Abstract
Recent studies exploring the effects of surgical robots on teamwork are revealing challenges not reflected in clinical studies. This study is a sub analysis of observational data collected from 89 procedures utilising the da Vinci systems. Previous analyses had demonstrated interactions between flow disruptions and contextual factors. This study sought a more granular analysis to provide better insight for improvement. Raters sub-classified disruptions, based upon the original notes, grouped according to four operative phases (pre-robot; docking; surgeon on console; undocking; and finish). The need for repeated utterances; additional supplies retrieval; fogging or matter on the endoscope and procedure-specific training were particularly disruptive. Variations across phases reflect differing demands across the operative course. Combined qualitative and quantitative observational methodologies can identify otherwise undocumented sources of process variation and potential failure. Future observational frameworks should attempt to merge human reliability analysis, a priori modelling, and post hoc analyses of observational data.
Practioner Summary
Robotic surgery introduces new challenges into the operating room. Direct observation was used to classify and identify flow disruptions in order to diagnose problems in need of improvement. This technique complements other error prediction and system diagnostic methods which may not account for the complexity and transparency of health care.
Keywords: Health care ergonomics, teamwork, human–machine systems, complex systems, patient safety
Introduction
Despite a relatively swift uptake of robotic surgery for a wide variety of procedures, and some recognised adoption and performance challenges (Lucas, Pattison, and Sundaram 2012), until recently few studies had explored the teamwork, environmental and organisational demands of this new technology (Allers et al. 2016; Nyssen and Blavier 2015; Randell et al. 2016; Tiferes et al. 2016). Indeed, though human factors and ergonomics are growing considerations for medical device designers and regulators, the variability of use cases, environments, organisations, users, techniques and other devices make the wider systems implications of new technologies difficult to predict or understand holistically. Health care work systems are usually opaque, complex, highly variant, poorly defined and bear only cursory comparisons with other high-risk or high technology work systems such as aviation, despite continued comparisons (Kapur et al. 2016). One way to address the gap between what should happen in the operating room (OR) (‘work as imagined’) and what really happens in the OR (‘work as done’) (Xiao et al. 2010) is through direct observation of surgical and other clinical processes (Blandford et al. 2015; Guerlain et al. 2005; Gurses et al. 2012). In previous work, we used direct observation to explore some of the use problems, which we describe as surgical flow disruptions (FDs), associated with the da Vinci robotic surgery system, in relation to surgical context (Catchpole et al. 2015) and trainee skill development (Jain et al. 2016). In this sub-analysis of those data, we refine the original analysis, looking in detail at the sub-set of the data to provide more interpretive depth that will inform future approaches to the integration of the da Vinci and other robotic systems into surgical environments.
Successful robotic surgery requires surgeons to learn a new set of manual skills, which has been partially addressed through the introduction and increasing use of simulation (Abboudi et al. 2013; Kelly et al. 2012). However, the OR support staff – scrub nurses/techs, circulating nurses/‘runners’ – also require robot-specific skills such as docking the robot to the laparoscopic ports, changing instruments on the robot and even in turning around the OR for the next case. As the surgeon works at a console away from the operating table, this can degrade the shared understanding amongst the team members of the surgical progress and requirements that demands new approaches to teamwork, communication and situation awareness (Randell et al. 2016). Even the required size and layout of the room is altered by the size of the robot, associated control consoles and the data and power cables necessary for function (Ahmad et al. 2016; Allers et al. 2016). This means that a limited number of ORs in any surgical suite may be suitable for robotic surgery. Organisationally, it is necessary to manage the staff shift rosters to ensure team members with sufficient robotic skills are available and those skills are maintained and developed amongst OR staff. These broader systems implications and requirements for robotic surgery, combined with a general dearth of observational studies examining these effects, means that the specific requirements for successful robotic surgery are not well established and may not always be explicitly stated or understood.
Direct observation studies have helped to understand the complex interactions between individuals, tasks, technology, environment, workspace and organisation (Catchpole et al. 2007; Shouhed et al. 2012; Wiegmann et al. 2007), which can be linked to a variety of patient outcomes (Catchpole et al. 2006; de Leval et al. 2000; Wiegmann et al. 2007), and can be used diagnostically to understand where inefficiencies or accidents might arise and where improvements might be made(Allers et al. 2016; Henrickson et al. 2009). The measurement of surgical FDs is sensitive to different intraoperative technologies, surgical errors (Wiegmann et al. 2007), surgical experience (Catchpole et al. 2015; Tang et al. 2004) and the effectiveness of the supporting team (Mishra et al. 2008; Morgan et al. 2015a; Robertson et al. 2014). In essence, the small, irregular deviations from optimal care are artefacts of processes that provide a ‘window on to the system’ to understand where the resilience required for normal system function has momentarily failed, with recurrent problems suggesting particular fragility or mismatches between goals and work system configuration. Detailed analysis of these data affords diagnosis for feedback and system improvement (Morgan et al. 2015b), identifying opportunities to redesign tasks, training, working environment, organisational support and technologies to improve safety, efficiency, quality and patient outcomes. Given sufficient measurement rigour, these metrics can also be used to evaluate the strength and quality of interventions (Catchpole et al. 2014; McCulloch et al. 2017). Consequently, direct, prospective observation and systems analysis methods have demonstrated the value of looking deeper into complex, systems to identify threats to safety and to develop improvement initiatives before accidents occur. For robotic surgery, this raises the possibility of being able to identify strategies for advanced skill acquisition that reduce learning curves, improve teamwork, offer deeper insights into performance enhancements in robotic technologies, and thus reduce the expense of robotic surgery while improving the safety and quality of care.
In an initial analysis, we published direct observational data of FD in robotic surgery (Catchpole et al. 2015). Flow disruption rate varied with surgeon experience, training cases, surgical type, the model of robot and patient characteristics and were predominantly related to training (eg pauses due to inexperience either with the surgery or the robot), equipment (eg problems with sutures or camera fogging), communication (eg missed communications) and co-ordination problems (eg waiting for a team member to arrive). Variations of frequency and type across phases reflected the changing team, technology and training demands within an operation. This provided evidence of the range of contextual parameters that effect intraoperative performance, and initial indications as to where improvements might be made. Though the broad categories used in the observation–classification framework limited the conclusions about improvements that could be drawn, this had been anticipated in the study design. When each FD was counted, a short note detailing the nature of the observation was also recorded. This provided the ability to re-classify, sub-classify or explore the more detailed qualities of each FD, for a more granular understanding of the events and what they might suggest about the function and weaknesses of the system of work.
While most studies have focused on the statistical quantities of FDs, in this paper we study these more detailed notes, applying a more refined analysis to provide better insight into direct areas for focus and improvement. Taking the four most frequent FD categories in our previously explored robotic surgery data – equipment, communication, co-ordination and training – we sought to review and sub-classify these FDs in order to improve the level of detail in the classification, and thus improve system diagnostics.
Method
This was a sub-analysis of the results from 89 directly observed robotic surgeries, in multiple ORs, across several specialties (Urology, Gynaecology, Nephrology and Cardiac Surgery) using two different surgical robot types (the Intuitive Surgical da Vinci S and Si). The Si claims better imagery, touch screens and allows the connection of a second ‘training’ console, which provides dual surgeon operations. The observations were a convenience sample of 102 successive surgeries, with 13 removed due to incomplete data, conducted between June 2012 and July 2014 in a 968 bed non-profit tertiary care medical centre with approximately 500 robotic surgeries conducted per year. Institutional Review Board approval (Pro00028833) was obtained from Cedars-Sinai Medical Center to allow the direct observation of robotic surgeries for the purpose of identification and characterisation of FDs. In order to gather the most process data possible, a waiver of informed consent was granted by the IRB, provided patient identifiers (including outcomes) were not collected.
The observational data were separated into four surgical phases. Phase one started once the patient entered the OR (pre-robot); phase two began once the abdomen was insufflated (including robot docking); phase three started once the surgeon was on the robotic console (the main surgical intervention); and phase 4 began once the surgeon was off-console (including robot undocking). During each observation, the phase, description of the FD and the FD classification were recorded. Phase durations and patient and team co-variates were also recorded but were not used in the current analysis. FDs are defined as ‘deviations from the natural progression of an operation thereby potentially compromising safety or efficiency’ (Wiegmann et al. 2007). Data collection relies on the ability of experienced observers to identify deviations from the course of an operation, based on an understanding of the usual operative course, which observers obtained from watching at least ten previous surgeries. Observers were then trained to write a short note or description of the event that they observed for post hoc discussion, inter-rater comparisons and to ensure consistency of classification. This open-ended data collection method served as a detailed record of the events, and thus could also be used qualitatively for further analysis. After previous studies in a range of surgeries, (eg Catchpole et al. 2014; Henrickson et al. 2009) initial classification was based on nine categories – communication, coordination, external factors, training, equipment, environment, patient factors, surgeon decision-making and robotic instrument changes. Classification was performed at the time of observation and subsequently used in the analysis. Our previous analysis revealed that FDs were weighted to four of the nine categories: communication, co-ordination, equipment and training. The current analysis focused on the FD descriptions found within these four most frequent.
The new analysis followed a grounded theory approach to thematic analysis and common in HCI studies (Adams, Lunt, and Cairns 2008), composing new sub-classifications within the four most frequent FD categories of communication, coordination, equipment and training. First two researchers independently read through the first 200 FD descriptions of a given category and developed subcategories based upon reoccurring themes in the observational data. Once each researcher independently completed the task, the two together discussed, agreed and merged the categories and finalised a set of sub-categories, as well as definitions and examples for each subcategory. Two coders then independently sub-classified all FDs, within the given category, based upon the original notes describing each observation. Any discrepancies between the two raters were given to a third rater to independently arbitrate. These were not the same researchers who observed the operations, maintaining independence from the data collection process. Any remaining discrepancies were discussed and decided upon with arbitration from clinical human factors expertise (KC) and surgical expertise (JA and CS). Inter-rater reliability was calculated using Cohen’s κ to determine if there was appropriate agreement between the two raters. This found 9 sub-categories within the communication FD category, 12 within co-ordination, 14 within equipment and 10 within training (Table 1). Each sub-category was evaluated within the context of operative phase. The training sub-category, alone, was evaluated within the context of training or non-training cases.
Table 1.
Flow disruption definitions and examples.
| Category | Sub category | Example | Note as collected |
|---|---|---|---|
| Communication | Repeat information | Surgeon has to repeat a request to a nurse | Surgeon asks nurse to change flow to 1, repeats several times |
| Misunderstanding | Miscommunications on what tasks need to be done | Resident irrigates when surgeon wants suction | |
| Clarification | ST asks for clarification on the size of sutures needed | Nurse clarifies suture request with surgeon | |
| 2-rater (%) Agreement: κ = 0.763, p < 0.001 | Unacknowledged communication | Surgeon calls for lights to be adjusted several times. Request is never completed | Nurse complains that the lights are off multiple times, while she fixes gas problem but nobody turns on for her |
| Problems relating to microphone | Surgeon 1 says Surgeon 2 is echoing and unable to hear him | Microphone not on, scrub tech says it is off because of feedback issues | |
| External distraction | Resident unable to hear surgeon due to nurses talking | Anaesthesiologist asks Nurse to look in chart for creatinine, but Nurse on phone | |
| Pertinent discussion | Surgeons discuss bleed and suture | Surgeon tells circulating nurse that a vessel clamp has been removed for about a minute, forgot to call it out when he took it off | |
| Conflict | Surgeons argue about what equipment is needed | Surgeon tells staff to shut up | |
| Accommodation for noise | Surgeon 1 leaves console to say something to Surgeon 2 | Surgeon gets off console to ask about needle drivers with second surgeon | |
| Coordination | Equipment adjustment or reposition | Surgeon instruments because they are positioned improperly | Second surgeon wants instruments in left arm switched to right arm |
| Equipment movement | Nurse moves tower because it is too close to sterile robot arm | Nurse can’t get past equip to plug in machine or grab gloves, because path blocked | |
| Coordination around robot | Reposition other equipment to accommodate the robot | Reposition light, because it is in the way of the robot | |
| Accommodation for patient | Reposition patient’s leg because it is in the way of the robot arm | Patient`s legs repositioned and a monitor so the robot doesn’t hit while docking | |
| Accommodation for missing, inadequate, or depleted equipment | Gas runs out and the tank needs to be changed | CO2 gas ran out | |
| Retrieval of equipment | Surgeon needs indigo so nurse leaves room to retrieve it. | Surgeon needs dye, An doesnt have, Nurse leaves to get | |
| 2-rater (%) Agreement: κ = 0.637, p < 0.001 | Personnel support | Nurse cannot switch gas tanks so ST has to come in and help | Surgeon asks for patient vital update, Anaesthesiologist in restroom, Nurse answers vitals are okay |
| Personnel unavailable | Nurse is calling for ST. St is out of the room | ST calls for Nurse, but she’s gone | |
| Training support | Surgeon explains how much tissue to cauterise | Camera needs to be focused, Surgeon has to point nurse to where the settings are on the robot | |
| Waiting | Waiting for surgeon to arrive. All set and ready for surgery to start | Waiting on Surgeon to arrive, all set up and ready for surgery to start | |
| Human error | CN grabbed the wrong equipment for patient bed | Surgeon says ST put instruments in wrong side | |
| Troubleshooting | Surgeon has to stop and figure out what’s wrong with Bipolar. It is not plugged in | Surgeon says bovie still not working; ‘are we grounded’ | |
| Equipment | Visual problems | Had to clean camera because it fogged up | Camera blurry/foggy, Surgeon and Surgeon 2 can’t see |
| Awareness of robot arm | Robot arms hit bedside assist | Robot arms hitting each other | |
| Robot inoperative | Robot shows error message, ‘Recoverable fault’ | Robot shows error message ‘recoverable fault’ | |
| Equipment or Instrument Inoperative | Pedal for suction is not working properly | Suction device not working, nurse messes with it (3 mins) | |
| Inadequate equipment or instruments | Surgeon complains bipolar scissors are not cutting well. They are dull | Cutting suture, but scissors are really dull and takes several tries (out of robot). Second surgeon: ‘well those are sharp’ | |
| Human slip | Surgical team member accidently unplugs console | Tech accidentally unplugs console; replugs | |
| 2-rater (%) Agreement: κ = 0.633, p < 0.001 | Unfamiliarity with equipment | Surgical team member not sure how to adjust white balance on camera | Surgeon forgot how to focus camera |
| Robotic surgery limitations | Surgeon has to get off console to adjust robot | Surgeon says he can’t quite reach what he needs to with grasper | |
| Sutures | Surgeon breaks suture thread while suturing valve | Suture breaks; remove and replace | |
| Clips and port slips | Surgeon is trying to clip, but the clip is not holding; right port came out | Trocar popped out, had to rearrange and insert it again | |
| Insufflation problems | Pneumoperitoneum (CO2 pressurisation to create working space in the body cavity) is not holding pressure | Surgeon asks why pressure is so low, ‘are we leaking’ | |
| Sanitation | Instrument dropped on the floor and is now contaminated | Nurse touches sanitary suction. Has to go get a new one | |
| Training | Procedure specific | Surgeon explains to resident where to cut mesh | Surgeon telling scrub tech where to clip vas deferens, having to guide him a number of times says ‘just let me guide you in’ |
| Instrument verbal instruction | Surgeon explains how to best use monocular scissors | Surgeon ‘push hard and rotate straight’ for uterine manipulation | |
| Robot surgical instruction | Surgeon explains to resident suturing procedures | Surgeon taking over controls to show Surgeon 2 how to position the robot arms to help him dissect easier | |
| Robot technical instruction | Surgeon explains to resident how to position robot arms | Surgeon teaching Res how to move the robot arms to dock, moves over to Res side to demonstrate | |
| General Verbal Instruction | Surgeon describing how to approach something | Surgeon says to resident ‘do your side first, not mine’ | |
| 2-rater (%) Agreement: κ = 0.711, p < 0.001 | Anatomy discussion | Surgeon showing where to cut to stop bleeding and points out arteries | Surgeon describes structures to student and resident; tells Surgeon 2 to avoid ovary and structures around it; must be careful w/pt in reproductive years |
| Port placement | Surgeon explains to resident how to place trocar | Surgeon explains how to determine where to make trocar incisions; marks incision sites | |
| Position of anatomy | Surgeon instructs resident how to move the bowel out of the way | Surgeon leaves robot to instruct nurse on how to antevert the uterus | |
| Operating room arrangement | Surgeon instructs resident how to place face tray over patient | Surgeon explains to CN how to best position tower cart | |
| Halt action | Surgeon tells resident ‘stop, don’t do too much!’ | Surgeon says ‘stop stop, you’re holding it too far. hold it towards middle’ |
Results
Summary of overall results
In the 89 operations studied (45 Urology, 30 Gynecology, 10 Nephrology, 4 Cardiac), 50 of which were training cases (17 urology, 28 gynecology, 2 Nephrology, 3 Cardiac). A total of 4229 FDs were identified. The FD categories analysed here (communication, coordination, equipment and training) accounted for 3003 of these FDs (71% of the total), divided across 4 operative phases: Pre robot (339 FD), robot docking (406 FD), main surgical intervention (1964 FD) and procedure completion (293 FD). Co-ordination (890 FD) and equipment (880 FD) were the most frequently observed problems, with training (700) and communication (533) FDs less frequent but numerous nonetheless. This distribution also varied within each operative phase, with most communication (72%), equipment (76%), training (70%) and coordination (48%) FDs occurring in phase 3 (Figure 1). Proportionally, a greater number of coordination FDs occurred in phase 1, reflecting the operational demands of preparing for the subsequent surgical intervention. Results confirmed there was significantly good agreement (κ > .63) among the raters on all four categories for the subsequent sub-classifications (Table 1).
Figure 1.
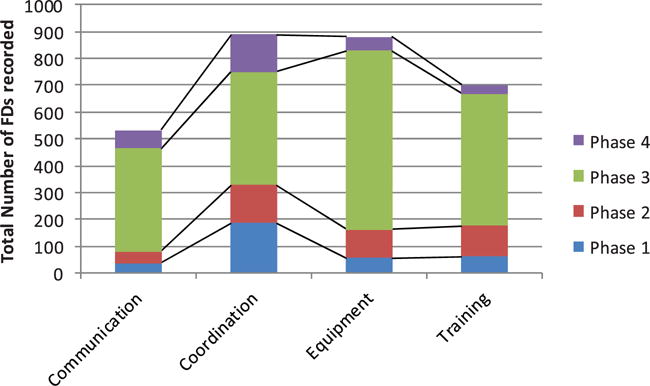
Flow disruption types by operative phase.
Communication
Communication FDs relate to instances where any miscommunication impacted surgical progress. These were sub-classified into nine sub-categories (Table 1). They were dominated by a consistent need for repeated utterances (Figure 2), occurring on average 3–4 times per operation. They were also proportionally consistent across the operation (Figure 3), accounting for approximately 60% of all communication problems regardless of the operative phase. This reflected the acoustic challenges of the OR – face masks which attenuate sound, reflective surfaces creating echoic sounds and masking sounds (alarms, monitors, instrumentation) – coupled with the particular challenges of robotic surgery, where the surgeon is separated from the rest of the team, and their voice is attenuated by the console which wraps around the surgeon’s head. This lead to a frequently observed behaviour where, upon not being heard the first time, the surgeon removed their head from the robotic console (breaking their concentration on the surgical task) in order to repeat their verbal message. While a microphone and speaker system has been deployed within the console design to address this problem, this did not always function appropriately and when it was not switched off, was responsible for other, equipment-related FDs.
Figure 2.
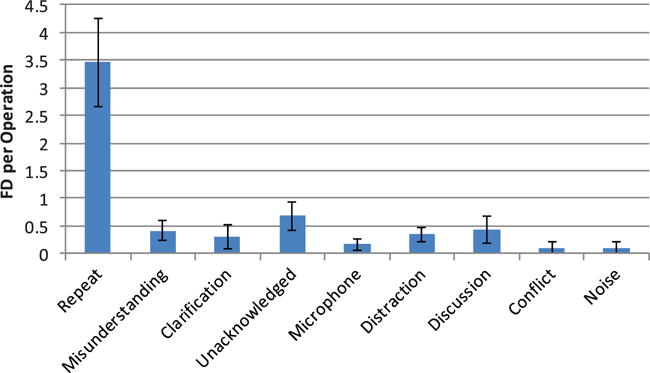
Average communication flow disruptions per operation.
Figure 3.
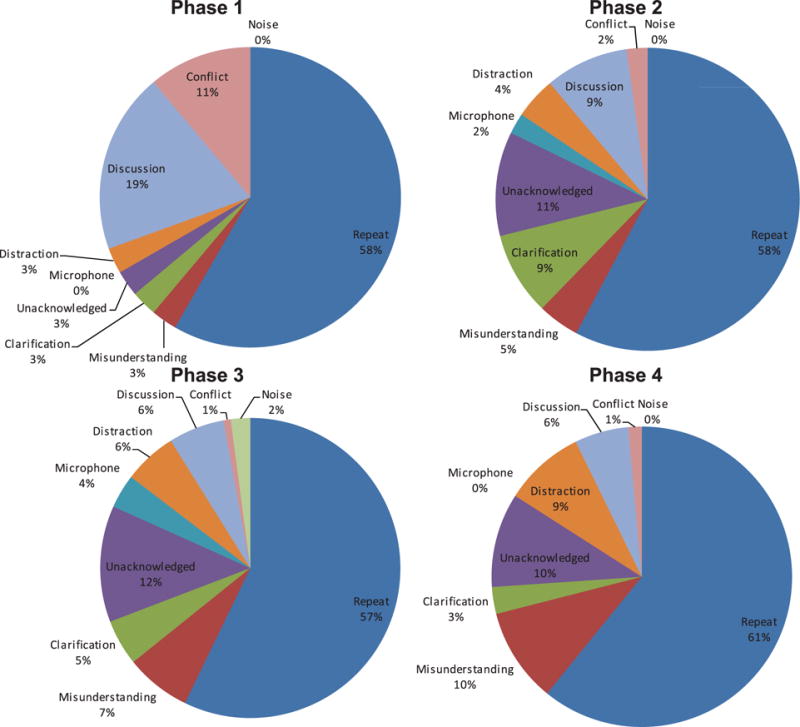
Distribution of communication flow disruptions across operative phases.
The next most frequent communication problem related to unacknowledged communications, which accounted for about 10% of the communication FDs in operative phases 2, 3 and 4. Given the problems of being heard in the OR, acknowledging team members might be important for catching missed communications but was not a well-practiced or reinforced behaviour. In contrast, in phase 1 conflict and discussion-related communication FDs were frequently experienced. This illustrates the agreements that need to be reached during the planning of the surgical approach; in essence, establishing a shared view of progress and requirements for the rest of the operation.
Co-ordination
Coordination-related FDs were instances where lapses in teamwork preparation or conduct affected surgical flow. Essentially, these are failures to have the right people, with the right things, in the right place, at the right time. Co-ordination FDs were classified into 12 sub-categories (Table 1). The need for supplies retrieval was the most frequent FD (Figure 4) and was generally consistent across all operative phases (Figure 5), accounting for 20–25% of the total number of coordination FDs. Requirements for supporting or teaching team members were more frequent in the later surgical phases, with non-attributed human errors (errors for which no other cause was apparent) following a similar pattern. This suggests that technical knowledge (and performance pressure) may be greater in phases 3 and 4 of the surgery, reflecting the high relative demands for clinical and process skills in the more technical parts of the operation. Coordination FDs related to waiting on staff were expected to be higher, since there is a well-known challenge with ensuring all staff, supplies and equipment are ready for the start of the case. However, waiting still accounts for approximately 10% of these FDs, and on average occurred at least once per case. In situations when supplies could not be retrieved, the need to accommodate problems with or a lack of, supplies was relatively consistent across cases. In a typical exchange, a surgeon would ask if an item is present, an OR team member would respond that this item was not available (perhaps after spending some time looking) and the surgeon would then take an alternative approach or strategy.
Figure 4.
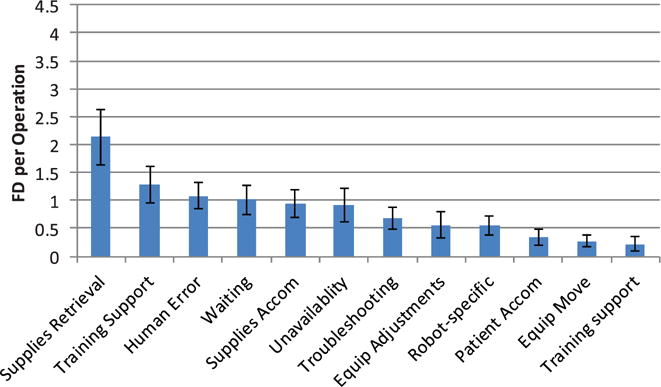
Average coordination flow disruptions per operation.
Figure 5.
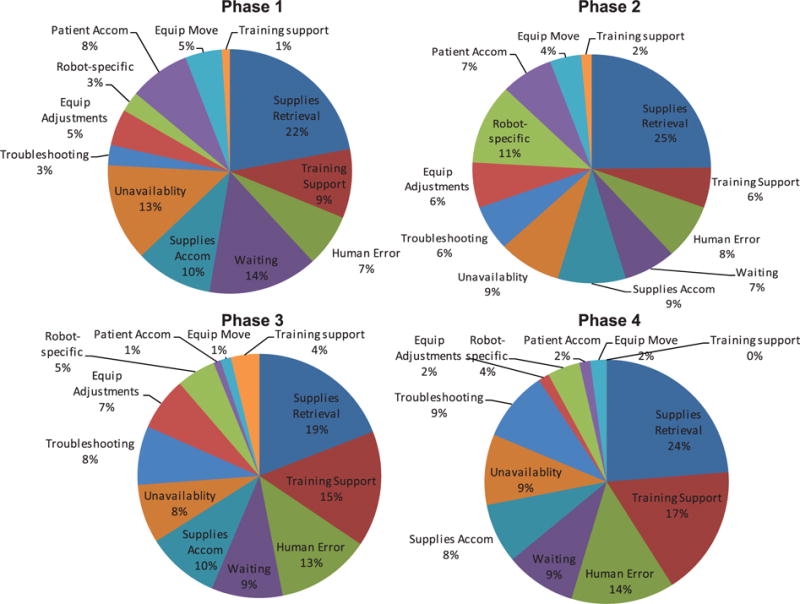
Distribution of coordination flow disruptions across operative phases.
Equipment
Equipment-related FDs are any equipment issue that affects the progress of the surgery. Overall, there was a considerable variation in equipment-related problems over the four phases (Figure 6), reflecting differences in equipment usage over the operative course. Visual problems – fogging, tissue or blood on the camera – was the most frequent problem (Figure 7), specifically while using the robot. Fogging occurs when water condenses on the lens in the warm, moist atmosphere inside the body, if not properly adjusted from the cool and dry OR environment. Blood, fat or other tissue may also adhere to the lens they touch or can be transferred from the camera port. Both situations require removal of the camera, which is wiped and re-inserted. Working in the tight confines of the lower abdomen requires care and skill on re-insertion to ensure the lens does not to pick up more adherent matter. Inoperative equipment – not switched on, not configured correctly or for another reason could not be operated correctly – is also a feature across phases 2, 3 and 4. Phase one is dominated by problems associated with equipment unfamiliarity. These disruptions appear in the absence of a surgeon or others experienced in the technical demands of room and equipment configuration for robotic surgery. Other less frequent but notable problems were: breaking sutures, which results from over-tension due to a lack of haptic feedback with the robot (and possibly, as staff reports suggested, the recent purchase of a lower quality suture material); problems with the robot arm, which on average occurred every case and usually resulting from a lack of awareness about the location of the arm (leading it to strike other team members or clash with other OR equipment), or simply that the robot was inoperative due to configuration or other unidentified problems.
Figure 6.
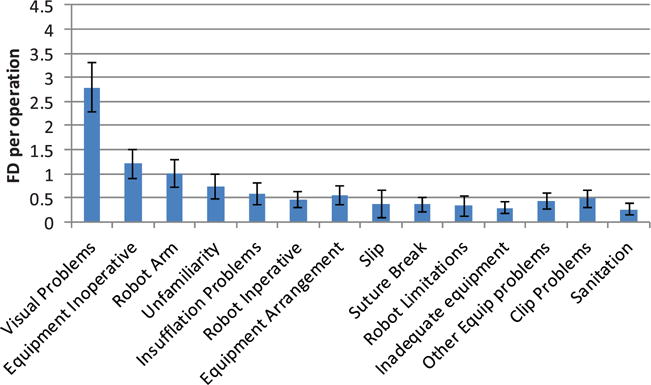
Equipment-related flow disruptions.
Figure 7.
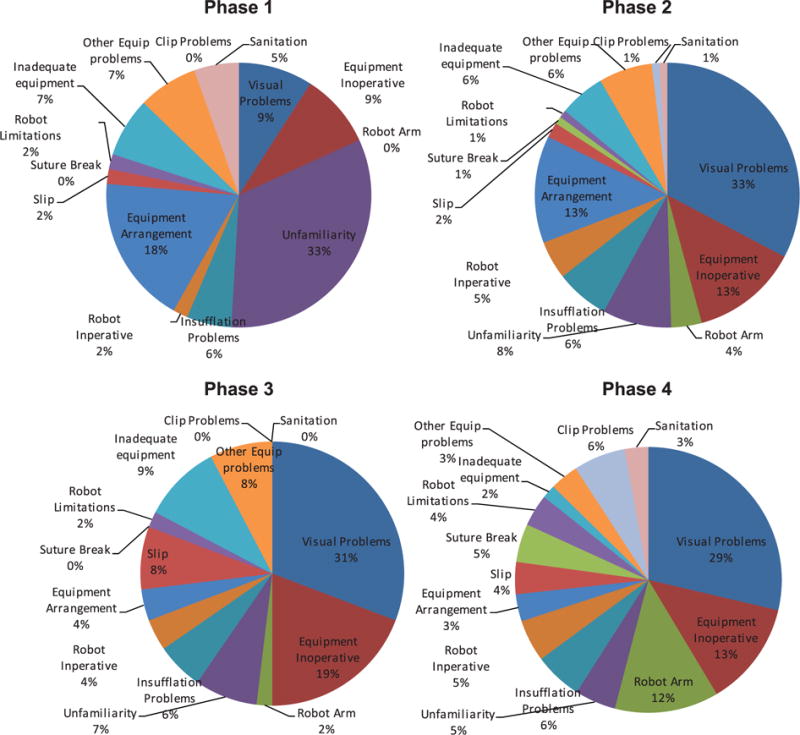
Equipment-related flow disruptions by operative phase.
Training
The training-related FDs – instruction by the attending surgeon to fellows, residents or medical students – were classified into 10 categories (Table 1). Most frequent were those relating specifically to the procedure, followed by instrument and specific robotic surgery instruction (Figure 8). There were also pauses to discuss anatomical issues – reflecting patient-specific issues and awareness of position inside the body. There was considerable variation across phases (Figure 9), illustrating the different skill demands and learning needs across the operation. Instrument preparation was most important in phase one, with technical aspects of robot docking and port placement in phase two. Procedure-specific instruction dominates in phases three and four, but also requires instrumentation and robotic instruction. Figure 10 shows the relative distribution between training and non-training cases. 89% of the training FDs occurred in the 50 training cases (56% of the 89 cases studied). Proportionally, halt actions and procedure-specific training FDs remain approximately the same, while training cases require a greater proportion of robotic and verbal instruction.
Figure 8.
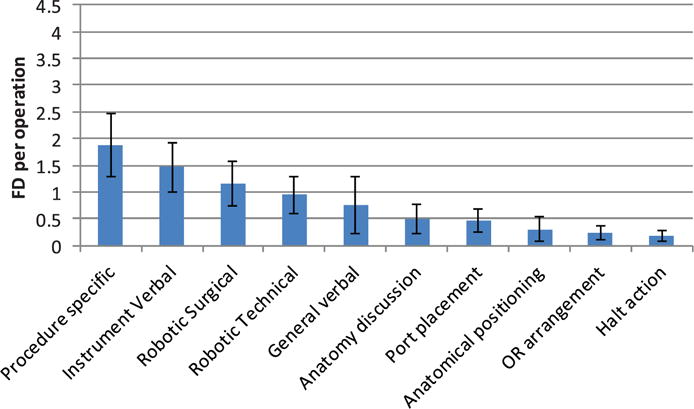
Training-related flow disruptions.
Figure 9.
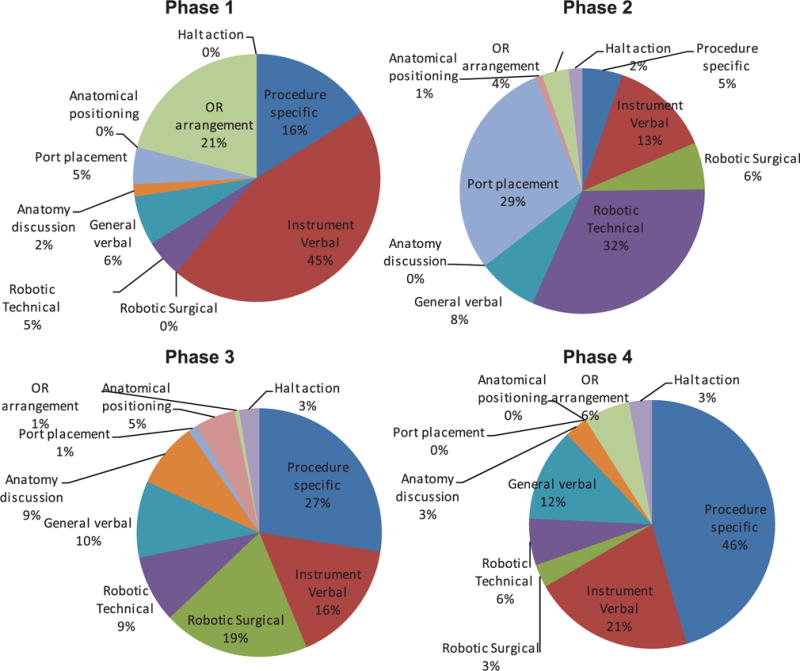
Training-related flow disruptions by operative phase.
Figure 10.
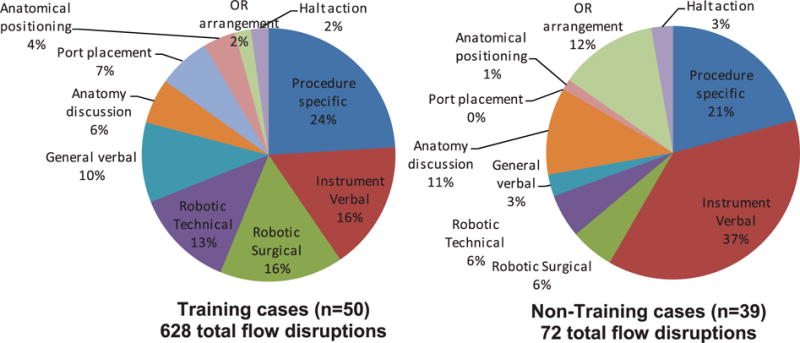
Training-related flow disruptions in training and non-training cases.
Discussion
We used the sub-categorisation of FDs to develop our findings from a previous analysis of these data to provide insight into system function, safe and efficient operation and variations in work demands across the different phases of the operation. Coordination FDs were dominated by the need to find equipment located outside of the OR. Sometimes this was because the needs for the surgery were not effectively planned or communicated prior to the need identified. Sometimes this was an adaptive clinical strategy (in response to patient anatomy, the course of the operation or other newly identified contextual factors) to address changes in the expected course of the operation. The storage of robotic surgical instrumentation and supplies outside the OR (due to the size, diversity and the need to use the OR for other operations) predisposed this problem, with the additional time for retrieval requiring the team to anticipate needs ahead of time to avoid disruptions. More generally, this illustrates the change in task and other demands associated with the introduction of new technology, beyond surgical skills. The complexity and task specificity of the equipment places increased demands on human/system interactions, in contrast to the traditional scalpel-and-suture approach, which is arguably more resilient as a consequence.
Equipment-related disruptions were predominantly related to visual problems, while on the console, specifically around improper insertion of the camera and a lack of warming facility to avoid fogging. This could be particularly disruptive and recurrent, requiring multiple manual lens cleanings. Even though this might only take 30s, and the robot automatically returned the camera to the previous location (unlike traditional laparoscopy, where the surgeon has to reposition the camera), it is nevertheless disruptive and the effects accumulate if repeated cleanings are required. These problems are also encountered in traditional laparoscopy, where the operation of the camera is manual and so allows more direct control, where a lens warmer (which avoids fogging) may be easier to use and where there is usually more space to manoeuvre the camera to avoid matter adherence. Similar approaches may be applicable to robotic surgery. More worryingly, there were a range of low-level technological failures that were difficult for teams (and the observers) to identify the causes of. These are not generally reported in analyses of robotic failures, which tend to focus on gross issues (Lucas, Pattison, and Sundaram 2012) and might not be easily identified through prospective hazard analysis. It is not immediately clear what would be required to resolve them.
Communication FDs were related to the need to repeat of information. Information repeat often occurred to compensate for a noisy OR, or due to problems with the microphone from the spatial separation of the surgeon from the operating table and the other team members. Team members continually monitored and supported each other, creating resilience and reliability through collaboration and coordination. Training FDs mostly occurred when a resident was present and gaining experience on the robot. In this respect, they may well be necessary, as they typically resulted from discussions related to the procedure, specifically, discussing how to carry out the surgical step with the robot. The operating team continually adapts to a range of contextual pressures including the patient, other team members (such as trainees), their shared expertise both with the equipment and with the procedure, a surgeon’s individual preferences and on-the-fly adaptations (such as anatomical differences or compensating for deficient supplies or equipment) required to complete the tasks.
As others have noted, though robotic surgery may have benefits, or at least be equivalent to more traditional laparoscopy (Deutsch et al. 2012; Paraiso et al. 2011), it also creates brittleness by relying on communication and coordination, which are already suspected as being a particular weakness of surgical delivery (Nyssen and Blavier 2015). It also changes roles, with the surgical assistant (usually a resident) and scrub techs, at least for part of the operation, becoming more like technicians (Randell et al. 2016). For example, their role is not to hand the surgeon an instrument, but to change equipment on the robot. The need to have an OR team skilled in robotic surgery requires the development of those specific skills and the management of schedules to ensure that staff skilled in robotic surgery are available to support the surgery. Within the highly fluid teams, production pressures and resource constraints of health care delivery, this is an additional challenge and expense that has seldom been discussed within the robotic surgery literature. Checklists, or teamwork training, both of which have proven extremely popular as interventions, have often used evaluations based on reductionist approaches that are insufficient to describe functioning and integration of the complex adaptive system of healthcare delivery (Catchpole and Russ 2015). Clearly, many of the problems here would not be amenable to these types of interventions, so we will be seeking to extend our observations to other robotic surgery centres, and to begin to work with the surgical teams on developing their own solutions to the challenges we have found. This is a preferable approach to ‘top-down’ implementation, especially as regards adoption and sustainability of health care interventions (Dixon-Woods, McNicol, and Martin 2012), where clinical and human factors/ergonomics experts working alongside quality improvement experts can be particularly valuable (Hignett et al. 2015). More suggested solutions can be found in Table 2.
Table 2.
Suggested solutions to most frequent flow disruptions.
| Disruption | Solutions |
|---|---|
| Repeat commands | Improve acoustics by reducing masking sounds and treating walls and ceiling with acoustically absorbent materials Improvements to microphone technology to allow the surgeon to communicate when their head is inside the console Training and emphasis on ‘read back’ to acknowledge commands |
| Supplies retrieval | Improve OR equipment storage Improve accessibility to equipment Improve process for bringing equipment into the OR Improve prediction of equipment required for case Improve communication regarding required equipment Use Lean-style stock management principles |
| Visual problems | Provide lens warmer for camera Improve camera insertion technique Improve camera control |
| Procedure specific & instrument training | Surgical cognitive task analysis Training needs analysis Provide improved resources for procedural learning & instrument training Develop simulation to include procedural learning & instrument training Require minimum knowledge and skills prior to participating in surgical robotics procedures |
By including a range of specialties and robot models we aimed to generate results that were generalisable and not specific to one team or type of surgery. However, the distribution of operations studied was not even across the specialties, nor was the distribution of training cases within each specialty. Layout in different ORs was not studied, and to our knowledge, did not have a substantial impact on FDs. However, this would be worthy of further study. In previous studies, we found differences between surgical types in phases 2 and 3, but not 1 and 4. In these studies we also found a small difference between S and Si models in phase four. We are currently exploring these factors in a further analysis.
This study demonstrates the deployment of a combined qualitative (descriptive) and quantitative (frequencies and rates) methodology, which was necessary given the substantial, but otherwise undocumented, sources of process variation and potential failure. This helps to identify the different sources of disruption, and in this case reveals that much of the disruption is weighted towards a relatively small number of major issues. The FDs do not capture all events, but nevertheless appear to capture a great many deviations from optimal. Some FDs can be more severe than others, which is challenging to quantify, and requires the application of a linear model to a stochastic system – for example, a phone call in one context can have little impact; but in another might prove catastrophic. Some disruptions are resolved immediately; some are never resolved; some reappear or are compensated for but add additional cognitive load that may be difficult to detect; and some lead to new disruptions. In comparison to similar studies conducted in other types of surgery, communication and coordination FDs are similarly frequent, while equipment FDs are far more prevalent in robotic surgery than have been found in other types of surgery (eg cardiac, trauma, laparoscopic and vascular) less dependent on technology (Catchpole et al. 2007, 2014; McCulloch et al. 2009). While the relationship between FDs and accidents is not clear, in high-risk surgeries a concatenation effect has been noted. The cognitive demands required to address the FD predisposes to errors, which can lead to further FDs and further erosion of cognitive resources, leading to a further downward spiral. Alternatively, the co-incidence of multiple FDs with other critical moments can create more serious situations. In congenital heart surgery, these small, seemingly innocuous problems that were not compensated for lead to effects on mortality and morbidity (de Leval et al. 2000). We are examining this concatenation or ‘snowball’ effect in further analyses across a number of surgeries using FD data.
Future work also aims to investigate the relationship between FDs and the probability of one FD leading to additional FDs in the context of robotic surgery, and will continue to explore the potential for more sophisticated deterministic descriptions of the relationship between patient factors, clinical (technical/process) expertise, training, external performance shaping factors and the process, operative duration and patient outcome. This also builds on the evidence base that human factors scientists are familiar with, but which is less well understood in the healthcare profession, that technology can affect a complex range of human–system interactions and cannot simply be substituted into a system of care. Here, we have found evidence of a range of task, teamwork, environmental and organisational affects related to robotic surgery that should form part of a human factors integration plan. Unfortunately, and despite increasing awareness in the health care device industry of some aspects of user-centred design, these considerations are rarely included in the procurement or implementation of new technologies in healthcare. There is yet much work to do.
Through a sub analysis of existing observational data of FDs in robotic surgery, we have found a number of recurrent mismatches between work demands and the ability of humans within the system to address them. We have found a frequent need to pause surgery for repeated communications, obscured video imagery, unavailable or non-functioning equipment and supplies or for teaching purposes. We have also elucidated how direct observation offers a number of advantages over human reliability analysis and other a priori models of process. This contributes to a growing literature seeking to understand the challenges faced by health care practitioners working at the sharp end of care, how blunt-end parameters affect performance and how we might learn from and address those challenges in more sophisticated ways than is the norm in the health care industry.
Acknowledgments
Our sincere thanks to all the surgeons, OR staff and residents who participated and allowed us to observe their operations.
Funding
This work was supported by National Institute of Biomedical Imaging & Biomedical Engineering [grant number R03EB017447] (Catchpole/Anger) and the UCLA Medical Student Training in Aging Research Program- the National Institute on Aging [grant number T35AG026736], the John A. Hartford Foundation, and the Lillian R. Gleitsman Foundation.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abboudi H, Khan MS, Aboumarzouk O, Guru KA, Challacombe B, Dasgupta P, Ahmed K. Current Status of Validation for Robotic Surgery Simulators: A Systematic Review. BJU International. 2013;111(2):194–205. doi: 10.1111/j.1464-410X.2012.11270.x. [DOI] [PubMed] [Google Scholar]
- Adams A, Lunt P, Cairns P. A Qualitative Approach to HCI Research. In: Cairns P, Cox A, editors. Research Methods for Human-Computer Interaction. Cambridge, UK: Cambridge University Press; 2008. pp. 138–157. [Google Scholar]
- Ahmad N, Hussein AA, Cavuoto L, Sharif M, Allers JC, Hinata N, Guru KA. Ambulatory Movements, Team Dynamics and Interactions during Robot-assisted Surgery. BJU International. 2016;118(1):132–9. doi: 10.1111/bju.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers JC, Hussein AA, Ahmad N, Cavuoto L, Wing JF, Hayes RM, Guru KA. Evaluation and Impact of Workflow Interruptions during Robot-assisted Surgery. Urology. 2016;92:33–7. doi: 10.1016/j.urology.2016.02.040. [DOI] [PubMed] [Google Scholar]
- Blandford A, Berndt E, Catchpole K, Furniss D, Mayer A, Mentis H, Randell R. Strategies for Conducting Situated Studies of Technology Use in Hospitals. Cognition Technology & Work. 2015;17(4):489–502. doi: 10.1007/s10111-014-0318-7. [DOI] [Google Scholar]
- Catchpole K, Russ S. The Problem with Checklists. BMJ Quality & Safety. 2015;24(9):545–549. doi: 10.1136/bmjqs-2015-004431. [DOI] [PubMed] [Google Scholar]
- Catchpole KR, Giddings AE, de Leval MR, Peek GJ, Godden PJ, Utley M, Dale T. Identification of Systems Failures in Successful Paediatric Cardiac Surgery. Ergonomics. 2006;49(5–6):567–588. doi: 10.1080/00140130600568865. [DOI] [PubMed] [Google Scholar]
- Catchpole KR, Giddings AE, Wilkinson M, Hirst G, Dale T, de Leval MR. Improving Patient Safety by Identifying Latent Failures in Successful Operations. Surgery. 2007;142(1):102–110. doi: 10.1016/j.surg.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Catchpole K, Ley E, Wiegmann D, Blaha J, Shouhed D, Gangi A, Gewertz B. A Human Factors Subsystems Approach to Trauma Care. JAMA Surgery. 2014;149(9):962–968. doi: 10.1001/jamasurg.2014.1208. [DOI] [PubMed] [Google Scholar]
- Catchpole K, Perkins C, Bresee C, Solnik MJ, Sherman B, Fritch J, Anger JT. Safety, Efficiency and Learning Curves in Robotic Surgery: A Human Factors Analysis. Surgical Endoscopy. 2015;30(9):3749–61. doi: 10.1007/s00464-015-4671-2. [DOI] [PubMed] [Google Scholar]
- Deutsch GB, Sathyanarayana SA, Gunabushanam V, Mishra N, Rubach E, Zemon H, DeNoto G., III Robotic Vs. Laparoscopic Colorectal Surgery: An Institutional Experience. Surgical Endoscopy and Other Interventional Techniques. 2012;26(4):956–963. doi: 10.1007/s00464-011-1977-6. [DOI] [PubMed] [Google Scholar]
- Dixon-Woods M, McNicol S, Martin G. Ten Challenges in Improving Quality in Healthcare: Lessons from the Health Foundation’s Programme Evaluations and Relevant Literature. BMJ Quality & Safety. 2012;21(10):876–884. doi: 10.1136/bmjqs-2011-000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerlain S, Adams RB, Turrentine FB, Shin T, Guo H, Collins SR, Calland JF. Assessing Team Performance in the Operating Room: Development and Use of a ‘Black-box’ Recorder and Other Tools for the Intraoperative Environment. The Journal of the American College of Surgeons. 2005;200(1):29–37. doi: 10.1016/j.jamcollsurg.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Gurses AP, Kim G, Martinez EA, Marsteller J, Bauer L, Lubomski LH, Thompson D. Identifying and Categorising Patient Safety Hazards in Cardiovascular Operating Rooms Using an Interdisciplinary Approach: A Multisite Study. BMJ Quality & Safety. 2012;21(10):810–818. doi: 10.1136/bmjqs-2011-000625. [DOI] [PubMed] [Google Scholar]
- Henrickson SE, Wadhera RK, ElBardissi AW, Wiegmann DA, Sundt TM. Development and Pilot Evaluation of a Preoperative Briefing Protocol for Cardiovascular Surgery. Journal of the American College of Surgeons. 2009;208(6):1115–1123. doi: 10.1016/j.jamcollsurg.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hignett S, Jones EL, Miller D, Wolf L, Modi C, Shahzad MW, Catchpole K. Human Factors and Ergonomics and Quality Improvement Science: Integrating Approaches for Safety in Healthcare. BMJ Quality & Safety. 2015;24(4):250–254. doi: 10.1136/bmjqs-2014-003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Fry BT, Hess LW, Anger JT, Gewertz BL, Catchpole K. Barriers to Efficiency in Robotic Surgery: The Resident Effect. Journal of Surgical Research. 2016;205(2):296–304. doi: 10.1016/j.jss.2016.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N, Parand A, Soukup T, Reader T, Sevdalis N. Aviation and Healthcare: A Comparative Review with Implications for Patient Safety. JRSM Open. 2016;7(1):2054270415616548. doi: 10.1177/2054270415616548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DC, Margules AC, Kundavaram CR, Narins H, Gomella LG, Trabulsi EJ, Lallas CD. Face, Content, and Construct Validation of the Da Vinci Skills Simulator. Urology. 2012;79(5):1068–1072. doi: 10.1016/j.urology.2012.01.028. [DOI] [PubMed] [Google Scholar]
- de Leval MR, Carthey J, Wright DJ, Reason JT. Human Factors and Cardiac Surgery: A Multicenter Study. Journal of Thoracic and Cardiovascular Surgery. 2000;119(4):661–672. doi: 10.1016/S0022-5223(00)70006-7. [DOI] [PubMed] [Google Scholar]
- Lucas SM, Pattison EA, Sundaram CP. Global Robotic Experience and the Type of Surgical System Impact the Types of Robotic Malfunctions and Their Clinical Consequences: An FDA MAUDE Review. BJU International. 2012;109(8):1222–1227. doi: 10.1111/j.1464-410X.2011.10692.x. discussion 1227. [DOI] [PubMed] [Google Scholar]
- McCulloch P, Morgan L, New S, Catchpole K, Roberston E, Hadi M, Griffin D. Combining Systems and Teamwork Approaches to Enhance the Effectiveness of Safety Improvement Interventions in Surgery: The Safer Delivery of Surgical Services (S3) Program. The Annals of Surgery. 2017;265(1):90–96. doi: 10.1097/SLA.0000000000001589. [DOI] [PubMed] [Google Scholar]
- Mishra A, Catchpole K, Dale T, McCulloch P. The Influence of Non-Technical Performance on Technical Outcome in Laparoscopic Cholecystectomy. Surgical Endoscopy and Other Interventional Techniques. 2008;22(1):68–73. doi: 10.1007/s00464-007-9346-1. [DOI] [PubMed] [Google Scholar]
- Morgan L, Hadi M, Pickering S, Robertson E, Griffin D, Collins G, New S. The Effect of Teamwork Training on Team Performance and Clinical Outcome in Elective Orthopaedic Surgery: A Controlled Interrupted Time Series Study. BMJ Open. 2015a;5(4):e006216. doi: 10.1136/bmjopen-2014-006216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan L, Pickering SP, Hadi M, Robertson E, New S, Griffin D, McCulloch P. A Combined Teamwork Training and Work Standardisation Intervention in Operating Theatres: Controlled Interrupted Time Series Study. BMJ Quality & Safety. 2015b;24(2):111–119. doi: 10.1136/bmjqs-2014-003204. [DOI] [PubMed] [Google Scholar]
- Nyssen AS, Blavier A. Investigating Expertise, Flexibility and Resilience in Socio-technical Environments: A Case Study in Robotic Surgery. In: Hollnagel E, Braithwaite J, Wears RL, editors. Resilient Health Care. Farnham: Ashgate; 2015. pp. 97–100. [Google Scholar]
- Paraiso MFR, Jelovsek JE, Frick A, Chen CCG, Barber MD. Laparoscopic Compared with Robotic Sacrocolpopexy for Vaginal Prolapse: A Randomized Controlled Trial. Obstetrics & Gynecology. 2011;118(5):1005–1013. doi: 10.1097/AOG.0b013e318231537c. [DOI] [PubMed] [Google Scholar]
- Randell R, Honey S, Alvarado N, Pearman A, Greenhalgh J, Long A, Dowding D. Embedding Robotic Surgery into Routine Practice and Impacts on Communication and Decision Making: A Review of the Experience of Surgical Teams. Cognition, Technology & Work. 2016;18(2):423–437. doi: 10.1007/s10111-016-0368-0. [DOI] [Google Scholar]
- Robertson ER, Hadi M, Morgan LJ, Pickering SP, Collins G, New S, Catchpole KC. Oxford NOTECHS II: A Modified Theatre Team Non-technical Skills Scoring System. Plos One. 2014;9(3):e90320. doi: 10.1371/journal.pone.0090320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouhed D, Catchpole K, Ley EJ, Blaha J, Blocker RC, Duff S, Wiegmann D. Flow Disruptions during Trauma Care. Journal of the American College of Surgeons. 2012;215(3):S99–S100. [Google Scholar]
- Tang B, Hanna GB, Bax NM, Cuschieri A. Analysis of Technical Surgical Errors during Initial Experience of Laparoscopic Pyloromyotomy by a Group of Dutch Pediatric Surgeons. Surgical Endoscopy. 2004;18(12):1716–1720. doi: 10.1007/s00464-004-8100-1. [DOI] [PubMed] [Google Scholar]
- Tiferes J, Hussein AA, Bisantz A, Kozlowski JD, Sharif MA, Winder NM, Guru KA. The Loud Surgeon behind the Console: Understanding Team Activities during Robot-Assisted Surgery. Journal of Surgical Education. 2016;73(3):504–512. doi: 10.1016/j.jsurg.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Wiegmann DA, ElBardissi AW, Dearani JA, Daly RC, Sundt TM. Disruptions in Surgical Flow and Their Relationship to Surgical Errors: An Exploratory Investigation. Surgery. 2007;142(5):658–665. doi: 10.1016/j.surg.2007.07.034. [DOI] [PubMed] [Google Scholar]
- Xiao T, Sanderson P, Clayton S, Venkatesh B. The ETTO Principle and Organisational Strategies: A Field Study of ICU Bed and Staff Management. Cognition Technology & Work. 2010;12(2):143–152. doi: 10.1007/s10111-010-0147-2. [DOI] [Google Scholar]


