by k or more links to another k-core member. Any given connected component can have only one 2-core, but many 3-cores or 4-cores. A 3-core will have to be a part of a 2-core, a 4-core will have to be part of a 3-core, and so forth. There are many measures that have been developed to measure the properties of social networks. Any edition of Wasserman, Stanley, and Faust, Katherine, Social Network Analysis: Methods and Applications (Structural Analysis in the Social Sciences), (First Edition 1994) Cambridge University Press, Cambridge, UK, West 20th St., New York, USA, Melbourne, Madrid, ISBN 978-0521387071 is a good reference for such measures.
Compliance with Ethics Guidelines
Conflict of Interest
Britt Skaathun is supported by NIH Research Training Grant #T32AI7384-26.
John Schneider is supported by National Institute for Allergy and Infectious Disease grant R01 AI120700.
Tetyana I Vasylyeva is supported by the Clarendon Fund and Hertford College of the University of Oxford.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Samuel R Friedman, National Development and Research Institutes, Inc., New York.
Leslie Williams, National Development and Research Institutes, Inc., New York.
April M Young, Department of Epidemiology, University of Kentucky College of Public Health, Lexington, KY.
Jennifer Teubl, National Development and Research Institutes, Inc., New York.
Dimitrios Paraskevis, Department of Hygiene, Epidemiology and Medical Statistics, Medical School, National and Kapodistrian University of Athens, Athens, Greece.
Evangelia Kostaki, Department of Hygiene, Epidemiology and Medical Statistics, Medical School, National and Kapodistrian University of Athens, Athens, Greece.
Carl Latkin, Department of Health, Behavior, and Society, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD.
Danielle German, Department of Health, Behavior, and Society, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD.
Pedro Mateu-Gelabert, National Development and Research Institutes, Inc., New York.
Honoria Guarino, National Development and Research Institutes, Inc., New York.
Tetyana I Vasylyeva, Department of Zoology, University of Oxford, Oxford, United Kingdom.
Britt Skaathun, Division of Global Public Health, University of California San Diego, California, USA.
John Schneider, University of Chicago, Department of Medicine and Center for HIV Elimination, Chicago, United States.
Ania Korobchuk, Alliance for Public Health, Kyiv, Ukraine.
Pavlo Smyrnov, Alliance for Public Health, Kyiv, Ukraine.
Georgios Nikolopoulos, University of Cyprus, Medical School, Nicosia, Cyprus.
References
- 1.Valente TW. Social networks and health: Models, methods, and applications. Oxford University Press; 2010. [Google Scholar]
- 2.Friedman SR, Bolyard M, Maslow C, Mateu-Gelabert P, Sandoval M. Harnessing the power of social networks to reduce HIV risk. Focus. 2005 Jan;20(1):5–6. [PubMed] [Google Scholar]
- 3.Friedman SR, Sandoval M, Mateu-Gelabert P, Rossi D, Gwadz M, Dombrowski K, Smyrnov P, Vasylyeva T, Pouget ER, Perlman DC. Theory, Measurement and Hard Times: Some issues for HIV/AIDS research. AIDS & Behavior. 2013;17(6):1915–1925. doi: 10.1007/s10461-013-0475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young AM, Rudolph AE, Su AE, King L, Jent S, Havens JR. Accuracy of name and age data provided about network members in a social network study of people who use drugs: implications for constructing sociometric networks. Annals of Epidemiology. 2016;26(11):802–9. doi: 10.1016/j.annepidem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudolph AE, Young AM, Havens JR. A rural/urban comparison of privacy and confidentiality concerns associated with providing sensitive location information in epidemiologic research involving persons who use drugs. Addictive Behaviors. 2017;74:106–111. doi: 10.1016/j.addbeh.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wylie JL, Jolly A. Patterns of chlamydia and gonorrhea infection in sexual networks in Manitoba, Canada. Sex Transm Dis. 2001;28:14–24. doi: 10.1097/00007435-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Magiorkinis G, Angelis K, Mamais I, Katzourakis A, Hatzakis A, Albert J, et al. The global spread of HIV-1 subtype B epidemic. Infect Genet Evol. 2016;46:169–179. doi: 10.1016/j.meegid.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelis K, Albert J, Mamais I, Magiorkinis G, Hatzakis A, Hamouda O, et al. Global Dispersal Pattern of HIV Type 1 Subtype CRF01_AE: A Genetic Trace of Human Mobility Related to Heterosexual Sexual Activities Centralized in Southeast Asia. J Infect Dis. 2015 Jun 1;211(11):1735–44. doi: 10.1093/infdis/jiu666. [DOI] [PubMed] [Google Scholar]
- 9.Paraskevis D, Magiorkinis G, Magiorkinis E, Ho SY, Belshaw R, Allain JP, Hatzakis A. Dating the origin and dispersal of hepatitis B virus infection in humans and primates. Hepatology. 2013;57(3):908–16. doi: 10.1002/hep.26079. [DOI] [PubMed] [Google Scholar]
- 10.German D, Grabowski MK, Beyrer C. Enhanced use of phylogenetic data to inform public health approaches to HIV among men who have sex with men. Sex Health. 2016 Sep 2; doi: 10.1071/SH16056. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurt CB, Dennis AM. Putting it all together: lessons from the Jackson HIV outbreak investigation. Sex Transm Dis. 2013;40:213–5. doi: 10.1097/OLQ.0b013e318284e3d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasylyeva TI, Friedman SR, Paraskevis D, Magiorkinis G. Integrating molecular epidemiology and social network analysis to study infectious diseases: towards a socio-molecular era for public health. Infect Genet Evol. 2016;46:248–255. doi: 10.1016/j.meegid.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panichsillapakit T, Smith DM, Wertheim JO, Richman DD, Little SJ, Mehta SR. Prevalence of Transmitted HIV Drug Resistance Among Recently Infected Persons in San Diego, CA 1996–2013. J Acquir Immune Defic Syndr. 2016;71(2):228–36. doi: 10.1097/QAI.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georgios K, Nikolopoulos GK, Pavlitina E, Muth SQ, Schneider J, Psichogiou M, Williams LD, et al. A network intervention that locates and intervenes with recently HIV-infected persons: The Transmission Reduction Intervention Project (TRIP) Scientific Reports. 2016 Dec 5;6:38100. doi: 10.1038/srep38100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman SR, Downing MJ, Smyrnov P, Nikolopoulos G, Schneider JA, Livak B, et al. Socially-integrated transdisciplinary HIV prevention. AIDS and Behavior. 2013 Oct 29;18(10) doi: 10.1007/s10461-013-0643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skaathun B. Dissertation. University of Chicago; 2016. Social Network Optimization for HIV Preventive Care. [Google Scholar]
- 17.Friedman SR, Flom PL, Kottiri BJ, Neaigus A, Sandoval M, Curtis R, et al. Consistent condom use in the heterosexual relationships of young adults who live in a high-HIV-risk neighborhood and do not use “hard drugs”. AIDS Care. 2001;13:285–296. doi: 10.1080/09540120120043937. [DOI] [PubMed] [Google Scholar]
- 18.Friedman SR, Flom PL, Kottiri BJ, Neaigus A, Sandoval M, Fuld J, et al. Consistent condom use among drug-using youth in a high-HIV-risk neighborhood. AIDS Care. 2002;14:493–507. doi: 10.1080/09540120208629668. [DOI] [PubMed] [Google Scholar]
- 19.Friedman SR, Jose B, Neaigus A, Goldstein M, Curtis R, Ildefonso G, et al. Consistent condom use in relationships between seropositive injecting drug users and sex partners who do not inject drugs. AIDS. 1994;8:357–361. doi: 10.1097/00002030-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Neaigus A, Friedman SR, Jose B, Goldstein M, Curtis R, Ildefonso G, Des Jarlais DC. High-Risk Personal Networks and Syringe Sharing as Risk Factors for HIV Infection among New Drug Injectors. Journal of Acquired Immune Deficiency Syndromes. 1996;11:499–509. doi: 10.1097/00042560-199604150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Friedman SR, Curtis R, Neaigus A, Jose B, Des Jarlais DC. Social Networks, Drug Injectors’ Lives, and HIV/AIDS. New York: Kluwer/Plenum; 1999. [Google Scholar]
- 22.Friedman SR, Neaigus A, Jose B, Curtis R, Goldstein M, Ildefonso G, et al. Sociometric Risk Networks and HIV Risk. American Journal of Public Health. 1997;87(8):1289–1296. doi: 10.2105/ajph.87.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman SR, Kottiri BJ, Neaigus A, Curtis R, Vermund SH, Des Jarlais DC. Network-related mechanisms may help explain long-term HIV-1-seroprevalence levels that remain high but do not approach population-group saturation. American Journal of Epidemiology. 2000;152(10):913–922. doi: 10.1093/aje/152.10.913. http://aje.oxfordjournals.org/content/152/10/913.full. [DOI] [PubMed] [Google Scholar]
- 24.Gyarmathy VA, Neaigus A. The effect of personal network exposure on injecting equipment sharing among IDUs in Budapest, Hungary. Connections. 2006;27(1):25–38. [PMC free article] [PubMed] [Google Scholar]
- 25.Weeks MR, Clair S, Borgatti SP, Radda K, Schensul JJ. Social networks of drug users in high-risk sites: Finding the connections. AIDS and Behavior. 2002;6(2):193–206. [Google Scholar]
- 26.Yang C, Latkin C, Muth SQ, Rudolph A. Injection Drug Users’ Involvement In Drug Economy: Dynamics of Sociometric and Egocentric Social Networks. Connect (Tor) 2013;33(1):24–34. [PMC free article] [PubMed] [Google Scholar]
- 27.Wendel T, Khan B, Dombrowski K, et al. Dynamics of methamphetamine markets in New York City: Final technical report to the National Institute of Justice (Document No: 23611) 2011. [Google Scholar]
- 28.Kuhns LM, Birkett M, Muth S, et al. Methods for collection of participant-aided sociograms for the study of social, sexual and substance-using networks among young men who have sex with men. Connections. 2015;35(1) doi: 10.17266/35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothenberg R, Baldwin J, Trotter R, Muth S. The risk environment for HIV transmission: results from the Atlanta and Flagstaff network studies. Journal of urban health: bulletin of the New York Academy of Medicine. 2001;78(3):419–432. doi: 10.1093/jurban/78.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudolph AE, Latkin C, Crawford ND, Jones KC, Fuller CM. Does Respondent Driven Sampling Alter the Social Network Composition and Health-Seeking Behaviors of Illicit Drug Users Followed Prospectively? Plos One. 2011;6(5):e19615. doi: 10.1371/journal.pone.0019615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Go VF, Latkin C, Le Minh N, et al. Variations in the Role of Social Support on Disclosure Among Newly Diagnosed HIV-Infected People Who Inject Drugs in Vietnam. AIDS and Behavior. 2015:1–10. doi: 10.1007/s10461-015-1063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Go V, Frangakis C, Minh N, et al. Efficacy of a Multi-level Intervention to Reduce Injecting and Sexual Risk Behaviors among HIV-Infected People Who Inject Drugs in Vietnam: A Four-Arm Randomized Controlled Trial. PloS One. 2014;10(5):e0125909–e0125909. doi: 10.1371/journal.pone.0125909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potterat JJ, Rothenberg RB, Muth SQ. Network structural dynamics and infectious disease propagation. Int J STD AIDS. 1999;10(3):182–185. doi: 10.1258/0956462991913853. [DOI] [PubMed] [Google Scholar]
- 34.Curtis KM, Misshula E, Riggs Marshall DM., IV Dynamics of Methamphetamine Markets in New York City: Final Technical Report to the National Institute of Justice. 2011 Oct; Document # 236122. Available at http://snrg-nyc.org/wp-content/uploads/2012/07/Meth-Report.pdf.
- 35.Dombrowski K, Khan B, Wendel T, McLean K, Misshula E, Curtis R. Estimating the size of the methamphetamine-using population in New York City using network sampling techniques. Advances in applied sociology. 2012 Dec 1;2(4):245. doi: 10.4236/aasoci.2012.24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odell KM, Russell RC. Soundex phonetic comparison system. cf U.S. Patents 1261167 (1918), 1435663 (1922) [Google Scholar]
- 37.Shannon CE. A mathematical theory of communication, Part I, Part II. Bell Syst Tech J. 1948;27:623–56. [Google Scholar]
- 38.Lait AJ, Randell B. Newsletter Contents. 17. SIGGNL; 1998. An Assessment of Name Matching Algorithm, Society of Indexers Genealogical Group. [Google Scholar]
- 39.Hopkins C, Young AM. SPIDER: Semi-automated Processing of Ineterconnected Dyads using Entity Resolution [computer program] 2017 Available at: https://www.cra.com/spider.
- 40.LinkAlyzer. 2001 ( http://socioworks.com/productsall/linkalyzer/) [computer program]
- 41.Young AM, Rudolph AE, Havens JR. Network-Based Research on Rural Opioid Use: an Overview of Methods and Lessons Learned. Current HIV/AIDS Reports. doi: 10.1007/s11904-018-0391-2. https://doi.org/10.1007/s11904-018-0391-2. [DOI] [PMC free article] [PubMed]
- 42.Kottiri BJ, Friedman SR, Neaigus A, Curtis R, Des Jarlais DC. Risk networks and racial/ethnic differences in the prevalence of HIV infection among injection drug users. JAIDS. 2002;30:95–104. doi: 10.1097/00042560-200205010-00013. [DOI] [PubMed] [Google Scholar]
- 43.Dombrowski K, Khan B, Curtis R, Friedman S. Topological and Historical Considerations for Infectious Disease Transmission among Injecting Drug Users in Bushwick, Brooklyn (USA) World Journal of AIDS. 2013;3:1–9. doi: 10.4236/wja.2013.31001. Published Online March 2013 ( http://www.scirp.org/journal/wja) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dombrowski K, Khan B, McLean K, Curtis R, Wendel T, Misshula E, Friedman SR. A Reexamination of Connectivity Trends via Exponential Random Graph Modeling in Two IDU Risk Networks. Substance Use and Misuse. 2013 Dec;48(14):1485–1497. doi: 10.3109/10826084.2013.796987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dombrowski K, Khan B, Habecker P, Hagan H, Friedman SR, Saad M. The Interaction of Risk Network Structures and Virus Natural History in the non-Spreading of HIV among People Who Inject Drugs in the Early Stages of the Epidemic. AIDS Behav. 2017 Apr;21(4):1004–1015. doi: 10.1007/s10461-016-1568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan B, Dombrowski K, Saad M, Mclean K, Friedman SR. Network firewall dynamics and the subsaturation stabilization of HIV. Discrete Dynamics in Nature and Society. 2013;2013(151) doi: 10.1155/2013/720818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman SR, Williams LD, Pavlatina E, Paraskevis D, Schneider J, Skaathun B, et al. Transmission Reduction Intervention Project Collaboration Group. Networks of recently-infected people who inject drugs (PWID) in Athens, Greece, June 2013 – July 2015: epidemiological insight from a project to reduce. HIV transmission International AIDS Conference; Durban. 2016; TUPEC230. [Google Scholar]
- 48.Friedman SR, Bolyard M, Sandoval M, Mateu-Gelabert P, Maslow C, Zenilman JM. The relative prevalence of different STIs in HIV-discordant sexual partnerships: Data from a risk network study in a high-risk New York neighborhood. Sexually Transmitted Infections. 2008;84(1):17–8. doi: 10.1136/sti.2007.026815. [DOI] [PubMed] [Google Scholar]
- 49.Friedman SR, Bolyard M, Khan M, Maslow C, Sandoval M, Mateu-Gelabert P, et al. Group Sex Events and HIV/STI Risk in an Urban Network. J Acq Immun Syn. 2008;49(4):440–446. doi: 10.1097/qai.0b013e3181893f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Latkin C, Yang C, Tobin K, Hulbert A. Factors associated with recruiting an HIV seropositive risk network member among injection drug users. AIDS Behav. 2010 Oct;14(5):1137–41. doi: 10.1007/s10461-010-9676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latkin C, Donnell D, Liu TY, Davey-Rothwell M, Celentano D, Metzger D. The dynamic relationship between social norms and behaviors: the results of an HIV prevention network intervention for injection drug users. Addiction. 2013;108(5):934–43. doi: 10.1111/add.12095. Epub 2013 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latkin CA, Kuramoto SJ, Davey-Rothwell MA, Tobin KE. Social norms, social networks, and HIV risk behavior among injection drug users. AIDS Behav. 2010 Oct;14(5):1159–68. doi: 10.1007/s10461-009-9576-4. Epub 2009 May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Latkin C, Yang C, Tobin K, Hulbert A. Factors associated with recruiting an HIV seropositive risk network member among injection drug users. AIDS Behav. 2010 Oct;14(5):1137–41. doi: 10.1007/s10461-010-9676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tobin KE, Hua W, Costenbader EC, Latkin CA. The association between change in social network characteristics and non-fatal overdose: results from the SHIELD study in Baltimore, MD, USA. Drug Alcohol Depend. 2007 Feb 23;87(1):63–8. doi: 10.1016/j.drugalcdep.2006.08.002. Epub 2006 Sep 7. [DOI] [PubMed] [Google Scholar]
- 55.Latkin CA, Hua W, Tobin K. Social network correlates of self-reported non-fatal overdose. Drug Alcohol Depend. 2004 Jan 7;73(1):61–7. doi: 10.1016/j.drugalcdep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Davey-Rothwell MA, Kuramoto SJ, Latkin CA. Social networks, norms, and 12-step group participation. Am J Drug Alcohol Abuse. 2008;34(2):185–93. doi: 10.1080/00952990701877086. [DOI] [PubMed] [Google Scholar]
- 57.Davey MA, Latkin CA, Hua W, Tobin KE, Strathdee S. Individual and social network factors that predict entry to drug treatment. Am J Addict. 2007 Jan…feb;16(1):38–45. doi: 10.1080/10601330601080057. [DOI] [PubMed] [Google Scholar]
- 58.Tsang MA, Schneider JA, Sypsa V, Schumm P, Nikolopoulos GK, Paraskevis D, et al. Network characteristics of people who inject drugs within a new HIV epidemic following austerity in Athens, Greece. Journal of Acquired Immune Deficiency Syndromes (1999) 2015;69(4):499–508. doi: 10.1097/QAI.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knowlton AR, Hua W, Latkin C. Social support networks and medical service use among HIV-positive injection drug users: implications to intervention. AIDS Care. 2005 May;17(4):479–92. doi: 10.1080/0954012051233131314349. [DOI] [PubMed] [Google Scholar]
- 60.Maragh-Bass AC, Denison JA, Thorpe RJ, Jr, Knowlton AR. The interactive effects of social support and physical functioning on HIV medical outcomes among African Americans whom inject drugs. J Ethn Subst Abuse. 2017 Feb;15:1–19. doi: 10.1080/15332640.2016.1264337. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.https://www.ncbi.nlm.nih.gov/m/pubmed/25504312
- 62.Smyrnov P, Williams LD, Korobchuk A, Sazonova Y, Nikolopoulos GK, Skaathun B, Morgan E, Schneider J, Vasylyeva TI, Friedman SR. Risk network approaches to locating undiagnosed HIV cases in Odessa, Ukraine. J Int AIDS Soc. 2018 Jan;21(1) doi: 10.1002/jia2.25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan E, Skaathun B, Nikolopoulos GK, Paraskevis D, Friedman S, Schneider JA. A network intervention to locate newly infected persons within MSM networks in Chicago. AIDS Behav. doi: 10.1007/s10461-018-2202-6. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mateu-Gelabert P, Jessell L, Goodbody E, Kim D, Gile K, Teubl J, et al. High enhancer, downer, withdrawal helper: multifunctional nonmedical benzodiazepine use among young adult opioid users in New York City. International Journal on Drug Policy. 2017;46:17–27. doi: 10.1016/j.drugpo.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedman SR, Mateu-Gelabert P, Ruggles KV, Goodbody E, Sykes C, Jessell L, et al. Sexual risk and transmission behaviors, partnerships and settings among young adult opioid users in New York City. AIDS & Behavior. 2017;21(4):994–1003. doi: 10.1007/s10461-016-1672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]