Abstract
We examined whether the pattern of middle- to late-life systemic inflammation was associated with white matter structural abnormalities in older adults. 1,532 participants (age=76.5; SD=5.4) underwent 3T brain MRI to quantify white matter hyperintensity (WMH) volume and whole-brain WM microstructural integrity (fractional anisotropy, mean diffusivity). High-sensitivity C-reactive protein (CRP), a marker of systemic inflammation, was measured at three visits (21 and 14 years before, and concurrent with, neuroimaging). Participants were categorized into one of six groups based on their 21-year pattern of low (<3mg/L) versus elevated (≥3mg/L) CRP. Compared to the group with low CRP at all three visits, the group which transitioned from low to elevated CRP during midlife demonstrated greatest WMH volume and poorest WM microstructural integrity, after adjusting for demographic variables and cardiovascular risk factors. Participants with high CRP at all visits also demonstrated greater WM structural abnormalities, but only after accounting for differential attrition. These results suggest that increasing and persistent inflammation in the decades spanning middle- to late-life may promote WM disease in older adults.
Keywords: Inflammation, Brain, White matter disease, Aging, Magnetic resonance imaging, Diffusion tensor imaging
1. Introduction
Cerebral white matter (WM) abnormalities are common among older adults and are independently associated with cognitive decline and dementia (Hahn et al., 2013; Knopman et al., 2015). Understanding the biological antecedents of these WM changes in older adults has become a priority in recent years. Although systemic inflammation has been associated with WM abnormalities in older adults, it remains unclear whether systemic inflammation is a potential driver of late-life WM changes, or merely an associated feature (Wersching et al., 2010; Zhu et al., 2017). With few exceptions (i.e., Bettcher et al., 2015; Metti et al., 2014), previous studies have been limited by the cross-sectional assessment of inflammatory biomarkers, capturing only a snapshot of what is likely a dynamic inflammatory process. Accordingly, it remains unknown how the trajectory and chronicity of systemic inflammation in the decades leading up to older adulthood relates to MRI-defined markers of WM structure in older adults.
Given current theories which implicate aberrant immune functioning and chronic inflammation as potential drivers of neurodegenerative disease, it is essential to develop an understanding of how the long-term trajectory of systemic inflammation influences structural brain abnormalities in older adults (Carret-Rebillat et al., 2015; Cunningham and Hennessy, 2015). Using a large community sample of white and African American adults, the objective of the current study was to examine how the 21-year longitudinal pattern of high-sensitivity C-reactive protein (CRP), a widely-used marker of systemic inflammation, relates to the development of white matter hyperintensities (WMH) and diffusion tensor imaging- (DTI) defined WM microstructural abnormalities in non-demented older adults. Specifically, we evaluated the relationship between discrete dynamic patterns of ascending, descending, and persistent mid- to late-life systemic inflammation and measures of WM structure in older adults. We tested the hypothesis that individuals with chronically elevated CRP and a higher average CRP level would have greater WM structural abnormalities as older adults. In light of evidence for sex-and race-based differences in peripheral immune function (Kim et al., 2010; Oertelt-Prigione, 2012), effect modification by these demographic factors, as well as APOE ε4 status, was also examined.
2. Methods
2.1 Study Population
We conducted an analysis of data from the Atherosclerosis Risk in Communities (ARIC) Study, an ongoing community-based, prospective study, which initially enrolled 15,792 adults between ages 45 and 65 from four communities within the U.S.: Washington County, MD; Forsyth County, NC; northwestern suburbs of Minneapolis, MN; and Jackson, MS (all African American) (The ARIC Investigators, 1989). Following a baseline visit (1987–89), participants were invited back for three additional in-person visits occurring approximately three years apart until Visit 4 (1996–1998), and a 5th visit (Visit 5, 2011–2013) approximately 14 years later. The ARIC study protocols were approved by the Institutional Review Boards at each site. All participants gave written informed consent.
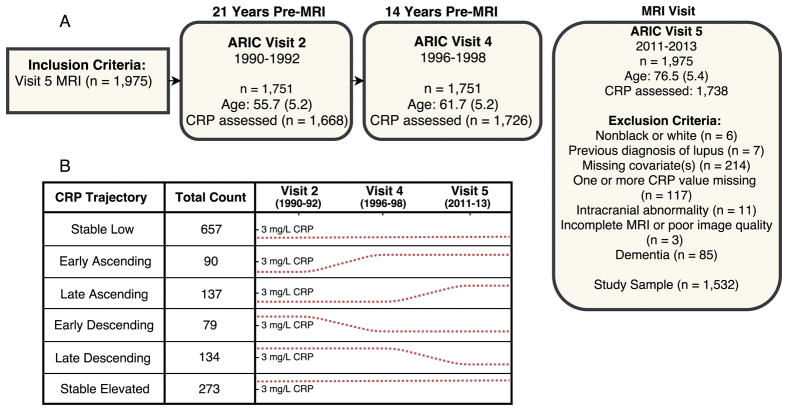
Brain MRIs were conducted on a subset of 1,978 participants at Visit 5. Selection of participants was based on safety inclusion criteria, previous participation in ARIC brain MRI study (2004–06), and evidence of cognitive impairment. An age-stratified random sample of participants without cognitive impairment was also included (Knopman et al., 2015). Participant inclusion and exclusion criteria are described in detail in Figure 1.
Figure 1. Study flowchart and C-reactive protein trajectory grouping.
(A) Study design and primary inclusion and exclusion criteria. (B) Participants were assigned to one of six C-reactive protein (CRP) trajectory groups based on CRP levels at Visits 2, 4, and 5. The dotted line denotes CRP levels at each time-point. A line above the tic mark indicates a CRP level ≥3 mg/L.
2.2 Inflammatory Markers
High-sensitivity CRP was measured at Visits 2, 4, and 5 from blood stored at −70°C. Visit 2 CRP (mg/L) was measured from serum using an immunoturbidimetric assay on the Roche Modular P chemistry analyzer (Roche Diagnostics, Indianapolis, IN). Visit 4 CRP was measured from plasma using the nephelometric method on the Siemens Dade Behring BN II analyzer (Siemens Healthcare Diagnostics, Deerfield IL). Visit 5 CRP levels were measured from plasma using an immunoturbidimetric assay on the Beckman Coulter Olympus AU400e analyzer (Beckman Coulter Inc., Brea, CA). Laboratory calibration studies conducted to evaluate potential CRP measurement differences between laboratories, assay method, instrument, specimen type, and time of measurement found differences that were not large enough to warrant calibration (bias <10%) (Parrinello et al., 2015).
Each participant was categorized as having “low” or “elevated” CRP levels at each visit using a cut-off of 3 mg/L. A CRP level at or above 3 mg/L is suggestive of ongoing systemic inflammation (Castoldi et al., 2007; Nyström, 2007). Using this “low” versus “elevated” CRP dichotomization, participants were categorized into one of six groups (see Figure 1), each with a distinct trajectory of mid to late-life CRP levels.
Stable low: low CRP levels (<3 mg/L) at all three visits
Early ascending: low CRP at Visit 2, and elevated CRP (≥3 mg/L) at Visits 4 and 5
Late ascending: low CRP at Visits 2 and 4, and elevated CRP at Visit 5
Early descending: elevated CRP at Visit 2, and low CRP at Visits 4 and 5
Late descending: elevated CRP at Visits 2 and 4, and low CRP at Visit 5
Stable elevated: elevated CRP at Visits 2, 4, and 5.
Each of the six pre-specified patterns represents an aging/inflammation phenotype that has been described previously in the aging literature (e.g., Franceschi et al., 2007). For example, it has been shown that systemic inflammation increases with age in a subset of individuals (Jenny et al., 2012). Such a transition to a state of elevated systemic inflammation may occur during midlife in some individuals, or later in life in others (Cohen-Manheim et al., 2015; Metti et al., 2014). Conversely, there is evidence for aging/inflammation phenotypes characterized by reduced inflammation with increasing age (Bettcher et al., 2015; Jenny et al., 2012). There were 162 participants with mid- to late-life CRP patterns that did not fit into one of the six pre-defined groups (i.e., the low/elevated/low and elevated/low/elevated groups). These participants were excluded from the primary analyses.
2.3 Brain MRI
The ARIC Visit 5 3T MRI acquisition sequence has been described in detail previously (Knopman et al., 2015). All participants were assessed using the following sequences: MP-RAGE, Axial T2*GRE, Axial T2 FLAIR, and Axial DTI. FLAIR images were used to calculate WMH volume (mm3). WMH volume was quantified using a computer-aided segmentation program to segment FLAIR images (FLAIR-histoseg) and measure the volumetric burden of leukoaraiosis (Raz et al., 2013). Analyses were adjusted for total intracranial volume. WMH volumes were log-transformed to correct for skewing.
DTI was used to calculate the fractional anisotropy (FA) and mean diffusivity (MD) of lobar WM, as described in detail previously (Power et al., 2017) (see Supplementary Methods). Lower FA and higher MD reflect the reduced microstructural integrity (Acosta-Cabronero and Nestor, 2014). We calculated FA and MD values for total brain WM using the voxel-weighted average of six ROIs (frontal, temporal, parietal, and occipital lobes, anterior and posterior corpus callosum). Standardized DTI values were reported for all analyses.
2.4 Assessment of Confounders and Mediators
Age, race (white/African American), sex, and educational attainment were self-reported at Visit 1. APOE was genotyped using the TaqMan assay (Applied Biosystems, Foster City, CA). History of long-term anti-inflammatory medication use (e.g., nonsteroidal anti-inflammatory drugs [NSAIDs], arthritis medication) was self-reported at Visit 5. Dementia diagnosis was adjudicated by an expert committee based on cognitive, neuroimaging, and functional data at Visit 5 (Knopman et al., 2016). Physiological variables and vascular risk factors were assessed concurrent with the initial CRP measurement at Visit 2. Body mass index (BMI; kg/m2) was calculated from recorded height and weight. Enzymatic methods were used to measure triglycerides and total cholesterol. High-density lipoprotein (HDL) was calculated after dextran-magnesium precipitation (Warnick et al., 1982). Alcohol use, and cigarette smoking status (current/former/never) were self-reported.
The following medical conditions were assessed at Visits 2, 3, 4, and 5: hypertension was defined as systolic or diastolic blood pressure >140 mm Hg and >90 mm Hg, respectively, or use of antihypertensive medication; coronary heart disease was defined based on participant self-report at Visit 1, and adjudicated at subsequent visits based on medical record documentation of previous myocardial infarction, coronary artery bypass graft or angioplasty, or myocardial infarction as determined by ECG; diabetes was defined as fasting glucose ≥126 mg/dl or a non-fasting glucose of ≥200 mg/dl, participant report of physician-diagnosed diabetes, or current use of diabetes medication including insulin; cancer diagnosis was ascertained using information from cancer registries and ARIC hospital surveillance. The presence of chronic inflammatory conditions (i.e., arthritis, gout) was assessed at Visit 4 based on self-report of physician diagnosis.
2.5 Statistical Analysis
Participants were compared on demographic and clinical characteristics using ANOVA and chi-square tests. Using the stable low group as the reference, covariate-adjusted linear regression was used to examine relationships between CRP trajectory and continuous measures of WM structure. An additional model adjusted for Visit 5 CRP level to determine whether CRP trajectory was associated with WM structure independent of CRP level at the time of MRI. We performed a series of exploratory analyses to further examine how longitudinal patterns of systemic inflammation relate to late-life WM structure. First, we examined whether a variable CRP trajectory (i.e., an elevated/low/elevated or low/elevated/low CRP pattern) was associated with greater WM structural abnormalities. Additionally, to assess whether the initial change in mid- to late-life inflammatory status was associated with later WM structure, we compared participants with stable low and stable elevated CRP patterns to a group (ascending combined) composed of participants with increasing CRP levels (i.e., early ascending, late ascending, and low/elevated/low) and another group (descending combined) composed of participants with decreasing CRP levels (early decreasing, late decreasing, and elevated/low/elevated). Additionally, we examined the association between WM structure and 21-year average CRP level. The mean of each participant’s three CRP values was derived, then log-transformed to correct for skewness.
To account for possible confounding, we adjusted regression models for demographic and physiological variables, APOE ε4 status (present/absent), smoking and alcohol use, anti-inflammatory medication use, and medical comorbidity at Visit 2 (concurrent with the first measurement of CRP). In a second model, we additionally adjusted for medical comorbidity occurring after Visit 2, which we believed may lie in the causal pathway between inflammation and reduced WM structure. This included all diagnoses of hypertension, coronary heart disease, diabetes, and cancer occurring up to the time of MRI. Multiplicative interaction terms were used to examine effect modification by race, sex, and APOE ε4 allele status. Follow-up stratified analyses were only performed in the case of a statistically significant interaction.
Sampling weights were incorporated to account for the ARIC brain MRI sampling strategy. Therefore, the primary results reflect estimates from the ARIC Visit 5 population. We conducted several sensitivity analyses. First, we examined the effects of excluding participants with prevalent clinical stroke before Visit 5. Second, we examined the effect of excluding participants with one or more abnormally high CRP measurement (>10 mg/L), suggestive of an acute inflammatory response. Lastly, we examined the effects of differential attrition due to death or dropout using inverse probability of attrition weighting (IPAW). This technique corrects for sampling bias resulting from attrition by up-weighting observed cases with characteristics that make death and drop-out before Visit 5 more likely. A two-sided p value <.05 was used as the cutoff for statistical significance. Analyses were conducted using Stata Version 14 (StataCorp, College Station, Tex., USA).
3. Results
3.1 Participant Characteristics
A total of 1,532 participants were included (28% African American, 61% female). Demographic and clinical characteristics of the study sample are presented stratified by CRP trajectory (Table 1). Overall, CRP levels increased from Visit 2 (median CRP: 1.85 mg/L) to Visit 4 (median CRP: 2.25 mg/L) and declined slightly between Visits 4 and 5 (median CRP: 1.92 mg/L). However, there was evidence for considerable between-subject heterogeneity (Supplementary Fig. 1).
Table 1.
Baseline (Visit 2) participant characteristics stratified by mid- to late-life C-reactive protein trajectory
| 21-Year Longitudinal Pattern of C-Reactive Protein | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | Stable Low (n = 657) | Early Ascending (n = 90) | Late Ascending (n = 137) | Early Descending (n = 79) | Late Descending (n = 134) | Stable Elevated (n = 273) |
| Demographic Variables | ||||||
| Age, mean (SD), y | 55.7 (5.2) | 55.7 (5.4) | 55.4 (5.2) | 56.8 (5.3) | 55.6 (5.0) | 54.7 (5.1)* |
| Female, No. (%) | 335 (51.0) | 66 (73.3)* | 65 (47.5) | 43 (54.4) | 108 (80.6)* | 206 (75.5)* |
| African American | 150 (22.8) | 31 (34.4)* | 30 (21.9) | 21 (26.6) | 38 (28.4) | 122 (44.7)* |
| Education | ||||||
| Less than high school | 79 (12.0) | 11 (12.2) | 10 (7.3) | 8 (10.1) | 11 (8.2) | 52 (19.1)* |
| High school/GED/vocational | 275 (41.9) | 40 (44.4) | 61 (44.5) | 31 (39.2) | 55 (41.0) | 114 (41.8) |
| College/graduate/professional | 303 (46.1) | 39 (43.3) | 66 (48.2) | 40 (50.6) | 68 (50.8) | 107 (39.2) |
| Apolipoprotein E ε4 alleles | ||||||
| 0 | 429 (65.3) | 70 (77.8)* | 105 (76.6) | 62 (78.5)* | 96 (71.6) | 216 (79.1)* |
| 1 | 208 (31.6) | 18 (20.0) | 30 (21.9) | 15 (19.0) | 33 (24.6) | 52 (19.1) |
| 2 | 20 (3.0) | 2 (2.2) | 2 (1.5) | 2 (2.5) | 5 (3.7) | 5 (1.8) |
| Physiological & Lab Variables | ||||||
| Body mass index, kg/m2 | 26.0 (3.9) | 27.5 (4.3)* | 25.4 (3.4) | 28.3 (5.0)* | 29.7 (5.6)* | 31.2 (6.1)* |
| Systolic blood pressure, mm Hg | 115.5 (15.7) | 119.2 (16.8)* | 114.6 (12.1) | 118.0 (15.6) | 117.0 (14.4) | 120.2 (16.7)* |
| Diastolic blood pressure, mm Hg | 72.2 (10.4) | 74.3 (11.7) | 72.7 (8.9) | 73.3 (10.5) | 72.5 (9.0) | 76.1 (10.7)* |
| Total cholesterol, mg/dl | 206.4 (36.7) | 207.2 (38.3) | 206.5 (37.7) | 206.5 (39.4) | 216.6 (43.3)* | 206.9 (32.7) |
| HDL, mg/dl | 52.3 (17.5) | 54.4 (16.1) | 53.6 (17.1) | 49.7 (17.2) | 53.3 (17.6) | 53.3 (16.3) |
| LDL, mg/dl | 130.8 (33.5) | 126.4 (36.9) | 131.3 (38.4) | 130.6 (34.6) | 137.0 (40.9)* | 127.7 (32.6) |
| Cardiovascular Disease | ||||||
| Hypertension | 135 (20.6) | 22 (24.4) | 21 (15.3) | 24 (30.4) | 44 (32.8)* | 110 (40.3)* |
| Diabetes mellitus | 26 (4.0) | 3 (3.3) | 4 (2.9) | 6 (7.6) | 13 (9.7)* | 26 (9.5)* |
| Coronary heart disease | 16 (2.5) | 2 (2.3) | 1 (0.8) | 3 (4.0) | 2 (1.5) | 8 (3.0) |
| Heart failure | 4 (0.6) | 2 (2.2) | 3 (2.2) | 2 (2.5) | 3 (2.3) | 14 (5.2) |
| Inflammatory Conditions | ||||||
| Arthritis a | 204 (31.1) | 36 (40.0) | 47 (34.3) | 31 (39.2) | 55 (41.0)* | 135 (49.5)* |
| Gout a | 21 (3.2) | 3 (3.3) | 10 (7.3)* | 6 (7.6)* | 4 (3.0) | 22 (8.1)* |
| Cancer | 37 (5.6) | 8 (8.9) | 9 (6.6) | 7 (9.0) | 7 (5.2) | 13 (4.8) |
| Medication | ||||||
| Anti-inflammatory (regular use)b | 88 (13.4) | 14 (15.6) | 21 (15.3) | 10 (12.7) | 18 (13.4) | 56 (20.5)* |
| Cholesterol lowering (last 2 weeks) | 35 (5.3) | 4 (4.4) | 7 (5.1) | 4 (5.1) | 2 (1.5) | 12 (4.4) |
| Cigarette Smoking Status | ||||||
| Current | 74 (11.3) | 21 (23.3)* | 23 (16.8) | 8 (10.1) | 17 (12.7) | 45 (16.5)* |
| Former | 263 (40.0) | 27 (30.0) | 62 (45.3) | 30 (38.0) | 49 (36.6) | 92 (33.7) |
| Never | 320 (48.7) | 42 (46.7) | 52 (38.0) | 41 (51.9) | 68 (50.8) | 136 (49.8) |
| Alcohol Consumption | ||||||
| Current | 393 (59.8) | 61 (67.8) | 98 (71.5)* | 44 (55.7) | 78 (58.2) | 148 (54.2) |
| Former | 113 (17.2) | 6 (6.7) | 21 (15.3) | 15 (19.0) | 20 (14.9) | 50 (18.3) |
| Never | 151 (23.0) | 25 (25.6) | 18 (13.1) | 20 (25.3) | 36 (26.9) | 75 (27.5) |
Difference between group and the Stable Low (referent group) statistically significant (p < .05)
Assessed at Visit 4 (1996–1998)
Assessed at Visit 5 (2011–2013)
3.2 21-Year Pattern of CRP and Late-Life White Matter Structure
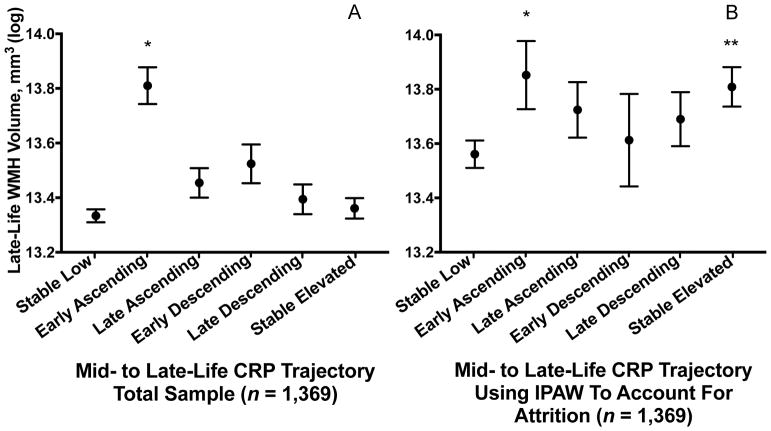
After adjusting for confounders, the group with early ascending CRP levels displayed greater WMH volume (0.35 standard deviations (SD); 95% confidence interval [CI]: 0.07, 0.64; p=.015) compared to the stable low group (Figure 2A). A comparison of beta coefficients indicated that this effect was similar to that of five years of additional age (at study baseline) on WMH volume. After accounting for differential rates of death and drop-out using IPAW, participants in both the early ascending (0.22 SD; CI: 0.02, 0.43; p=.035) and stable elevated (0.19 SD; CI: 0.05, 0.33; p=.008) groups demonstrated significantly greater WMH volume compared to the stable low group (Figure 2B). We found no evidence for effect modification by age, sex, or APOE ε4 allele status (p-interactions >.05). These associations were not measurably changed after additionally adjusting for prevalent medical comorbidity occurring up to Visit 5 (Supplementary Fig. 2). Additionally, our results remained similar after excluding participants with clinical stroke (n=48) and abnormally high CRP levels (>10 mg/L; n=226), and after adjusting for CRP levels at the time of MRI (Supplementary Fig. 3–5).
Figure 2. Late-life white matter hyperintensity volume according to 21-year C-reactive protein trajectory.
Estimated late-life total white matter hyperintensity volume according to the trajectory of C-reactive protein levels from mid- to late-life in the (A) primary analysis and (B) after accounting for differential attrition due to death and drop-out using inverse probability of attrition weighting. Models are adjusted for age, sex, race-center, education, cigarette smoking and alcohol use status, BMI, total cholesterol, HDL, hypertension, diabetes, coronary heart disease, previous cancer, inflammatory disease, anti-inflammatory medication use, and APOE ε4 status.
*P < .05; ** P < .01 difference between group and the Stable Low (referent) group
Abbreviations: IPAW = inverse probability of attrition weighting; WMH = white matter hyperintensity.
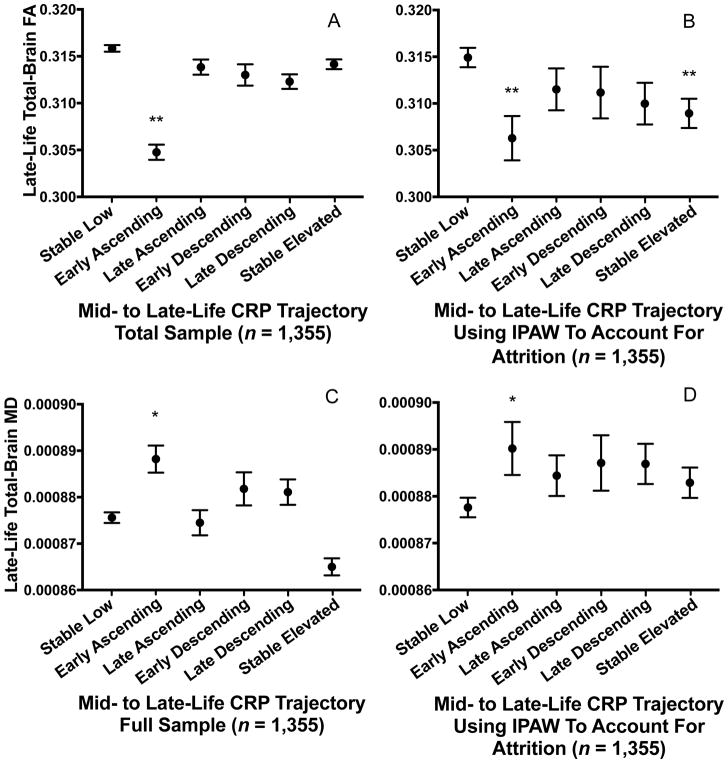
Participants with early ascending CRP levels displayed lower total FA (−0.46 SD; CI: −0.74, −0.17; p=.002; Figure 3A) and higher total MD (0.32 SD; CI: 0.06, 0.58; p=.016; Figure 3C) compared to the stable low group, after adjusting for confounders. The estimated differences in FA and MD associated with early ascending CRP levels were similar in magnitude to that of 16 and five years of additional age (at study baseline), respectively (based on a comparison of beta coefficients). After accounting for differential rates of death and drop-out using IPAW, participants in both the early ascending (−0.36 SD; CI: −0.58, −0.14, p=.001) and stable elevated (−0.25 SD; CI: −0.42, −0.09, p=.003) groups demonstrated significantly lower total FA (Figure 3B). However, this was not the case for measures of MD (Figure 3D). Interaction terms for race, age, and APOE ε4 allele status were not statistically significant (p-interactions >.05). All findings remained similar after additionally adjusting for prevalent medical comorbidity occurring before Visit 5, total WMH volume, and CRP levels at the time of MRI, and after excluding participants with clinical stroke and abnormally high CRP levels (>10 mg/L) (Supplementary Fig. 2–6).
Figure 3. Late-life fractional anisotropy and mean diffusivity according to 21-year C-reactive protein trajectory.
Estimated late-life total brain (A, B) fractional anisotropy and (C, D) mean diffusivity according to the trajectory of C-reactive protein levels from mid- to late-life in the (A, C) primary analysis of the total sample and (B, D) after accounting for differential attrition due to death and drop-out using inverse probability of attrition weighting. Models are adjusted for age, sex, race-center, education, cigarette smoking and alcohol use status, BMI, total cholesterol, HDL, hypertension, diabetes, coronary heart disease, previous cancer, inflammatory disease, anti-inflammatory medication use, and APOE ε4 status.
*P < .05; ** P < .01 difference between group and the Stable Low (referent) group
Abbreviations: FA = fractional anisotropy; IPAW = inverse probability of attrition weighting; MD = mean diffusivity.
3.2.1 Exploratory Analyses
The WM structure of the 162 participants with CRP trajectories that could not be categorized according to one of the six patterns (i.e., participants with a pattern of low/elevated/low or elevated/low/elevated CRP levels) did not differ from that of the reference group (Supplementary Fig. 7). Findings were similar when the low/elevated/low and elevated/low/elevated groups were combined and examined as distinct groups. When we repeated our analyses after collapsing participants with ascending and descending CRP trajectories into the ascending combined and descending combined groups, respectively, we found that only the ascending combined group demonstrated significantly greater WMH volume (0.20 SD; CI: 0.05, 0.34; p=.008), lower FA (−0.23 SD; CI: −0.36, −0.09; p=.001), and greater MD (0.16 SD; CI: 0.04, 0.27; p=.006), compared to the stable low group (Supplementary Fig. 8).
3.3 21-Year Average CRP and Late-Life White Matter Structure
After adjusting for confounders, higher 21-year average CRP level was associated with greater WMH volume and reduced WM microstructural integrity (Table 2). The associations of higher average CRP level with higher WMH volume (p-interaction=.030), lower total FA (p-interaction=.001), and higher total MD (p-interaction=.015) were stronger among participants with one or more copies of the APOE ε4 allele compared to participants who were APOE ε4 allele-negative. Interaction terms for race and sex were non-significant (p-interactions >.05). Our findings were not substantively changed in sensitivity analyses that adjusted for prevalent medical comorbidity occurring up to Visit 5 (Supplementary Table 1), accounted for differential attrition using IPAW (Supplementary Table 2), and excluded participants with clinical stroke (Supplementary Table 3). Associations between 21-year average CRP and WM microstructural integrity were attenuated, but remained statistically significant, after additionally adjusting for WMH volume (Supplementary Table 4).
Table 2.
Associations between 21-year average C-reactive protein and late-life white matter structure in the total sample and stratified by APOE ε4 allele status
| MRI Characteristics (Visit 5) | Total (n = 1,532) | APOE ε4 Negative (n = 1,104) | APOE ε4 Positive (n = 428) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| β (95% CI) a | p | β (95% CI) a | p | β (95% CI) a | p | |
| WMH Volume, mm3 (log transformed) b | 0.05 (0.00, 0.10) | .031 | 0.02 (−0.03, 0.08) | .402 | 0.10 (0.03, 0.18) | .008 |
| Total White Matter, FA b | −0.07 (−0.11, −0.02) | .004 | −0.03 (−0.08, 0.03) | .340 | −0.15 (−0.23, −0.08) | .001 |
| Total White Matter, MD b | 0.05 (0.01, 0.08) | .016 | 0.02 (−0.02, 0.07) | .330 | 0.09 (0.03, 0.15) | .004 |
Models are adjusted for age, sex, race-center, education, cigarette smoking and alcohol use status, BMI, total cholesterol, HDL, hypertension, diabetes, coronary heart disease, previous cancer, inflammatory disease, anti-inflammatory medication use, and APOE ε4 status.
Abbreviations: FA = fractional anisotropy; MD = mean diffusivity; WMH = white matter hyperintensity
β values represent the difference in standardized white matter hyperintensity volume, fractional anisotropy, and mean diffusivity per one unit higher log CRP (mg/L).
APOE ε4 allele by CRP interaction statistically significant
4. Discussion
In this study, we characterized the relationship between past inflammation, as assessed by CRP levels over a 21-year period, and late-life WM structure in a large, biracial community-dwelling sample. In addition to showing that a higher average CRP level over a 21-year period is associated with greater late-life WM structural abnormalities, our findings indicate that the longitudinal pattern of systemic inflammation from mid- to late-life may be an important determinant of subsequent WM structural abnormalities among older adults. Specifically, we found that a shift from normal to elevated (≥3mg/L) CRP levels in the 5th and 6th decade that is sustained into late-life is associated with greater WMH volume and reduced WM microstructural integrity in the 7th and 8th decade of life relative to those who maintained low CRP levels over this same period. This effect was robust, similar to that of 16 years and five years of additional age on total FA and WMH volume, respectively. Importantly, these associations were independent of potentially confounding demographic and physiological factors, cardiovascular and inflammatory disease, and anti-inflammatory medication use.
Our analysis of the combined CRP patterns indicates that, overall, individuals with increasing inflammation after midlife tend to have greater WM structural abnormalities in late-life. However, our primary analysis revealed that a pattern of systemic inflammation which begins in the fifth and sixth decade of life and persists into older adulthood (the 7th and 8th decade) is associated with greatest WM structural abnormalities. In addition, our findings indicate that participants who maintained elevated CRP at all three study visits (suggesting an even earlier transition to a state of systemic inflammation) also show reduced late-life WM structure. However, this association was only observed after accounting for sampling bias related to participant death and drop-out. Correcting for differential attrition is important in this context because individuals with the greatest levels of systemic inflammation and WM abnormalities are at increased risk for morbidity and mortality (Supplementary Table 5) (Varadhan et al., 2014; Windham et al., 2015). The incremental value of characterizing longitudinal patterns of CRP is underscored by our analyses showing that results were largely unchanged after adjusting for CRP level at the time of MRI. Thus, CRP trajectory accounted for additional variance in WM structure over and above that which is accounted for using a cross-sectional approach.
Although recent work from the ARIC study indicates that systemic inflammation in one’s 50s may be a risk factor for WM disease 20 years later (Walker et al., 2017), the current results indicate that not all participants with heightened systemic inflammation during this midlife period are at an elevated risk for structural WM abnormalities. In particular, we found that participants who had elevated levels of CRP during midlife followed by low CRP levels in subsequent years were not at an increased risk for developing WM abnormalities. These findings are consistent with those of a recent study, which demonstrated that participants with a 6-year decline in CRP during their 7th decade (a pattern represented in the present study by the late descending group) had better white matter microstructural integrity compared to participants who did not show declines in CRP during this time (Bettcher et al., 2015). Together, these results highlight the potential etiological importance of increasing and persistently elevated systemic inflammation and substantiate evidence from animal studies which indicates that prolonged systemic inflammation can induce downstream neurodegenerative and microangiopathic changes (Cunningham and Hennessy, 2015; Krstic et al., 2012).
While it is possible that the emergence of chronic disease during middle adulthood initiates and perpetuates a state of chronic low-grade inflammation, the current findings support an association between systemic inflammation and late-life WM structure that is independent of midlife cardiovascular risk factors and medical comorbidity. We also recognize that systemic inflammation may promote subsequent systemic disease which may, in turn, lead to neurologic dysfunction. Our finding that the relationship between systemic inflammation and WM structural abnormalities was robust to adjustment for potentially mediating medical conditions occurring during follow-up provides further support for the hypothesis that peripheral inflammatory signaling contributes to WM dysfunction independent of overt disease. Our results also substantiate previous work which shows that low-grade systemic inflammation alone may be enough to promote adverse neurological outcomes (Cunningham and Hennessy, 2015), as our results did not change measurably after excluding participants with one or more abnormally high CRP levels. Previous reports, which have demonstrated that systemic inflammation may directly contribute to cerebrovascular permeability, microcirculatory dysfunction (Abbott, 2000), and increased pro-inflammatory signaling within the central nervous system (Carret-Rebillat et al., 2015) provide a potential biological basis for the associations observed in the current study. However, causality cannot be directly inferred from our findings, as it remains possible that the associations between CRP trajectory and WM structure may be confounded or mediated by unmeasured or subclinical disease.
Our finding that individuals who demonstrate a higher average CRP level across a two-decade span also tend to show greater WM structural abnormalities during late-life indicates that the degree to which systemic inflammation is elevated may also influence late-life neurological health. The significantly stronger associations between average CRP level and WM structure among individuals with one or more copies of the APOE ε4 allele may be explained by a tendency for ε4 allele carriers to have a stronger neuroinflammatory response to peripheral and central inflammatory signaling (Lynch et al., 2003; Ophir et al., 2005). Together, the current findings add to the body of cross-sectional and longitudinal literature demonstrating a link between markers of systemic inflammation and WM structure in large population-based samples (Bettcher et al., 2015; Shoamanesh et al., 2015; Wersching et al., 2010). These findings further highlight WM macrostructural and microstructural changes as a potentially relevant biological mediator of the previously described relationship between midlife systemic inflammation and increased dementia risk (Schmidt et al., 2002).
Our findings highlight the value of examining the relationship between longitudinal markers of systemic inflammation and neurological endpoints, rather than using a one-time measure. Additional strengths of the current study include the large sample size, use of a community sample, inclusion of a large group of African American participants, and the ability to thoroughly account for potentially confounding demographic, physiological, and clinical variables. Although the current findings provide insights into the relationship between systemic inflammation and late-life WM changes, our results should be interpreted within the context of several limitations. First, this study lacks serial 3T MRIs, which precludes the assessment of WM changes. It is, therefore, possible that some WM abnormalities were present before the initial assessment of our exposure variable. Second, although CRP is a widely used marker of systemic inflammation, it represents only one marker of a complex immune network. The longitudinal examination of other inflammatory markers, including cytokines and chemokines, may provide additional insights. Third, systemic inflammation trajectory was estimated based on relatively few data points. A more frequent longitudinal assessment of inflammatory markers would likely allow for a more accurate characterization of systemic inflammation trajectory and permit the examination of other potentially informative characteristics, such as intra-individual variability (Metti et al., 2014). Lastly, although we demonstrated that the association of CRP with FA and MD was largely unchanged after adjusting for WMH volume, we were unable to restrict our analyses to normally appearing WM. Therefore, we cannot rule out the possibility that the associations between systemic inflammation and measures of WM microstructural integrity were driven, at least in part, by macroscopic alterations in WM structure. Despite these limitations, the current findings provide insight into the relationship between the longitudinal pattern of systemic inflammation and late-life WM abnormalities and offer additional support for the view that chronic systemic inflammation contributes to the development of structural brain changes in older adults.
Supplementary Material
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917 from the National Heart, Lung, and Blood Institute and the National Institute of Neurological Disorders and Stroke, with previous brain MRI examinations funded by R01-HL70825 from the National Heart, Lung, and Blood Institute. This study was also supported by contracts T32 AG027668 (Dr. Walker) and K24 AG052573 (Dr. Gottesman) from the National Institute on Aging, and K24DK106414 and R01DK089174 (Dr. Selvin) from the National Institute of Diabetes and Digestive and Kidney Diseases. The sponsors had no role in the design and conduct of the study; collection management, analysis and interpretation of the data; or preparation review, or approval of the manuscript. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Disclosure statement: DSK serves on a Data Safety Monitoring Board for the DIAN study; is an investigator in clinical trials sponsored by Lilly Pharmaceuticals, Biogen and the Alzheimer’s Treatment and Research Institute at the University of Southern California; and receives research support from the NIH. BGW receives research support from NIH, is an investigator/dementia expert on a CMS Coverage with Evidence Development (CED) study, and is an investigator in a clinical trial sponsored by ACADIA Pharmaceutical. CRJ serves on a scientific advisory board for Eli Lilly & Company and receives research support from NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation. RFG serves as Associate Editor for Neurology® and receives research support from NIH. The other authors declare no competing interests.
References
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000 doi: 10.1023/a:1007074420772. https://doi.org/10.1023/A:1007074420772. [DOI] [PubMed]
- Acosta-Cabronero J, Nestor PJ. Diffusion tensor imaging in Alzheimer’s disease: Insights into the limbic-diencephalic network and methodological considerations. Front Aging Neurosci. 2014;6:266. doi: 10.3389/fnagi.2014.00266. https://doi.org/10.3389/fnagi.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher BM, Yaffe K, Boudreau RM, Neuhaus J, Aizenstein H, Ding J, Kritchevsky SB, Launer LJ, Liu Y, Satterfield S, Rosano C. Declines in inflammation predict greater white matter microstructure in older adults. Neurobiol Aging. 2015;36:948–954. doi: 10.1016/j.neurobiolaging.2014.11.004. https://doi.org/10.1016/j.neurobiolaging.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carret-Rebillat A-S, Pace C, Gourmaud S, Ravasi L, Montagne-Stora S, Longueville S, Tible M, Sudol E, Chang RC-C, Paquet C, Mouton-Liger F, Hugon J. Neuroinflammation and Aβ accumulation linked to systemic inflammation are decreased by genetic PKR down-regulation. Sci Rep. 2015;5:8489. doi: 10.1038/srep08489. https://doi.org/10.1038/srep08489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoldi G, Galimberti S, Riva C, Papagna R, Querci F, Casati M, Zerbini G, Caccianiga G, Ferrarese C, Baldoni M, Valsecchi MG, Stella A. Association between serum values of C-reactive protein and cytokine production in whole blood of patients with type 2 diabetes. Clin Sci (Lond) 2007;113:103–8. doi: 10.1042/CS20060338. https://doi.org/10.1042/CS20060338. [DOI] [PubMed] [Google Scholar]
- Cohen-Manheim I, Doniger GM, Sinnreich R, Simon ES, Pinchas-Mizrachi R, Otvos JD, Kark JD. Increase in the Inflammatory Marker GlycA over 13 Years in Young Adults Is Associated with Poorer Cognitive Function in Midlife. PLoS One. 2015;10:e0138036. doi: 10.1371/journal.pone.0138036. https://doi.org/10.1371/journal.pone.0138036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Hennessy E. Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research. Alzheimers Res Ther. 2015;7:33. doi: 10.1186/s13195-015-0117-2. https://doi.org/10.1186/s13195-015-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. https://doi.org/10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Hahn K, Myers N, Prigarin S, Rodenacker K, Kurz A, Förstl H, Zimmer C, Wohlschläger AM, Sorg C. Selectively and progressively disrupted structural connectivity of functional brain networks in Alzheimer’s disease - Revealed by a novel framework to analyze edge distributions of networks detecting disruptions with strong statistical evidence. Neuroimage. 2013;81:96–109. doi: 10.1016/j.neuroimage.2013.05.011. https://doi.org/10.1016/j.neuroimage.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PHM, Ding J, Fried LP, Kritchevsky SB, Rifkin DE, Sarnak MJ, Newman AB. Long-term assessment of inflammation and healthy aging in late life: The cardiovascular health study all stars. Journals Gerontol - Ser A Biol Sci Med Sci. 2012;67 A:970–976. doi: 10.1093/gerona/glr261. https://doi.org/10.1093/gerona/glr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CX, Bailey KR, Klee GG, Ellington AA, Liu G, Mosley TH, Rehman H, Kullo IJ. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: The Mayo Clinic proteomic markers of arteriosclerosis study. PLoS One. 2010;5:e9065. doi: 10.1371/journal.pone.0009065. https://doi.org/10.1371/journal.pone.0009065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, Schneider ALC, Hengrui S, Alonso A, Coresh J, Albert MS, Mosley TH., Jr Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study. Alzheimer’s Dement. Diagnosis, Assess Dis Monit. 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. https://doi.org/10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, Jack CR, Graff-Radford J, Schneider ALC, Windham BG, Coker LH, Albert MS, Mosley TH, Coresh J, Roche KB, Selnes OA, McKhann G, Alonso A, Folsom AR, Eckfeldt J, Wagenknecht LE, Heiss G, Couper D, Wruck L. Vascular Imaging abnormalities and cognition: Mediation by cortical volume in nondemented individuals: Atherosclerosis risk in communities-neurocognitive study. Stroke. 2015;46:433–440. doi: 10.1161/STROKEAHA.114.007847. https://doi.org/10.1161/STROKEAHA.114.007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, Manalastas A, Hilfiker M, Pfister S, Schwerdel C, Riether C, Meyer U, Knuesel I. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J Neuroinflammation. 2012;9:151. doi: 10.1186/1742-2094-9-151. https://doi.org/10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. https://doi.org/10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- Metti AL, Yaffe K, Boudreau RM, Ganguli M, Lopez OL, Stone KL, Cauley JA. Change in inflammatory markers and cognitive status in the oldest-old women from the study of osteoporotic fractures. J Am Geriatr Soc. 2014;62:662–666. doi: 10.1111/jgs.12739. https://doi.org/10.1111/jgs.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metti AL, Yaffe K, Boudreau RM, Simonsick EM, Carnahan RM, Satterfield S, Harris TB, Ayonayon HN, Rosano C, Cauley JA. Trajectories of inflammatory markers and cognitive decline over 10 years. Neurobiol Aging. 2014;35:2785–2790. doi: 10.1016/j.neurobiolaging.2014.05.030. https://doi.org/10.1016/j.neurobiolaging.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T. C-reactive protein: a marker or a player? Clin Sci. 2007;113:79–81. doi: 10.1042/CS20070121. https://doi.org/10.1042/CS20070121. [DOI] [PubMed] [Google Scholar]
- Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev. 2012 doi: 10.1016/j.autrev.2011.11.022. https://doi.org/10.1016/j.autrev.2011.11.022. [DOI] [PubMed]
- Ophir G, Amariglio N, Jacob-Hirsch J, Elkon R, Rechavi G, Michaelson DM. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-κB signaling cascade. Neurobiol Dis. 2005;20:709–718. doi: 10.1016/j.nbd.2005.05.002. https://doi.org/10.1016/j.nbd.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Parrinello CM, Grams ME, Couper D, Ballantyne CM, Hoogeveen RC, Eckfeldt JH, Selvin E, Coresh J. Recalibration of blood analytes over 25 years in the Atherosclerosis Risk in Communities Study: Impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem. 2015;61:938–947. doi: 10.1373/clinchem.2015.238873. https://doi.org/10.1373/clinchem.2015.238873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Tingle JV, Reid RI, Huang J, Sharrett R, Coresh J, Griswold M, Kantarci K, Jack CR, Knopman DS, Gottesman RF, Mosley TH, Sharrett AR, Coresh J, Griswold M, Kantarci K, Jack CR, Knopman DS, Gottesman RF, Mosley TH, Sharrett R, Coresh J, Griswold M, Kantarci K, Jack CR, Knopman DS, Gottesman RF, Mosley TH. Midlife and late life vascular risk factors and white matter microstructural integrity: the ARIC-NCS study. JAHA. 2017;18:e005608. doi: 10.1161/JAHA.117.005608. https://doi.org/10.1161/JAHA.117.005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz L, Jayachandran M, Tosakulwong N, Lesnick TG, Wille SM, Murphy MC, Senjem ML, Gunter JL, Vemuri P, Jack CR, Miller VM, Kantarci K. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80:911–918. doi: 10.1212/WNL.0b013e3182840c9f. https://doi.org/10.1212/WNL.0b013e3182840c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. https://doi.org/10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, Wolf PA, DeCarli C, Romero JR, Seshadri S. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015;84:825–832. doi: 10.1212/WNL.0000000000001279. https://doi.org/10.1212/wnl.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- Varadhan R, Yao W, Matteini A, Beamer BA, Xue QL, Yang H, Manwani B, Reiner A, Jenny N, Parekh N, Daniele Fallin M, Newman A, Bandeen-Roche K, Tracy R, Ferrucci L, Walston J. Simple biologically informed infammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. Journals Gerontol - Ser A Biol Sci Med Sci. 2014;69 A:165–173. doi: 10.1093/gerona/glt023. https://doi.org/10.1093/gerona/glt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Power MC, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Windham BG, Selvin E, Jack CR, Gottesman RF. Midlife Systemic Inflammation, Late-Life White Matter Integrity, and Cerebral Small Vessel Disease. Stroke. 2017 doi: 10.1161/STROKEAHA.117.018675. STROKEAHA.117.018675. https://doi.org/10.1161/STROKEAHA.117.018675. [DOI] [PMC free article] [PubMed]
- Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982 [PubMed] [Google Scholar]
- Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, Conty M, Minnerup J, Ringelstein EB, Berger K, Deppe M, Knecht S. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74:1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. https://doi.org/10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- Windham BG, Deere B, Griswold ME, Wang W, Bezerra DC, Shibata D, Butler K, Knopman D, Gottesman RF, Heiss G, Mosley TH. Small brain lesions and incident stroke and mortality: A cohort study. Ann Intern Med. 2015;163:22–31. doi: 10.7326/M14-2057. https://doi.org/10.7326/M14-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Chai YL, Hilal S, Ikram MK, Venketasubramanian N, Wong B-S, Chen CP, Lai MKP. Serum IL-8 is a marker of white-matter hyperintensities in patients with Alzheimer disease. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2017;7:41–47. doi: 10.1016/j.dadm.2017.01.001. https://doi.org/10.1016/j.dadm.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.