Abstract
Major depressive disorder (MDD) is characterized by biased information processing that leads to difficulties regulating negative affect, which includes difficulty decreasing negative affect as well as maladaptively increasing negative affect via cognitive processes. To examine the underlying neural correlates, we scanned depressed and never-depressed adults as they completed a cognitive reappraisal task which required decreasing negative affect while viewing a negative image (down-regulation) and increasing negative affect while viewing a neutral image (emotion generation). Compared to control participants, MDD participants had less recruitment of the dorsal anterior cingulate (dACC) and supplementary motor area (SMA) during early phases of down-regulation, the latter associated with poorer negative affect regulation. Further, MDD participants exhibited greater recruitment of the right middle temporal gyrus (MTG) during emotion generation, which was associated with lower negative affect. Dysregulated negative affect in MDD may be due to impairments in efficiently activating the dACC and SMA to meet regulation demands, and maladaptive generation of negative affect that characterizes individuals with MDD may be counteracted by compensatory activation in the MTG. Elucidating neural mechanisms that underlie the generation of negative affect in the absence of external stimuli is an important extension of previous work examining dysfunctional emotional processes in MDD.
Keywords: depression, emotion regulation, negative affect, dorsal anterior cingulate, supplementary motor area, middle temporal gyrus
1. Introduction
Individuals with major depressive disorder (MDD) are impaired in regulating negative affect in two important ways: they have difficulty reducing negative emotions and they generate negative affect in the absence of external cues (Mathews and MacLeod, 2005). Several neuroimaging studies investigating MDD-associated dysfunction in reducing negative affect have used tasks in which participants are asked to reappraise negative stimuli in order to alter their affective experience, typically to decrease negative affect (e.g., Ochsner et al., 2012; Rive et al., 2013). Fewer studies have investigated the neural correlates of the cognitive processes by which depressed individuals increase or exacerbate their experience of negative affect, or even generate negative affect in response to a neutral or ambiguous stimulus (Gollan et al., 2008; Gotlib and Joormann, 2010; Nolen-Hoeksema et al., 2008). The present study was designed to elucidate the neural substrates of these two “top-down” emotion regulation processes in MDD: cognitive reappraisal to reduce and to generate negative affect.
Cognitive reappraisal involves reframing or reinterpreting the meaning of a stimulus in order to change one’s affective response (Gross, 2002; Rive et al., 2013). As a whole, studies examining the neural substrates of reappraisal in healthy individuals have implicated key frontal, cingulate, and parietal control regions that play a role in modulating the emotion-responsive activity of subcortical structures such as the amygdala (Etkin et al., 2015; Kohn et al., 2014). Neuroimaging studies examining depressed individuals as they use regulation strategies such as reappraisal in an attempt to decrease negative affect have identified overlapping regions but have also yielded mixed findings concerning differences between individuals with MDD and healthy comparison groups (Etkin et al., 2015). Whereas some investigators have found reduced amygdala activation during reappraisal, particularly in severe depression (Beauregard et al., 2006; Erk et al., 2010; Greening et al., 2014), other researchers have not found depression-associated differences in amygdala activation (Dillon & Pizzagalli, 2013; Johnstone et al., 2007; Sheline et al., 2009). Patterns of prefrontal activity indicate compensatory bilateral activation in MDD participants (Johnstone et al., 2007), in addition to heightened dorsal anterior cingulate cortex (dACC) and default mode network (DMN) regional activation during the down-regulation of negative affect (Beauregard et al., 2006; Sheline et al., 2009). In a systematic review of neural correlates of reappraisal of negative stimuli in MDD, Rive et al. (2013) concluded that findings concerning the directionality of medial prefrontal cortex (mPFC) activation are equivocal, but highlighted ventromedial PFC (vmPFC) and dACC as regions of consistent dysfunctional activation.
Fewer studies have investigated conditions in which individuals use cognitive processes to generate or increase negative affect. Most relevant studies focus on healthy individuals responding to prompts to elaborate on or increase the personal relevance of negative stimuli (Ochsner et al., 2004; Ray et al., 2005; Urry et al., 2006). These studies have generally found increases in amygdala activation when individuals are prompted with this instruction. They have also identified patterns of prefrontal and cingulate activation that are similar to those seen during reappraisal; in addition, actively increasing negative affect recruits greater left lateralized frontal regions, mPFC, and posterior cingulate cortex (PCC) (Ochsner et al., 2004), and stronger activation of dorsolateral PFC, ACC, and orbitofrontal cortex (Eippert et al., 2007).
Even fewer studies have reported results from a condition in which participants are instructed to generate negative affect in the absence of a negative stimulus (i.e., to a neutral image), and no study has yet examined this process in MDD. Importantly, individually-generated negative affect without an external negative cue is a strong parallel to maladaptive cognitive processes in MDD, such as negative cognitive biases that affect the interpretation of neutral or ambiguous external cues (i.e., selective focusing on the negative aspects of a situation or overgeneralizing to make broad negative conclusions) (Beck, 1963; Gotlib and Joormann, 2010), as well as some forms of rumination, in which negative affect occurs in response to thinking about internal states (Nolen-Hoeksema, 1991). Although these processes may become automatic or habitual over time (Beck, 1963), the instruction to deliberately generate negative affect should activate the underlying neural networks to a differential extent for those who habitually engage in these processes versus those who do not (as in Ray et al., 2005).
In a study of healthy individuals, Ochsner et al. (2009) used a top-down emotion generation paradigm and found that cognitively reappraising neutral images to be negative activated the amygdala, as well as lateral and medial PFC and dACC, temporal cortex, and putamen. Ray et al. (2005) also reported activation of the amygdala and ventrolateral PFC (vlPFC) in response to instructions to increase negative affect to a neutral image, particularly in individuals high in trait rumination. The authors suggest that ruminators more readily or more efficiently engage in the process of applying negative interpretations and amplifying negative affect. Even though there were no associations found between rumination and negative affect ratings in their task, the neuroimaging results revealed differential activation in networks that may underlie the habitual and maladaptive cognitive behaviors documented in ruminators. Finally, McRae, Misra, Prasad, et al. (2011) found amygdala activation when healthy adults were provided with narrative content to make a neutral stimulus more negative.
We used a modified version of a well-validated cognitive reappraisal neuroimaging paradigm (Ochsner et al., 2004, 2009) in which, during an “increase negative” condition, depressed and healthy control participants engaged in elaborative semantic processing of a neutral scene, enhancing or adding negative information that might fit in the image. Importantly, in the absence of a negative image, group differences in neural activation during this condition should be independent of any depression-related differences in bottom-up reactivity to a negative stimulus (McRae et al., 2011). Instead, when contrasted with an unregulated emotional response to viewing a neutral stimulus, activation to this condition should reflect top-down negative emotion generation from participant-generated appraisal (Ochsner et al., 2009). We also explored a “decrease negative” reappraisal condition similar to that used in previous studies, in contrast to unregulated or natural emotional responding to a negative stimulus.
We expected to find an MDD-related decrease in activation in medial and lateral PFC and dACC when participants used reappraisal, an explicit voluntary emotion regulation strategy, to decrease negative affect in response to negative images (Rive et al., 2013), and possibly increased activation in the PFC that may serve a compensatory role (Johnstone et al., 2007). We hypothesized further that similar brain regions would be engaged during the generation of negative affect to a neutral image. In addition, we expected to find MDD-associated increased activation in regions such as the PCC that are involved in self-relevant information processing, and in parietal and temporal regions (e.g., superior temporal gyrus, angular gyrus) that support altering semantic representations, which would be engaged when participants add negative content to a neutral image (Binder et al., 2009). We first conducted whole-brain analyses to identify group differences during the regulation conditions across a distributed regulatory network. We then followed up with a region-of-interest (ROI) approach specifically to probe group differences in amygdala activation, given previous equivocal findings and the use of ROI analyses in previous studies (e.g., Ochsner et al., 2004; Wager et al., 2008). Finally, previous studies have highlighted the temporally extended nature of regulation processes (Kalisch, 2009; Sheline et al., 2009), including depletion of regulatory strength over time (Schmeichel et al., 2006); perhaps not surprisingly, therefore, sustained emotional engagement to negative stimuli has been documented in depression (Siegle et al., 2002). Thus, we contrasted early versus late runs of the task in the hope of clarifying mixed findings concerning the directionality of MDD-related anomalies in prefrontal activation during reappraisal.
2. Methods
2.1 Participants
Participants (n=46) were recruited via community and online advertisements and were invited to complete the assessment interview if they met initial inclusion criteria based on a telephone screen. All participants were required to be between the ages of 18-59 years, female, have no current or past psychosis, mania, or hypomania, have no substance abuse or dependence symptoms in the past 6 months, meet MRI safety criteria including no history of major head trauma, and to be free of psychotropic medication. We included only female participants in this study given evidence of sex differences in neural responses to negative stimuli (Cahill et al., 2001; Canli et al., 2002; McRae et al., 2008). We also included only unmedicated depressed individuals given the effects of medication on emotion regulation processes and neural activation (McRae et al., 2014; Rive et al., 2015, 2013). All procedures were approved by the Stanford Institutional Review Board and participants provided written informed consent.
2.2 Procedures
2.2.1 Diagnostic assessment and self-report measures
At their initial session, participants completed a structured diagnostic interview (Structured Clinical Interview for the DSM-IV; First et al., 1997) with a trained interviewer to establish a current primary diagnosis of MDD (depressed group) or no current or past history of any Axis I disorders (control group; CTL). These criteria were verified within two weeks of the scan session if more than a month had passed since initial interview (17 CTL and 9 MDD participants). The Hamilton Depression Rating Scale, 17-item version (Hamilton, 1967, HDRS-17; 1960) was also administered. Participants provided demographic information and completed the Beck Depression Inventory-II (BDI-II; Beck et al., 1996).
2.2.2 fMRI task
The scan task was programmed using E-Prime software (PST Inc.) and was composed of 6 runs with 12 trials each. During each trial, either a negative or a neutral image from the International Affective Picture System (IAPS; Lang et al., 2008) was presented for 2000 msec. The negative and neutral pictures were matched for valence intensity across the instruction conditions. A cue word was then presented under the image, and the image and word together were displayed for 8000 msec. There were three cue words that were explained outside the scanner: “look” cued the participant to “have whatever thoughts and feelings you would naturally have” in response to the image, “decrease” cued the down-regulation of negative affect in response to a negative image (“think of the picture in a way that helps you feel less negative”), and “increase” cued the generation of negative affect to a neutral image (“think of something to tell yourself that makes you feel more negative about the picture”). These instructions were derived from similar tasks (Ochsner et al., 2009, 2004). The combination of image valence (negative or neutral) and instruction cue word were pseudo-randomly ordered and counterbalanced to create four experimental conditions with a total of 18 trials each: Decrease Negative (to negative image), Look Negative, Increase Negative (to neutral image), and Look Neutral. Following each cued regulation phase, participants used a button box to rate how negative they were feeling in that moment from 1-5 (1=not at all; 5=very) during a 4000-msec rating window. Between each trial were periods of rest lasting 1000 msec, followed by 8000 msec of a Flanker-type arrows task in which participants pressed a key in response to the direction of the middle arrow, intended to serve as a “wash-out” phase during which they were distracted from processing the emotional content of the previous trial.
Prior to the scan, participants were provided with task instructions and practiced the task on a computer, with an opportunity to ask questions and receive feedback on strategy use to ensure comprehension and task compliance. Following the scan, participants rated the difficulty they experienced when regulating their affect in the two regulation conditions (Decrease Negative and Increase Negative).
2.3 fMRI image acquisition
Neuroimaging data were acquired on a 3 Tesla GE Healthcare Discovery MR 750 system (GE Healthcare Systems, Milwaukee, WI) and analyzed with the FMRIB Software Library version 5.0.6 (FSL; www.fmrib.ox.ac.uk/fsl) using FEAT version 6.0.0 (fMRI Expert Analysis Tool). A T2*-sensitive gradient spiral in/out pulse sequence (Glover and Law, 2001) was used for whole brain functional imaging with the following parameters: 6 scans each 5:48 long, 30 interleaved 4 mm thick axial slices, field of view (FOV)=220 mm (64×64), repetition time (TR)=2000 ms, echo time (TE)=30 ms, flip angle=80, in plane voxel size 3.4 mm2. A T1-weighted spiral spoiled gradient high resolution structural image was acquired for 160 interleaved 1 mm thick axial slices, FOV=220 mm (192×256), TR/TE/TI=8.5/3.4/400 ms,. flip angle = 15, in plane voxel size 0.86 mm2.
2.4 Data analysis
Responses to self-report measures were analyzed with repeated measures analyses of variance (ANOVAs) as appropriate; similarly, demographic and clinical variables were compared with χ2 tests or t-tests, with degrees of freedom adjusted using the Welch-Satterthwaite method in the event of inhomogeneity of variance, determined by the Levene’s test.
Standard preprocessing steps applied to functional images included discarding the first 4 volumes from each functional run, applying a 100 Hz high pass filter, and performing slice timing correction, brain extraction using BET, and motion correction using MCFLIRT. All included subjects had <3mm total motion translation in x, y, and z planes. Data were spatially smoothed using a 5 mm FWHM Gaussian kernel. Functional datasets were coregistered to each participant’s high-resolution structural image using a 6-parameter transformation and then normalized to Montreal Neurological Institute (MNI) standard space at 2mm3 resolution using a 12-parameter transformation. Functional data was analyzed in a three stage pipeline: first a whole-brain general linear model at the single subject level, second combining runs within a single subject in a fixed effects model, and third comparing across subjects in the MDD vs. CTL groups in a random effects model.
Single-subject analyses used a generalized linear model that modeled the 4 experimental conditions as 8 second epochs during which the IAPS image and instruction cue were presented simultaneously: Decrease Negative, Look Negative, Increase Negative, and Look Neutral. Other events modeled at the first level included image viewing (without cue), rating phase, and the arrows task. Boxcar regressors of these events were convolved with a canonical double-gamma hemodynamic response function. Phases where the participant viewed a fixation cross during rest served as the baseline.
Voxelwise patterns in activation were assessed across the whole brain modeling subject as a random effect using FMRIB’s Local Analysis of Mixed Effects (FLAME; Woolrich et al., 2004). Group (MDD, CTL) differences during reappraisal were examined by modeling group by regulation condition interactions for two contrasts of interest, selected to examine the neural correlates of top-down emotion regulation to change one’s affective experience, controlling for unregulated or spontaneous affective responses to a same-valence stimulus: Decrease Negative > Look Negative and Increase Negative > Look Neutral. Significant interactions were decomposed by extracting percent signal change for each participant and cluster and then analyzing using repeated measures analyses of variance (ANOVAs) and pairwise comparisons. Finally, we computed correlations between percent signal change and both in-scanner affect ratings and post-task ratings of regulation difficulty. We also modeled the first 3 runs of the task and the second 3 runs separately in order to investigate group differences in early- versus late-task (early > late or late > early) patterns of neural activation. Statistical images for these higher level analyses were thresholded at Z>3.1 and cluster probability p<0.01, corrected for whole-brain multiple comparisons using Gaussian random-field theory (Worsley et al., 1992).
In addition to our whole-brain analyses, we conducted an ROI analysis for bilateral amygdala clusters, defined by the Harvard-Oxford Subcortical Atlas and thresholded at 90% extent. For these analyses, we applied a more liberal corrected voxelwise threshold of p<0.05 and cluster threshold of p<0.05 to ensure that we would detect potential group differences in this small region.
3. Results
3.1 Participant demographic and clinical characteristics
Three control participants were excluded due to unusable fMRI task data (one for excessive motion, one for the presence of artifacts, and one for premature termination of task), and 7 control participants were excluded due to technical malfunction during the behavioral task. The final sample used in analysis consisted of 16 females with current MDD and 20 healthy CTL females. The participants had a mean age of 33.2 years; the MDD and CTL groups did not differ with respect to age or other key demographic variables and all had at minimum of a high school education (see Table 1). The sample was approximately half Caucasian and the majority was right-handed. Twelve MDD participants met criteria for at least one current comorbid anxiety disorder (6 social phobia, 7 generalized anxiety, 1 panic disorder, 2 post-traumatic stress disorder, and 3 specific phobia), and all but one MDD participants reported >2 previous depressive episodes. All were free of psychotropic medication at the time of scan for at least 1 year; 6 had a history of medication use for MDD. As expected, the MDD group had significantly higher scores than did the CTL group on the HDRS-17 and BDI-II (Table 1).
Table 1.
Demographic information and group comparison
| Variable | CTL (n=20) | MDD (n=16) | Statistics |
|---|---|---|---|
| Age (in years) | 32.8 +/− 11.8 | 33.7 +/− 10.6 | t(34)= −0.26, p=.80 |
| Race/Ethnicity | 13 Caucasian, 3 Asian, 2 African-American, 1 Hispanic, 1 Mixed race | 10 Caucasian, 4 Asian, 1 African-American, 1 Hispanic | χ2(4)=2.2, p=.70 |
| English language | 79% Native English | 75% Native English | χ2(1)=0.08, p=.78 |
| Education | 40% <4 year college, 35% 4 year college, 25% at least some graduate school | 37.5% <4 year college, 31.3% 4 year college, 31.3% at least some graduate school | χ2(2)=0.18, p=.92 |
| Handedness | 75% right handed | 94% right handed | χ2(1)=2.3, p=.13 |
| MDD duration (in years) | N/A | 18.4 +/− 13.0 | N/A |
| HDRS-17 score | 1.8 +/− 2.3 | 16.5 +/− 5.2 | t(19.8)=−10.4, p<.001 |
| BDI-II score | 3.6 +/− 4.3 | 34.3 +/− 7.9 | t(21.9)=−13.9, p<.001 |
Note: CTL = Nondepressed control group; MDD = Major Depressive Disorder group; HDRS-17 = Hamilton Depression Rating Scale, 17-item version; BDI-II = Beck Depression Inventory-II
3.2 Behavioral results
In-scan behavioral data were not available for 2 of the 16 depressed participants due to technical difficulties with the button box. MDD and CTL participants did not differ significantly in their negative affect ratings during any of the regulation conditions (Table 2); a repeated measures ANOVA yielded no significant main effect or interactions involving diagnostic group, ps>.42. As expected, across the entire sample affect ratings were significantly more negative during Look Negative vs. Look Neutral (p<.001), and we found the predicted decreases in negative affect during the down-regulation task: negative affect ratings were significantly lower during Decrease Negative than during Look Negative trials (p<.001). We also found significant increases in negative affect during top-down emotion generation: ratings during Increase Negative were significantly greater than during Look Neutral (p<.001). Ratings during Decrease Negative and during Increase Negative did not differ significantly from each other (p=.98), potentially due to the range of the rating scale we used.
Table 2.
Affect ratings during task
| Task condition | CTL (n=20) | MDD (n=14) | Pairwise comparison |
|---|---|---|---|
| Decrease Negative | 2.77 | 2.57 | F(1,32)=0.84, p=.37 |
| Look Negative | 3.42 | 3.15 | F(1,32)=1.57, p=.22 |
| Increase Negative | 2.67 | 2.66 | F(1,32)=0.002, p=.97 |
| Look Neutral | 1.18 | 1.36 | F(1,32)=2.43, p=.13 |
Note. CTL = Nondepressed control group; MDD = Major Depressive Disorder; Sample size for MDD group for these ratings is 14 (2 participants’ data are missing due to button box malfunction)
When examining affect ratings separately for early versus late runs, there was similarly no significant effect of diagnostic group (ps>.43); across all subjects, affect ratings in response to negative stimuli became more negative over time (Decrease Negative and Look Negative ps<.001). To examine ratings of difficulty following regulation instructions during Decrease Negative and Increase Negative conditions, we conducted a 2 (diagnostic group) by 2 (task condition) repeated measures ANOVA with diagnostic group as a between-subjects factor and task condition as a within-subject factor. Difficulty ratings did not differ significantly by diagnostic group or task condition (main effects and interaction ps>.2).
3.3 Decreasing negative affect to a negative image: whole brain results
3.3.1 Group differences across the entire task
When examining all 6 runs of the task combined, there were no regions in which there were group differences in activation when comparing the Decrease Negative and Look Negative conditions for negative stimuli (the main effect of this contrast across all participants is presented in the Supplement).
3.3.2 Group differences in early vs. late phases of the task
Next we examined whether the MDD and CTL participants exhibited hypothesized different patterns of activation from the first 3 runs to the second 3 runs of the task, due to differences in habituation, burden of the reappraisal task, or delayed engagement of regulatory resources. This analysis yielded a significant three-way interaction of group, condition, and time for two clusters: dACC (peak −4, 30, 24), extending dorsally to paracingulate (228 voxels), F(1,34)=16.63, p<.001, and left supplemental motor area (SMA) extending to the precentral gyrus (peak −2, −14 56, 153 voxels), F(1,34)=20.87, p<.001 (Figure 1A). Decomposing these interactions indicated that whereas dACC activation for the contrast Decrease Negative > Look Negative significantly increased over time for the MDD group (F(1,34)=13.40, p=.001), this activation marginally decreased over time in the CTL group, F(1,34)=4.1, p=.051 (Figure 1B). In addition, the CTL group had higher activation than did the MDD group in the early runs (F(1,34)=20.12, p<.001). Similarly, SMA activation increased over time for the MDD group (F(1,34)=18.05, p<.001), but decreased over time in the CTL group (F(1,34)=4.43, p=.043) (Figure 1C). The two groups significantly differed during both early (p=.005) and late (p=.007) runs: whereas the CTL group had higher activation in the early runs, the MDD group had higher activation in the late runs.
Figure 1.
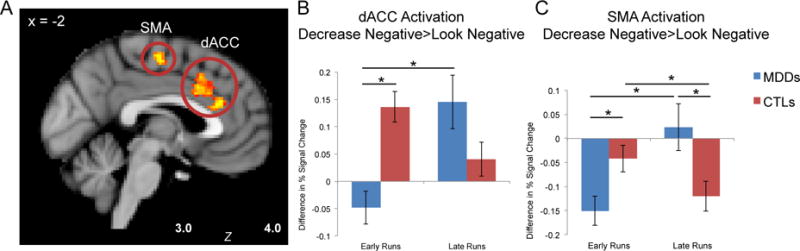
Significant activation for interaction of group, condition, and time, depicting regions in which activation was greater for the CTL than for MDD group in the Decrease Negative > Look Negative contrast, for the early runs (1-3) vs. the late runs (4-6) of the task. Panel A: This contrast yielded two significant clusters: in supplementary motor area (SMA) and in dorsal anterior cingulate cortex (dACC). Panel B: In the early runs of the task, MDD participants’ dACC activation was less during Decrease Negative vs. Look Negative, and significantly lower than activation in the CTL group. The dACC activation increased for the MDD group over the task, while the CTL group showed a marginal decrease. Panel C: Similarly for the SMA, the MDD group showed less activation than did the CTL group during Decrease Negative vs. Look Negative in early runs of the task, and the MDD group significantly increased activation by the later runs; in contrast, the CTL group had higher SMA activation initially, which decreased over time. * p<.05
3.3.3 Correlations with affect ratings and self-report measures
There were no significant correlations between dACC activation and negative affect ratings or scores on self-report measures. Higher SMA activation in the Decrease Negative > Look Negative contrast during early runs was significantly correlated with larger decreases in negative affect during those runs in the MDD group (r=−.83, p<.001) but not in the CTL group (r=−.06, p=.82). In addition, higher SMA activation in the late runs was correlated with less overall difficulty regulating for the CTL participants, as reported post-task (r=−.54, p=.014).
3.4 Decreasing negative affect to a negative image: amygdala ROI results
There was no evidence of amygdala activation at our thresholds for the contrast of Look Negative > Decrease Negative. For Look Negative > Baseline, there was evidence of right amygdala activation across all participants during the first half of the task (155 voxels, peak 24, 0, −10). During the latter half of the task, however, and when averaging across all 6 runs, there was no amygdala activation that reached threshold. MDD and CTL participants did not differ significantly in amygdala activation during Look Negative trials, and percent signal change did not correlate with the self-reported affect or ratings of regulation difficulty. The contrast of Decrease Negative > Baseline did not yield significant amygdala activation, nor, as expected, did the contrast of Decrease Negative > Look Negative.
3.5 Generating negative affect to a neutral image: whole brain results
3.5.1 Group differences across the entire task
No regions showed significant activation for CTL > MDD when contrasting the Increase Negative and Look Neutral conditions across the 6 runs of the task (the main effect of this contrast across all participants is presented in the Supplement). For the comparison of MDD > CTL, a cluster in the right middle temporal gyrus emerged (peak 42, −56, 12; 219 voxels), F(1,34)=14.83, p<.001. Local maxima extended through the temporal lobe including the supramarginal gyrus, planum temporale, and bordering on the angular gyrus. Post-hoc pairwise comparisons on the extracted percent signal change from this cluster showed that MDD individuals exhibited greater activation than did CTLs during the Increase Negative condition (F(1,34)=4.37, p=.044); in contrast, there were no group differences for Look Neutral (F(1,34)=0.12, p=.73) (see Figure 2). Moreover, both the CTL and MDD groups showed significant differences in activation between the two conditions, but in opposite directions: whereas CTLs had greater MTG cluster activation for Look Neutral compared to Increase Negative (p=.002), MDD participants had greater MTG cluster activation for Increase Negative compared to Look Neutral (p=.033).
Figure 2.
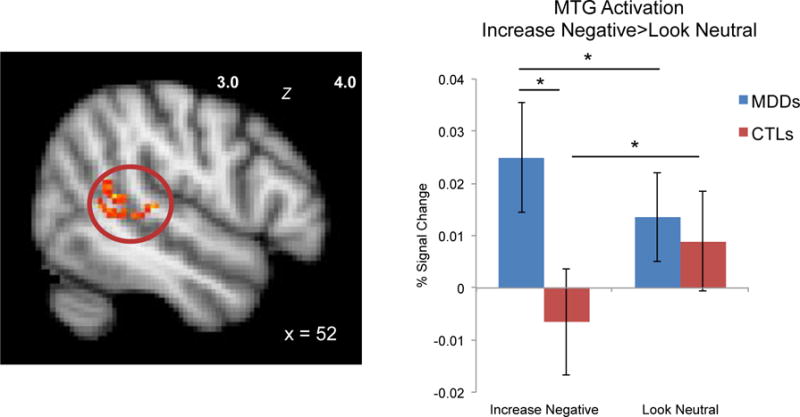
MDD and CTL groups differ significantly in right middle temporal gyrus (MTG) activation during Increase Negative emotion generation. * p<.05
3.5.2 Group differences in early vs. late phases of the task
Next, we examined whether the MDD and CTL participants exhibited different patterns of activation from the first 3 runs to the second 3 runs. This analysis yielded a significant three-way interaction of group, condition, and time for one cluster in the cerebellum, peak −8, −48, −16 (172 voxels), F(1,34)=34.71, p<.001. Posthoc tests indicated that the group differences were driven by the Increase Negative condition: at a trend level, the CTL group had greater activation than did the MDD group during the early runs (p=.069), while the reverse pattern was true for the late runs (p=.065). Whereas the CTL individuals decreased activation for both conditions over the course of the task (p=.028 for Look Neutral, p=.002 for Increase Negative), the MDD group showed decreased activation at a trend level for only the Look Neutral condition (p=.052), not the Increase Negative condition (p=.27).
3.5.3 Correlations with affect ratings and self-report measures
Across the entire sample, affect ratings during the Increase Negative condition were negatively correlated with activation in the MTG cluster specifically during that condition, r=−.34, p=.048 (Figure 3), but were not correlated with MTG activation during Look Neutral or the contrast of Increase Negative > Look Neutral (ps>.05). The interaction of group and affect ratings did not predict activation (p=.69). Greater self-reported difficulty with emotion regulation during the Increase Negative condition was correlated with greater MTG activation during Increase Negative trials (r=.43, p=.009) (Figure 3); there was no interaction with group (p=.49). Finally, affect ratings and difficulty ratings were not correlated with cerebellar activation (ps>.15).
Figure 3.
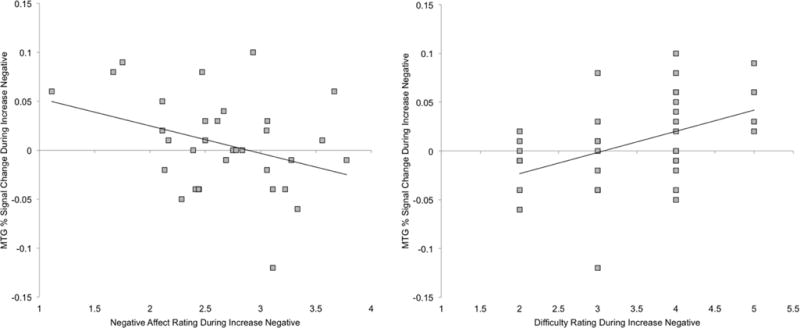
Activation in right middle temporal gyrus (MTG) during Increase Negative is inversely correlated with negative affect ratings and positively correlated with difficulty increasing negative affect across all participants, suggesting that higher activation is protective against excessive negative affect generation.
3.6 Generating negative affect to a neutral image: amygdala ROI results
When comparing Increase Negative > Look Neutral across all 6 runs of the task for CTL and MDD groups combined, we found significant activation in a 162-voxel cluster in the right amygdala (peak 24, 4, −14) and a 274-voxel cluster in the left amygdala (peak −14, −6, −12; Figure S2). In addition, a left amygdala cluster (297 voxels, peak −12, −8, −14) showed significant activation within the first 3 runs of the task both at the combined group level and within the MDD group; however, the MDD and CTL groups did not differ significantly with respect to the percent signal change (ps>.4). Moreover, the percent signal change in amygdala clusters for Increase Negative > Look Neutral was not correlated with affect ratings or with self-reported difficulty with emotion regulation within either group. Finally, as expected, there was no significant amygdala activation for Look Neutral > Increase Negative.
4. Discussion
This study was designed to examine the neural substrates of top-down modulation of negative affect in MDD, specifically decreasing negative affect through reappraisal and generating negative affect in the absence of an external trigger. We found that during reappraisal of negative images in order to decrease negative affect, depressed participants differed from their nondepressed counterparts with respect to the patterns of activation in dACC and SMA over the course of the task. During Decrease Negative compared to Look Negative trials, depressed individuals exhibited less activation in these areas than did CTLs during early phases of the task, but in contrast to the CTLs, who decreased their activation over the course of the task, MDD participants increased activation during regulation. In addition, during a novel condition in studies of depressed individuals – the generation of negative affect in response to a neutral stimulus – MDD participants activated a temporal lobe region including the MTG to a significantly greater extent than did CTLs; moreover, this activation was related to lower negative affect and to greater difficulty generating negative affect. These findings increase our understanding of mechanisms that underlie dysfunctional reappraisal in MDD by highlighting two potential neural sources of impaired regulation of negative affect: inefficiency in recruiting dACC and SMA during the down-regulation of negative affect and a greater reliance on MTG to compensate for increases in negative affect.
During both Increase Negative and Decrease Negative trials, our task successfully activated canonical reappraisal-related neural regions (e.g., Buhle et al., 2014), including frontoparietal and lateral and medial PFC regions (vlPFC, dACC, and superior parietal lobule; see Supplement). We also explored amygdala activation given its frequent identification in a priori analyses in previous studies (e.g., Ochsner et al., 2004; Ray et al., 2005); however, we found no MDD-associated differences in activation in this structure. Across all participants, we observed bilateral amygdala activation during the top-down generation of negative affect that persisted for the duration of the task; in contrast, amygdala activation in response to viewing a negative image decreased over time and was not significantly modulated by reappraisal. Persistent amygdala activation during the generation of negative affect could be due to participants’ elaborative efforts working in opposition to habituation effects and indicates that our task effectively engaged this emotion-responsive region. In addition, both groups reported more negative affect during both the Look Negative and Decrease Negative conditions over the course of the task. Thus, viewing the negative images seems to have been increasingly unpleasant over time.
Although all participants’ negative affect ratings changed significantly as a function of regulation instructions, the MDD and CTL groups did not differ either in their ratings in any task condition or in the change in their affect during the regulation conditions relative to the baseline look conditions. This null finding is consistent with evidence that unmedicated depressed individuals can engage in instructed and trained emotion regulation (Dillon and Pizzagalli, 2013; Ehring et al., 2010).
Despite exhibiting equivalent changes in negative affect during Decrease Negative compared to Look Negative trials, MDD and CTL participants differed in their patterns of activation in the dACC and SMA. Both of these regions have been implicated in emotional down-regulation (Etkin et al., 2011; Kohn et al., 2014); they function as hubs for the execution of reappraisal by connecting and integrating information from prefrontal cognitive control regions and subcortical emotion-responsive regions. Indeed, the SMA has been linked to regulation success (Kohn et al., 2014; Wager et al., 2008), and researchers have highlighted its role in cognitive control processes, particularly in support of the selection and execution of voluntary responses, as might be seen in reappraisal (see also Etkin et al., 2011). Depressed individuals in this study did not activate the dACC or SMA during the reappraisal of negative stimuli to the same extent as CTL participants during the early phases of the task; moreover, they increased activation in these regions over time, whereas CTL participants decreased activation. Higher early activation of the SMA during reappraisal was associated with greater decreases in negative affect for the MDD group. Previous studies have documented associations between ACC activation and reappraisal success (Ochsner et al., 2002; Phan et al., 2005; Wager et al., 2008), supporting our interpretation that activating these regions during reappraisal is important for effective affect modulation. The relatively lower dACC and SMA activation in MDD participants when reappraising vs. viewing negative stimuli in the early phases of the task might reflect less effectively regulated negative affect, as has been documented in MDD (Davidson et al., 2002) By the latter half of the task, the MDD individuals appear to have corrected this imbalance, suggesting that although they are not able to modulate the dACC and SMA to meet reappraisal demands initially, they can do so over time. Their increased activation over time during reappraisal, however, diverged notably from the CTL participants’ pattern of decreasing activation in these regions, with no accompanying impact on the effectiveness of their reappraisal efforts, perhaps due to increased efficiency and reduced effort needed during ongoing down-regulation for the CTL group.
Perhaps the most important contribution of this study concerns the difference between MDD and CTL participants during the instructed generation of negative affect in response to a neutral image. Compared with the CTL participants, MDD participants had higher activation in a temporal lobe cluster that included the posterior right middle temporal gyrus and supramarginal gyrus. A similar middle temporal cluster was identified in a study in healthy individuals during reappraisal to generate negative affect (Ochsner et al., 2009). Across both CTL and MDD groups, higher activation in this cluster was associated with lower negative affect and with greater difficulty following the Increase Negative instructions. When instructed to deliberately generate a negative interpretation of a neutral stimulus, the MDD individuals who reported the most difficulty and the least change in affect were those who activated the MTG most strongly, suggesting that the MTG serves a compensatory function, particularly in the MDD group. Alternatively, across both groups, participants who are least likely to generate negative affect may engage this region more, including during affect regulation. Activation in this region has been reported in previous studies of emotion regulation, including as part of a set of DMN regions underlying reappraisal (Sheline et al., 2009), during up-regulation to a negative image (Eippert et al., 2007), and during down-regulation (Buhle et al., 2014; Kim and Hamann, 2007), pointing to its role in executing reappraisal efforts that involve reinterpreting the meaning of a stimulus and, thereby, changing its semantic representation (Buhle et al., 2014; Kohn et al., 2014).
We should note four limitations of this study. First, several aspects of the regulation task design may have influenced the results (see Ochsner et al., 2012, for a review of these issues). For example, our presentation of the stimulus image two seconds before the regulation cue likely allowed a natural emotional response to unfold before participants attempted to change it, making the regulation potentially more difficult, although more generalizable to real-world scenarios (Gross, 2002). Moreover, there is evidence that cues intended to increase affect are more effective when presented after the image (Ochsner et al., 2012). However, some of the null amygdala results may have been due to habituation during the initial image viewing phase. Second, our sample was composed of unmedicated female MDD individuals with moderate depression, thus limiting the generalizability of our findings to more severely depressed or medicated samples (Dillon and Pizzagalli, 2013; Rive et al., 2013). As a related point, our sample included left-handed participants, which may have introduced some laterality differences in activation. We should note, however, that the proportion of left-handed participants was comparable in the MDD and CTL groups; further, when including handedness as a covariate, our analyses remained significant (ps<=.001 for all group by condition interactions). Third, contrary to our expectations, the MDD and CTL groups did not differ in self-reported affect in the Increase Negative condition that was intended to assess dysregulated emotional processes that characterize MDD. The relatively small 5-point range of the negative affect rating scale may have limited variability in reported affect. It is also noteworthy that although the MDD individuals did not report the predicted higher levels of negative affect, patterns of neural activation point to group differences in the processes that underlie the generation of negative affect. Finally, the reappraisal instructions were phrased slightly differently in the two regulation conditions, which may have introduced variability in the strategies that individuals applied. For example, the lack of group differences in semantic processing regions activated during the Decrease Negative condition may have been due to participants using distancing strategies (i.e., thinking that the picture was not real) instead of reinterpreting the meaning of the picture itself.
Despite these limitations, this study is important in identifying differences between depressed and nondepressed participants in the neural mechanisms that underlie two specific types of dysfunctional affective processes posited to be characteristic of MDD: impaired reappraisal to decrease negative affect and maladaptive top-down increases in negative affect. Participants with MDD were characterized by ineffective recruitment of dACC and SMA in early phases of the down-regulation task followed by increasing activation in these regions over time, and by heightened recruitment of MTG during top-down emotion generation, highlighting two possible compensatory strategies in MDD. The group differences identified in this study point both to sources of dysfunctional emotion regulation and to potential mechanisms that underlie the maladaptive increases in negative affect documented in MDD.
Supplementary Material
Highlights.
This novel fMRI paradigm prompts both regulation and generation of negative affect
Depressed subjects vs controls show inefficient neural activation during regulation
Groups differ in middle temporal gyrus activation during negative affect generation
Emotion regulation in depression may require compensatory neural activation
Acknowledgments
Funding for the current study was provided by the National Institute of Mental Health grants R01-MH59259, R01-MH101495, F32-MH090617, T32-MH019938, and by NARSAD grant 19018.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
LFR and IHG contributed to study conception and design, EGD analyzed the data and drafted the manuscript, and all authors contributed to interpretation of the data and critical revisions of the manuscript.
Conflict of interest
The authors report no conflicts of interest with regard to the present work.
References
- Beauregard M, Paquette V, Lévesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Beck AT. Thinking and Depression: Idiosyncratic Content and Cognitive Distortions. Arch Gen Psychiatry. 1963;9:324–333. doi: 10.1001/archpsyc.1963.01720160014002. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proclam Natl Acad Sci. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Pizzagalli DA. Evidence of successful modulation of brain activation and subjective experience during reappraisal of negative emotion in unmedicated depression. Psychiatry Res - Neuroimaging. 2013;212:99–107. doi: 10.1016/j.pscychresns.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T, Tuschen-Caffier B, Schnülle J, Fischer S, Gross JJ. Emotion regulation and vulnerability to depression: spontaneous versus instructed use of emotion suppression and reappraisal. Emotion. 2010;10:563–572. doi: 10.1037/a0019010. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. User’s guide for the structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version. Am Psychiatr Pub 1997 [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gollan JK, Pane HT, McCloskey MS, Coccaro EF. Identifying differences in biased affective information processing in major depression. Psychiatry Res. 2008;159:18–24. doi: 10.1016/j.psychres.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305.Cognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening SG, Osuch EA, Williamson PC, Mitchell DGV. The neural correlates of regulating positive and negative emotions in medication-free major depression. Soc Cogn Affect Neurosci. 2014;9:628–637. doi: 10.1093/scan/nst027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/S0048577201393198. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: Time matters. Neurosci Biobehav Rev. 2009;33:1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation - An ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. 2008 doi: 10.1016/j.epsr.2006.03.016. [DOI] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- McRae K, Misra S, Prasad AK, Pereira SC, Gross JJ. Bottom-up and top-down emotion generation: Implications for emotion regulation. Soc Cogn Affect Neurosci. 2011;7:253–262. doi: 10.1093/scan/nsq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Gr Process Intergr Relations. 2008;11:143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Rekshan W, Williams LM, Cooper N, Gross JJ. Effects of antidepressant medication on emotion regulation in depressed patients: An iSPOT-D report. J Affect Disord. 2014;159:127–132. doi: 10.1016/j.jad.2013.12.037. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100:569–582. doi: 10.1037/0021-843X.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspect Psychol Sci. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, Mcrae K, Cooper JC, Weber J, Gabrieli JDE, Gross JJ. Bottom-up and top-down processes in emotion generation: Common and distinct neural mechanisms. Psychol Sci. 2009;20:1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JDE, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn Affect Behav Neurosci. 2005;5:156–68. doi: 10.3758/CABN.5.2.156. [DOI] [PubMed] [Google Scholar]
- Rive MM, Mocking RJT, Koeter MWJ, van Wingen G, de Wit SJ, van den Heuvel OA, Veltman DJ, Ruhé HG, Schene AH. State-dependent differences in emotion regulation between unmedicated bipolar disorder and major depressive disorder. JAMA psychiatry. 2015;72:687–696. doi: 10.1001/jamapsychiatry.2015.0161. [DOI] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies Neurosci Biobehav Rev. 2013;37:2529–53. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Demaree HA, Robinson JL, Pu J. Ego depletion by response exaggeration. J Exp Soc Psychol. 2006;42:95–102. doi: 10.1016/j.jesp.2005.02.005. [DOI] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/S0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NK, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist Ma, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Smith SM. Constrained linear basis sets for HRF modelling using Variational Bayes. Neuroimage. 2004;21:1748–1761. doi: 10.1016/j.neuroimage.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


