Abstract
Coral reefs are experiencing increasing anthropogenic impacts that result in substantial declines of reef-building corals and a change of community structure towards other benthic invertebrates or macroalgae. Reefs around Zanzibar are exposed to untreated sewage and runoff from the main city Stonetown. At many of these sites, sponge cover has increased over the last years. Sponges are one of the top spatial competitors on reefs worldwide. Their success is, in part, dependent on their strong chemical defenses against predators, microbial attacks and other sessile benthic competitors. This is the first study that investigates the bioactive properties of sponge species in the Western Indian Ocean region. Crude extracts of the ten most dominant sponge species were assessed for their chemical defenses against 35 bacterial strains (nine known as marine pathogens) using disc diffusion assays and general cytotoxic activities were assessed with brine shrimp lethality assays. The three chemically most active sponge species were additionally tested for their allelopathic properties against the scleractinian coral competitor Porites sp.. The antimicrobial assays revealed that all tested sponge extracts had strong antimicrobial properties and that the majority (80%) of the tested sponges were equally defended against pathogenic and environmental bacterial strains. Additionally, seven out of ten sponge species exhibited cytotoxic activities in the brine shrimp assay. Moreover, we could also show that the three most bioactive sponge species were able to decrease the photosynthetic performance of the coral symbionts and thus were likely to impair the coral physiology.
Introduction
Coral reefs worldwide have experienced substantial losses of coral cover and species diversity over the past decades in response to various anthropogenic drivers [1–3]. These declines in coral cover have resulted in shifts of benthic community composition [4–6]. Non reef-building taxa that can cope better with anthropogenic stressors, such as climate change, eutrophication, sedimentation, and disease prevalence, continue to increase in abundance [4,5,7–10]. Additionally, organisms other than hard corals are released from top-down control through overfishing and can undergo uncontrolled growth due to the absence of their predators [10–13]. Sponges are one of the top spatial competitors for reef-building scleractinian corals and their abundance, as well as biomass, on reefs worldwide is steadily increasing [6,14,15]. In the Caribbean, sponge biomass and coverage is now equal to or even exceeding that of corals [10,16–18]. Their great success can be partly explained by their ability to feed on a wide variety of nutritional sources [19], the very low energetic costs of their filter-feeding activities [20] and their strong chemical defenses [18].
Sponges produce the greatest diversity of secondary metabolites among benthic marine organisms [21] with more than 5300 currently described [22]. The primary interest in sponge secondary metabolites has been related to their potential pharmacological activity, but a growing number of studies have started to investigate the ecological functions of these compounds. Their secondary metabolites give sponges the ability to deter predators [23–25], inhibit pathogenic microbes [25–27] and demonstrate competitive dominance towards other sessile benthic organisms [15,28,29].
A crucial factor contributing to the competitive success of sponges is their ability to combat microbial attacks. Many sponges defend their surface from colonization by fouling organisms as well as from potential pathogenic bacteria by producing secondary metabolites with antimicrobial properties [25,30–32]. Marine organisms are constantly exposed to potentially harmful bacteria. In the Indian Ocean bacterial abundances range from 6x 104 ml-1 to 2.5x 106 cells ml-1 in the surrounding seawater [33–35]. Sponges are additionally exposed to large quantities of microbes passing through their bodies due to their filter feeding activities [20]. Given the exposure of sponges to high numbers of bacteria in the marine environment and the relatively low incidence of infection with diseases, chemical compounds in sponges are likely crucial in providing effective defenses against the invasion of pathogenic microbes after damage or injury [7,25,36–38].
Over the last 20 years the prevalence and severity of marine diseases has increased substantially, particularly impacting reef building corals [5,39,40]. Coral diseases have been correlated with environmental stress caused by human activities and environmental alterations associated with global climate change [39–41]. Diseased corals in the Caribbean were almost exclusively found in anthropogenically impacted areas [42] and some coral diseases are assumed to be caused by human faecal bacteria [43,44]. Sponges on the other hand, seem to be less susceptible to environmental conditions that are stressful for corals or might have better antimicrobial defenses since sponge diseases are much less prevalent [5,7,45].
In addition to their strong defenses against microbial attacks, many sponges are assumed to be competitively superior over other reef organisms [28,46–48]. One of the main factors shaping the community composition of sessile, benthic assemblages is the competition for space [49]. Especially on coral reefs, free substratum space with adequate irradiance for photosynthesis and exposure to food-providing water currents is one of the most limiting resources for benthic organisms [50,51]. The high biodiversity on coral reefs results in high frequency of competitive interactions between sessile organisms of the same and of different species [52,53]. Sponges have not only the ability of rapidly overgrowing benthic reef organisms but they also release chemical compounds that can harm and kill other competitors [15,28,46,48]. Cytotoxic secondary metabolites produced by sponges may be able to inhibit the growth of other competing organisms by impairing their cell division and thus provide sponges with an advantage during competition for space on crowded coral reef substratum [54,55]. The bioactivity, especially the cytotoxicity, of sponge extracts seems to be a good proxy for their ability to overgrow corals in the field [56]. The four most bioactive sponges in the Spermonde Archipelago were also reported to cause necrosis of corals in more than 85% of interactions observed in situ [15]. Bioactive compounds are released through tissue contact, via sponge mucus or directly into the surrounding water causing bleaching and tissue necrosis in neighbouring corals thereby reducing their chances of survival [28,46,57]. Several sponges have already been identified to use allelopathy in order to inhibit the growth of other benthic organisms or were even able to cause bleaching and tissue necrosis in neighbouring corals [15,28,46,48,58]. The competitive abilities of sponges may be further enhanced with increasing anthropogenic disturbances that are stressful to corals but tolerable for sponges [59]. In the Western Indian Ocean (WIO) corals were greatly affected by the 1997/1998 El Niño Southern Oscillation (ENSO) resulting in mass coral bleaching and a decline in coral cover [60–62]. This decline of coral species has led to a shift of the ecological balance in favour of other space-competing functional groups especially corallimorpharians [4,63,64]. Additionally, the reefs on Zanzibar´s West Coast are heavily exposed to untreated sewage and runoff from the main city which could represent a potential source for the introduction of a variety of bacteria and pathogens [65,66]. Multiple drainage pipes, some extending up to 55m from the coast along the sea bottom, come from the 2289 septic tanks, and discharge daily 2.2 x 106 l of wastewater [65]. Over the last 12 years an increase of the amount of 15N in common benthic organisms, and an increased amount of fecal indicator bacteria (i.e. Enterococcus (ENT)) suggest that water quality has deteriorated [65,67–70].
In this study we provide the first evaluation of chemical competitive defenses in Western Indian Ocean (WIO) reef sponges. Our aim was to examine the organic extracts from the most abundant sponge species on reefs around the West Coast of Zanzibar for ecologically significant antimicrobial and cytotoxic activities. Additionally, extracts of the three most active sponge species, Pseudoceratina sp., Callyspongia sp. and Haliclona atra, were tested for their allelopathic properties in field experiments. Corals of the genus Porites were chosen for the field experiments since the reefs at Bawe Island are dominated by large monostands of branching and massive Porites following the El Niño in 1997/ 1998 and a Crown-of-Thorn Starfish (COTS) outbreak in 2002–2006 [8,71–74]. Furthermore, all three sponge species were observed to grow adjacent or even in between branching Porites corals, making the study of interactions among these two organisms ecologically relevant.
Material and methods
Ethics statement
This research was completed in accordance with permits issued by the Research Committee of the Zanzibarian Government.
Study site
The field study was conducted from September to December 2014 at reefs around Bawe Island, Zanzibar (Unguja), Tanzania (S1 Fig). Bawe (06° 09´25.56” S, 39° 08´0.96” E) is located on the west side of the island in the Zanzibar channel, about 7km from the capital Stonetown [71]. The reef at Bawe is heavily influenced by fishing activities and untreated sewage discharge from Stonetown and its harbour [8,65,66,75].
Collection and extraction procedure
Based on a sponge community survey [76], the ten most abundant sponge species were chosen for the investigation of their antimicrobial and cytotoxic properties. This included Callyspongia aerizusa, Callyspongia sp., Haliclona atra, Haliclona fascigera, Biemna sp., Paratetilla sp., Pseudoceratina sp. Scopalina hapalia, Plakortis kenyensis and Tetrapocillon minor. The sponge species were identified by Dr. Nicole de Voogd and vouchers have been registered in the sponge collection of the Naturalis Biodiversity Center in Leiden, Netherlands. Specimens of the selected sponge species were sampled opportunistically by scuba-divers at 10m depth. Replicates (3–5 individuals) of each sponge species were collected and transferred into zip block bags filled with ambient seawater. There was a minimum distance of 20 m between replicates to avoid collection of clones. Samples were immediately transferred in coolers to the laboratory facilities at the Institute of Marine Sciences (IMS, Stonetown). After gently removing dripping water, sponge pieces were weighed to the nearest 0.1 g to determine wet weight (WW), cut into small pieces and secondary metabolites were extracted three times with 99.9% Ethanol. The extracts were filtered to remove particles and the filtrate was kept at ‐20°C for storage and transport. Samples were filtered again and evaporated under reduced pressure using a rotary evaporator (water bath temperature 35°C) at the laboratories of the ICBM, University of Oldenburg. The crude extracts were transferred into preweighted glass vials and evaporated to complete desiccation with a Speed Vac. Natural extract concentrations were calculated as mg extract per gram of sponge wet weight (see Table 1). Extracts from 3–5 replicate sponge individuals per species were pooled and used for the experiments to get a significant estimate of the response parameters for this population. This has been done in many studies on chemical defense (e.g. [76–80]). All extracts were stored at -20°C until use.
Table 1. The most abundant sponge species at Bawe Island, Zanzibar, their percent coverage and natural extract yield.
The data for the benthic cover of the different sponge species were obtained by a previous study [76].
| Order | Species | Number of Replicates | Extract yield [mg g (WW)-1] a | Benthic cover at 10m depth[%] |
|---|---|---|---|---|
| Haplosclerida | Haliclona fascigera | 5 | 13.00 (± 6.38) | 0.17 (±0.90) |
| Haplosclerida | Haliclona atra | 3 | 26.51 (± 4.53) | 2.33 (±4.70) |
| Haplosclerida | Callyspongia aerizusa | 5 | 19.35 (± 10.14) | 0.37 (±1.32) |
| Haplosclerida | Callyspongia sp. | 3 | 22.90 (± 2.18) | 0.13 (±0.48) |
| Verongida | Pseudoceratina sp. | 3 | 31.06 (± 18.63) | 0.00 (±0.00) |
| Homosclerophorida | Plakortis kenyensis | 3 | 16.80 (± 13.25) | 0.01 (±0.03) |
| Scopalinida | Scopalina hapalia | 3 | 17.14 (± 5.86) | 0.04 (±0.14) |
| Poecilosclerida | Biemna sp. | 3 | 18.29 (± 2.84) | 2.88 (±4.20) |
| Tetractinellida | Paratetilla sp. | 3 | 26.31 (± 3.66) | 0.01 (±0.04) |
| Poecilosclerida | Tetrapocillon minor | 4 | 18.39 (± 7.37) | 0.11 (±0.24) |
a Extracts yields in mg extract per g WW (wet weight) are given as the mean of 3–5 extractions (± STD).
Bacterial panel
The agar disc-diffusion assay was used to assess antibacterial activities of the sponge crude extracts. 35 bacterial strains, representing a wide phylogenetic range (see Table 2), were tested including nine known pathogens associated with marine diseases (Aurantimonas coralicida, Acitenobacter pitiii, three strains of Vibrio alginolyticus, Vibrio owensii, two strains of Vibrio coralliilyticus and Vibrio shilonii). Bacteria were considered pathogens if their closest match was a bacterial strain associated with a marine disease and they further possessed sequence similarities of >98% to known pathogens in the 16S NCBI BLAST database.
Table 2. Description of the 35 bacterial strains used in the antimicrobial assay, including nine known pathogens for marine diseases percent similarity indicates how close the bacterial sequence of the isolate is to the closest strain in the NCBI BLAST databank.
| No. | Phylum | Class | Family | Accession no. of bacterial isolate | Species (closest NCBI hit) | Accession no. of closest NCBI hit | Similarity of the closest NCBI hit | |
|---|---|---|---|---|---|---|---|---|
| 1701 | Actinobacteria | Actinobacteria | Micrococcaceae | MG551787 | Kocuria halotolerance | NR_044025 | 98.888 | |
| 1744 | Actinobacteria | Actinobacteria | Micrococcaceae | MG551810 | Micrococcus aloeverae | NR_134088 | 99.666 | |
| 1682 | Actinobacteria | Actinobacteria | Nocardiaceae | MG551778 | Rhodococcus corynebacterioides | NR_119107 | 99.343 | |
| 1656 | Actinobacteria | Actinobacteria | Streptomycetaceae | MG551768 | Streptomyces flavoviridis | NR_041218 | 100 | |
| 1733 | Proteobacteria | Alphaproteobacteria | Rhodobacteraceae | Loktanella pyoseonensis | NR_115100 | 98.684 | ||
| 1636 | Bacteroidetes | Flavobacteria | Flavobacteriaceae | MG551762 | Aquimarina gracilis | NR_113781 | 99.302 | |
| 1686 | Firmicutes | Bacilli | Bacillales Family XII. Incertae | MG551781 | Exiguobacterium profundum | NR_043204 | 99.549 | |
| 1694 | Proteobacteria | Alphaproteobacteria | Rhodobacteraceae | MG551784 | Paracoccus zeaxanthinifaciens | NR_025218 | 99.892 | |
| 1754 | Proteobacteria | Gammaproteobacteria | Rhodobacteraceae | MG551813 | Ruegeria areniliticus | NR_109635 | 99.678 | |
| 1668 | Proteobacteria | Alphaproteobacteria | Rhodobacteraceae | MG551772 | Ruegeria areniliticus | NR_109635 | 97.439 | |
| 1792 | Proteobacteria | Alphaproteobacteria | Rhodobacteraceae | MG551832 | Pseudovibrio denitrificans | NR_113946 | 99.784 | |
| 1721 | Proteobacteria | Gammaproteobacteria | Alteromonadaceae | MG551801 | Microbulbifer variabilis | NR_041021 | 99.78 | |
| 1633 | Proteobacteria | Gammaproteobacteria | Pseudoalteromonadaceae | MG551852 | Pseudoalteromonas phenolica | NR_113299 | 99.785 | |
| 1783 | Proteobacteria | Gammaproteobacteria | Pseudoalteromonadaceae | MG551825 | Pseudoalteromonas piscicida | NR_114190 | 99.251 | |
| 1810 | Proteobacteria | Gammaproteobacteria | Enterobacteriaceae | MG551842 | Pantoea eucrina | NR_116246 | 99.299 | |
| 1703 | Proteobacteria | Gammaproteobacteria | Pseudoalteromonadaceae | MG551789 | Pseudomonas pseudoalcaligenes | NR_037000 | 98.168 | |
| 1652 | Proteobacteria | Gammaproteobacteria | Vibrionaceae | MG551766 | Vibrio maritimus | NR_117551 | 98.28 | |
| 1809 | Proteobacteria | Alphaproteobacteria | Rhodobacteraceae | MG551841 | Ruegeria areniliticus | NR_109635 | 98.072 | |
| 1767 | Proteobacteria | Gammaproteobacteria | Pseudoalteromonadaceae | MG551817 | Pseudomonas luteoviolacea | NR_026221 | 99.466 | |
| 1727 | Actinobacteria | Actinobacteria | Micrococcaceae | MG551804 | Kocuria sediminis | NR_118222 | 99.024 | |
| 1726 | Proteobacteria | Gammaproteobacteria | Pseudoalteromonadaceae | MG551803 | Pseudoalteromonas piscicida | NR_114190 | 99.679 | |
| 1722 | Proteobacteria | Gammaproteobacteria | Halomonadaceae | MG551802 | Halomonas aquamarina | NR_042063 | 99.465 | |
| 1334 | Proteobacteria | Gammaproteobacteria | Alteromonadaceae | MG711595 | Aliagarivorans marinus | NR_044585 | 98 | |
| 1348 | Proteobacteria | Gammaproteobacteria | Vibrionaceae | MG711594 | Vibrio maritimus | NR_117551 | 98 | |
| 0852 | Proteobacteria | Gammaproteobacteria | Moraxellaceae | MG551849 | Acitenobacter soli | NR_044454 | 99 | |
| 1659 | Proteobacteria | Gammaproteobacteria | Pseudoalteromonadaceae | MG551769 | Pseudomonas luteoviolacea | NR_114237 | 99.678 | |
| Known pathogens for marine diseases | Info about pathogens | |||||||
| 1678 | Proteobacteria | Gammaproteobacteria | Moraxellaceae | MG551777 | Acinetobacter pitii | NR_117930 | 99.663 | Fish pathogen [82] and human pathogen (Pneumonia; [83]) |
| 1621 | Proteobacteria | Gammaproteobacteria | Vibrionaceae | MG551759 | Vibrio alginolyticus | NR_113781 | 99.302 | Marine pathogen, associated with several diseases in fish and shrimp [84–88] |
| 1645 | Proteobacteria | Gammaproteobacteria | Vibrionaceae | MG551765 | Vibrio owensii | NR_117424 | 99.569 | Tissue loss disease "Montipora White Syndrome" in the Hawaiian reef coral Montipora capitata [89] |
| 1675 | Proteobacteria | Gammaproteobacteria | Vibrionaceae | MG551775 | Vibrio alginolyticus | NR_113781 | 98.783 | Marine pathogen, associated with several diseases in fish and shrimp [84–88] |
| 1761 | Proteobacteria | Gammaproteobacteria | Vibrionaceae | MG551816 | Vibrio coralliitycus | NR_117892 | 99.57 | Bacterial bleaching and rapid tissue destruction [90–92] |
| 1644 | Proteobacteria | Gammaproteobacteria | Vibrionaceae | MG551764 | Vibrio alginolyticus | NR_113781 | 99.785 | Marine pathogen, associated with several diseases in fish and shrimp [84–88] |
| WHV0001 | Proteobacteria | Alphaproteobacteria | Aurantimonadaceae | Aurantimonas coralicida | AY065627 | DSMZ, Germany | White plague type II disease [93] | |
| WHV0002 | Proteobacteria | Gammaproteobacteria | Vibrionaceae | Vibrio shilonii | ATCC BAA-91 | DSMZ, Germany | Bacterial bleaching [94,95] | |
| WHV0003 | Proteobacteria | Gammaproteobacteria | Vibrionaceae | Vibrio coralliilyticus | AJ440005 | DSMZ, Germany | Bacterial bleaching and rapid tissue destruction [90–92] | |
The bacterial strains were formerly isolated from the crustose coralline alga Hydrolithon reinboldii and from two sponges, namely Rhabdastrella globostellata (no.1334 and 1348) and Pseudoceratina sp. (no.0852) from Guam, except for three marine pathogens (WHV0001, WHV0002 and WHV0003) that were ordered from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, DMSZ, Braunschweig, Germany. The isolation, conservation and identification of the various bacterial strains have been performed by our colleagues [25,81]. The comparison of the sequence data from the isolated strains with sequences in the NCBI BLAST database was according to methods described by our colleagues [81].
Antimicrobial assay
Bacterial strains were grown in liquid marine broth medium for 24 hours at 25°C before each experiment. Crude extracts of the different sponge species were dissolved in aliquots of ethanol at natural concentrations (see Table 1). 15 μl of crude extract were added to sterile filter paper (Ø 6 mm, Whatman) and the solvent was allowed to completely evaporate. Control filters were prepared in the same manner with 15 μl of solvent only. Each one of the 196 Marine broth agar plates (1.5% agar, 3.75 g l-1 Difco Marine Broth 2216, filtered deionized water) was inoculated with 200 μl of liquid culture of the respective test strains and spread evenly to provide a uniform bacteria lawn. Up to seven extract discs and one solvent control disc per plate were randomly assigned to the different plates and placed on the surface of agar plates with the extract side facing the plate. Three to six replicates of each pooled extract were tested, depending on the abundance of the sponges in the field during collection. Following a 24 h and 48 h incubation period, growth inhibition zones were scored as clear halos around the discs. The radius of the inhibition zone (without disc) was measured to the nearest 0.5 mm.
Permutational multivariate analyses of variance (PERMANOVA) obtaining Monte Carlo p-values (due to too low numbers of permutations) were conducted with the Primer software (Version 6.1.13) and the PERMANOVA+ add-on (Version 1.03). The analyses were used to test for significant activities of sponge extracts and to compare activities against environmental and pathogenic bacteria [96,97]. Results were categorized as no effect (0), weak inhibition (0–1mm), moderate inhibition (>1 to 3mm), strong inhibition (>3 to 7mm) and very strong inhibition (>7 to 15mm) after [98].
Brine shrimp lethality assay
Brine shrimp (Artemia salina) eggs were placed in a hatching tank containing seawater with strong aeration under continuous light at 24°C for 12 hours. Photophilic nauplii were collected approximately 12 hours after hatching. A stock solution of 10mg ml-1 was prepared for each pooled extract of the ten sponge species. From the stock solutions 1000 and 100 μl were transferred to individual petri dishes and solvents were allowed to evaporate. The respective amount of ethanol served as control. After the solvents had evaporated, 5 ml of filtered (0.45 μm), autoclaved, seawater was added to each petri dish. The petri dishes were placed on a shaker for 30 minutes to ensure that the extracts dissolved in the seawater. Subsequently, ten brine shrimps were added to each petri dish and the total volume was adjusted to 10 ml resulting in a final extract concentration of 1 mg, 0.1 mg and 0.01 mg sponge crude extract per 10 ml seawater. Larvae were not fed during the experiments as they still rely on their yolk-sac [99] and can survive for up to 48 hours without food [100]. Toxicity was determined after 48 hours (instar III/IV stage) of exposure by counting the surviving nauplii. This time was chosen since most extracts displayed an increasing activity up to 48 hours of exposure [101]. In addition, Artemia nauplii have been shown to exhibit their greatest sensitivity to sponge compounds in the second and third instar larval phase [100,102]. Extracts and controls were prepared in triplicates. Larvae were considered dead if no internal or external movement could be observed.
A PERMANOVA obtaining Monte Carlo p-values (due to too low numbers of permutations) was conducted with the Primer software (Version 6.1.13) and the PERMANOVA+ add-on (Version 1.03) to test for significant differences in mortality rates of brine shrimp between the individual sponge extracts and the control (n = 6 for each sponge species and the control).
Allelopathic activities of sponge extracts
Field experiments determined the allelopathic activities of sponge extracts under natural conditions. Assays with sponge extracts incorporated into phytagel were adapted from past studies of sponge overgrowth experiments and sponge-coral interactions [28,48]. We incorporated sponge extracts of Pseudoceratina sp., Callyspongia sp. and Haliclona atra at natural concentrations into phytagel strips and placed them in contact around branches of the corals of the genus Porites. Treatment phytagel strips with natural gravimetric concentrations of sponge extracts and control phytagel strips with only the extract solvent were prepared as described in [48]. Ethanol was used as solvent to extract allelopathic compounds of Pseudoceratina sp., Callyspongia sp. and H. atra. The phytagel was poured into a rectangular mold backed with a gaze. The phytagel mixture was allowed to cool and harden onto the gaze before being cut into strips with a small square patch of phytagel in the center of each strip. The gaze strips were transported in zip block bags on the same day to the reef of Bawe and fixed with rubber bands around coral branches of different Porites individuals at depths of 4–6 m. Six colonies that displayed no signs of bleaching were chosen for the experiment and marked with numbered tags.
For each sponge extract, one control (solvent only) and one treatment (with extract) strip was attached to adjacent coral branches. For extracts of H. atra and Pseudoceratina sp. six replicate coral colonies, and for the extracts of Callyspongia sp. four replicate colonies were used (the remaining two strips were lost during the dive limiting this to 4 replicates). Coral colonies were from the same fringing reef and treatment occurred between 13:00 and 15:00 h. Measurements of the photosynthetic efficiency of the zooxanthellae were taken by a diving PAM fluorometer (Walz, Germany) after 16–18 hours, before sunrise of the next day, in order to estimate the maximum quantum yield of the dark-adapted coral tissue. Under each phytagel patch, 6–8 measurements were taken, as well as from coral branches of the same colony that had neither treatment nor control phytagel strips attached to account for effects of shading, abrasion and the physical presence of the gel. For all readings of the PAM fluorometer, a clip was used that kept the probe at a distance of 1 cm from the coral surface. PAM fluorometry is a non-invasive technique and even though the use of the maximum photosynthetic yield as a proxy for intra- and interspecific comparisons of coral health under different environmental conditions is contentious, comparisons of coral tissue from the same coral branch are accepted [103,104]. Permutational multivariate analyses of variance (PERMANOVA) were conducted with the Primer software (Version 6.1.13) and the PERMANOVA+ add-on (Version 1.03). The analysis was used to test for significant differences of the effects of sponge extracts and for the effects of the phytagel with the solvent [96,97].
Results
Antimicrobial assay
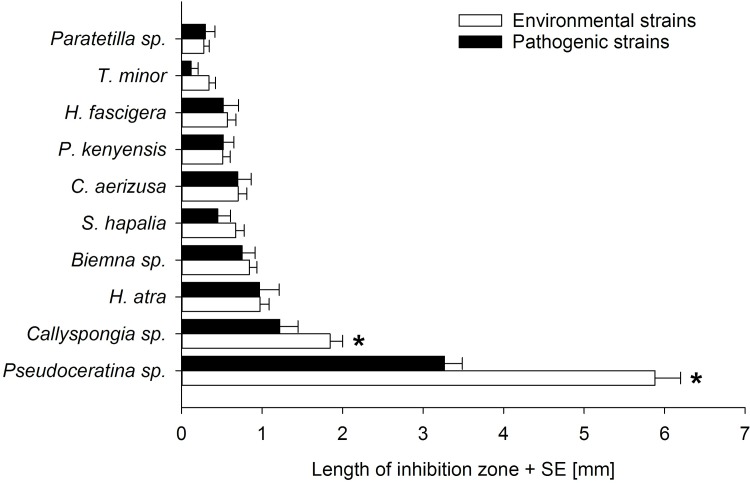
All tested sponge extracts showed antimicrobial activity while solvent control discs never inhibited bacterial growth (Table 3). Antimicrobial effects of the extracts varied widely with respect to the bacterial strains. The extract of Pseudoceratina sp. had the strongest antimicrobial activity in terms of the size of the inhibition zones, as well as the number of bacteria inhibited (inhibited all strains). Extracts of Callyspongia sp. and Haliclona atra revealed the second and third most antibacterial activity (71% and 49% of all strains inhibited, respectively; Table 3). In contrast, Paratetilla sp. and Tetrapocillon minor were the least chemically defended species inhibiting only 11% of the bacterial strains and also showing the smallest inhibition zones. The other five sponge species displayed all moderate antibacterial activity with 23–34% of the bacterial strains inhibited (Table 3). When comparing the activity of the different sponge extracts against environmental and pathogenic bacterial strains, most sponge species were equally defended against potential pathogens. Only Pseudoceratina sp. and Callyspongia sp. were better defended against environmental bacteria with respect to the size of the inhibition zones (Fig 1; PERMANOVA, p < 0.05, S1 Table). Detailed results of the disc diffusion assays are presented in S2 Table.
Table 3. Degree of antimicrobial activity by crude extracts of ten sponge species.
| Sponge species | Number of bacterial strains inhibited (total 35) | ||||
|---|---|---|---|---|---|
| Weak | Moderate | Strong | Very strong | Sum of strains inhibited (% active) |
|
| T. minor | 0 | 4 | 0 | 0 | 4 (11%) |
| Paratetilla sp. | 0 | 4 | 0 | 0 | 4 (11%) |
| H. fascigera | 1 | 7 | 0 | 0 | 8 (23%) |
| P. kenyensis | 1 | 8 | 0 | 0 | 9 (26%) |
| S. hapalia | 1 | 6 | 2 | 0 | 9 (26%) |
| C. aerizusa | 2 | 8 | 1 | 0 | 11 (31%) |
| Biemna sp. | 3 | 9 | 0 | 0 | 12 (34%) |
| H. atra | 8 | 7 | 2 | 0 | 17 (49%) |
| Callyspongia sp. | 2 | 18 | 5 | 0 | 25 (71%) |
| Pseudoceratina sp. | 2 | 6 | 19 | 9 | 35 (100%) |
Radius of inhibition zone: 0 no effect; >0–1mm: weak inhibition; >1–3mm: moderate inhibition; >3–7mm: strong inhibition; >7–15mm: very strong inhibition (after Lippert et al. 2003).
Fig 1. Length of inhibition zones (mean radius, mm + SE) for environmental and pathogenic bacterial strains.
Bacterial inhibition by sponge crude extracts for environmental and pathogenic bacterial strains were compared. * indicates a significant difference between the inhibition of environmental vs. pathogenic bacterial strains (PERMANOVA, p < 0.05).
S2 Fig shows the antimicrobial activities of the tested sponge species against all Vibrio species (environmental as well as pathogenic strains). We choose to display the antimicrobial activities of all sponge species against Vibrio spp. because many species of this genus have been recognized as significant pathogens for marine organisms, including sponges [105–109].
Brine shrimp lethality assay
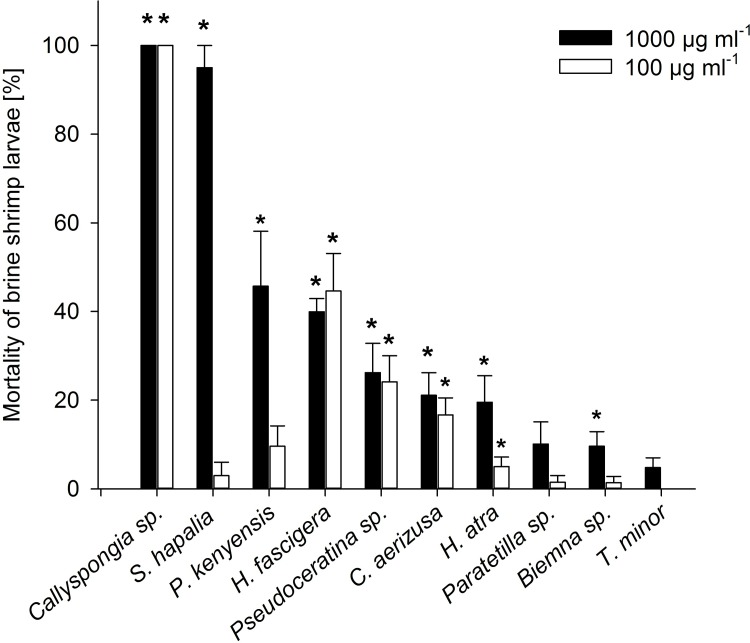
The results of the brine shrimp bioassay showed that seven out of the ten sponge species contained cytotoxic compounds (Fig 2; PERMANOVA, p < 0.05, S3 Table). The highest lethality was found for extracts of Callyspongia sp. at both concentrations, which exhibited Artemia mortality rates of 100% after just 12 hours (data not shown). Scopalina hapalia caused high mortality with 95% dead nauplii at a concentration of 1000 μg ml-1, but only non-significant mortality (5%) at a concentration of 100μg ml-1. H. fascigera, Pseudoceratina sp., C. aerizusa, H. atra and P. kenyensis all showed moderate lethality rates between 10.1% and 45% at the two test concentrations. No significant mortality rates were obtained from extracts of Biemna sp., Paratetilla sp. and T. minor. No mortality of Artemia larvae could be detected in controls. Detailed results of the brine shrimp assays can be found in S4 Table.
Fig 2. Mortality rates (+ SE) of the brine shrimp larvae in the lethality assay.
The mortality rates of the brine shrimp larvae are displayed in response to exposure to the different sponge crude extract concentrations at 1000μg ml-1 and 100μg ml-1 after 48 hours (mean + SE, n = 6). * indicates a significant mortality rate compared to control (PERMANOVA, p < 0.05).
Allelopathic activities of sponge extracts
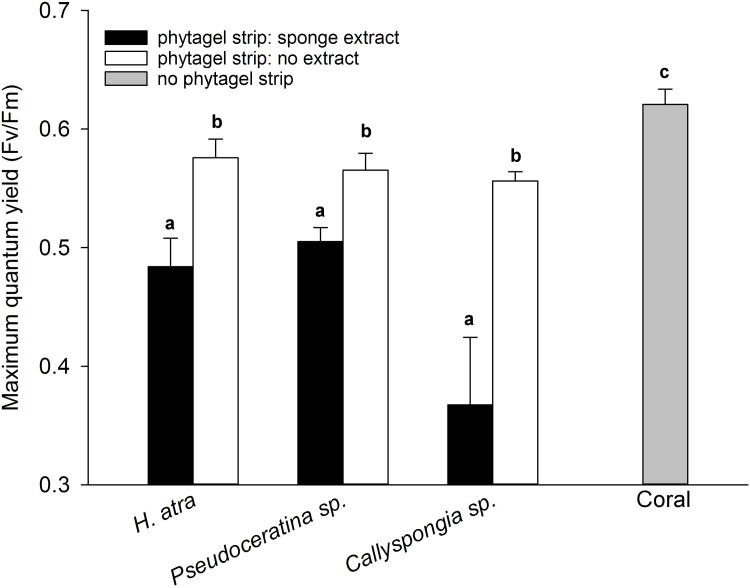
Effects of crude extracts from three Zanzibarian sponge species on the branching coral Porites sp. are presented in Fig 3. PAM readings were taken under each sponge extract and control gel band. Control phytagel strips had significant effects on the maximum photosynthetic yield of corals. Nonetheless, phytagel strips with sponge extracts exhibited stronger impairments on the corals photosynthetic performance and were also significantly different from the control strips. The extracts of all three investigated sponges, H. atra, Callyspongia sp. and Pseudoceratina sp., showed significant effects on the photosynthetic yield of branching Porites corals (PERMANOVA, p = 0.0001, df = 2). Metabolites produced by Callyspongia sp. had the most pronounced effect on the photosynthetic yield, decreasing the maximum quantum yield by 41% (PERMANOVA, p = 0.0039, t = 5.6076, df = 3). Extracts of H. atra and Pseudoceratina sp. had comparable negative effects on the effective quantum yield, reducing it by 22% and 19% each (PERMANOVA, p = 0.0014, t = 5.1455, df = 5 for H. atra and p = 0.0018, t = 6.1752, df = 5 for Pseudoceratina sp.). Detailed results of the effects of the different sponge extracts on the maximum quantum yields of individual Porites corals are presented in S5 Table.
Fig 3. In situ allelopathic effects of sponge extracts on the photosynthetic yield of a branching Porites coral.
Phytagel strips containing natural concentrations of sponge secondary metabolites reduced the maximum photosynthetic quantum yield (bars) of the symbiotic algae (zooxanthellae) in a branching Porites coral after 16–18 h of exposure (mean + SE, n = 6, except Callyspongia sp., n = 4). Letters indicate significant differences between treatment, control strips and unexposed coral tissue (control coral).
Sponge metabolites in the gel did not only affect the maximum photosynthetic yield of the Porites corals, but caused also bleaching of the underlying coral tissue. Coral tissue exposed to the metabolites of the extract of Callyspongia sp. and H. atra showed the most visibly bleaching.
Discussion
Sponges are regarded as the richest source of secondary metabolites with diverse bioactive properties, contributing to nearly 40% of all marine biomedical compounds discovered [110,111]. This study is the first that investigated the chemical ecology of sponges from East-Africa, revealing high bioactivity with regards to antimicrobial, cytotoxic and allelopathic effects.
Antimicrobial assay
All investigated sponge species exhibited antimicrobial activity in varying degrees depending on the number of inhibited test strains (11% up to 100% of strains inhibited). These results are in accordance with other studies examining the antimicrobial activity of sponges in which all sponge species from the Indian Ocean [112,113] and Antarctica [98] displayed antimicrobial activity. All sponges, except for Pseudoceratina sp. and Callyspongia sp., were equally active against known coral pathogens as well as against bacteria encountered in their environment. However, both of them displayed also moderate to high antimicrobial activities against pathogens, including several Vibrio strains. In contrast to our study sponges in the Caribbean displayed weaker inhibition rates for seawater bacteria compared to bacteria inhabiting necrotic sponge tissue or known pathogens [114]. However, other than the use of antimicrobial compounds, sponges possess efficient defense mechanisms that recognize pathogens and initiate an immune response. Sponges distinguish between infectious and non-infectious bacteria through molecular responses, receptor molecules and membrane proteins [115–117]. Bacteria associated with sponges are able to produce molecules which act on the sponge cells by inhibiting its immune and apoptotic system [118–121]. Growth inhibition, which was tested in the present study, is only one of the three stages in the colonization process by bacteria or potential pathogens (attachment, growth and swarming). Secondary metabolites of sponges might interfere with each of the three different colonization stages [27,32].
The strong activity against the variety of bacterial strains exhibited by Pseudoceratina sp. in this study indicates that this sponge species produces broad-spectrum antimicrobial compounds. Sponges of the genus Pseudoceratina contain bromotyrosine alkaloids and sterols of the aplystane type which possess cytotoxic [122,123], antimicrobial [124,125] as well as anti-HIV [126] and antimalarial [127] activities. Purealin C and its derivatives, which have been isolated from another Pseudoceratina species, also exhibit broadspectrum antimicrobial activities [128]. The two Callyspongia sponges displayed strong to moderate antimicrobial activity. Callyspongia species possess a variety of bioactive secondary metabolites, including Siphondiol [129], Akaterpin [130] and Utenine [131] displaying antimicrobial properties. The third most active sponge genus was Haliclona, with H. atra and H. fascigera inhibiting 17 (47%) and eight (22%) of the tested bacterial strains, respectively. Haliclona spp. produce Manzamine alkaloids with antitumor and antimalarial activities [132,133], as well as the antifungal and the antimicrobial compounds Papuamine, Haliclonadiamide and Halaminoles [134,135]. Additionally, extracts from several Indo-Pacific Haliclona species inhibited various bacterial strains, emphasizing the overall antimicrobial activity of this genus [112,136,137]. However, there are also some Haliclona species which possess weak or no antimicrobial activity [98,138,139]. The variability in sponge secondary metabolites of the same genus, or even of the same species, is not uncommon as their production is influenced by genetic traits as well as environmental factors and sponge-associated microbes [98,140–144]. Biemna sp. displayed moderate antimicrobial activity, inhibiting 12 (33%) of the tested bacterial strains. Sponges of the genus Biemna are a source for bioactive compounds with antimicrobial as well as cytotoxic activities, which include pyridoacridines (e.g. Labuanine), steroids (e.g. Ehrenasterol and Biemnasterol) as well as polycyclic alkaloids (e.g. Biemnadin, Hydroxyascididemin and Netamines; [136,137,145]). The sponges Plakortis kenyensis and Scopalina hapalia were active against nine (25%) of the examined bacterial strains. No reports of secondary metabolites of S. hapalia were found in the literature. The most prominent antimicrobial compounds isolated from plakinid sponges are Plakinidones, Plakortide, Manzamenone and Plakortin, which showed activity against several bacteria including Staphylococcus aureus, Escherichia coli and Bacillus subtilis [146–150]. No bioactive properties have been reported for the sponges Paratetilla sp. and Tetrapocillon minor. There is often no correlation between the antimicrobial activity of the sponge extract and the epibacterial abundance in sponges or ascidians [151,152]. Therefore, it would be interesting to test all colonization stages with the extracts of sponges from Zanzibar since T. minor was a sponge that inhibited the growth of only 11% of the tested strains but had always a very clean and smooth surface (personal observation). Instead of producing antimicrobial compounds, sponges also attract bacteria to their surfaces that repel other biofilm-forming bacteria and thus maintain a clean and smooth surface [153].
These examples also highlight one of the problems when evaluating sponge antimicrobial activities from the literature. Many of the antimicrobial activities reported for sponges used human pathogenic bacteria and not environmental bacteria to which the sponges are exposed to and did not test marine pathogens which pose a potential risk to the sponge holobiont. Our study on the contrary is one of a few that focused on marine environmental and marine pathogen bacteria to evaluate sponge antimicrobial defenses.
There is still a lack of knowledge about the main cause of sponge diseases and the role of pathogenic bacteria [45,154,155] which is why in the present study we utilised pathogenic bacteria known to cause diseases in corals and other marine organisms. Marine pathogens seem to affect not only one host (e.g. corals) but seem to be able to cause diseases also in other hosts if conditions are opportune. For example, cyanobacteria, which were associated with coral diseases, also replaced the Synechococcus/ Prochlorococcus clades in sponges affected by sponge orange band disease [154]. Environmental perturbations including urban runoff, nutrient enrichment, anthropogenic pollution and especially increase in temperature are linked to disease outbreaks in marine invertebrates [155–157]. The brown lesion disease in sponges is most likely caused by terrestrial pathogens used in pest control management of insects and fungi [158]. The high prevalence of antimicrobial defenses in the investigated sponges suggests that pathogens are a common threat to sponges on Zanzibar’s reefs.
The coral reefs surrounding Stonetown are especially exposed to untreated sewage and runoff from the main city [65,66]. Human fecal bacteria in wastewater are the etiological agent of white pox and black band disease in corals [43,44]. Even if wastewater does not harbour etiological agents for diseases of benthic invertebrates, the discharge of sewage introduces many opportunistic microbial taxa, such as e.g. Vibrionaceae and Rhodobacteraceae, that can alter the microbial community in coral as well as sponge holobionts resulting in the onset of disease [90,106–108,159,160]. Nutrient enrichment, especially increase in nitrogen, can facilitate the spread of diseases in corals because pathogens are normally nitrogen limited [39,161,162]. The reef around Bawe Island has higher phosphate and nitrate concentrations compared to other reefs around Zanzibar, which could help to increase pathogen fitness and virulence [66]. Around Bawe only a few cases of sponge diseases have been reported (e.g. necrotic tissue spots in H. fascigera; [161]), indicating that sponge antimicrobial defenses seem to be efficient in defending sponges from pathogens. The high antimicrobial activity of the investigated extracts might be a response of the sponges to a high prevalence of potential pathogens.
Brine shrimp lethality assay
The brine shrimp lethality assay is an easy, rapid and inexpensive bioassay to test for the general toxicity of organisms. Brine shrimp are highly sensitive organisms in regard to sponge crude extracts [163,164]. Additionally, there is a strong relationship between the toxicity of brine shrimp assays and the potential anti-tumor activities of extracts [101,165].
In the present study, seven (70%) of the tested sponge species showed cytotoxicity in the brine shrimp assay. In other studies, 59–75% of sponge species also showed cytotoxic activities in brine shrimp or other cytotoxicity assays in line with our results [164–166]. Extracts from all four sponges within the order Haplosclerida displayed significant cytotoxic activities in the brine shrimp assay with Callyspongia sp. showing the highest cytotoxic activity followed by the two Haliclona species. Their cytotoxic activities were comparable to other Haplosclerida species reported in the literature [167–169]. Significant cytotoxic activities were also detected in extracts of S. hapalia and P. kenyensis at the highest test concentration, which is again in line in literature reports [170–172]. The moderate cytotoxic activity of Pseudoceratina sp. can most likely be ascribed to bioactive bromotyrosine derivatives in the crude extract [172–175], while the lack of activity in Biemna sp. was contrary to previous studies [172,176–179].
Toxic sponge compounds are able to rapidly kill cells of competitors through apoptosis, autophagocytosis and necrosis [54,55,180], thereby inhibiting the growth of other space competing organisms. Allelochemicals with cytotoxic properties have the ability of inhibiting metabolic processes in various coral species as well as killing live coral tissue within a few days [46,181]. Other functions of cytotoxic compounds include the inhibition of photosynthesis in the corals symbiotic zooxanthellae [28]. Therefore, we investigated the allelopathic activities of the three most bioactive sponge species and assessed if the cytotoxic and antimicrobial properties provide them with a competitive advantage over corals.
Allelopathic activities of sponge extracts
Extracts of Callyspongia sp., Pseudoceratina sp. and H. atra, incorporated into phytagel, had rapid negative effects on the maximum photosynthetic quantum yield of the zooxanthella that live within the coral tissue. The three sponges revealed a decrease of the photosynthetic efficiency in the corals by 19–41%. Additionally, H. atra as well as Callyspongia sp. also caused bleaching in branching Porites corals. The allelopathic effects of the sponge extracts might have been underestimated since the bioactive compounds were evenly distributed in the gel instead of concentrated on its surface. Several studies have shown that compounds identified as allelopathic agents had higher concentrations on the surface of the producing organism [57,182–184]. This is in accordance with the optimal defense theory (ODT) which states that organisms concentrate defensive compounds in parts that are especially exposed to predation or parts that are important for the fitness of an organism, e.g. reproductive organs [185–188]. Furthermore, it is likely that some reported bioactivities are slightly underestimated because non-polar compounds would not be completely extracted by using ethanol. Due to the limited laboratory conditions, ethanol was the only available solvent that could be used for the extraction of secondary metabolites. Previous studies found bioactivities often in non-polar compounds as these do not easily dilute when exuded into the surrounding seawater [189,190]. Therefore, differences which have been detected in the yield as well as bioactivity of sponge and seaweed extracts might depend on the used solvent [26,191].
The maximum photosynthetic quantum yield is a proxy for the health of the zooxanthellae, which provide corals with energy for growth and reef formation [192,193]. Thus, impairments in the health of these symbiotic algae can lead to reduced growth and less available energy that corals could invest otherwise in recruitment or spatial competition. It has already been demonstrated that corals experience a reduction in their growth, fecundity, egg size, recruits and survival during competition with algae or other benthic organisms [59,194–198].
Some coral species seem to be more susceptible to allelopathic damage. Experiments with extracts and live fragments of several algae species demonstrated that corals of the branching genera Acropora and Pocillopora were especially sensitive to allelopathic agents while corals of the massive genus Porites were not as much affected [183,199,200]. Some coral species differed also in their recovery potential. Corals of the genus Porites recovered quickly while corals of the genus Pocillopora showed no signs of recovery after the removal of aggressive alga individuals in situ [201]. Many branching corals species are already more sensitive to natural and anthropogenic disturbances and experience high mortality rates on reefs worldwide which could be further intensified by aggressive spatial competitors [183,199,201].
The ten sponge species tested in this study were previously also tested for their deterrent properties against fish predation [76]. Although, no relationship could be found between the feeding deterrence of sponge extracts and their antimicrobial activity or toxicity, the two most bioactive sponges (H. atra and Pseudoceratina sp.) were also defended against fish predators. For two of them their role as a successful benthic competitor was confirmed by their high abundances on the reef. H. atra was the most abundant sponge species at 5m depth, while Pseudoceratina sp. exhibited patchy but high abundances on the reefs around Bawe Island at 10m depth ([76]; personal observation).
Conclusion
We demonstrated that sponges from Zanzibar possess strong growth inhibitory activities against tropical marine environmental as well as pathogenic bacterial strains. The remarkable antimicrobial activities could represent an adaptation to the high prevalence of bacteria caused by sewage outflow from Stonetown. Moreover, the cytotoxic activities and the strong allelopathic properties of Callyspongia sp., H. atra and Pseudoceratina sp. might indicate that they are important space competitors of scleractinian corals on the reef. The bioactive compounds might exert negative effects on the fecundity, reproduction or even the coral microbiome making corals even more vulnerable towards further natural or anthropogenic disturbances and pathogenesis.
The increased sewage input in combination with other global and local stressors, such as climate change, destructive fishing practices or damage to the reef through tourism activities, will most likely result in more frequently occurring sponge-coral interactions. The chemical defenses in the investigated sponges might be one reason explaining their increasing abundances from < 1% up to 7.5% on the reef at Bawe Island in recent years [8,72,76]. Therefore, the reef management around Zanzibar has to focus on mitigating anthropogenic caused disturbances such as overfishing and especially focus on the establishment of a wastewater treatment facility.
Supporting information
(TIF)
(TIFF)
(DOCX)
(XLSX)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
We would like to thank staff and students at the IMS in Zanzibar, the ZMT and the members of the environmental biochemistry group at the ICBM in Wilhelmshaven (especially M Möller, S Nietzer and G Steinert), University of Oldenburg. We greatly appreciate the field and diving assistance of NS Jiddawi, MS Shalli, FE Belshe, S Bröhl and U Pint. We would like to thank JG Plass-Johnson and FE Belshe for insightful comments on the manuscript. Stephanie B Helber acknowledges funding of the SUTAS project (SAW-2013-ZMT-4) by the German Leibniz Association (WGL) and PJ Schupp internal funding by the ICBM, University of Oldenburg.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded by the German Leibniz Association (WGL) and had the Grant number SAW-2013-ZMT-4 to Peter Schupp. Additional funding was provided by internal funds of the Institute for Chemistry and Biology of the Marine Environment (ICBM) to Peter Schupp, University of Oldenburg, Germany. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JBC, et al. Coral reefs in the Anthropocene. Nature. 2017;546: 82–90. doi: 10.1038/nature22901 [DOI] [PubMed] [Google Scholar]
- 2.Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science (80-). 2003;301: 958–960. doi: 10.1126/science.1086050 [DOI] [PubMed] [Google Scholar]
- 3.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429: 827–833. doi: 10.1038/nature02691 [DOI] [PubMed] [Google Scholar]
- 4.Norström A V., Nyström M, Lokrantz J, Folke C. Alternative states on coral reefs: Beyond coral-macroalgal phase shifts. Mar Ecol Prog Ser. 2009;376: 293–306. doi: 10.3354/meps07815 [Google Scholar]
- 5.Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS. Could some coral reefs become sponge reefs as our climate changes? Glob Chang Biol. 2013;19: 2613–2624. doi: 10.1111/gcb.12212 [DOI] [PubMed] [Google Scholar]
- 6.Pawlik JR, Burkepile DE, Thurber RV. A vicious circle? Altered carbon and nutrient cycling may explain the low resilience of Caribbean coral reefs. Bioscience. 2016;66: 470–476. doi: 10.1093/biosci/biw047 [Google Scholar]
- 7.Ruetzler K. Sponges on coral reefs: a community shaped by competitive cooperation. Boll Mus Ist Biol Univ Genova. 2003;68: 85–148. Available: http://www.narcis.nl/publication/RecordID/oai:uva.nl:158420 [Google Scholar]
- 8.Muhando C a, Kuguru BL, Wagner GM, Mbije NE, Ohman MC. Environmental effects on the distribution of corallimorpharians in Tanzania. Ambio. 2002;31: 558–561. doi: 10.1639/0044-7447(2002)031\{[\}0558:EEOTDO]2.0.CO;2 [PubMed] [Google Scholar]
- 9.Ward-Paige CA, Risk MJ, Sherwood OA, Jaap WC. Clionid sponge surveys on the Florida Reef Tract suggest land-based nutrient inputs. Mar Pollut Bull. 2005;51: 570–579. doi: 10.1016/j.marpolbul.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 10.Loh T-L, McMurray SE, Henkel TP, Vicente J, Pawlik JR. Indirect effects of overfishing on Caribbean reefs: sponges overgrow reef-building corals. PeerJ. 2015;3: e901 doi: 10.7717/peerj.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes TP, Reed DC, Boyle M-J. Herbivory on coral reefs: community structure following mass mortalities of sea urchins. J Exp Mar Bio Ecol. 1987;113: 39–59. [Google Scholar]
- 12.Loh T-L, Pawlik JR. Bitten down to size: Fish predation determines growth form of the Caribbean coral reef sponge Mycale laevis. J Exp Mar Bio Ecol. Elsevier B.V.; 2009;374: 45–50. doi: 10.1016/j.jembe.2009.04.007 [Google Scholar]
- 13.Slattery M, Lesser MP. Allelopathy in the tropical alga Lobophora variegata (Phaeophyceae): Mechanistic basis for a phase shift on mesophotic coral reefs? J Phycol. 2014;50: 493–505. doi: 10.1111/jpy.12160 [DOI] [PubMed] [Google Scholar]
- 14.Barnes DKA, Bell JJ. Coastal sponge communities of the West Indian Ocean: Morphological richness and diversity. Afr J Ecol. 2002;40: 350–359. doi: 10.1046/j.1365-2028.2002.00388.x [Google Scholar]
- 15.de Voogd NJ, Becking LE, Hoeksema BW, Noor A, van Soest RWM. Sponge interactions with spatial competitors in the Spermonde Archipelago. Bolletino di Mus e Ist di Biol dell’Universita di Genova. 2004;68: 253–261. Available: http://www.narcis.nl/publication/RecordID/oai:uva.nl:158420 [Google Scholar]
- 16.Diaz MC, Rützler K. Sponges: An essential component of Caribbean coral reefs. Bull Mar Sci. 2001;69: 535–546. Available: http://www.scopus.com/inward/record.url?eid = 2-s2.0-0035681585&partnerID = 40&md5 = 06c39bf986cf0c184e882cd505ae8956 [Google Scholar]
- 17.Maliao RJ, Turingan RG, Lin J. Phase-shift in coral reef communities in the Florida Keys National Marine Sanctuary (FKNMS), USA. Mar Biol. 2008;154: 841–853. doi: 10.1007/s00227-008-0977-0 [Google Scholar]
- 18.Pawlik JR. The chemical ecology of sponges on Caribbean reefs: Natural products shape natural systems. Bioscience. 2011;61: 888–898. doi: 10.1525/bio.2011.61.11.8 [Google Scholar]
- 19.Pawlik JR, Mcmurray SE, Erwin P, Zea S. A review of evidence for food limitation of sponges on Caribbean reefs. Mar Ecol Prog Ser. 519: 265–283. doi: 10.3354/meps11093 [Google Scholar]
- 20.Gili J-M, Coma R. Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol. 1998;13: 316–321. [DOI] [PubMed] [Google Scholar]
- 21.Faulkner JD. Marine natural products: metabolites of marine invertebrates. Nat Prod Rep. 1984;1: 551–598. [Google Scholar]
- 22.Sinko J, Rajchard J, Balounova Z, Fikotova L. Biologically active substances from water invertebrates: A review. Vet Med (Praha). 2012;57: 177–184. [Google Scholar]
- 23.Burns E, Ifrach I, Carmeli S, Pawlik JR, Ilan M. Comparison of anti-predatory defenses of Red Sea and Caribbean sponges. I. Chemical defense. Mar Ecol Prog Ser. 2003;252: 105–114. doi: 10.3354/meps252115 [Google Scholar]
- 24.Pawlik JR, Charnas B, Toonen RJ, Fenical W. Defenses of Caribbean sponges against predatory reef fish. 1. Chemical deterrency. Mar Ecol Prog Ser. 1995;127: 183–194. doi: 10.3354/meps127183 [Google Scholar]
- 25.Rohde S, Nietzer S, Schupp PJ. Prevalence and mechanisms of dynamic chemical defenses in tropical sponges. PLoS One. 2015;10: e0132236 doi: 10.1371/journal.pone.0132236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amade P, Charroin C, Baby C, Vacelet J. Antimicrobial activities of marine sponges from the Mediterranean Sea. Mar Biol. 1987;94: 271–275. [Google Scholar]
- 27.Kelly SR, Garo E, Jensen PR, Fenical W, Pawlik JR. Effects of Caribbean sponge secondary metabolites on bacterial surface colonization. Aquat Microb Ecol. 2005;40: 191–203. doi: 10.3354/ame040191 [Google Scholar]
- 28.Pawlik JR, Steindler L, Henkel TP, Beer S, Ilan M. Chemical warfare on coral reefs: Sponge metabolites differentially affect coral symbiosis in situ. Limnol Oceanogr. 2007;52: 907–911. [Google Scholar]
- 29.Madduppa H, Schupp PJ, Faisal MR, Sastria MY, Thoms C. Persistent outbreaks of the “black disease” sponge Terpios hoshinota in Indonesian coral reefs. Mar Biodivers. Marine Biodiversity; 2017;47: 149–151. doi: 10.1007/s12526-015-0426-5 [Google Scholar]
- 30.Wahl M. Marine epibiosis. I. Fouling and antifouling: some basic aspects. Mar Ecol Prog Ser. 1989;58: 175–189. [Google Scholar]
- 31.Littler MM, Littler DS. Impact of CLOD pathogen on Pacific coral reefs. Science (80-). 1995;267: 1356–13660. [DOI] [PubMed] [Google Scholar]
- 32.Kelly SR, Jensen PR, Henkel TP, Fenical W, Pawlik JR. Effects of Caribbean sponge extracts on bacterial attachment. Aquat Microb Ecol. 2003;31: 175–182. doi: 10.3354/ame031175 [Google Scholar]
- 33.Sorokin YI, Kopylov AI, Mamaeva N V. Abundance and dynamics of microplankton in the central tropical Indian Ocean. Mar Ecol Prog Ser. 1985;24: 27–41. [Google Scholar]
- 34.Wiebinga CASJ, Veldhuis MJW, de Baar HJW. Abundance and productivity of bacterioplankton in relation to seasonal upwelling in the northwest Indian Ocean. Deep Sea Res I. 1997;44: 451–476. [Google Scholar]
- 35.Goosen NK, van Rijswijk P, de Bie M, Peene J, Kromkamp J. Bacterioplankton abundance and production and nanozooplankton abundance in Kenyan coastal waters (western Indian Ocean). Deep Res Part II Top Stud Oceanogr. 1997;44: 1235–1250. doi: 10.1016/S0967-0645(97)00016-7 [Google Scholar]
- 36.Thoms C, Ebel R, Proksch P. Activated chemical defense in Aplysina sponges revisited. J Chem Ecol. 2006;32: 97–123. doi: 10.1007/s10886-006-9355-x [DOI] [PubMed] [Google Scholar]
- 37.Hernández-Delgado EA, Sandoz B, Bonkosky M, Mattei H. Impacts of non-point source sewage pollution on Elkhorn coral, Acropora palmata (Lamarck), assemblages of the southwestern Puerto Rico shelf. Proc 11th Int Coral Reef Symp. 2008; 7–11. [Google Scholar]
- 38.Futch CJ, Griffin DW, Banks K, Lipp EK. Evaluation of sewage source and fate on southeast Florida coastal reefs. Mar Pollut Bull. 2011;62: 2308–2316. doi: 10.1016/j.marpolbul.2011.08.046 [DOI] [PubMed] [Google Scholar]
- 39.Bruno JF, Petes LE, Harvell CD, Hettinger A. Nutrient enrichment can increase the severity of coral diseases. Ecol Lett. 2003;6: 1056–1061. doi: 10.1046/j.1461-0248.2003.00544.x [Google Scholar]
- 40.Gochfeld DJ, Aeby GS. Antibacterial chemical defenses in Hawaiian corals provide possible protection from disease. Mar Ecol Prog Ser. 2008;362: 119–128. doi: 10.3354/meps07418 [Google Scholar]
- 41.Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, et al. Emerging Marine Diseases—Climate Links and Anthropogenic Factors. Science (80-). 1999;285: 1505–1510. doi: 10.1126/science.285.5433.1505 [DOI] [PubMed] [Google Scholar]
- 42.Green EP, Bruckner AW. The significance of coral disease epizootiology for coral reef conservation. Biol Conserv. 2000;96: 347–361. [Google Scholar]
- 43.Frias-Lopez J, Zerkle AL, Bonheyo GT, Fouke BW. Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces. Appl Environ Microbiol. 2002;68: 2214–2228. doi: 10.1128/AEM.68.5.2214-2228.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller E, Peters EC, et al. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc Natl Acad Sci. 2002;99: 8725–8730. doi: 10.1073/pnas.092260099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luter HM, Whalan S, Webster NS. Prevalence of tissue necrosis and brown spot lesions in a common marine sponge. Mar Freshw Res. 2010;61: 484–489. doi: 10.1071/MF09200 [Google Scholar]
- 46.Porter JW, Targett NM. Allelochemical interactions between sponges and corals. Biol Bull. 1988;175: 230–239. doi: 10.2307/1541563 [Google Scholar]
- 47.López-Victoria M, Zea S, Weil E. Competition for space between encrusting excavating Caribbean sponges and other coral reef organisms. Mar Ecol Prog Ser. 2006;312: 113–121. doi: 10.3354/meps312113 [Google Scholar]
- 48.Engel S, Pawlik JR. Allelopathic activities of sponge extracts. Mar Ecol Prog Ser. 2000;207: 273–281. doi: 10.3354/meps207273 [Google Scholar]
- 49.Dayton PK. Competition, disturbance, and community organization: ThepProvision and subsequent utilization of space in a rocky intertidal community. Ecol Monogr. 1971;41: 351–389. [Google Scholar]
- 50.Jackson JBC, Buss LEO. Allelopathy and spatial competition among coral reef invertebrates. Proc Natl Acad Sci U S A. 1975;72: 5160–5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birrell CL, McCook LJ, Willis BL, Diaz-Pulido GA. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr Mar Biol An Annu Rev. 2008;46: 25–63. [Google Scholar]
- 52.Van Veghel MLJ, Cleary DFR, Bak RPM. Interspecific nteractions and competitive ability of the polymorphic reef-building coral Montastrea annularis. Bull Mar Sci. 1996;58: 792–803. [Google Scholar]
- 53.Knowlton N, Jackson JBC. Beyond baselines: Rethinking priorities for ocean conservation. PLoS Biol. 2008;6: e54 doi: 10.1371/journal.pbio.0060054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aerts LAM. Sponge- coral interactions on Caribbean reefs. 1999. doi: 10.1159/000144207
- 55.Folmer F, Jaspars M, Dicato M, Diederich M. Marine cytotoxins: callers for the various dances of death. Gastroenterol Hepatol. 2009;2: 34–50. [Google Scholar]
- 56.Aerts LAM, van Soest RWM. Quantification of sponge/coral interactions in a physically stressed reef-community, NE Colombia. Mar Ecol Prog Ser. 1997;148: 125–134. [Google Scholar]
- 57.Uriz MJ, Becerro MA, Tur JM, Turon X. Location of toxicity within the Mediterranean sponge Crambe crambe (Demospongiae: Poecilosclerida). Mar Biol. 1996;124: 583–590. [Google Scholar]
- 58.Turon X, Becerro MA, Uriz MJ, Llopis J. Small-scale association measures in epibenthic communities as a clue for allelochemical interactions. Oecologia. 1996;108: 351–360. doi: 10.1007/BF00334661 [DOI] [PubMed] [Google Scholar]
- 59.Chadwick NE, Morrow KM. Competition among sessile organisms on coral reefs Coral Reefs: An Ecosystem in Transition; Dubinsky Z and Stambler N (eds). 2011. pp. 347–371. doi: 10.1007/978-94-007-0114-4 [Google Scholar]
- 60.McClanahan TR, Ateweberhan M, Graham NAJ, Wilson SK, Ruiz Sebastiàn C, Guillaume MMM, et al. Western Indian Ocean coral communities:bleaching responses and susceptibility to extinction. Mar Ecol Prog Ser. 2007;337: 1–13. doi: 10.3354/meps337001 [Google Scholar]
- 61.McClanahan TR, Graham NAJ, Darling ES. Coral reefs in a crystal ball: Predicting the future from the vulnerability of corals and reef fishes to multiple stressors. Curr Opin Environ Sustain. Elsevier B.V.; 2014;7: 59–64. doi: 10.1016/j.cosust.2013.11.028 [Google Scholar]
- 62.Obura DO. Resilience and climate change: Lessons from coral reefs and bleaching in the Western Indian Ocean. Estuar Coast Shelf Sci. 2005;63: 353–372. doi: 10.1016/j.ecss.2004.11.010 [Google Scholar]
- 63.Muhando CA, Kuguru BL, Wagner GM, Mbije NE, Öhman MC. Environmental effects on the distribution of corallimorpharians in Tanzania. AMBIO A J Hum Environ. 2002;31: 558–561. doi: 10.1639/0044-7447(2002)031\{[\}0558:EEOTDO]2.0.CO;2 [PubMed] [Google Scholar]
- 64.Kuguru BL, Mgaya YD, Öhman MC, Wagner GM. The reef environment and competitive success in the Corallimorpharia. Mar Biol. 2004;145: 875–884. doi: 10.1007/s00227-004-1376-9 [Google Scholar]
- 65.Moynihan MA, Baker DM, Mmochi AJ. Isotopic and microbial indicators of sewage pollution from Stone Town, Zanzibar, Tanzania. Mar Pollut Bull. Elsevier Ltd; 2012;64: 1348–1355. doi: 10.1016/j.marpolbul.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 66.Limbu SM, Kyewalyanga MS. Spatial and temporal variations in environmental variables in relation to phytoplankton composition and biomass in coral reef areas around Unguja, Zanzibar, Tanzania. Springerplus. Springer International Publishing; 2015;4: 1–8. doi: 10.1186/2193-1801-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heikoop JM, Dunn JJ, Risk MJ, Tomascik T, Schwarcz HP, Sandeman IM, et al. d 15N and d 13C of coral tissue show significant inter-reef variation. Coral Reefs. 2000;19: 189–193. [Google Scholar]
- 68.Noble RT, Moore DF, Leecaster MK, McGee CD, Weisberg SB. Comparison of total coliform, fecal coliform, and enterococcus bacterial indicator response for ocean recreational water quality testing. Water Res. 2003;37: 1637–1643. doi: 10.1016/S0043-1354(02)00496-7 [DOI] [PubMed] [Google Scholar]
- 69.Risk MJ. The reef crisis and the reef science crisis: nitrogen isotopic ratios as an objective indicator of stress. Mar Pollut Bull. 2009;58: 787–788. doi: 10.1016/j.marpolbul.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 70.Baker DM, Jordán-Dahlgren E, Maldonado MA, Harvell DC. Sea fan corals provide a stable isotope baseline for assessing sewage pollution in the Mexican Caribbean. Limnol Oceanogr. 2010;55: 2139–2149. doi: 10.4319/lo.2010.55.5.2139 [Google Scholar]
- 71.Bergman KC, Öhman MC. Coral reef structure at Zanzibar Island, Tanzania. Marine Science Development in Tanzania and Eastern Africa Proceedings of the 20th Anniversary Conference on Advances in Marine Sciences in Tanzania. 2001. pp. 263–275. Available: http://gridnairobi.unep.org/chm/EAFDocuments/Tanzania/bergman_p263-276.pdf
- 72.Muthiga N, Costa A, Motta H, Muhando CA, Mwaipopo R, Schleyer M. 6. Status of coral reefs in East Africa: Kenya, Tanzania, Mozambique and South Africa In Wilkinson C (ed), Status of coral reefs of the world, Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre, Townsville, Australia: 1998. pp. 91–104. [Google Scholar]
- 73.Muhando CA, Lanshammar F. Ecological effects of the Crown-of-Thorns Starfish removal programme on Chumbe Island Coral Park, Zanzibar, Tanzania. Proceedings of the 11th International Coral Reef Symposium. 2008. pp. 1127–1131.
- 74.Muhando CA, Mohammed MS. Coral reef benthos and fisheries in Tanzania before and after the 1998 bleaching and mortality event. West Indian Ocean J Mar Sci. 2002;1: 43–52. [Google Scholar]
- 75.Lokrantz J, Nyström M, Norström A V., Folke C, Cinner JE. Impacts of artisanal fishing on key functional groups and the potential vulnerability of coral reefs. Environ Conserv. 2010;36: 327–337. doi: 10.1017/S0376892910000147 [Google Scholar]
- 76.Helber SB, de Voogd NJ, Muhando CA, Rohde S, Schupp PJ. Anti-predatory effects of organic extracts of 10 common reef sponges from Zanzibar. Hydrobiologia. Springer International Publishing; 2017;790: 247–258. doi: 10.1007/s10750-016-3036-8 [Google Scholar]
- 77.Hay ME, Kappel QE, Fenical W. Synergisms in plant defenses against herbivores: Interactions of chemistry, calcification, and plant quality. Ecology. 1994;75: 1714–1726. [Google Scholar]
- 78.Jormalainen V, Honkanen T, Heikkilä N. Feeding preferences and performance of a marine isopod on seaweed hosts: cost of habitat specialization. Mar Ecol Prog Ser. 2001;220: 219–230. [Google Scholar]
- 79.Ankisetty Sridevi Nandiraju S, Win H, Park YC, Amsler Charles D. McClintock James B. Baker JA. Chemical investigation of predator-deterred macroalgae from the Antarctic peninsula. J Nat Prod. 2004;67: 1295–1302. doi: 10.1021/np049965c [DOI] [PubMed] [Google Scholar]
- 80.Erickson AA, Paul VJ, Alstyne KL Van, Kwiatkowski LM. Palatability of macroalgae that use different types of chemical defenses. J Chem Ecol. 2006;32: 1883–1895. doi: 10.1007/s10886-006-9116-x [DOI] [PubMed] [Google Scholar]
- 81.Steinert G, Whitfield S, Taylor MW, Thoms C, Schupp PJ. Application of diffusion growth chambers for the cultivation of marine sponge-associated bacteria. Mar Biotechnol. 2014;16: 594–603. doi: 10.1007/s10126-014-9575-y [DOI] [PubMed] [Google Scholar]
- 82.Li J, Cao J, Wang X, Liu N, Wang W, Luo Y. Acinetobacter pittii, an emerging new multi-drug resistant fish pathogen isolated from diseased blunt snout bream (Megalobrama amblycephala Yih) in China. Appl Microbiol Biotechnol. Applied Microbiology and Biotechnology; 2017;101: 6459–6471. doi: 10.1007/s00253-017-8392-4 [DOI] [PubMed] [Google Scholar]
- 83.Larcher R, Pantel A, Arnaud E, Sotto A, Lavigne J-P. First report of cavitary pneumonia due to community-acquired Acinetobacter pittii, study of virulence and overview of pathogenesis and treatment. J Antimicrob Chemother. BMC Infectious Diseases; 2005;56: 893–898. doi: 10.1093/jac/dki335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee K-K. Pathogenesis studies on Vibrio alginolyticus in the grouper, epinephelus malabaricus, Bloch et Schneider. Microb Pathog. 1995;19: 39–48. doi: 10.1016/S0882-4010(85)90000-2 [DOI] [PubMed] [Google Scholar]
- 85.Anguiano-Beltrán C, Searcy-Bernal R, Lizárraga-Partida ML. Pathogenic effects of Vibrio alginolycticus on larvae and postlarvae of the red abalone Haliotis rufescens. Dis Aquat Organ. 1998;33: 199–122. doi: 10.3354/dao033119 [Google Scholar]
- 86.Liu PC, Lin JY, Hsiao PT, Lee KK. Isolation and characterization of pathogenic Vibrio alginolyticus from diseased cobia Rachycentron canadum. J Basic Microbiol. 2004;44: 23–28. doi: 10.1002/jobm.200310316 [DOI] [PubMed] [Google Scholar]
- 87.Xie Z-Y, Hu C-Q, Chen C, Zhang L-P, Ren C-H. Investigation of seven Vibrio virulence genes among Vibrio alginolyticus and Vibrio parahaemolyticus strains from the coastal mariculture systems in Guangdong, China. Lett Appl Microbiol. 2005;41: 202–207. doi: 10.1111/j.1472-765X.2005.01688.x [DOI] [PubMed] [Google Scholar]
- 88.Lin YC, Chen JC, Chen YY, Yeh ST, Chen LL, Huang CL, et al. Crowding of white shrimp Litopenaeus vananmei depresses their immunity to and resistance against Vibrio alginolyticus and white spot syndrome virus. Fish Shellfish Immunol. Elsevier Ltd; 2015;45: 104–111. doi: 10.1016/j.fsi.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 89.Ushijima B, Smith A, Aeby GS, Callahan SM. Vibrio owensii induces the tissue loss disease Montipora White Syndrome in the Hawaiian reef coral Montipora capitata. PLoS One. 2012;7: e46717 doi: 10.1371/journal.pone.0046717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ben-Haim Y, Rosenberg E. A novel Vibrio sp. pathogen of the coral Pocillopora damicornis. Mar Biol. 2002;141: 47–55. doi: 10.1007/s00227-002-0797-6 [Google Scholar]
- 91.Ben-Haim Y, Thompson FL, Thompson CC, Cnockaert MC, Hoste B, Swings J, et al. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int J Syst Evol Microbiol. 2003;53: 309–315. doi: 10.1099/ijs.0.02402-0 [DOI] [PubMed] [Google Scholar]
- 92.Ushijima B, Videau P, Burger AH, Shore-Maggio A, Runyon CM, Sudek M, et al. Vibrio coralliilyticus strain OCN008 is an etiological agent of acute montipora white syndrome. Appl Environ Microbiol. 2014;80: 2102–2109. doi: 10.1128/AEM.03463-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Denner EBM, Smith GW, Busse H-J, Schumann P, Narzt T, Polson SW, et al. Aurantimonas coralicida gen. nov., sp. nov., the causative agent of white plague type II on Caribbean scleractinian corals. Int J Syst Evol Microbiol. 2003;53: 1115–1122. doi: 10.1099/ijs.0.02359-0 [DOI] [PubMed] [Google Scholar]
- 94.Kushmaro A, Banin E, Loya Y, Stackebrandt E, Rosenberg E. Vibrio shiloi sp. nov., the causative agent of bleaching of the coral Oculina patagonica. Int J Syst Evol Microbiol. 2001;51: 1383–1388. doi: 10.1099/00207713-51-4-1383 [DOI] [PubMed] [Google Scholar]
- 95.Ben-Haim Y, Banim E, Kushmaro A, Loya Y, Rosenberg E. Inhibition of photosynthesis and bleaching of zooxanthellae by the coral pathogen Vibrio shiloi. Environ Microbiol. 1999;1: 223–229. doi: 10.1046/j.1462-2920.1999.00027.x [DOI] [PubMed] [Google Scholar]
- 96.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Aust Ecol. 2001;26: 32–46. [Google Scholar]
- 97.McArdle BH, Anderson MJ. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology. 2001;82: 290–297. [Google Scholar]
- 98.Lippert H, Brinkmeyer R, Mülhaupt T, Iken K. Antimicrobial activity in sub-Arctic marine invertebrates. Polar Biol. 2003;26: 591–600. doi: 10.1007/s00300-003-0525-9 [Google Scholar]
- 99.Pelka M, Danzl C, Distler W, Petschelt A. A new screening test for toxicity testing of dental materials. J Dent. 2000;28: 341–345. [DOI] [PubMed] [Google Scholar]
- 100.Lewis GE. Testing the toxicity of extracts of southern African plants using brine shrimp (Artemia salina). S Afr J Sci. 1995;9: 382–384. [Google Scholar]
- 101.Carballo JL, Hernández-inda ZL, Pérez P, García-Grávalos MD. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002;5: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sánzchez- Fortún S, Sanz F, Barahona M V. Acute toxicity of several organophosphorous insecticides and potection by cholinergic antagonists and 2-PAM on Artemia salina larvae. Arch Environ Contam Toxicol. 1996;31: 391–398. [DOI] [PubMed] [Google Scholar]
- 103.Beer S, Ilan M, Eshel A, Weil A, Brickner I. Use of pulse amplitude modulated (PAM) fluorometry for in situ measurements of photosynthesis in two Red Sea faviid corals. Mar Biol. 1998;131: 607–612. [Google Scholar]
- 104.Ralph PJ, Gademann R, Larkum AWD, Kühl M. Spatial heterogeneity in active chlorophyll fluorescence and PSII activity of coral tissues. Mar Biol. 2002;141: 639–646. doi: 10.1007/s00227-002-0866-x [Google Scholar]
- 105.Austin B, Austin DA. No Title Bacterial fish pathogens. Springer, Dordrecht; 2012. pp. 357–341. [Google Scholar]
- 106.Thurber RV, Willner-Hall D, Rodriguez-Mueller B, Desnues C, Edwards RA, Angly F, et al. Metagenomic analysis of stressed coral holobionts. Environ Microbiol. 2009;11: 2148–2163. doi: 10.1111/j.1462-2920.2009.01935.x [DOI] [PubMed] [Google Scholar]
- 107.Stabili L, Cardone F, Alifano P, Corriero G, Gaino E. Epidemic mortality of the sponge Ircinia variabilis (Schmidt, 1862) associated to proliferation of a Vibrio bacterium. Invertebr Microbiol. 2012;64: 802–813. doi: 10.1007/s00248-012-0068-0 [DOI] [PubMed] [Google Scholar]
- 108.Sweet M, Bulling M, Cerrano C. A novel sponge disease caused by a consortium of micro-organisms. Coral Reefs. 2015;34: 871–883. doi: 10.1007/s00338-015-1284-0 [Google Scholar]
- 109.Rønneseth A, Castillo D, D’Alvise P, Tønnesen Ø, Haugland G, Grotkjær T, et al. Comparative assessment of Vibrio virulence in marine fish larvae. J Fish Dis. 2017; [DOI] [PubMed] [Google Scholar]
- 110.Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat Prod Rep. 2015;32: 116–211. doi: 10.1039/c4np00144c [DOI] [PubMed] [Google Scholar]
- 111.Bhatnagar I, Kim S-K. Immense essence of excellence: Marine microbial bioactive compounds. Mar Drugs. 2010;8: 2673–2701. doi: 10.3390/md8102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ely R, Supriya T, Naik CG. Antimicrobial activity of marine organisms collected off the coast of South East India. J Exp Mar Bio Ecol. 2004;309: 121–127. doi: 10.1016/j.jembe.2004.03.010 [Google Scholar]
- 113.Dobretsov S, Dahms H-U, Qian P-Y. Antibacterial and anti-diatom activity of Hong Kong sponges. Aquat Microb Ecol. 2005;38: 191–201. doi: 10.3354/ame038191 [Google Scholar]
- 114.Newbold RW, Jensen PR, Fenical W, Pawlik JR. Antimicrobial activity of Caribbean sponge extracts. Aquat Microb Ecol. 1999;19: 279–284. doi: 10.3354/ame019279 [Google Scholar]
- 115.Perovic-Ottstadt S, Adell T, Proksch P, Wiens M, Korzhev M, Gamulin V, et al. A (1->3)-ß-D-glucan recognition protein from the sponge Suberites domuncula. Eur J Biochem. 2004;271: 1924–1937. doi: 10.1111/j.1432-1033.2004.04102.x [DOI] [PubMed] [Google Scholar]
- 116.Wiens M, Korzhev M, Perović-Ottstadt S, Luthringer B, Brandt D, Klein S, et al. Toll-like receptors are part of the innate immune defense system of sponges (Demospongiae: Porifera). Mol Biol Evol. 2007;24: 792–804. doi: 10.1093/molbev/msl208 [DOI] [PubMed] [Google Scholar]
- 117.Fu W, Zhang J, Zheng C, Liu J, An Z, Liu H, et al. Molecular cloning of partial 14-3-3 genes in the marine sponge Hymeniacidon perleve and its role in differentiating infectious and non-infectious bacteria. Chinese Sci Bull. 2013;58: 766–776. doi: 10.1007/s11434-012-5400-z [Google Scholar]
- 118.Thomas T, Rusch D, Demaere MZ, Yung PY, Lewis M, Halpern A, et al. Functional genomic signatures of sponge bacteria reveal unique and shared features of symbiosis Int Soc Microb Ecol. Nature Publishing Group; 2010;4: 1557–1567. doi: 10.1038/ismej.2010.74 [DOI] [PubMed] [Google Scholar]
- 119.Siegl A, Kamke J, Hochmuth T, Richter M, Chunguang L, Dandekar T, et al. Single-cell genomics reveals the lifestyle of Poribacteria, a candidate phylum symbiotically associated with marine sponges. Int Soc Microb Ecol. 2011;5: 61–70. doi: 10.1038/ismej.2010.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gardères J, Taupin L, Saïdin JB, Dufour A, Le Pennec G. N-acyl homoserine lactone production by bacteria within the sponge Suberites domuncula (Olivi, 1792) (Porifera, Demospongiae). Mar Biol. 2012;159: 1685–1692. [Google Scholar]
- 121.Gardères J, Henry J, Bernay B, Ritter A, Zatylny-Gaudin C, Wiens M, et al. Cellular effects of bacterial N-3-oxo dodecanoyl-L-homoserine lactone on the sponge Suberites domuncula (Olivi, 1792): Insights into an intimate inter- kingdom dialogue. PLoS One. 2014;9: e97662 doi: 10.1371/journal.pone.0097662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsukamoto S, Kato H, Hirota H, Fusetani N. Ceratinamides A and B: New antifouling dibromotyrosine from the marine sponge Pseudoceratina purpurea. Tetrahedron. 1996;52: 8181–8186. doi: 10.1002/chin.199636223 [DOI] [PubMed] [Google Scholar]
- 123.Buchanan MS, Carroll AR, Fechner GA, Boyle A, Simpson M, Addepalli R, et al. Aplysamine 6, an alkaloidal inhibitor of isoprenylcysteine carboxyl methyltransferase from Peudoceratina sp. J Nat Prod. 2008;71: 1066–1067. doi: 10.1021/np0706623 [DOI] [PubMed] [Google Scholar]
- 124.Fusetani N. Marine Natural Products: Chemical Diversity. Nat Prod Rep. 2008;2: 1–22. doi: 10.1002/9780470048672.wecb371 [DOI] [PubMed] [Google Scholar]
- 125.Takada N, Watanabe R, Suenaga K, Yamada K, Ueda K, Kita M, et al. Zamamistatin, a significant antibacterial bromotyrosine derivative, from the Okinawan sponge Pseudoceratina purpurea. Tetrahedron Lett. 2001;42: 5265–5267. doi: 10.1016/S0040-4039(01)00993-5 [Google Scholar]
- 126.Ross SA, Weete JD, Schinazi RF, Wirtz SS, Tharnish P, Scheuer PJ, et al. Mololipids, a new series of anti-HIV bromotyramine-derived compounds from a sponge of the order Verongida. J Nat Prod. 2000;63: 501–503. doi: 10.1021/np980414u [DOI] [PubMed] [Google Scholar]
- 127.Lebouvier N, Jullian V, Desvignes I, Maurel S, Parenty A, Dorin-Semblat D, et al. Antiplasmodial activities of homogentisic acid derivative protein kinase inhibitors isolated from a Vanuatu marine sponge Pseudoceratina sp. Mar Drugs. 2009;7: 640–653. doi: 10.3390/md7040640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salim AA, Khalil ZG, Capon RJ. Structural and stereochemical investigations into bromotyrosine-derived metabolites from southern Australian marine sponges, Pseudoceratina spp. Tetrahedron. Elsevier Ltd; 2012;68: 9802–9807. doi: 10.1016/j.tet.2012.09.008 [Google Scholar]
- 129.López S, Fernández- Trillo F, Midón P, Castedo L, Saá C. First stereoselective syntheses of (—) -Siphonodiol and (—) -Tetrahydrosiphonodiol, bioactive polyacetylenes from marine sponges. J Org Chem. 2005;70: 6346–6352. doi: 10.1021/jo050807f [DOI] [PubMed] [Google Scholar]
- 130.Fukami A, Ikeda Y, Kondo S, Naganawa H, Takeuchi T, Furuya S, et al. Akaterpin, a novel bioactive triterpene from the marine sponge Callyspongia sp. Tetrahedron Lett. 1997;38: 1201–1202. doi: 10.1016/S0040-4039(97)00016-6 [Google Scholar]
- 131.Yamada A, Kitamura H, Yamaguchi K, Fukuzawa S, Kamijima C, Yazawa K, et al. Development of chemical substances regulating biofilm formation. Bulletin of the Chemical Society of Japan. 1997. pp. 3061–3069. doi: 10.1246/bcsj.70.3061 [Google Scholar]
- 132.Sakai R, Higa T, Jefford CW, Bernardinelli G. Manzamine A, a Novel Antitumor Alkaloid from a Sponge. J Am Chem Soc. 1986;108: 6404–6405. doi: 10.1021/ja00280a055 [Google Scholar]
- 133.Kobayashi M, Chen Y-J, Aoki S, In Y, Ishida T, Kitagawa I. Four new ß-Carboline alkaloids isolated from two Okinawan marine sponges of Xestospongia sp. and Haliclona sp. Tetrahedron. 1995;51: 3727–3736. [Google Scholar]
- 134.Baker BJ, Scheuer PJ, Shoolery JN. Papuamine, an antifungal pentacyclic alkaloid from a marine sponge, Haliclona sp. Am Chem Soc. 1988;110: 966–968. [Google Scholar]
- 135.Clark RJ, Garson MJ, Hooper JNA. Antifungal alkyl amino alcohols from the tropical marine sponge Haliclonan sp. J Nat Prod. 2001;64: 1568–1571. Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11754615 [DOI] [PubMed] [Google Scholar]
- 136.Crews P, Harrison B. New triterpene-ketides (Merotriterpenes), Haliclotriol A and B, from an Indo-Pacific Haliclona sponge. Tetrahedron. 2000;56: 9039–9046. doi: 10.1016/S0040-4020(00)00758-4 [Google Scholar]
- 137.Thompson JE, Walker RP, Faulkner JD. Screening and bioassays for biologically-active substances from forty marine sponge species from San Diego, California, USA. Mar Biol. 1985;88: 11–21. [Google Scholar]
- 138.Bergquist PR, Bedfort JJ. The incidence of antibacterial activity in marine Demospongiae; systematic and geographic considerations. Mar Biol. 1978;46: 215–221. [Google Scholar]
- 139.Rifai S, Fassouane a., El-Abbouyi a., Wardani a., Kijjoa a., Van Soest R. Screening of antimicrobial activity of marine sponge extracts. J Mycol Med. 2005;15: 33–38. doi: 10.1016/j.mycmed.2004.11.001 [Google Scholar]
- 140.Edrada RA, Wray V, Berg A, Grafe U, Sudarsono, Brauers G, et al. Novel spiciferone derivatives from the fungus Drechslera hawaiiensis isolated from the marine sponge Callyspongia aerizusa. Z Naturforsch C. 2000;55: 218–221. [DOI] [PubMed] [Google Scholar]
- 141.Uriz MJ, Turon X, Becerro MA, Galera J, Lozano J. Patterns of resource allocation to somatic, defensive, and reproductive functions in the Mediterranean encrusting sponge Crambe crambe (Demospongiae, Poecilosclerida). Mar Ecol Prog Ser. 1995;124: 159–170. [Google Scholar]
- 142.Kim TK, Garson MJ, Fuerst JA. Marine actinomycetes related to the “Salinospora” group from the Great Barrier Reef sponge Pseudoceratina clavata. Environ Microbiol. 2005;7: 509–518. doi: 10.1111/j.1462-2920.2005.00716.x [DOI] [PubMed] [Google Scholar]
- 143.Rohde S, Gochfeld DJ, Ankisetty S, Avula B, Schupp PJ, Slattery M. Spatial variability in secondary metabolites of the Indo-Pacific sponge Stylissa massa. J Chem Ecol. 2012;38: 463–475. doi: 10.1007/s10886-012-0124-8 [DOI] [PubMed] [Google Scholar]
- 144.Turon X, Martí R, Uriz MJ. Chemical bioactivity of sponges along an environmental gradient in a Mediterranean cave. Sci Mar. 2009;73: 387–397. doi: 10.3989/scimar.2009.73n2387 [Google Scholar]
- 145.Govinden- Soulange J, Marie D, Kauroo S, Beesoo R, Ramanjooloo A. Antibacterial properties of marine sponges from Mauritius. Trop J Pharm Res. 2014;13: 249–254. [Google Scholar]
- 146.Youssef DTA, Badr JM, Shaala LA, Mohamed GA, Bamanie FH. Ehrenasterol and biemnic acid; new bioactive compounds from the Red Sea sponge Biemna ehrenbergi. Phytochem Lett. Phytochemical Society of Europe; 2015;12: 296–301. doi: 10.1016/j.phytol.2015.04.024 [Google Scholar]
- 147.Higgs MD, Faulkner JD. Plakortin, an antibiotic from Plakortis halichondrioides. J Org Chem. 1978;43: 3454–3457. doi: 10.1021/jo00412a006 [Google Scholar]
- 148.Kushlan DM, Faulkner JD. A novel perlactone from the Caribbean sponge Plakortis angulospiculatus. J Nat Prod. 1991;54: 1451–54. [DOI] [PubMed] [Google Scholar]
- 149.Mohammed R, Peng J, Kelly M, Yousaf M, Winn E, Odde S, et al. Polyketide-peroxides from a Species of Jamaican Plakortis (Porifera: Demospongiae). Aust J Chem. 2010;63: 877–885. doi: 10.1071/CH09665 [Google Scholar]
- 150.Kubota T, Ishiguro Y, Takahashi-Nakaguchi A, Fromont J, Gonoi T, Kobayashi J. Manzamenones L-N, new dimeric fatty-acid derivatives from an Okinawan marine sponge Plakortis sp. Bioorganic Med Chem Lett. Elsevier Ltd; 2013;23: 244–247. doi: 10.1016/j.bmcl.2012.10.109 [DOI] [PubMed] [Google Scholar]
- 151.Becerro MA, Lopez NI, Turon X, Uriz MJ. Antimicrobial activity and surface bacterial film in marine sponges. J Exp Mar Bio Ecol. 1994;179: 195–205. [Google Scholar]
- 152.Wahl M, Jensen PR, Fenical W. Chemical control of bacterial epibiosis on ascidians. Mar Ecol Prog Ser. 1994;110: 45–57. [Google Scholar]
- 153.Krug PJ. Defense of benthic invertebrates against surface colonization by larvae: A chemical arms race. Prog Mol Subcell Biol. 2006;42: 1–53. doi: 10.1007/3-540-30016-3_1 [DOI] [PubMed] [Google Scholar]
- 154.Angermeier H, Kamke J, Abdelmohsen UR, Krohne G, Pawlik JR, Lindquist NL, et al. The pathology of sponge orange band disease affecting the Caribbean barrel sponge Xestospongia muta. FEMS Microbiol Ecol. 2011;75: 218–230. doi: 10.1111/j.1574-6941.2010.01001.x [DOI] [PubMed] [Google Scholar]
- 155.Cebrian E, Uriz MJ, Garrabou J, Ballesteros E. Sponge mass mortalities in a warming mediterranean sea: Are cyanobacteria-harboring species worse off? PLoS One. 2011;6: e20211 doi: 10.1371/journal.pone.0020211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lesser MP, Bythell JC, Gates RD, Johnstone RW, Hoegh-Guldberg O. Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. J Exp Mar Bio Ecol. 2007;346: 36–44. doi: 10.1016/j.jembe.2007.02.015 [Google Scholar]
- 157.Di Camillo CG, Bartolucci I, Cerrano C, Bavestrello G. Sponge disease in the Adriatic Sea. Mar Ecol. 2012;34: 62–71. doi: 10.1111/j.1439-0485.2012.00525.x [Google Scholar]
- 158.Cervino JM, Winiarski-Cervino K, Polson SW, Goreau T, Smith GW. Identification of bacteria associated with a disease affecting the marine sponge Ianthella basta in New Britain, Papua New Guinea. Mar Ecol Prog Ser. 2006;324: 139–150. doi: 10.3354/meps324139 [Google Scholar]
- 159.Piskorska M, Smith GW, Weil E. Bacteria associated with the coral Echinopora lamellosa (Esper 1795) in the Indian Ocean- Zanzibar region. African J Environ Sci Technol. 2007;1: 93–98. [Google Scholar]
- 160.Ziegler M, Roik A, Porter A, Zubier K, Mudarris MS, Ormond R, et al. Coral microbial community dynamics in response to anthropogenic impacts near a major city in the central Red Sea. Mar Pollut Bull. The Authors; 2015;105: 629–640. doi: 10.1016/j.marpolbul.2015.12.045 [DOI] [PubMed] [Google Scholar]
- 161.Voss JD, Richardson LL. Nutrient enrichment enhances black band disease progression in corals. Coral Reefs. 2006;25: 569–576. doi: 10.1007/s00338-006-0131-8 [Google Scholar]
- 162.Redding JE, Myers-Miller RL, Baker DM, Fogel M, Raymundo LJ, Kim K. Link between sewage-derived nitrogen pollution and coral disease severity in Guam. Mar Pollut Bull. Elsevier Ltd; 2013;73: 57–63. doi: 10.1016/j.marpolbul.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 163.Harvell D, Jordán-Dahlgren E, Merkel S, Rosenberg E, Raymundo LJ, Smith GW, et al. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography. 2007;20. [Google Scholar]
- 164.Richelle-Maurer E, Braekman J-C, de Kluijver MMJ, Devijver C, Feio S, Gaspar H, et al. Distribution of antimicrobial and cytotoxic activities in marine sponges. Boll dei Musei Instituti Biol dell’Universita di Genova. 2002;66–67: 165–166. [Google Scholar]
- 165.Soapi K, Feussner K, Aalbersberg WG. Antimicrobial and cytotoxic activities of marine plants and invertebrates from the coast of Espirito Santo in Vanuatu. South Pacific J Nat Appl Sci. 2013;31: 89–95. [Google Scholar]
- 166.Uriz MJ, Martin D, Rosell D. Relationship of biological and taxonomic characteristics to chemically mediated bioactivity in Mediterranean littoral sponges. Mar Biol. 1992;113: 287–297. [Google Scholar]
- 167.Park T, Mansoor TA, Shinde PB, Bao B, Hong J, Jung JH. New cerebrosides from a marine Sponge Haliclona (Reniera) sp. Pharm Soc Japan. 2009;57: 106–111. [DOI] [PubMed] [Google Scholar]
- 168.Mansoor TA, Shinde PB, Luo X, Hong J, Lee C, Sim CJ, et al. Renierosides, cerebrosides from a marine sponge Haliclona (Reniera) sp. J Nat Prod. 2007;70: 1481–1486. doi: 10.1021/np070078u [DOI] [PubMed] [Google Scholar]
- 169.Teruya T, Kobayashi K, Suenaga K, Kigoshi H. Cyclohaliclonamines A-E: dimeric, trimeric, tetrameric, pentameric, and hexameric 3-alkyl pyridinium alkaloids from a marine sponge Haliclona sp. J Nat Prod. 2006;69: 135–7. doi: 10.1021/np050308+ [DOI] [PubMed] [Google Scholar]
- 170.Sata N, Abinsay H, Yoshida WY, Horgen DF, Sitachitta N, Kelly M, et al. Lehualides A—D, metabolites from a Hawaiian sponge of the genus Plakortis. J Nat Prod Prod. 2005;68: 1400–1403. [DOI] [PubMed] [Google Scholar]
- 171.Epifanio R de A, Pinheiro LS, Alves NC. Polyketides from the marine sponge Plakortis angulospiculatus. J Braz Chem Soc. 2005;16: 1367–1371. [Google Scholar]
- 172.de Voogd NJ. Indonesian sponges: Biodiversity and mariculture potential. 2005. [Google Scholar]
- 173.Lira NS, Montes RC, Tavares JF, Sobral M, Cunha EVL, de Athayde-filho PF. Brominated compounds from marine sponges of the genus Aplysina and a compilation of their 13C NMR spectral data. Mar Drugs. 2011;9: 2316–2368. doi: 10.3390/md9112316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Su J-H, Chen Y-C, El-Shazly M, Du Y-C, Su C-W, Tsao C-W, et al. Towards the small and the beautiful: a small dibromotyrosine derivative from Pseudoceratina sp. sponge exhibits potent apoptotic effect through targeting IKK/NFκB signaling pathway. Mar Drugs. 2013;11: 3168–85. doi: 10.3390/md11093168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shaala LA, Youssef DTA, Badr JM, Sulaiman M, Khedr A. Bioactive secondary metabolites from the Red Sea marine verongid sponge Suberea Species. Mar Drugs. 2015;13: 1621–1631. doi: 10.3390/md13041621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zeng C-M, Ishibashi M, Kobayashi J. Biemnasterol, a new cytotoxic sterol with the rare 22,25- diene side chain, isolated from the marine sponge Biemna sp. J Nat Prod. 1993;56: 2016–2018. [DOI] [PubMed] [Google Scholar]
- 177.Aoki S, Wei H, Matsui K, Rachmat R, Kobayashi M. Pyridoacridine alkaloids inducing neuronal differentiation in a neuroblastoma cell line, from marine sponge Biemna fortis. Bioorganic Med Chem. 2003;11: 1969–1973. doi: 10.1016/S0968-0896(03)00086-5 [DOI] [PubMed] [Google Scholar]
- 178.Sorek H, Rudi A, Gueta S, Reyes F, Martin MJ, Aknin M, et al. Netamines A-G: seven new tricyclic guanidine alkaloids from the marine sponge Biemna laboutei. Tetrahedron. 2006;62: 8838–8843. doi: 10.1016/j.tet.2006.06.063 [Google Scholar]
- 179.Sapar A, Noor A, Soekamto NH, Ahmad A. Rapid screening for cytotoxicity and group identification of secondary metabolites in methanol extracts from four sponge species found in Kapoposang Island, Spermonde Archipelago, Indonesia. Kuroshio Sci. 2014;8: 25–31. [Google Scholar]
- 180.Singh A, Thakur NL. Significance of investigating allelopathic interactions of marine organisms in the discovery and development of cytotoxic compounds. Chem Biol Interact. Elsevier Ltd; 2016;243: 135–147. doi: 10.1016/j.cbi.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 181.Chaves-Fonnegra A, Feldheim KA, Secord J, Lopez J V. Population structure and dispersal of the coral-excavating sponge Cliona delitrix. Mol Ecol. 2015;24: 1447–1466. doi: 10.1111/mec.13134 [DOI] [PubMed] [Google Scholar]
- 182.Kubanek J, Whalen KE, Engel S, Kelly SR, Henkel TP, Fenical W, et al. Multiple defensive roles for triterpene glycosides from two Caribbean sponges. Oecologia. 2002;131: 125–136. doi: 10.1007/s00442-001-0853-9 [DOI] [PubMed] [Google Scholar]
- 183.Rasher DB, Stout EP, Engel S, Kubanek J, Hay ME. Macroalgal terpenes function as allelopathic agents against reef corals. Proc Natl Acad Sci U S A. 2011;108: 17726–17731. doi: 10.1073/pnas.1108628108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Andras TD, Alexander TS, Gahlena A, Parry RM, Facundo M, Kubanek J, et al. Seaweed allelopathy against coral: Surface distribution of a Seaweed metabolite by imaging mass spectrometry. J Chem Ecol. 2012;38: 1203–1214. doi: 10.1007/s10886-012-0204-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Becerro MA, Paul VJ, Starmer J. Intracolonial variation in chemical defenses of the sponge Cacospongia sp. and its consequences on generalist fish predators and the specialist nudibranch predator Glossodoris pallida. Mar Ecol Prog Ser. 1998;168: 187–196. [Google Scholar]
- 186.Schupp PJ, Eder C, Paul V, Proksch P. Distribution of secondary metabolites in the sponge Oceanapia sp. and its ecological implications. Mar Biol. 1999;135: 573–580. [Google Scholar]
- 187.Rohde S, Schupp PJ. Allocation of chemical and structural defenses in the sponge Melophlus sarasinorum. J Exp Mar Bio Ecol. 2011;399: 76–83. doi: 10.1016/j.jembe.2011.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Freeman CJ, Gleason DF. Chemical defenses, nutritional quality, and structural components in three sponge species: Ircinia felix, I. campana, and Aplysina fulva. Mar Biol. 2010;157: 1083–1093. doi: 10.1007/s00227-010-1389-5 [Google Scholar]
- 189.Sotka EE, Forbey J, Horn M, Poore AGB, Raubenheimer D, Whalen KE. The emerging role of pharmacology in understanding consumer-prey interactions in marine and freshwater systems. Integr Comp Biol. 2009;49: 291–313. doi: 10.1093/icb/icp049 [DOI] [PubMed] [Google Scholar]
- 190.Angulo-Preckler C, Spurkland T, Avila C, Iken K. Antimicrobial activity of selected benthic Arctic invertebrates. Polar Biol. 2015;38: 1941–1948. [Google Scholar]
- 191.Cronin G, Lindquist N, Hay ME, Fenical W. Effects of storage and extraction procedures on yields of lipophilic metabolites from the brown seaweeds Dictyota ciliolata and D. menstrualis. Mar Ecol Prog Ser. 1995;119: 265–273. [Google Scholar]
- 192.Muscatine L, Mccloskey LR, Marian RE. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol Oceanogr. 1981;26: 601–611. [Google Scholar]
- 193.Davies SP. The role of zooxanthellae in the nutritional energy requirements of Pocillopora eydouxi. Coral Reefs. 1984;2: 181–186. doi: 10.1007/BF00263571 [Google Scholar]
- 194.Romano SL. Long-term effects of interspecific aggression on growth of the reef-building corals Cyphastrea ocellina (Dana) and Pocillopora damicomis (Linnaeus). J Exp Mar Bio Ecol. 1990;140: 135–146. [Google Scholar]
- 195.Tanner JE. Interspecific competition reduces fitness in scleractinian corals. J Exp Mar Bio Ecol. 1997;214: 19–34. doi: 10.1016/S0022-0981(97)00024-5 [Google Scholar]
- 196.Tanner JE. Competition between scleractinian corals and macroalgae: an experimental investigation of coral growth, survival and reproduction. J Exp Mar Bio Ecol. 1995;190: 151–168. [Google Scholar]
- 197.Idjadi JA, Karlson RH. Spatial arrangement of competitors influences coexistence of reef-building corals. Ecology. 2007;88: 2449–2454. [DOI] [PubMed] [Google Scholar]
- 198.Foster NL, Box SJ, Mumby PJ. Competitive effects of macroalgae on the fecundity of the reef-building coral Montastraea annularis. Mar Ecol Prog Ser. 2008;367: 143–152. doi: 10.3354/meps07594 [Google Scholar]
- 199.Carpenter KE, Abrar M, Aeby GS, Aronson RB, Banks S, Bruckner A, et al. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science (80-). 2008;321: 560–563. doi: 10.1126/science.1159196 [DOI] [PubMed] [Google Scholar]
- 200.Diaz‐Pulido G, Gouezo M, Tilbrook B, Dove S, Anthony K. High CO2 enhances the competitive strength of seaweeds over corals. Ecol Lett. 2011;14: 156–162. doi: 10.1111/j.1461-0248.2010.01565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bonaldo RM, Hay ME. Seaweed-coral interactions: Variance in seaweed allelopathy, coral susceptibility, and potential effects on coral resilience. PLoS One. 2014;9: e85786 doi: 10.1371/journal.pone.0085786 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIFF)
(DOCX)
(XLSX)
(DOCX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.