Abstract
Background/Aims
Healthy volunteers in phase 1 clinical trials contribute to the development of safe drugs and other biologics and accept risks and burdens without anticipated health benefits from participation. Although emerging data have shown that healthy volunteers are influenced by risk, some still worry that financial incentives lead them to take on unreasonable risk. Yet little is known about healthy volunteers’ preferences and how they make choices about enrolling in research studies.
Methods
We surveyed 654 healthy volunteers at the end of their participation in a phase 1 Pfizer trial in the United States, Belgium, and Singapore to examine their reported willingness to enroll in studies of different types, with various procedures, and with possible side effects.
Results
The majority of respondents were willing to join many kinds of studies, but fewer were willing to participate in first-in-human vaccine studies or studies of psychiatric drugs than in other study types. With regard to procedures, a substantial proportion were unwilling to participate in studies that involved invasive procedures, such as a lumbar puncture (45.4%) and bone marrow biopsy (42.3%), but willing to participate in studies with less invasive procedures such as a computed tomography scan of the heart (86.8%), magnetic resonance imaging (87.4%), and skin allergy testing (86.8%). Although there was some variation by gender and region, the majority were willing to participate in studies with side effects like pain (80%) or nausea and vomiting (64%), but only a minority were willing to join if the research drug would result in their having a 1 in a million chance of death (34.4%), a small chance of kidney damage (16.7%), or influence how their mind works (23.2%; Figure 4).
Conclusions
Our results suggest that healthy volunteers are willing to participate in a wide range of types of phase 1 clinical trials, and express preferences for low risk and familiar studies and study procedures, preferences which are partially affected by offers of payment.
Keywords: Phase 1 clinical trials, research ethics, decision making, healthy volunteers, informed consent
Introduction
Healthy volunteers in phase 1 clinical trials contribute to the development of safe drugs and other biologics by accepting the possibility of risks from study participation without anticipated health benefits from the investigational products. The incidence of serious adverse events is low.1–3 Serious adverse events occasionally occur, however, as in the recent tragedy of the phase 1 clinical trial of BIA 10-2474 in France, in which a participant died and six were hospitalized.4 The literature on participation in phase 1 trials for healthy volunteers has focused primarily on the ethics of financial compensation,5,6 and whether participation disadvantages the poor7 and attracts unrepresentative cohorts.8 Evidence suggests that although there are often multiple motivations, healthy volunteers are primarily motivated by financial reward,9,10 and commentators worry that they are “people who need money and have a lot of time to spare: the unemployed, college students, contract workers, ex-cons, or young people living on the margins who have decided that testing drugs is better than punching a clock with the wage slaves.”11
An emerging empirical literature suggests that healthy volunteers often do indeed have low incomes and a high rate of unemployment.12, 13 Evidence also suggests that while healthy volunteers are primarily motivated by the offer of payment,14 they do pay attention to the risks associated with study interventions and potential side effects, as well as to the logistical burdens of participation.15–19 However, many previous studies are dated, focused on one geographic region, or have small sample sizes.17–19 Through a survey of a large cohort of participants in phase 1 Pfizer trials in the United States, Belgium and Singapore, we examine the socio-demographics and enrollment preferences of healthy volunteers. The current analysis probes further into research enrollment decision-making and describes how the type of study, study procedures, and possible side effects of study interventions affect healthy volunteers’ willingness to participate in research.
Methods
Study design
This analysis is part of a larger cross-sectional, descriptive and exploratory survey study of healthy volunteers in phase 1 drug development studies at Pfizer Clinical Research Units. The overall study purpose is to better characterize the socio-demographic characteristics, motivations, and decision making processes of healthy phase 1 clinical trial volunteers. Data were obtained through two surveys, one administered after consent for a research study but before the study began (entrance survey), and the second survey administered at the end of the study before discharge (exit survey). This paper focuses on questions in the exit survey that ask respondents about their willingness to participate in studies of different types of interventions, studies with certain procedures, and studies of drugs with particular side effects.
Study sample
The sample is healthy volunteers who are participants in a phase 1 trial at one of three Clinical Research Units run by Pfizer Inc. in New Haven, Connecticut USA, Brussels, Belgium and Singapore, Singapore between September 2009 and March 2011. An entrance survey was offered to those who completed an informed consent session for a phase 1 study. Participants who completed the phase 1 trial were invited to complete the exit survey prior to discharge. This analysis focuses on exit survey responses.
Survey instrument
The survey instruments were developed by the authors through an iterative process that included a comprehensive literature review, draft survey development, cognitive pre-testing, and final revisions. The surveys were pretested with 12 healthy volunteers participating in Vaccine Research Center studies at the U.S. National Institutes of Health and revised accordingly. Surveys in the United States and Singapore were administered in English. The same survey was translated into French and Flemish by the National Institutes of Health (NIH) Office of Research Services and reviewed for accuracy at the Brussels Clinical Research Unit. These were offered to Belgian participants. Certain demographic questions (e.g., income and education response categories) were adapted for each location. Survey questions were designed to determine study participants’ motivations, socio-demographic characteristics, and factors they consider in making research enrollment decisions, including how willing they are to participate in different studies. Paper and pencil questionnaires were self-administered in the respective Clinical Research Units. The exit survey took on average about 15 minutes to complete. Respondents were not compensated for completing the surveys. Completed surveys were sent without identifying information to the NIH for data entry and analysis.
For this analysis, participants were asked to indicate their willingness to participate in different types of research, research with different possible side effects, and research involving certain procedures. For five study types typical of phase 1 studies at Clinical Research Units with healthy volunteers, and a list of eight possible side effects (see Box 1), they were asked to indicate if they would be definitely willing, probably willing, probably not willing, or definitely not willing to participate in these studies. In response to a list of thirteen procedures that might be part of a research study (Box 1), they were asked to respond either: “I would not join the study”; “I would join if I was interested”; “I would join if I were offered enough money”; or “I am unsure whether I would join.” A subsequent question asked respondents whether more money; a very important study; a study with few total procedures; or a particular doctor performing the procedure would make them more willing overall to enroll in a study that included these procedures, would not change their willingness, or if they were not sure. Short explanations were provided for many of the trial types and procedures listed on the survey. The questions in this exploratory survey were meant to capture volunteers’ willingness to participate based on their understanding of each procedure or type of study.
Box 1. Types of studies, study procedures, and side effects listed in survey questions.
| Study types | Study procedures | Possible side effects of study drug |
|---|---|---|
| A First in Human study (a trial of a drug that has never before been tested in people) | A skin allergy test—a scratch in your forearm to test for allergies | You will have your head shaved |
| A marketed drug study (continued research on a drug that is already approved and being used by doctors) | A CT scan of your heart—a special kind of X-ray that takes many pictures | You have a chance of gaining some weight |
| A vaccine study (research on a new vaccine that has never been tested in people) | A lumbar puncture—putting a needle into your spinal cord to take out some spinal fluid | You will feel moderate pain for about an hour |
| A lifestyle intervention (a study of how improving your exercise and eating habits affects your health) | A biopsy of the skin on your arm—removing a pea-size piece of skin | You will feel like vomiting for about a day (and may actually vomit) |
| A study of a drug for psychiatric patients | A nerve and muscle test that involves 10 small electric shocks to your arm through a needle or a pad on the skin of your arm | You have a 1 in one million chance of dying |
| A PET scan of your brain—a brain scan after you get an injection of a radioactive sugar | You have a small chance of kidney damage | |
| An MRI—a scan that takes pictures of parts of your body using magnets instead of radiation | You will temporarily lose interest in sex | |
| Transcranial magnetic stimulation—when magnets are used to produce electric currents in your brain | You may experience side effects that affect the way your mind works | |
| An endoscopy—putting a tube down your throat (after numbing it) to look into your stomach | ||
| A bone marrow biopsy—putting a needle into your hip bone to withdraw some bone marrow fluid | ||
| Both a CT scan of your heart AND a lumbar puncture |
Data analysis / statistical methods
Data were entered into Excel and a random 10% double keyed to check for accuracy. Frequency distributions and simple descriptive statistics describe responses to the survey questions (see Box 1). Categorical data (e.g., response options, socio-demographic variables) were compared among groups by chi-square or Fisher’s exact tests. Ordered categories (e.g., willingness) were analyzed by the Kruskal-Wallis test. Continuous variables (e.g., age) were compared between groups by the t-test or Wilcoxon rank-sum test, as appropriate. The logistic regression analyses involved multivariable (for binary response variables) and multinomial (for polytomous response variables) models. Binary response variables were simplified to willing (definitely and probably) and not willing (probably not and definitely not); polytomous response options could not be simplified and were kept distinct (eg, would not join the study vs would join if interested vs would join if offered enough money vs unsure) for use in multinomial models.
Considering the wide range of subjects from distinct regions of the world, we explored the effects of socio-demographic characteristics on the responses of interest. Univariable, multivariable, and multinomial logistic regression models were used to assess, and adjust for, the effect of each socio-demographic characteristic on willingness to participate in studies with different types of interventions, procedures, and side effects. Socio-demographic characteristics that were analyzed included region, gender, income, education, employment, age, and previous research experience and these were considered a comprehensive set (to the extent reasonable) of explanatory variables for these culturally diverse cohorts. A p-value <0.05 and an odds ratio (OR) with 95% confidence intervals (CI) excluding 1.0 were considered statistically significant. Given the exploratory nature of the study, formal corrections were not carried out for multiple comparisons. Data were analyzed using SAS v9.2 (SAS Institute, Inc, Cary, NC).
Human subjects protection
This survey study was approved by the Combined NeuroScience Institutional Review Board at the National Institute of Health, the Erasme Ethics Committee (Comite d’Ethique Hospitalo-Facultaire Erasme-ULB) in Brussels, and the Parkway International Ethics Committee in Singapore. The front page of each survey included a written description of the nature and purpose of the study, a statement that participation was voluntary, that data would be anonymous, and that participants could choose not to complete the survey or answer certain questions without repercussions for their participation in the Pfizer phase 1 study.
Results
Sample population
Six hundred fifty four participants completed the exit survey (Of these, five hundred forty four (82.2%) had also completed the entrance survey). Respondents were predominantly male (86.9%) and single (68.0%) with an average age of about 35 years. Approximately two thirds had at least some college education (65.2%) and were employed full or part time (63.2%). About half reported an annual income equivalent to or more than $25,000 USD (52.1%). Most respondents (98.2%) reported excellent or good health and many (78.8%) reported previous clinical research experience (Table 1). Outside of Belgium, where all respondents were assumed to have public insurance, 48.0% in the United States and 29.5% in Singapore did not have health insurance. Socio-demographics are similar for the cohorts who completed both surveys and only the entrance survey, except those who completed both surveys were more likely to be Belgian (p=0.0185), Caucasian (p=0.0153), male (p=0.0040), slightly older (p=0.0074) and have previous research experience (p<0.0001).
Table 1.
Socio-demographic characteristics of survey respondents who completed the entrance only or both entrance and exit surveys
| Completed Only Entrance Survey (N=655) % (n) |
Completed Both Surveys (N=544) % (n) |
P-value | ||
|---|---|---|---|---|
| Gender | Female | 19.5% (120) | 13.1% (69) | 0.0040* |
| Male | 80.5% (496) | 86.9% (459) | ||
|
| ||||
| Age | Years, mean ± SD | 34.1 ± 9.8 | 35.5 ± 9.5 | 0.0074* |
| median (IQR) | 33.0 (25.0-42.0) | 35.0 (27.0-43.0) | ||
|
| ||||
| Educationa | High School or less | 35.9% (226) | 34.9% (184) | 0.7578 |
| Some College or more | 64.1% (404) | 65.2% (344) | ||
|
| ||||
| Employment | Employed | 57.5% (307) | 63.2% (281) | 0.0770 |
| Not employed | 42.5% (227) | 36.9% (164) | ||
|
| ||||
| Incomeb | <$ 25,000 | 47.0% (263) | 47.9% (230) | 0.8033 |
| >$25,000 | 53.0% (297) | 52.1% (250) | ||
|
| ||||
| Race | Asian | 27.1% (162) | 27.5% (138) | 0.0153* |
| Black or African | 25.8% (154) | 22.3% (112) | ||
| White or Caucasian | 36.6% (219) | 43.8 % (220) | ||
| Mixed or Other | 10.5% (63) | 6.4 % (32) | ||
|
| ||||
| Marital Status | Divorced | 9.4% (59) | 10.3% (54) | 0.6609 |
| Separated | 1.9% (12) | 1.7% (9) | ||
| Single | 71.1% (446) | 68.0% (355) | ||
| Married | 17.5% (110) | 19.9% (104) | ||
|
| ||||
| Type of Community | City | 59.7% (374) | 56.9% (300) | 0.6291 |
| Rural | 8.8% (55) | 10.6% (56) | ||
| Small town | 12.4% (78) | 13.7% (72) | ||
| Suburban | 19.1% (120) | 18.8% (99) | ||
|
| ||||
| Proximity from Study Site | Within 20 miles | 38.9% (243) | 35.2% (186) | 0.1995 |
| >20 miles | 61.1% (381) | 64.8% (342) | ||
|
| ||||
| Type of Healthcare Coveragec | Public/Government | 43.0% (195) | 37.0% (129) | 0.3268 |
| Private | 15.4% (71) | 16.7% (57) | ||
| Other | 6.4% (29) | 5.9% (20) | ||
| None/Out of pocket | 35.2% (160) | 40.5% (138) | ||
|
| ||||
| Health Status | Excellent | 68.2% (426) | 71.0% (370) | |
| Fair | 1.8% (11) | 0.6% (3) | 0.3656 | |
| Good | 30.1% (188) | 28.2% (147) | ||
| Poor | 0 | 0.2% (1) | ||
|
| ||||
| Participation in Previous Studies | None | 36.6% (218) | 21.2% (107) | <0.0001* |
| At least 1 | 63.4% (377) | 78.8% (398) | ||
|
| ||||
| Geographic Region | Belgium | 30.7% (201) | 37.3% (203) | 0.0185* |
| Singapore | 24.7% (162) | 25.6% (139) | ||
| US | 44.6% (292) | 37.1% (202) | ||
| Completed Exit Survey (n=654) | ||||
| Q: [Considering what I had to do,] I thought the amount of money was: | More than fair | 11.9% (76) | ||
| Fair/just about right | 71.1% (456) | |||
| Unfair/too low | 17.0% (109) | |||
| Q: Would you say that being in this research study was: | Better than I expected | 30.9% (198) | ||
| About the same | 65.2% (418) | |||
| Worse than I expected | 3.9% (25) | |||
For all data, percentages (%) are based on the total number of responses to each question as the denominator, which may vary. Age was not approximately normally distributed; thus, median and inter-quartile range (IQR) are reported in addition to mean and standard deviation (SD).
Levels of education were specific to each region (i.e., Belgium, Singapore, or the US); responses are reported according to US categories.
Income ranges were specific to each region’s respective currency (e.g., Euros in the Belgian questionnaire; Singapore $ in Singapore) and are reported in US categories.
Participants in Singapore and the U.S. (but not Belgium) were asked how they paid for their health care; participants were asked to select all that apply, so multiple options were applicable.
statistically significant at p<0.05
Exit survey respondents (n=654) reported that participating in the phase 1 study was at least as good as they had expected (96.1%) and that they thought the amount of money offered for the study was fair or more than fair (83.0%; Table 1).
Respondents in New Haven were participants in one of 22 Pfizer phase 1 trials being conducted during the survey period. These included imaging studies (4), single and multiple ascending dose studies (5), first-in-human drug studies (2), food effect studies (3), pharmacokinetic studies (2) drug-drug interaction studies (3) and bioavailability/bioequivalence studies (3). The studies involved dosing of medications or radiotracers (oral, subcutaneous, or intravenous), single and multiple period admissions, intravenous and arterial line placements, vital signs, venous and some arterial blood draws, electrocardiograms, telemetry, urine and stool samples, scans (including magnetic resonance imaging and positron emission tomography, and in one case, serial cerebrospinal fluid collection. Financial compensation for participation ranged from $1000 USD (for a no dosing methodology study) to $4650 USD (for a first in human intravenous dosing study with telemetry and electrocardiograms). The median amount offered was about $2700 USD.
Willingness to participate in studies testing different types of interventions
The majority of respondents said they were definitely or probably willing to enroll in five proposed types of research: 1) first-in-human testing of a drug; 2) further testing of a marketed drug; 3) first-in-human testing of a vaccine; 4) testing of a lifestyle intervention; and 5) testing of psychiatric drugs. There were some differences by study type. Nearly all were definitely or probably willing to participate in studies of marketed drugs (99.5%) and lifestyle intervention studies (97.2%), and substantially fewer in first-in-human drug studies (73.1%), studies of psychiatric drugs (70.7%), and first-in-human vaccine studies (65.2%; Figure 1).
Figure 1. Willingness to join studies of different types of interventions.
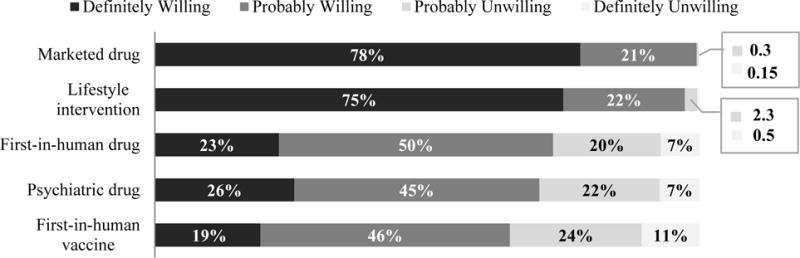
Controlling for socio-demographic variables, females indicated less willingness than males to participate in first-in-human drug studies (OR=0.36, 95% CI: 0.19-0.68) and vaccine studies (OR=0.37, 95% CI: 0.20-0.70). Those who earned less than $25,000 were more willing to participate in vaccine studies than those who earned more (OR=1.57, 95% CI: 1.04-2.38). Respondents from Singapore were more willing to participate in studies involving a psychiatric drug than those in the U.S. (OR=2.84, 95% CI: 1.54-5.23) or Belgium (OR=2.85, 95% CI: 1.51-5.36). No other socio-demographic characteristic or previous research experience affected willingness of respondents to participate in different types of studies.
Willingness to enroll in studies involving certain procedures
Responses about willingness to participate in studies involving a range of procedures (Box 1), showed that a substantial proportion were unwilling to participate in studies that involved invasive procedures, such as a lumbar puncture (45.4%), bone marrow biopsy (42.3%), and the combination of a computed tomography (CT) scan of heart and lumbar puncture (41.5%; Figure 2). In contrast, most respondents were willing to participate in less invasive procedures such as a computed tomography scan of the heart (86.8%), magnetic resonance imaging (87.4%), and skin allergy testing (86.8%; Figure 2).
Figure 2. Willingness to participate in studies with differentm procedures*.
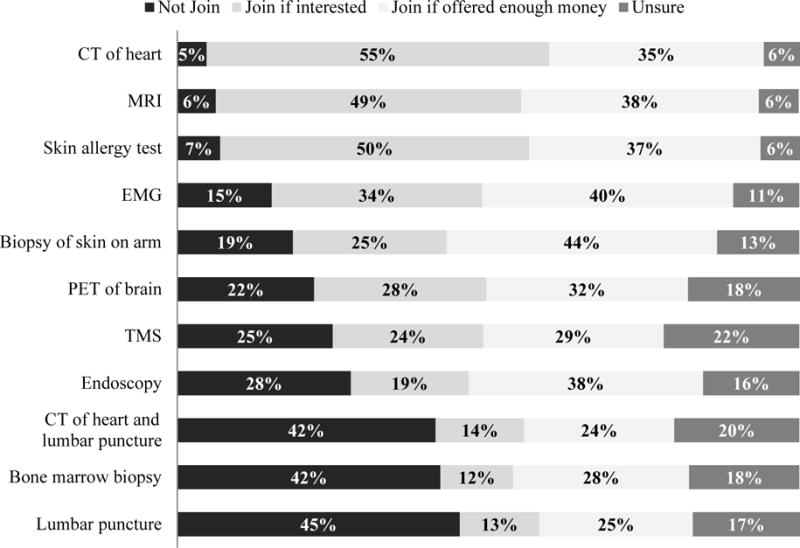
*Each procedure was described briefly in the survey. CT=Computerized Tomography; MRI=Magnetic Resonance Imagine; EMG= electromyogram; PET= positron emission tomography; TMS= Transcranial magnetic stimulation
A minority of participants (24.1-43.5% depending on the procedure) indicated that they would be willing to accept each procedure if enough money were offered (Figure 2). This group is heterogeneous with respect to socio-demographic characteristics. More respondents from Singapore would join if offered enough money for at least one procedure than respondents from the United States and Belgium (p=0.0130). In multinomial models adjusting for socio-demographic variables, willingness to participate in studies with various procedures varied by region and gender. Compared with respondents in the United States, respondents in Singapore were more willing to join if offered enough money than to not join for a study involving lumbar puncture (OR=2.47, 95% CI: 1.32-4.65); or a combination of lumbar puncture and computed tomography scan of the heart (OR=3.04, 95% CI: 1.58-5.86). Female respondents were less willing than males to join if interested than to not join a study that involved both a computed tomography scan of the heart and lumbar puncture (OR=0.21, 95% CI: 0.05-0.96). No socio-demographic variable was significantly associated with respondents’ willingness to participate in studies involving a bone marrow biopsy.
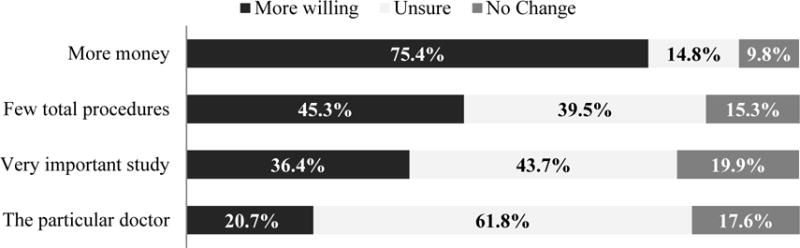
When asked if certain factors would increase their willingness to accept procedures overall, 75.4% of the respondents said more money would, 45.3% said fewer total procedures, 36.4% said a very important study, and 20.7% said a particular doctor performing the procedure would make them more willing to enter a study with any of the listed procedures (Figure 3). No socio-demographic variable was significantly associated with increased willingness when offered more money. Belgian participants indicated no change in willingness for a study with few total procedures (OR=4.23, 95% CI: 2.51-7.13) or if a particular doctor were performing the procedure (OR=2.36, 95% CI: 1.24-4.46) more often than US participants.
Figure 3. Factors that affect willingness to join studies with certain procedures.
Willingness to participate in studies with possible side effects
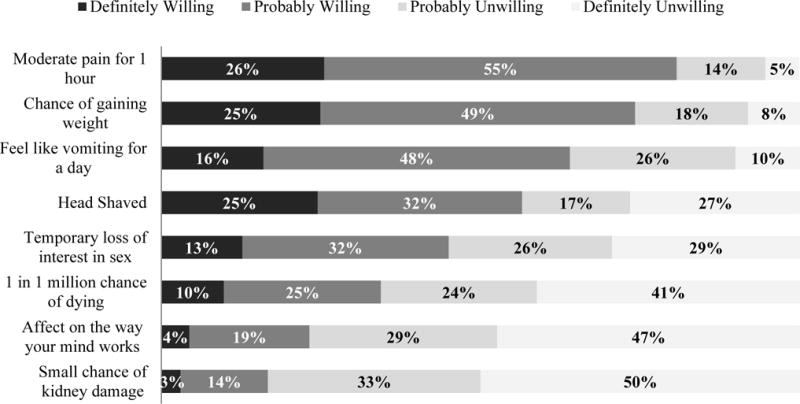
The majority was definitely or probably willing to join a study if the research drug being tested required the participant to shave his head (56.5%) or might result in weight gain (74.2%), moderate pain for an hour (80.8%), or vomiting for about a day (64.0%). Substantially fewer participants were willing to join a study if the research drug would result in their having a one in a million chance of death (34.4%), a small chance of kidney damage (16.7%), or influence how their mind works (23.2%; Figure 4).
Figure 4. Willingness to join studies of drugs with certain side effects.
In multivariable models adjusting for other socio-demographic variables, women were less willing than men to take a research drug if it required the participant to shave her head (OR=0.06, 95% CI: 0.02-0.14), might result in weight gain (OR=0.25, 95% CI: 0.13-0.47), or had a one in a million chance of death (OR=0.24, 95% CI: 0.10-0.57). Respondents in Belgium were more willing than those in the US to take a research drug if it required them to shave their heads (OR=1.84, 95% CI: 1.11-3.03), endure moderate pain for an hour (OR=3.80, 95% CI: 1.97-7.34), or resulted in vomiting for a day (OR=2.30, 95% CI: 1.42-3.72). No other socio-demographic characteristics were associated with willingness to take a research drug causing various side effects mentioned in the survey.
Discussion
This is the largest survey to date of healthy volunteers’ expressed preferences about participation in phase 1 clinical trials. Our data show that healthy volunteers are willing to participate in many kinds of phase 1 trials, as well as trials with diverse side effects and procedures. Three important findings emerge from our data: 1) Healthy volunteers report greater willingness to participate in trials that involve familiar and low risk procedures and interventions; 2) Willingness to participate in various types of trials or trials involving procedures or side effects did not vary in this cohort by income or previous research experience, but did vary by gender and region; and 3) Offers of money have a complicated role in reported willingness to participate.
First, in reporting willingness to participate, volunteers’ preferences were for familiar and low risk studies and procedures. Respondents were more willing to enroll in studies where the risks were known or thought to be low, such as studies of marketed drugs or lifestyle interventions, and less willing to join first-in-human drug or vaccine studies with uncertain risks, although more than half were still willing to join these latter types of studies. It is unclear, however, why respondents were less willing to participate in vaccine studies than any other kind, although aversion to vaccines has been noted previously. In a national survey during the swine flu pandemic in 2009, for example, a significantly higher number of people would be willing to accept an unapproved drug than an unapproved vaccine (54.4% v. 8.7%).20 In another survey of older Americans (≥50 years old), more respondents were willing to participate in a randomized-controlled trial of a new drug than in a randomized-controlled trial of a vaccine for Alzheimer’s disease (92% v. 55%).21 Misperceptions about vaccine safety and resultant vaccine hesitancy are also well documented.22, 23 Some individuals may be wary of vaccines because of misperceptions about risk, such as the perception that the vaccine may cause the disease24, 25 after “being exposed to the virus” inside.26
In this cohort, willingness to accept certain research procedures or side effects also reflects a preference for low risk and familiar procedures and interventions. Volunteers were least willing to enroll in studies with invasive procedures (e.g. lumbar puncture and bone marrow biopsy), or with severe and irreversible side effects (e.g. kidney damage and death). Respondents were also less willing to participate in studies in which the intervention might lead to effects on their minds. Perhaps potential effects on the mind are perceived as having more fundamental effects on respondents’ lives than physical effects, or maybe such effects are invisible and more frightening. Indeed, data show that the majority of Americans would not be willing to take psychiatric drugs even though they believe them to be effective.27 A noticeable proportion of individuals selected “unsure” for each procedure, with a higher proportion doing so for more invasive procedures. Perhaps those who chose “unsure” were leaning towards not willing to join, but did not want to commit to that answer. Nevertheless, perception of risk, regardless of its accuracy, appears to have influenced respondents’ selections about willingness to participate.
Familiarity also seems to factor into willingness to participate. For example, more respondents were willing to join a study with magnetic resonance imaging, computed tomography scans of the heart, and skin allergy tests if they were interested in the study than if they were offered enough money, and a relatively small proportion of respondents were “unsure” about these procedures. These procedures might be more familiar than others, perhaps offer something seen as potentially interesting or useful, and may not be considered as risky as some of the other procedures.
In addition, respondents reported that more money, fewer total procedures, the importance of a study, and the doctor performing the procedure would influence their willingness to participate in a given study. Money is an important factor in healthy volunteers’ decisions to participate in phase 1 clinical trials, thus it is not surprising that more money might influence their decisions. That 20.7% of respondents reported that the particular doctor performing the procedure would influence their willingness to participate in a study is, on the other hand, less expected. It would be interesting to explore reasons behind these responses. Perhaps a doctor with stronger credentials, or a doctor the respondent has seen before, is perceived to lower the risk associated with the procedures.
Second, in this cohort, those with research experience and those with the lowest income made choices about willingness to participate similar to other healthy volunteers in our cohort. Those willing to join a study if offered enough money reflect a range of socioeconomic characteristics that are on the whole similar to those who would not be willing. Importantly, however, in the entire cohort there is disproportionate representation of low income individuals, i.e. half reported annual household incomes equivalent to less than $25,000 USD, a level that falls below the national average in these three high-income countries.28–30 So, although willingness to join did not vary by income and previous experience within the cohort, those who participate in phase 1 research tend to be predominantly low income and frequently have previous research experience. Within this cohort, reported willingness to participate in particular studies did however vary by gender and region. Females reported less willingness to participate in many kinds of studies. Females also appear to be more risk averse than men and reported less willingness to participate in studies that might change their appearance, for example when the intervention requires that they shave their heads or might result in weight gain. Belgians appear more tolerant of non-serious side effects, such as having their heads shaved, and are less likely to change their willingness to accept study procedures for more money or for interest. Singapore respondents were more willing than those in the United States to agree to study procedures for money, despite the fact that Singaporean respondents were as a group better off socioeconomically than those respondents in the United States: 11.4% made more than $100,000 a year (vs. 1.3% in the US) and had lower average previous experience (2.7 ±4.2 studies vs. 5.7 ±17.2 in the US).
Third, our data suggest a complicated role for payment in preferences about study enrollment and willingness to enroll. Notably, only a minority reported their willingness to join a study with certain study related procedures if offered enough money, many said they would join if they were interested, and a non-trivial percentage said they were unsure. For the more invasive procedures such as spinal taps or bone marrow biopsies, more than 40% indicated they would not join, and a small percentage indicated that they would not join a study for each of the procedures. At the same time, the majority said their willingness might change if offered enough money. Although these responses are consistent with the idea that respondents are discriminating about their enrollment choices and that the offer of money is an important but not the sole consideration,31 it is not entirely clear how to interpret these responses. The majority in this cohort had previous research experience and their responses could reflect familiarity with usual payment amounts. Further research could probe the extent to which concerns about risks and invasiveness of procedures could be overridden by offers of money, and what amount of money (if any) would make someone willing to enroll in a study they otherwise would not be willing to join.
Our study had several limitations. First, the number of respondents who completed the exit survey was only about half of those who completed the entrance survey. This was the result of participants who chose not to participate in the phase 1 study, did not complete the phase 1 study, or declined to complete the exit survey. This could have introduced some selection bias as these groups may have had different views about willingness to participate in research than those who did complete the phase 1 study and the exit survey. Second, demographic information was collected in the entrance survey, limiting cross comparisons between demographics and tradeoff questions to those who completed both surveys. However, there were few socio-demographic differences between those who completed only the entrance survey and those who completed both, and responses on the exit survey were similar between those who did and did not complete the entrance survey, providing further reassurance that cross comparisons are representative. Third, surveys were self-administered in the Clinical Research Units, and the order of the questions was not randomized which might have introduced bias related to survey fatigue, “satisficing” or anchoring.32 Fourth, as this was an exploratory study, a large number of statistical tests were conducted. Hence the relationships reported should be interpreted with caution and subjected to replication in confirmatory studies. Finally, the overall number of female respondents is low, accounting for only 13% of the exit cohort, and some of their responses differ from those of the males.
Conclusion
These data from a large international cohort of healthy volunteers verify that healthy volunteers have clear preferences about the kinds of studies and study procedures that they are willing or not willing to enroll in. Indeed, healthy volunteers appear to prefer research studies and research involving certain procedures and side-effects based on risk and familiarity. Their preferences reflect considerations beyond financial incentives when deciding about enrollment in phase 1 clinical trials, including risks, invasiveness of procedures, reversibility of side effects, and concern about unknown risks. Further examination of the reasoning behind healthy volunteer choices would be useful, and may inform the informed consent process. Research to better understand why volunteers are less willing to join vaccine studies and studies of psychiatric drugs would be useful, as well as understanding their acceptance of computed tomography scans and magnetic resonance imaging. Clarification and education may be beneficial when preferences about studies or procedures are based on misinformation. Further exploration of the relationship between income or previous research experience and willingness to participate in certain studies, as well as differences in the willingness of women and persons in different countries would be useful, so that trials can appropriately tailor their recruitment methods and informed consent processes.
Acknowledgments
The authors acknowledge the valuable contributions of the survey participants; the research staff in New Haven, Brussels, and Singapore; and fellows and students at the CC NIH Department of Bioethics.
Funding
This research is supported by the Intramural Research Program at the National Institutes of Health Clinical Center.
Footnotes
Declaration of conflicting interests
Dr. Emanuel is represented by the Leigh Bureau as a frequent paid event speaker at numerous conventions, committee meetings and professional healthcare gatherings. He is also venture partner with Oak HC/FT and a Nuna stockholder. All other authors declare that there is no conflict of interest.
Disclaimer: The views expressed are the authors’ and do not represent the views or policies of Pfizer Inc., NIH, DHHS, or the US government.
References
- 1.Emanuel EJ, Bedarida G, Macci K, et al. Quantifying the risks of non-oncology phase I research in healthy volunteers: meta-analysis of phase I studies. BMJ. 2015;350:h3271. doi: 10.1136/bmj.h3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutfullin A, Kuhlmann J, Wensing G. Adverse events in volunteers participating in phase I clinical trials: a single-center five-year survey in 1,559 subjects. Int J Clin Pharmacol Ther. 2005;43:217–226. doi: 10.5414/cpp43217. [DOI] [PubMed] [Google Scholar]
- 3.Wensing G, Ighrayeb IA, Boix O, et al. The safety of healthy volunteers in First-in-Man trials - an analysis of studies conducted at the Bayer in-house ward from 2000 to 2005. Int J Clin Pharmacol Ther. 2010;48:563–570. doi: 10.5414/cpp48563. [DOI] [PubMed] [Google Scholar]
- 4.Butler D, Callaway E. Scientists in the dark after French clinical trial proves fatal. Nature. 2016;529:263–264. doi: 10.1038/nature.2016.19189. [DOI] [PubMed] [Google Scholar]
- 5.Lemmens T, Elliott C. Guinea pigs on the payroll: the ethics of paying research subjects. Account Res. 1999;7:3–20. doi: 10.1080/08989629908573939. [DOI] [PubMed] [Google Scholar]
- 6.Czarny MJ, Kass NE, Flexner C, et al. Payment to healthy volunteers in clinical research: the research subject’s perspective. Clin Pharmacol Ther. 2010;87:286–293. doi: 10.1038/clpt.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNeill P. Paying people to participate in research: why not? A response to Wilkinson and Moore. Bioethics. 1997;11:390–396. doi: 10.1111/1467-8519.00079. [DOI] [PubMed] [Google Scholar]
- 8.Tishler CL, Bartholomae S. Repeat participation among normal healthy research volunteers: professional guinea pigs in clinical trials? Perspect Biol Med. 2003;46:508–520. doi: 10.1353/pbm.2003.0094. [DOI] [PubMed] [Google Scholar]
- 9.Tishler CL, Bartholomae S. The recruitment of normal healthy volunteers: a review of the literature on the use of financial incentives. J Clin Pharmacol. 2002;42:365–375. [PubMed] [Google Scholar]
- 10.Stunkel L, Grady C. More than the money: A review of the literature examining healthy volunteer motivations. Contemp Clin Trials. 2011;32:342–352. doi: 10.1016/j.cct.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott C. Guinea-pigging: healthy human subjects for drug safety trials are in demand. But is it a living? New Yorker. 2008;7:36–41. [PubMed] [Google Scholar]
- 12.Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health. 2011;101:2217–2222. doi: 10.2105/AJPH.2011.300279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grady C, Bedarida G, Sinaii N, Gregorio MA, Emanuel EJ. Motivations, enrollment decisions, and sociodemographic characteristics of healthy volunteers in phase 1 research. Clin Trials. doi: 10.1177/1740774517722130. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida L, Azevedo B, Nunes T, et al. Why healthy subjects volunteer for phase I studies and how they perceive their participation? Eur J Clin Pharmacol. 2007;63:1085–1094. doi: 10.1007/s00228-007-0368-3. [DOI] [PubMed] [Google Scholar]
- 15.Hassar M, Pocelinko R, Weintraub M, et al. Free-living volunteer’s motivations and attitudes toward pharmacologic studies in man. Clin Pharmacol Ther. 1977;21:515–519. doi: 10.1002/cpt1977215515. [DOI] [PubMed] [Google Scholar]
- 16.Cunny KA, Miller HW. Participation in clinical drug studies: motivations and barriers. Clin Ther. 1994;16:273–282. [PubMed] [Google Scholar]
- 17.Novak E, Seckman CE, Stewart RD. Motivations for volunteering as research subjects. J Clin Pharmacol. 1977;17:365–371. [PubMed] [Google Scholar]
- 18.Vrhovac R, Francetic I, Rotim K. Drug trials on healthy volunteers in Yugoslavia. Int J Clin Pharmacol Ther Toxicol. 1990;28:375–379. [PubMed] [Google Scholar]
- 19.Mtunthama N, Malamba R, French N, et al. Malawians permit research bronchoscopy due to perceived need for healthcare. J Med Ethics. 2008;34:303–307. doi: 10.1136/jme.2007.020461. [DOI] [PubMed] [Google Scholar]
- 20.Quinn SC, Kumar S, Freimuth VS, et al. Public willingness to take a vaccine or drug under Emergency Use Authorization during the 2009 H1N1 pandemic. Biosecur Bioterror. 2009;7:275–290. doi: 10.1089/bsp.2009.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vries R, Ryan KA, Stanczyk A, et al. Public’s approach to surrogate consent for dementia research: cautious pragmatism. Am J Geriatr Psychiatry. 2013;21:364–372. doi: 10.1016/j.jagp.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freed GL, Clark SJ, Butchart AT, et al. Parental vaccine safety concerns in 2009. Pediatrics. 2010;125:654–659. doi: 10.1542/peds.2009-1962. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy A, Lavail K, Nowak G, et al. Confidence about vaccines in the United States: understanding parents’ perceptions. Health Aff. 2011;30:1151–1159. doi: 10.1377/hlthaff.2011.0396. [DOI] [PubMed] [Google Scholar]
- 24.Farquhar C, John-Stewart GC, John FN, et al. Pediatric HIV type 1 vaccine trial acceptability among mothers in Kenya. AIDS Res Hum Retroviruses. 2006;22:491–495. doi: 10.1089/aid.2006.22.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills E, Jadad AR, Ross C, et al. Systematic review of qualitative studies exploring parental beliefs and attitudes toward childhood vaccination identifies common barriers to vaccination. J Clin Epidemiol. 2005;58:1081–1088. doi: 10.1016/j.jclinepi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Newman PA, Duan N, Roberts KJ, et al. HIV vaccine trial participation among ethnic minority communities: barriers, motivators, and implications for recruitment. J Acquir Immune Defic Syndr. 2006;41:210–217. doi: 10.1097/01.qai.0000179454.93443.60. [DOI] [PubMed] [Google Scholar]
- 27.Croghan TW, Tomlin M, Pescosolido BA, et al. American attitudes toward and willingness to use psychiatric medications. J Nerv Ment Dis. 2003;191:166–174. doi: 10.1097/01.NMD.0000054933.52571.CA. [DOI] [PubMed] [Google Scholar]
- 28.US Census Table H-1. Table H-1. Income limits for each fifth and top 5 percent of all households: 1967 to 2015. https://www2.census.gov/programs-surveys/cps/tables/time-series/historical-income-households/h01ar.xls (Accessed 24 April 2017)
- 29.World Atlas, Economics. 25 highest income earning countries in the world. http://www.worldatlas.com/articles/the-highest-incomes-in-the-world.html (Accessed 24 April 2017)
- 30.The World Bank. GDP per capita in USD. http://data.worldbank.org/indicator/NY.GDP.PCAP.PP.CD?order=wbapi_data_value_2012+wbapi_data_value+wbapi_data_value-last&sort=descow (Accessed 24 April 2017)
- 31.Bentley JP, Thacker PG. The influence of risk and monetary payment on the research participation decision making process. J Med Ethics. 2004;30:293–298. doi: 10.1136/jme.2002.001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaeffer NC, Dykema J. Questions for surveys: current trends and future directions. Public Opin Q. 2011;75:909–961. doi: 10.1093/poq/nfr048. [DOI] [PMC free article] [PubMed] [Google Scholar]


